Preparation and Characterization of Mono- and Biphasic Ca1−xAgxHPO4·nH2O Compounds for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
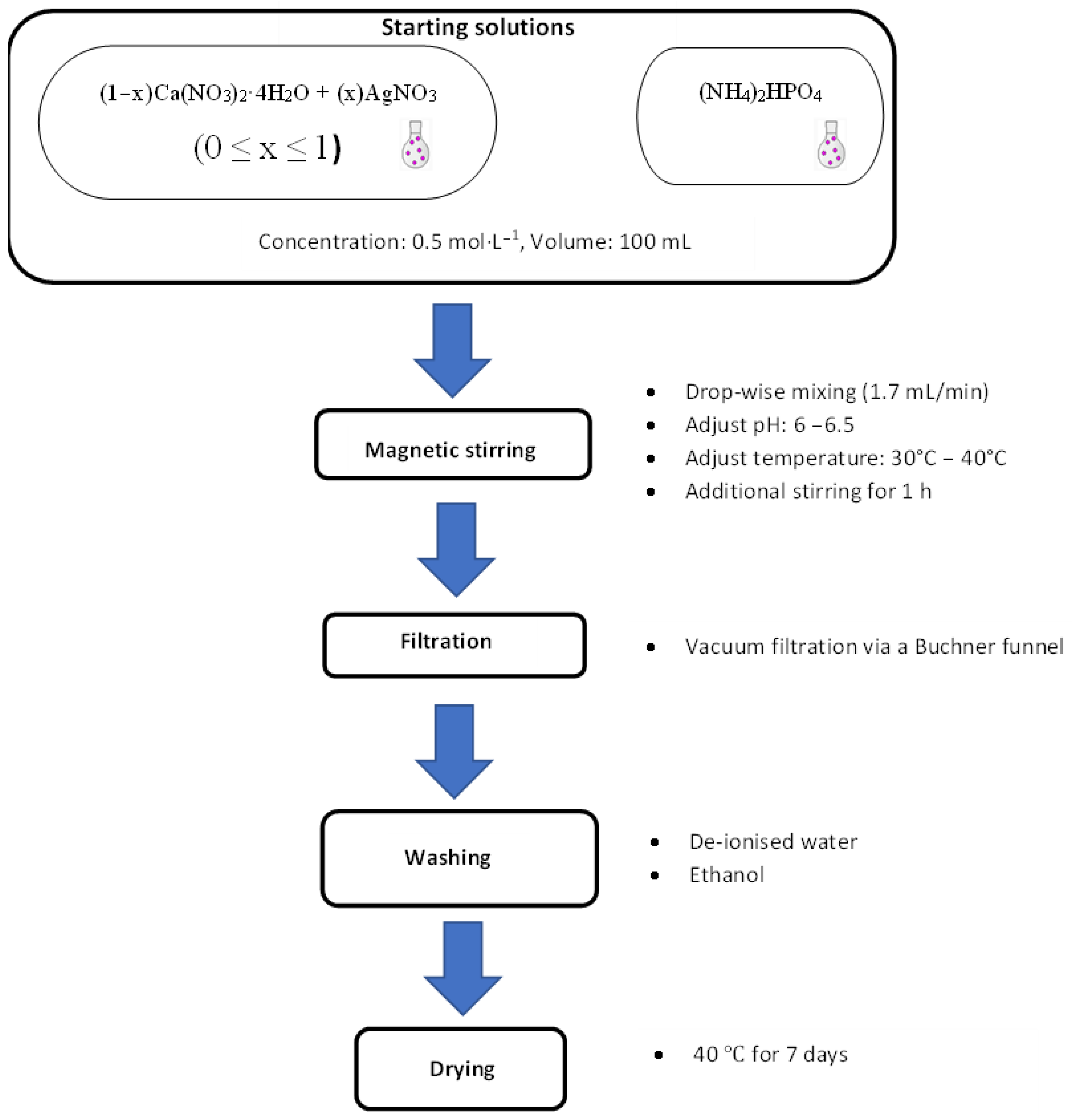
2.2. Synthesis of Ca1−xAgxHPO4·nH2O Compounds
2.3. Characterization Techniques
3. Results and Discussion
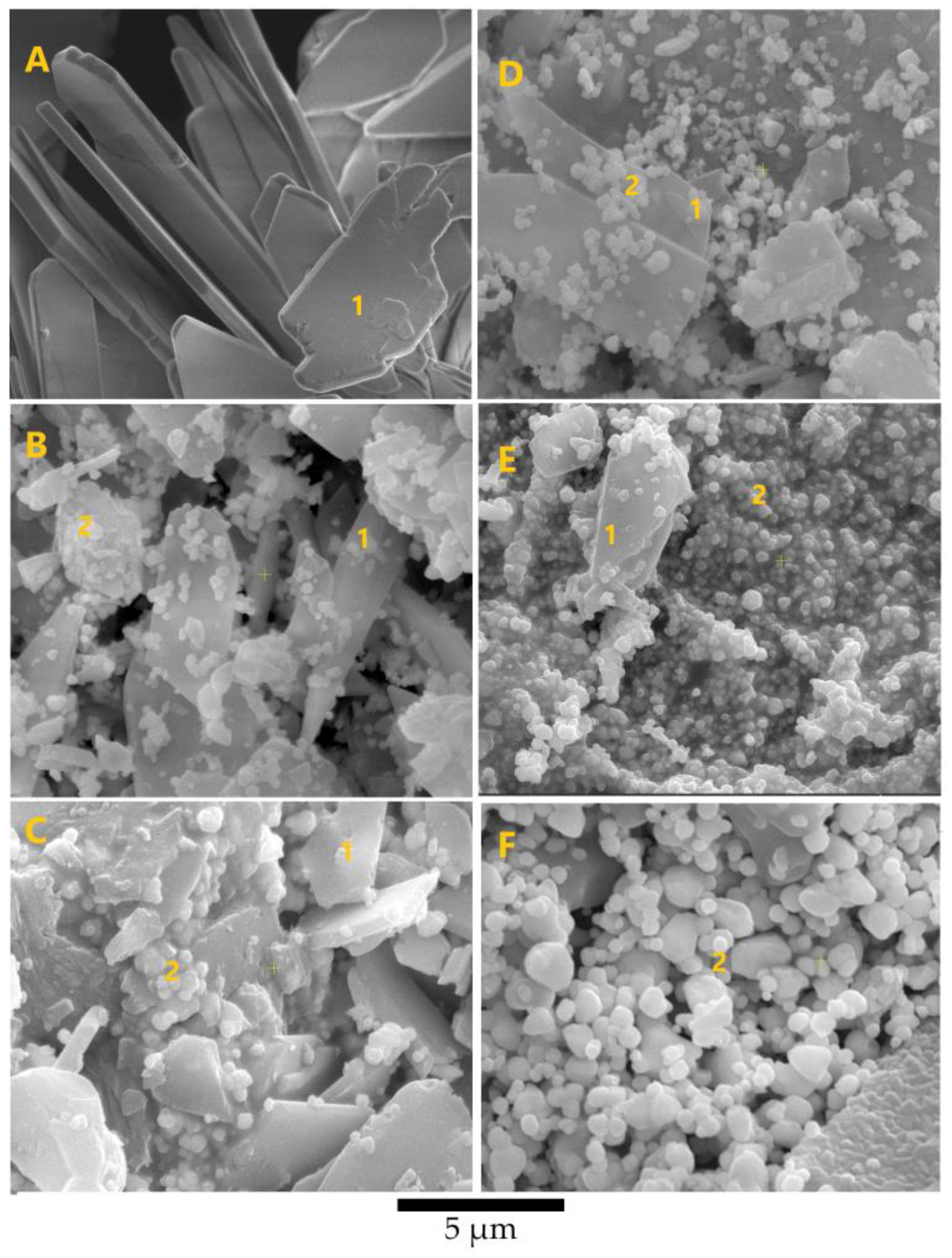
3.1. Mineralogical and Microstructural Analysis
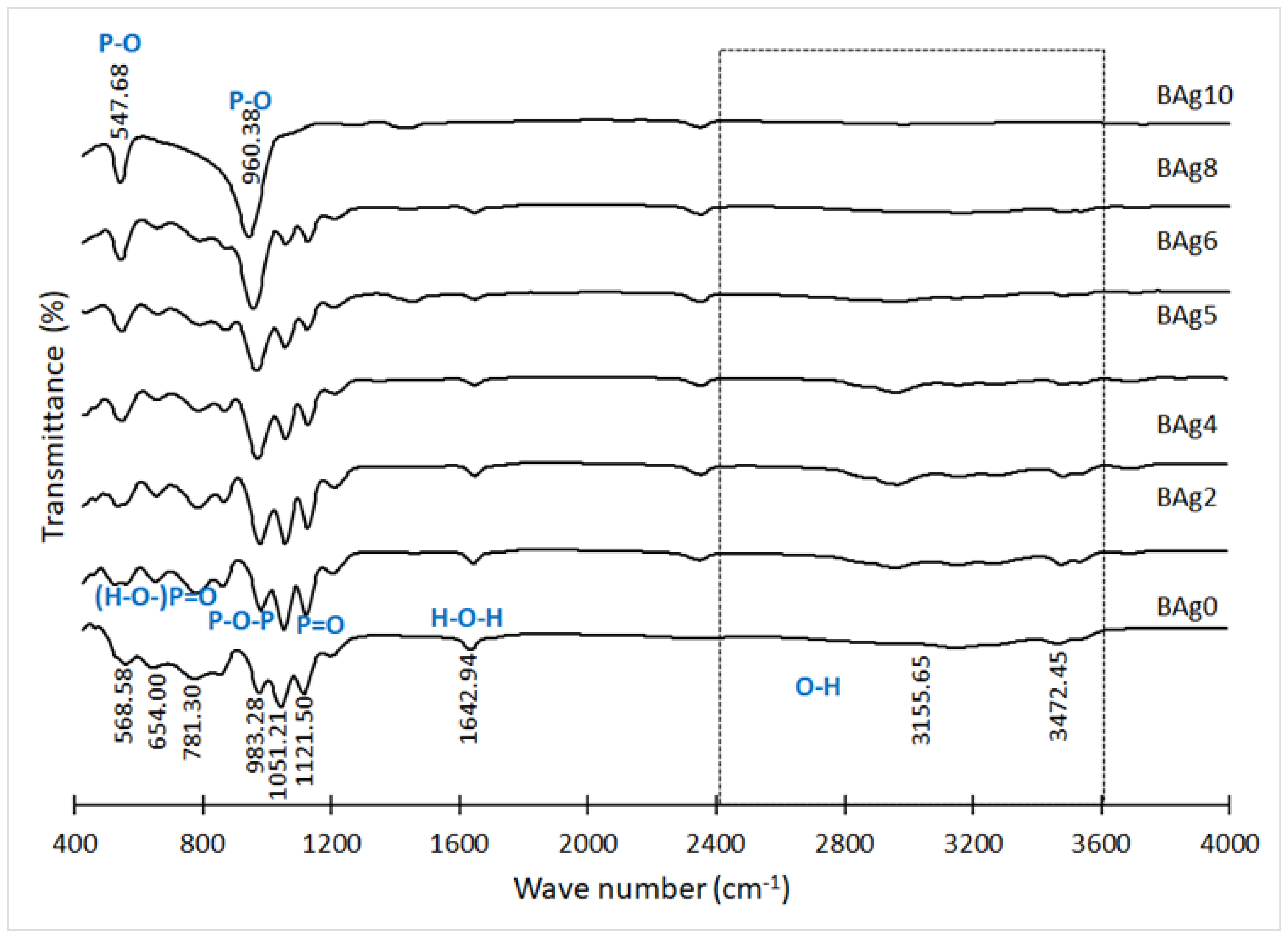
3.2. FTIR Spectrum of the Ca1−xAgxHPO4·nH2O Compounds
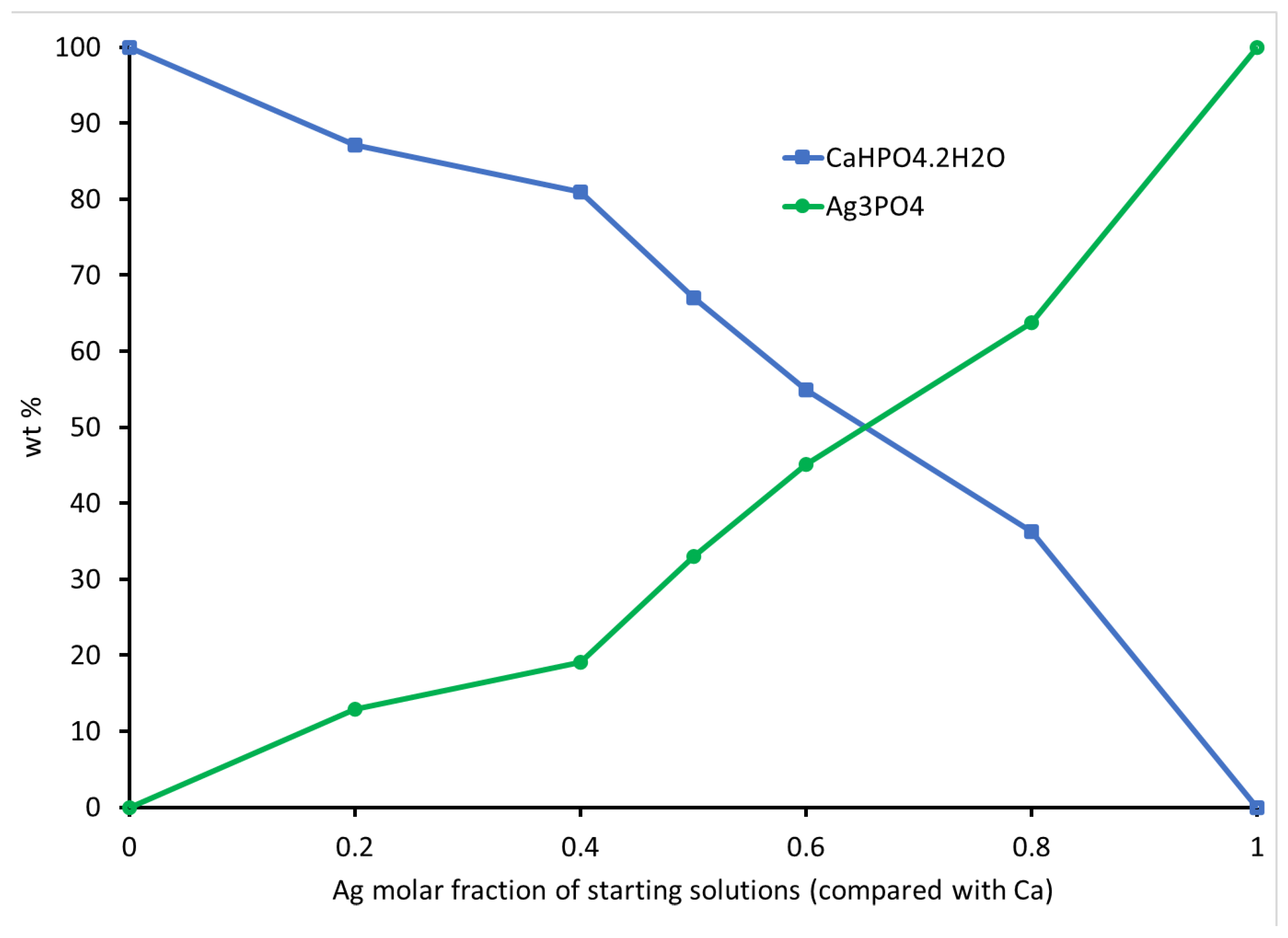
3.3. Elemental Analysis of CaHPO4·2H2O and Ag3PO4
3.4. Thermogravimetric Analysis (TGA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Khalifehzadeh, R.; Arami, H. Biodegradable calcium phosphate nanoparticles for cancer therapy. Adv. Colloid Interface Sci. 2020, 279, 102157. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wei, J.; Guo, H.; Chen, F.; Hong, H.; Liu, C. Self-setting bioactive calcium-magnesium phosphate cement with high strength and degradability for bone regeneration. Acta Biomater. 2008, 4, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Alshaaer, M.; Al-Kafawein, J.; Afify, A.; Hamad, N.; Saffarini, G.; Issa, K. Effect of Ca2+ Replacement with Cu2+ Ions in Brushite on the Phase Composition and Crystal Structure. Minerals 2021, 11, 1028. [Google Scholar] [CrossRef]
- Mouriño, V.; Cattalini, J.; Boccaccini, A. Metallic ions as therapeutic agents in tissue engineering scaffolds: An overview of their biological applications and strategies for new developments. J. R. Soc. Interface 2011, 9, 401–419. [Google Scholar] [CrossRef]
- Alshaaer, M.; Kailani, M.H.; Jafar, H.; Ababneh, N.; Awidi, A. Physicochemical and Microstructural Characterization of Injectable Load-Bearing Calcium Phosphate Scaffold. Adv. Mater. Sci. Eng. 2013, 2013, 149261. [Google Scholar] [CrossRef]
- Mert, I.; Mandel, S.; Tas, A.C. Do cell culture solutions transform brushite (CaHPO4 2H2O) to octacalium phosphate (Ca8(HPO4)2(PO4)4 5H2O)? In Advances in Bioceramics and Porous Ceramics IV; Narayan, R., Colombo, P., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 79–94. [Google Scholar]
