The Creation of an Average 3D Model of the Human Cartilaginous Nasal Septum and Its Biomimetic Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dissection
2.2. Shape Elucidation
2.3. 3D Model Creation
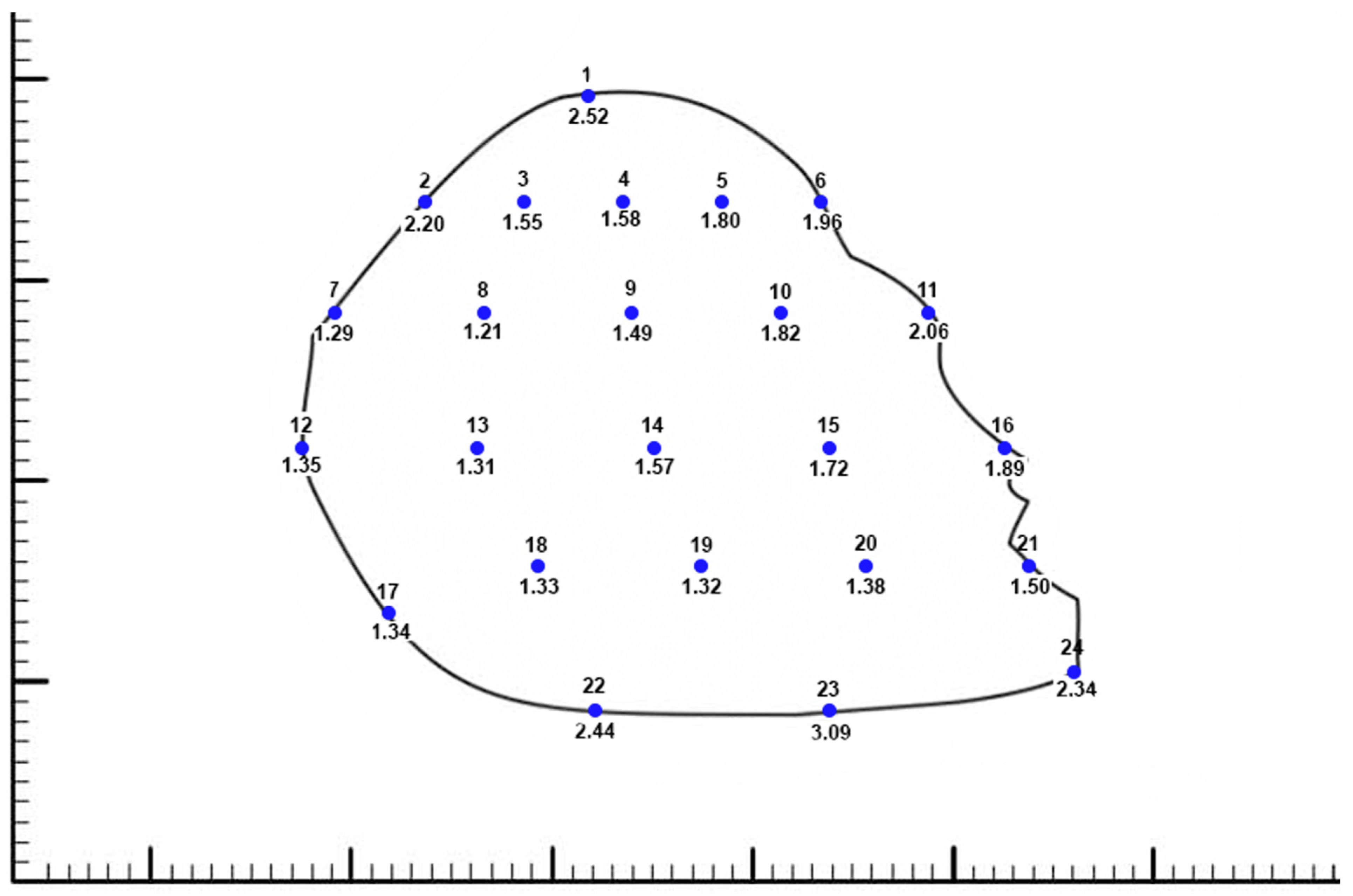
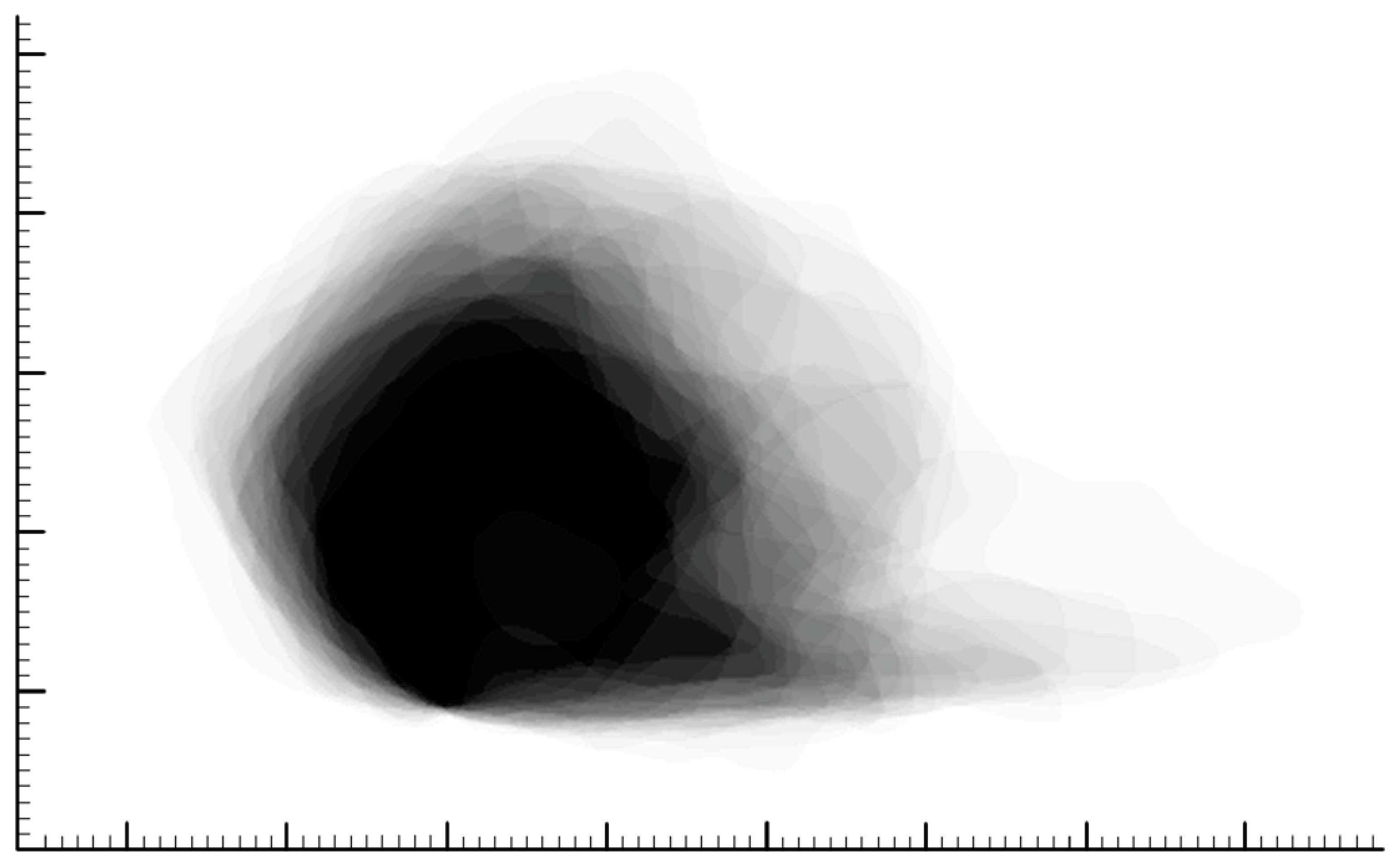
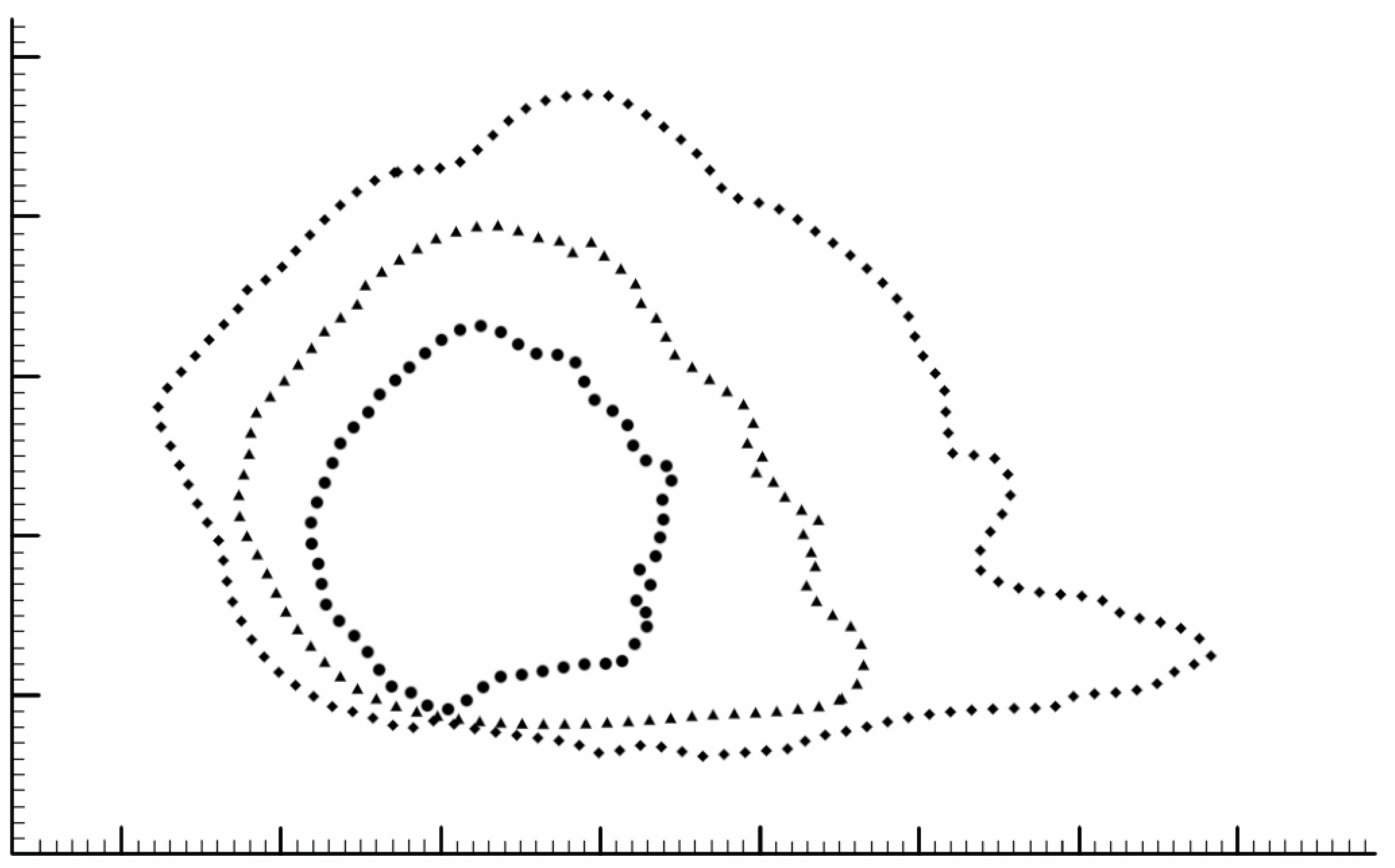
3. Results
4. Discussion
4.1. Shape and Dimensions
4.2. Finite Elemental Analysis
4.3. 3D Printing
4.4. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tasca, I.; Compadretti, G.C.; Losano, T.I.; Lijdens, Y.; Boccio, C. Extracorporeal septoplasty with internal nasal valve stabilisation. Acta Otorhinolaryngol. Ital. 2018, 38, 331–337. [Google Scholar] [CrossRef]
- Hwang, K.; Huan, F.; Kim, D.J. Mapping thickness of nasal septal cartilage. J. Craniofac. Surg. 2010, 21, 243–244. [Google Scholar] [CrossRef]
- Miles, B.A.; Petrisor, D.; Kao, H.; Finn, R.A.; Throckmorton, G.S. Anatomical variation of the nasal septum: Analysis of 57 cadaver specimens. Otolaryngol. Neck Surg. 2007, 136, 362–368. [Google Scholar] [CrossRef]
- Mowlavi, A.; Masouem, S.; Kalkanis, J.; Guyuron, B. Septal cartilage defined: Implications for nasal dynamics and rhinoplasty. Plast. Reconstr. Surg. 2006, 117, 2171–2174. [Google Scholar] [CrossRef]
- Tremp, M.; Schneider, J.; Raghu, R.B.N.; Goksel, A.; Saban, Y. A Systematic Analysis of the Nasal Septum in Crooked Noses and Suggested Treatment Algorithm According to Preservation Rhinoplasty (PR) Principles. Aesthetic Plast. Surg. 2023, 47, 1499–1507. [Google Scholar] [CrossRef]
- Lee, M.; Inman, J.; Callahan, S.; Ducic, Y. Fracture patterns of the nasal septum. Otolaryngol. Neck Surg. 2010, 143, 784–788. [Google Scholar] [CrossRef]