- Vallet-Regí, M. Our contributions to applications of mesoporous silica nanoparticles. Acta Biomater. 2022, 137, 44–52. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; García, A.; González-Jiménez, A.; Vallet-Regí, M. Antibacterial effect of 3D printed mesoporous bioactive glass scaffolds doped with metallic silver nanoparticles. Acta Biomater. 2023, 155, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.; Costerton, J. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 1671–1693. [Google Scholar] [CrossRef]
- Rosas, S.; Ong, A.; Buller, L.; Sabeh, K.; Law, T.; Roche, M.; Hernández, V. Season of the year influences infection rates following total hip arthroplasty. World J. Orthop. 2017, 8, 895–901. [Google Scholar] [CrossRef]
- Wei, S.; Ma, J.; Xu, L.; Gu, X.-S.; Ma, X.-L. Biodegradable materials for bone defect repair. Mil. Med. Res. 2020, 7, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Alshaaer, M.; Kailani, M.H.; Ababneh, N.; Abu Mallouh, S.A.; Sweileh, B.; Awidi, A. Fabrication of porous bioceramics for bone tissue applications using luffa cylindrical fibres (LCF) as template. Process. Appl. Ceram. 2017, 11, 13–20. [Google Scholar] [CrossRef]
- Nosrati, H.; Quang Svend Le, D.; Zolfaghari Emameh, R.; Perez, M.C.; Bünger, C.E. Nucleation and growth of brushite crystals on the graphene sheets applicable in bone cement. Boletín Española Cerámica Vidr. 2020, 61, 27–34. [Google Scholar] [CrossRef]
- Tamimi, F.; Le Nihouannen, D.; Eimar, H.; Sheikh, Z.; Komarova, S.; Barralet, J. The effect of autoclaving on the physical and biological properties of dicalcium phosphate dihydrate bioceramics: Brushite vs. monetite. Acta Biomater. 2012, 8, 3161–3169. [Google Scholar] [CrossRef] [PubMed]
- Hurle, K.; Oliveira, J.; Reis, R.; Pina, S.; Goetz-Neunhoeffer, F. Ion-doped Brushite Cements for Bone Regeneration. Acta Biomater. 2021, 123, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Dosen, A.; Giese, R.F. Thermal decomposition of brushite; CaHPO4·2H2O to monetite CaHPO4 and the formation of an amorphous phase. Am. Mineral. 2011, 96, 368–373. [Google Scholar] [CrossRef]
- Radwan, N.H.; Nasr, M.; Ishak, R.A.; Abdeltawa, N.F.; Awad, G.A. Chitosan-calcium phosphate composite scaffolds for control of postoperative osteomyelitis: Fabrication, characterization, and in vitro–in vivo evaluation. Carbohydr. Polym. 2020, 244, 116482. [Google Scholar] [CrossRef]
- Alshaaer, M.; Afify, A.; Moustapha, M.; Hamad, N.; Hammouda, G.; Rocha, F. Effects of the full-scale substitution of strontium for calcium on the microstructure of brushite: (CaxSr1–x)HPO4·nH2O system. Clay Miner. 2020, 55, 366–374. [Google Scholar] [CrossRef]
- Xue, Z.; Wang, Z.; Sun, A.; Huang, J.; Wu, W.; Chen, M.; Hao, X.; Huang, Z.; Lin, X.; Weng, S. Rapid construction of polyetheretherketone (PEEK) biological implants incorporated with brushite (CaHPO4·2H2O) and antibiotics for anti-infection and enhanced osseointegration. Mater. Sci. Eng. C 2020, 111, 110782. [Google Scholar] [CrossRef]
- Lu, B.Q.; Willhammar, T.; Sun, B.B.; Hedin, N.; Gale, J.D.; Gebauer, D. Introducing the crystalline phase of dicalcium phosphate monohydrate. Nat. Commun. 2020, 11, 1546. [Google Scholar] [CrossRef]
- Luo, J.; Engqvist, H.; Persson, C. A ready-to-use acidic, brushite-forming calcium phosphate cement. Acta Biomater. 2018, 81, 304–314. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Kilgus, D.; Howe, D.; Strang, A. Results of periprosthetic hip and knee infections caused by resistant bacteria. Clin. Orthop. Relat. Res. 2002, 404, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, A.A.; Abdulaziz, F.; Alyami, M.; Alotibi, S.; Sakka, S.; Mallouh, S.A.; Abu-Zurayk, R.; Alshaaer, M. The Effect of Full-Scale Exchange of Ca2+ with Zn2+ Ions on the Crystal Structure of Brushite and Its Phase Composition. Biomimetics 2023, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Laskus-Zakrzewska, A.; Zgadzaj, A.; Kolmas, J. Synthesis and physicochemical characterization of Zn-doped brushite. Ceram. Int. 2021, 47, 7798–7804. [Google Scholar] [CrossRef]
- Lowry, N.; Brolly, M.; Han, Y.; McKillop, S.; Meenan, B.; Boyd, A. Synthesis and characterisation of nanophase hydroxyapatite co-substituted with strontium and zinc. Ceram. Int. 2018, 44, 7761–7770. [Google Scholar] [CrossRef]