- Canzi, P.; Magnetto, M.; Marconi, S.; Morbini, P.; Mauramati, S.; Aprile, F.; Avato, I.; Auricchio, F.; Benazzo, M. New frontiers and emerging applications of 3D printing in ENT surgery: A systematic review of the literature. Acta Otorhinolaryngol. Ital. 2018, 38, 286–303. [Google Scholar] [CrossRef]
- Cao, Y.; Sang, S.; An, Y.; Xiang, C.; Li, Y.; Zhen, Y. Progress of 3D Printing Techniques for Nasal Cartilage Regeneration. Aesthetic Plast. Surg. 2022, 46, 947–964. [Google Scholar] [CrossRef]
- Zoccali, F.; Colizza, A.; Cialente, F.; Di Stadio, A.; La Mantia, I.; Hanna, C.; Minni, A.; Ralli, M.; Greco, A.; de Vincentiis, M. 3D Printing in Otolaryngology Surgery: Descriptive Review of Literature to Define the State of the Art. Healthcare 2022, 11, 108. [Google Scholar] [CrossRef]
- Paul, N.; Messinger, K.; Liu, Y.F.; Kwon, D.I.; Kim, C.H.; Inman, J.C. A Model to Estimate L-Strut Strength with an Emphasis on Thickness. JAMA Facial Plast. Surg. 2016, 18, 269–276. [Google Scholar] [CrossRef]
- Han, P.S.; Liu, Y.F.; Messinger, K.; Ardeshirpour, F.; Inman, J.C. Redefining the nasal septal L-strut: A quantitative analysis of septal arcs and angles. Laryngoscope 2018, 128, 1806–1810. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Lee, D.C.; Ha, D.H.; Kim, S.W.; Cho, D.W. Redefining the Septal L-Strut to Prevent Collapse. PLoS ONE 2016, 11, e0153056. [Google Scholar] [CrossRef]
- Mau, T.; Mau, S.T.; Kim, D.W. Cadaveric and engineering analysis of the septal L-strut. Laryngoscope 2007, 117, 1902–1906. [Google Scholar] [CrossRef] [PubMed]