- Honda, M.; Kawanobe, Y.; Nagata, K.; Ishii, K.; Matsumoto, M.; Aizawa, M. Bactericidal and Bioresorbable Calcium Phosphate Cements Fabricated by Silver-Containing Tricalcium Phosphate Microspheres. Int. J. Mol. Sci. 2020, 21, 3745. [Google Scholar] [CrossRef]
- Ewald, A.; Hösel, D.; Patel, S.; Grover, L.B.J.; Gbureck, U. Silver-doped calcium phosphate cements with antimicrobial activity. Acta Biomater. 2011, 7, 4064–4070. [Google Scholar] [CrossRef]
- Fadeeva, I.; Gafurov, M.; Kiiaeva, I.; Orlinskii, S.B.; Kuznetsova, L.M.; Filippov, Y.Y.; Fomin, A.S.; Davydova, G.A.; Selezneva, I.I.; Barinov, S.M. Tricalcium Phosphate Ceramics Doped with Silver, Copper, Zinc, and Iron (III) Ions in Concentrations of Less Than 0.5 wt.% for Bone Tissue Regeneration. BioNanoScience 2017, 7, 434–438. [Google Scholar] [CrossRef]
- Schierholz, J.; Lucas, L.; Rump, A.; Pulverer, G. Efficacy of silver-coated medical devices. J. Hosp. Infect. 1998, 40, 257–262. [Google Scholar] [CrossRef]
- Feng, Q.; Wu, J.; Chen, G.; Cui, F.; Kim, T.; Kim, J. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Zheng, Y.; Barbaro, K.; Lebedev, V.n.; Aksenov, S.M.; Borovikova, E.Y.; Gafurov, M.R.; Fadeeva, I.V.; Lazoryak, B.I.; Giacomo, G.D.; et al. Dependence of antimicrobial properties on site-selective arrangement and concentration of bioactive Cu2+ ions in tricalcium phosphate. Ceram. Int. 2023, 49, 21308–21323. [Google Scholar] [CrossRef]
- Singh, B.; Dubey, A.; Kumar, S.; Saha, N.; Basu, B.; Gupta, R. In vitro biocompatibility and antimicrobial activity of wet chemically prepared Ca10−xAgx(PO4)6(OH)2 (0.0 ≤ x ≤ 0.5) hydroxyapatites. Mater. Sci. Eng. C 2011, 31, 1320–1329. [Google Scholar] [CrossRef]
- Drake, P.; Hazelwood, K. Exposure-related health effects of silver and silver compounds: A review. Ann. Occup. Hyg. 2005, 49, 575–585. [Google Scholar] [PubMed]
- Patil, S.B.; Jena, A.; Bhargava, P. Influence of Ethanol Amount During Washing on Deagglomeration of Co-Precipitated Calcined Nanocrystalline 3YSZ Powders. Int. J. Appl. Ceram. Technol. 2012, 10, E247–E257. [Google Scholar] [CrossRef]
- Gokcekaya, O.; Ueda, K.; Ogasawara, K.; Kanetaka, H.; Narushima, T. In vitro evaluation of Ag-containing calcium phosphates: Effectiveness of Ag-incorporated β-tricalcium phosphate. Mater. Sci. Eng. C 2017, 75, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Natale, L.C.; Alania, Y.; Rodrigues, M.C.; Simões, A.; de Souza, D.n.; de Lima, E.; Arana-Chavez, V.E.; Hewer, T.L.R.; Hiers, R.; Esteban-Florez, F.L.; et al. Synthesis and characterization of silver phosphate/calcium phosphate mixed particles capable of silver nanoparticle formation by photoreduction. Mater. Sci. Eng. C 2017, 76, 464–471. [Google Scholar] [CrossRef]
- Fathi, M.; El Yacoubi, A.; El Youbi, M.; El Idrissi, B.C. New silver loaded calcium phosphate bone cement: Characterization and phase composition. Phosphorus Sulfur Silicon Relat. Elem. 2022, 197, 758–765. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.; Tang, Z.; Liu, H.; Zhang, R.; Ge, J.; Yang, H.; Ni, X.; Lin, X.; Yang, L. Study on injectable silver-incorporated calcium phosphate composite with enhanced antibacterial and biomechanical properties for fighting bone cement-associated infections. Colloids Surf. B Biointerfaces 2023, 227, 113382. [Google Scholar] [CrossRef]
- Qureshi, F.; Nawaz, M.; Ansari, M.A.; Khan, F.A.; Berekaa, M.M.; Abubshait, S.A.; Al-Mutairi, R.; Paul, A.K.; Nissapatorn, V.; de Lourdes Pereira, M.; et al. Synthesis of M-Ag3PO4, (M = Se, Ag, Ta) Nanoparticles and Their Antibacterial and Cytotoxicity Study. Int. J. Mol. Sci. 2022, 23, 11403. [Google Scholar] [CrossRef]
- Piva, R.H.; Piva, D.H.; Pierri, J.; Montedo, O.R.K.; Morelli, M.R. Azeotropic distillation, ethanol washing, and freeze drying on coprecipitated gels for production of high surface area 3Y–TZP and 8YSZ powders: A comparative study. Ceram. Int. 2015, 41, 14148–14156. [Google Scholar] [CrossRef]
- Tortet, L.; Gavarri, J.R.; Nihoul, G. Study of Protonic Mobility in CaHPO4 · 2H2O (Brushite) and CaHPO4 (Monetite) by Infrared Spectroscopy and Neutron Scattering. J. Solid. State Chem. 1997, 132, 6–16. [Google Scholar] [CrossRef]