- Menapace, D.C.; Carlson, K.D.; Dragomir-Daescu, D.; Matsumoto, J.; Hamilton, G.S. Finite Element Analysis of the Septal Cartilage L-Strut. Facial Plast. Surg. Aesthetic Med. 2021, 23, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Messinger, K.; Inman, J.C. Yield Strength Testing in Human Cadaver Nasal Septal Cartilage and L-Strut Constructs. JAMA Facial Plast. Surg. 2017, 19, 40–45. [Google Scholar] [CrossRef]
- Kavanagh, K.R.; Cote, V.; Tsui, Y.; Kudernatsch, S.; Peterson, D.R.; Valdez, T.A. Pediatric laryngeal simulator using 3D printed models: A novel technique. Laryngoscope 2017, 127, E132–E137. [Google Scholar] [CrossRef]
- AlReefi, M.A.; Nguyen, L.H.P.; Mongeau, L.G.; Haq, B.U.; Boyanapalli, S.; Hafeez, N.; Cegarra-Escolano, F.; Tewfik, M.A. Development and validation of a septoplasty training model using 3-dimensional printing technology. Int. Forum Allergy Rhinol. 2017, 7, 399–404. [Google Scholar] [CrossRef]
- Khan, G.; Choi, Y.S.; Park, E.S.; Choi, Y.D. The Application of Three-Dimensional Simulation Program and Three-Dimensional Printing in Secondary Rhinoplasty. J. Craniofac. Surg. 2018, 29, e774–e777. [Google Scholar] [CrossRef]
- Joo, O.Y.; Kim, T.H.; Kim, Y.S.; Roh, T.S.; Lee, E.J.; Shim, J.H.; Cho, H.W.; Yun, I.S. Fabrication of 3D-Printed Implant for Two-Stage Ear Reconstruction Surgery and Its Clinical Application. Yonsei Med. J. 2023, 64, 291–296. [Google Scholar] [CrossRef]
- Watson, J.; Hatamleh, M.M. Complete integration of technology for improved reproduction of auricular prostheses. J. Prosthet. Dent. 2014, 111, 430–436. [Google Scholar] [CrossRef]