- Madsen, H.E.L. Influence of foreign metal ions on crystal growth and morphology of brushite (CaHPO4, 2H2O) and its transformation to octacalcium phosphate and apatite. J. Cryst. Growth 2008, 310, 2602–2612. [Google Scholar] [CrossRef]
- Batvandi, M.; Haghighatzadeh, A.; Mazinani, B. Synthesis of Ag3PO4 microstructures with morphology-dependent optical and photocatalytic behaviors. Appl. Phys. A 2020, 126, 571. [Google Scholar] [CrossRef]
- Botelho, G.; Sczancoski, J.; Andres, J.; Gracia, L.; Longo, E. Experimental and theoretical study on the structure, optical properties, and growth of metallic silver nanostructures in Ag3PO4. J. Phys. Chem. C 2015, 119, 6293–6306. [Google Scholar] [CrossRef]
- Alshaaer, M. Microstructural characteristics and long-term stability of wollastonite-based chemically bonded phosphate ceramics. Int. J. Appl. Ceram. Technol. 2020, 18, 319–331. [Google Scholar] [CrossRef]
- Alkhraisat, M.H.; Rueda, C.; Cabarcos, E.L. Strontium Ions Substitution in Brushite Crystals: The Role of Strontium Chloride. J. Funct. Biomater. 2011, 2, 31–38. [Google Scholar] [CrossRef]
- Alshaaer, M.; Issa, K.; Alanazi, A.; Mallouh, S.A.; Afify, A.S.; Moustapha, M.E.; Komnitsas, K. Gradual Replacement of Ca2+ with Mg2+ Ions in Brushite for the Production of Ca1−xMgxHPO4·nH2O Materials. Minerals 2021, 11, 284. [Google Scholar] [CrossRef]
- Hirsch, A.; Azuri, I.; Addadi, L.; Weiner, S.; Yang, K.; Curtarolo, S.; Kronik, L. Infrared Absorption Spectrum of Brushite from First Principles. Chem. Mater. 2014, 26, 2934–2942. [Google Scholar] [CrossRef]
- Alotibi, S.S.; Alshaaer, M. The Effect of Full-Scale Exchange of Ca2+ with Co2+ Ions on the Crystal Structure and Phase Composition of CaHPO4·2H2O. Crystals 2023, 13, 941. [Google Scholar] [CrossRef]
- Rajendran, K.K.C. Growth and Characterization of Calcium Hydrogen Phosphate Dihydrate Crystals from Single Dif-fusion Gel Technique. Cryst. Res. Technol. 2010, 45, 939–945. [Google Scholar] [CrossRef]
- Promnopas, S.; Promnopas, W.; Maisang, W.; Wannapop, S.; Thongtem, T.; Thongtem, S.; Wiranwetchayan, O. Synthesis of Ag3PO4/Ag4P2O7 by microwave-hydrothermal method for enhanced UV–visible photocatalytic performance. Sci. Rep. 2023, 13, 4742. [Google Scholar] [CrossRef] [PubMed]
- Alshaaer, M.; Cuypers, H.; Rahier, H.; Wastiels, j. Production of monetite-based Inorganic Phosphate Cement (M-IPC) using hydrothermal post curing (HTPC). Cem. Concr. Res. 2011, 41, 30–37. [Google Scholar] [CrossRef]
- Gashti, M.P.; Stir, M.; Jurg, H. Growth of strontium hydrogen phosphate/gelatin composites: A biomimetic approach. New J. Chem. 2016, 40, 5495–5500. [Google Scholar] [CrossRef]
- Alshaaer, M.; Cuypers, H.; Mosselmans, G.; Rahier, H.; Wastiels, J. Evaluation of a low temperature hardening Inorganic Phosphate Cement for high-temperature applications. Cem. Concr. Res. 2011, 41, 38–45. [Google Scholar] [CrossRef]
- Frost, R.L.; Palmer, S.J. Thermal stability of the ‘cave’ mineral brushite CaHPO4·2H2O—Mechanism of formation and decomposition. Thermochim. Acta 2011, 521, 14–17. [Google Scholar] [CrossRef]
- Martín-Gómez, A.; Navío, J.; Jaramillo-Páez, C.; Sánchez-Cid, P.; Hidalgo, M. Hybrid ZnO/Ag3PO4 photocatalysts, with low and high phosphate molar percentages. J. Photochem. Photobiol. A Chem. 2020, 388, 112196. [Google Scholar] [CrossRef]

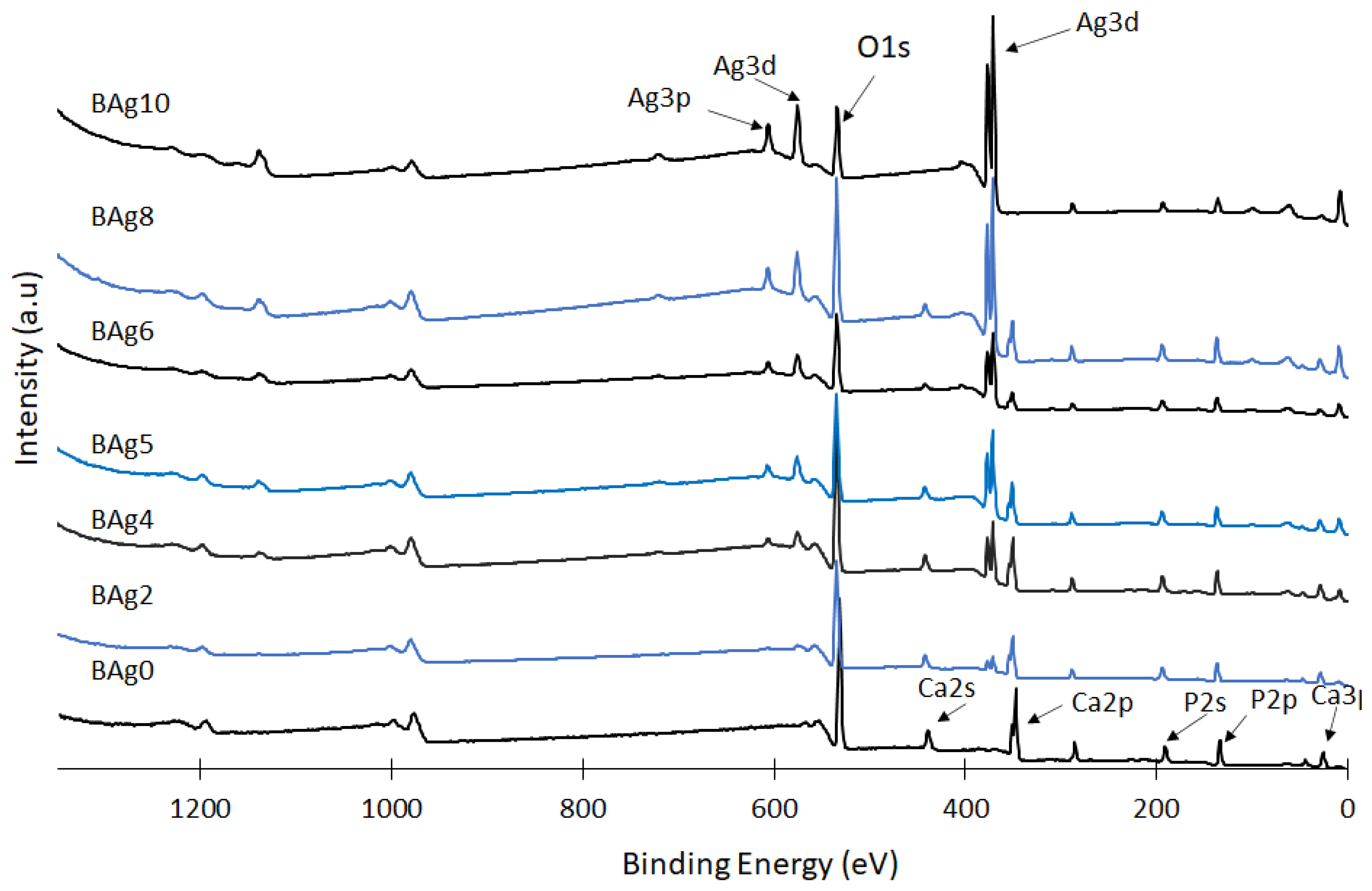
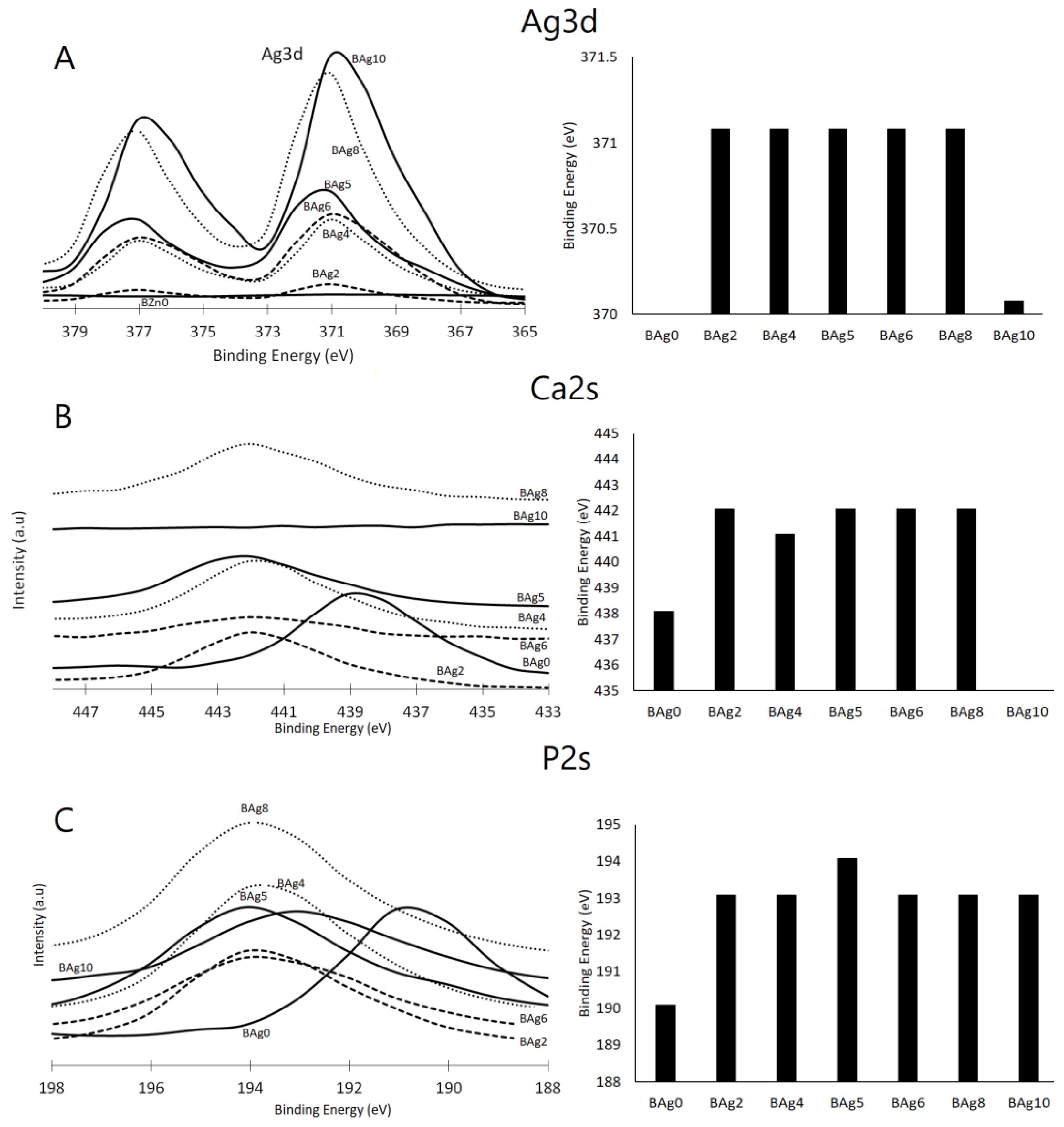

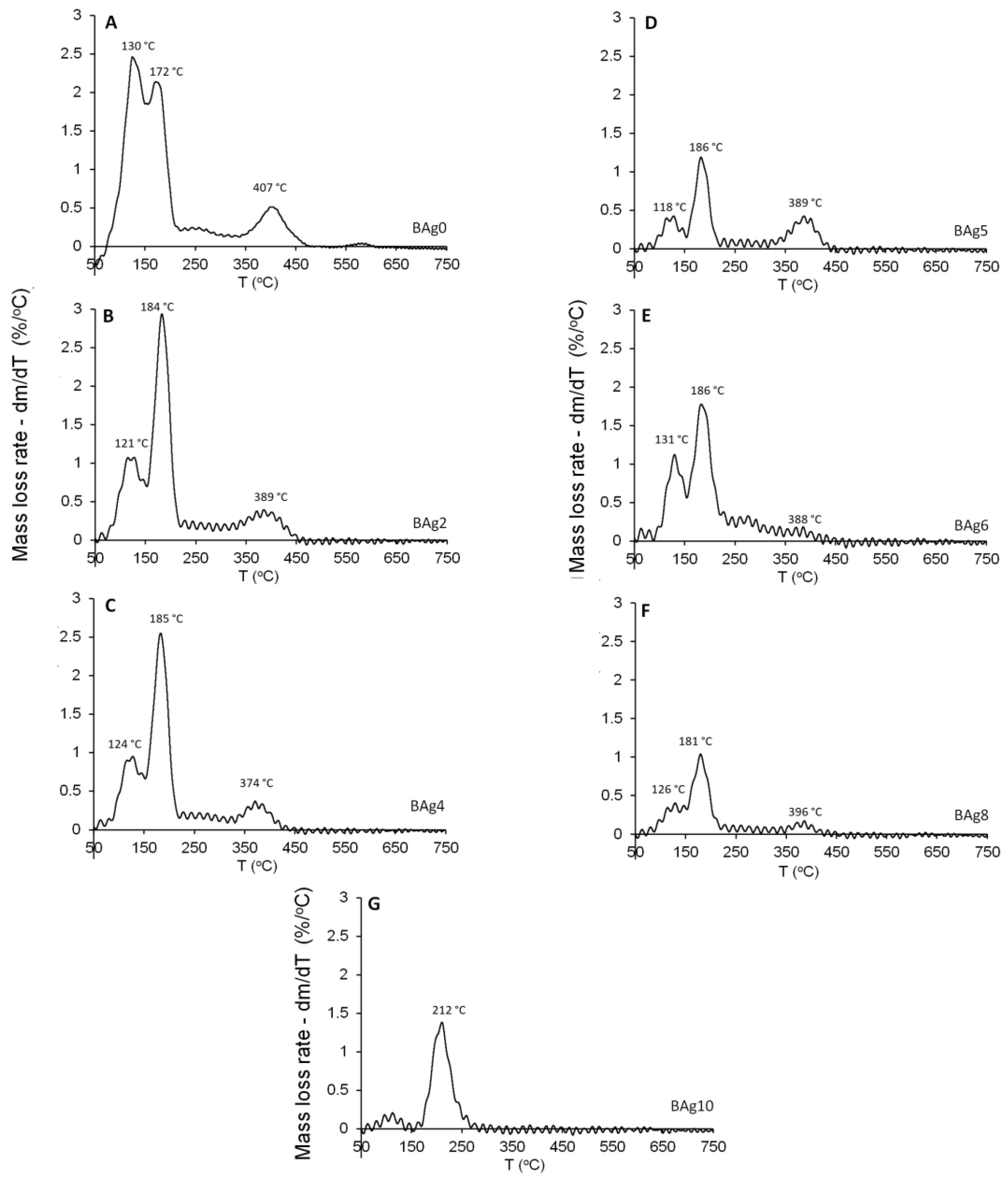
| ID | (NH4)2HPO4 | Ca(NO3)2·4H2O | AgNO3·6H2O | Ag/Ca Molar Ratio |
|---|---|---|---|---|
| BAg0 | 1 | 1 | 0 | 0 |
| BAg2 | 1 | 0.8 | 0.2 | 0.25 |
| BAg4 | 1 | 0.6 | 0.4 | 0.67 |
| BAg5 | 1 | 0.5 | 0.5 | 1.0 |
| BAg6 | 1 | 0.4 | 0.6 | 1.5 |
| BAg8 | 1 | 0.2 | 0.8 | 4 |
| BAg10 | 1 | 0 | 1 | - |
| Phase Composition | % wt. | Crystal Structure | a (Å) | b (Å) | c (Å) | ß° | Unit Cell Volume (Å3) | Crystallite Size (Å) * | |
|---|---|---|---|---|---|---|---|---|---|
| BAg0 | CaHPO4·2H2O | 100 | Monoclinic | 5.82 | 15.22 | 6.27 | 116.41 | 496.65 | 53 |
| BAg2 | CaHPO4·2H2O | 87.1 | Monoclinic | 5.81 | 15.19 | 6.24 | 116.41 | 493.43 | 50 |
| Ag3PO4 | 12.9 | Cubic | 6.00 | 6.00 | 6.00 | 90 | 216.43 | 21 | |