- Zhou, G.; Jiang, H.; Yin, Z.; Liu, Y.; Zhang, Q.; Zhang, C.; Pan, B.; Zhou, J.; Zhou, X.; Sun, H.; et al. In Vitro Regeneration of Patient-specific Ear-shaped Cartilage and Its First Clinical Application for Auricular Reconstruction. EBioMedicine 2018, 28, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Zhong, N.; Zhao, X. 3D printing for clinical application in otorhinolaryngology. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 4079–4089. [Google Scholar] [CrossRef] [PubMed]
- Zopf, D.A.; Mitsak, A.G.; Flanagan, C.L.; Wheeler, M.; Green, G.E.; Hollister, S.J. Computer Aided–Designed, 3-Dimensionally Printed Porous Tissue Bioscaffolds for Craniofacial Soft Tissue Reconstruction. Otolaryngol. Neck Surg. 2015, 152, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Choi, J.Y. Surgical outcomes and complications of septal extension graft supported by 3D printed polycaprolactone plate. Laryngoscope. 2020, 130, 1680–1685. [Google Scholar] [CrossRef]
- Kim, D.H.; Yun, W.S.; Shim, J.H.; Park, K.H.; Choi, D.; Park, M.I.; Hwang, S.H.; Kim, S.W. Clinical Application of 3-Dimensional Printing Technology for Patients with Nasal Septal Deformities: A Multicenter Study. JAMA Otolaryngol. Neck Surg. 2018, 144, 1145–1152. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, I.H.; Yun, W.S.; Shim, J.H.; Choi, D.; Hwang, S.H.; Kim, S.W. Long-term efficacy and safety of 3D printed implant in patients with nasal septal deformities. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 1943–1950. [Google Scholar] [CrossRef]
- Gadaleta, D.; Lee, D.Z.; Peng, M.W.; Cruickshank, N.; Shinde, R.; Hong, A.; Pennacchi, S.; Dawit, A.; Krein, H.; Udupa, J.K.; et al. Fabrication of custom PCL scaffold for nasal septal perforation repair. In Proceedings of the Medical Imaging 2018: Imaging Informatics for Healthcare, Research, and Applications, Houston, TX, USA, 10–15 February 2018; SPIE: Paris, France, 2018; Volume 10579, pp. 30–35. [Google Scholar] [CrossRef]