| BAg4 | CaHPO4·2H2O | 80.9 | Monoclinic | 5.81 | 15.18 | 6.24 | 116.43 | 492.91 | 65 |
| Ag3PO4 | 19.1 | Cubic | 6.01 | 6.01 | 6.01 | 6.01 | 217.03 | 33 | |
| BAg5 | CaHPO4·2H2O | 67 | Monoclinic | 5.81 | 15.21 | 6.26 | 116.41 | 495.65 | 56 |
| Ag3PO4 | 33 | Cubic | 6.01 | 6.01 | 6.01 | 6.01 | 217.03 | 34 | |
| BAg6 | CaHPO4·2H2O | 54.9 | Monoclinic | 5.81 | 15.21 | 6.26 | 116.41 | 495.65 | 70 |
| Ag3PO4 | 45.1 | Cubic | 6.00 | 6.00 | 6.00 | 90 | 216.00 | 30 | |
| BAg8 | CaHPO4·2H2O | 36.3 | Monoclinic | 5.82 | 15.22 | 6.27 | 116.41 | 496.65 | 130 |
| Ag3PO4 | 63.7 | Cubic | 6.00 | 6.00 | 6.00 | 90 | 216.00 | 18 | |
| BAg10 | Ag3PO4 | 100 | Cubic | 6.00 | 6.00 | 6.00 | 6.00 | 216.00 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulaziz, F.; Issa, K.; Alyami, M.; Alotibi, S.; Alanazi, A.A.; Taha, T.A.M.; Saad, A.M.E.; Hammouda, G.A.; Hamad, N.; Alshaaer, M. Preparation and Characterization of Mono- and Biphasic Ca1−xAgxHPO4·nH2O Compounds for Biomedical Applications. Biomimetics 2023, 8, 547. https://doi.org/10.3390/biomimetics8070547
Abdulaziz F, Issa K, Alyami M, Alotibi S, Alanazi AA, Taha TAM, Saad AME, Hammouda GA, Hamad N, Alshaaer M. Preparation and Characterization of Mono- and Biphasic Ca1−xAgxHPO4·nH2O Compounds for Biomedical Applications. Biomimetics. 2023; 8(7):547. https://doi.org/10.3390/biomimetics8070547
Chicago/Turabian StyleAbdulaziz, Fahad, Khalil Issa, Mohammed Alyami, Satam Alotibi, Abdulaziz A. Alanazi, Taha Abdel Mohaymen Taha, Asma M. E. Saad, Gehan A. Hammouda, Nagat Hamad, and Mazen Alshaaer. 2023. "Preparation and Characterization of Mono- and Biphasic Ca1−xAgxHPO4·nH2O Compounds for Biomedical Applications" Biomimetics 8, no. 7: 547. https://doi.org/10.3390/biomimetics8070547
APA StyleAbdulaziz, F., Issa, K., Alyami, M., Alotibi, S., Alanazi, A. A., Taha, T. A. M., Saad, A. M. E., Hammouda, G. A., Hamad, N., & Alshaaer, M. (2023). Preparation and Characterization of Mono- and Biphasic Ca1−xAgxHPO4·nH2O Compounds for Biomedical Applications. Biomimetics, 8(7), 547. https://doi.org/10.3390/biomimetics8070547