- Di Gesù, R.; Acharya, A.P.; Jacobs, I.; Gottardi, R. 3D printing for tissue engineering in otolaryngology. Connect. Tissue Res. 2020, 61, 117–136. [Google Scholar] [CrossRef]
- Onerci Altunay, Z.; Bly, J.A.; Edwards, P.K.; Holmes, D.R.; Hamilton, G.S.; O’Brien, E.K.; Carr, A.B.; Camp, J.J.; Stokken, J.K.; Pallanch, J.F. Three-dimensional printing of large nasal septal perforations for optimal prosthetic closure. Am. J. Rhinol. Allergy 2016, 30, 287–293. [Google Scholar] [CrossRef]
- Neuman, M.K.; Briggs, K.K.; Masuda, K.; Sah, R.L.; Watson, D. A compositional analysis of cadaveric human nasal septal cartilage. Laryngoscope 2013, 123, 2120–2124. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, P.S.; Punjabi, N.; Goese, M.; Inman, J.C. The Creation of an Average 3D Model of the Human Cartilaginous Nasal Septum and Its Biomimetic Applications. Biomimetics 2023, 8, 530. https://doi.org/10.3390/biomimetics8070530
Han PS, Punjabi N, Goese M, Inman JC. The Creation of an Average 3D Model of the Human Cartilaginous Nasal Septum and Its Biomimetic Applications. Biomimetics. 2023; 8(7):530. https://doi.org/10.3390/biomimetics8070530
Chicago/Turabian StyleHan, Peter S., Nihal Punjabi, Mickey Goese, and Jared C. Inman. 2023. "The Creation of an Average 3D Model of the Human Cartilaginous Nasal Septum and Its Biomimetic Applications" Biomimetics 8, no. 7: 530. https://doi.org/10.3390/biomimetics8070530
APA StyleHan, P. S., Punjabi, N., Goese, M., & Inman, J. C. (2023). The Creation of an Average 3D Model of the Human Cartilaginous Nasal Septum and Its Biomimetic Applications. Biomimetics, 8(7), 530. https://doi.org/10.3390/biomimetics8070530





