Clinical Effectiveness of 3D-Milled and 3D-Printed Zirconia Prosthesis—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Search Strategy
2.4. Article Screening
2.5. Data Extraction
2.6. Statistical Analysis
3. Results
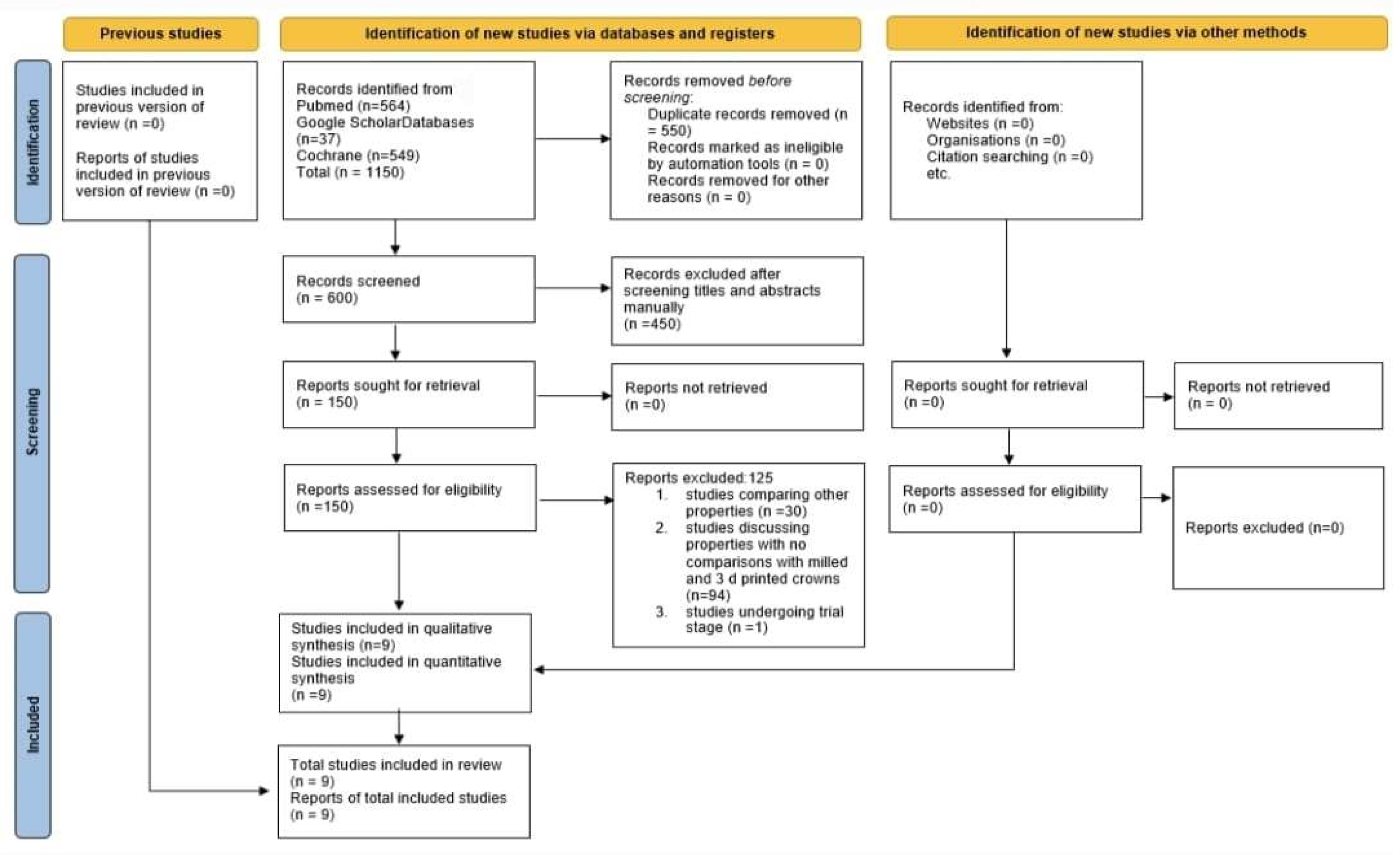
3.1. Selection of the Studies
3.2. Characteristics of Finalized Studies
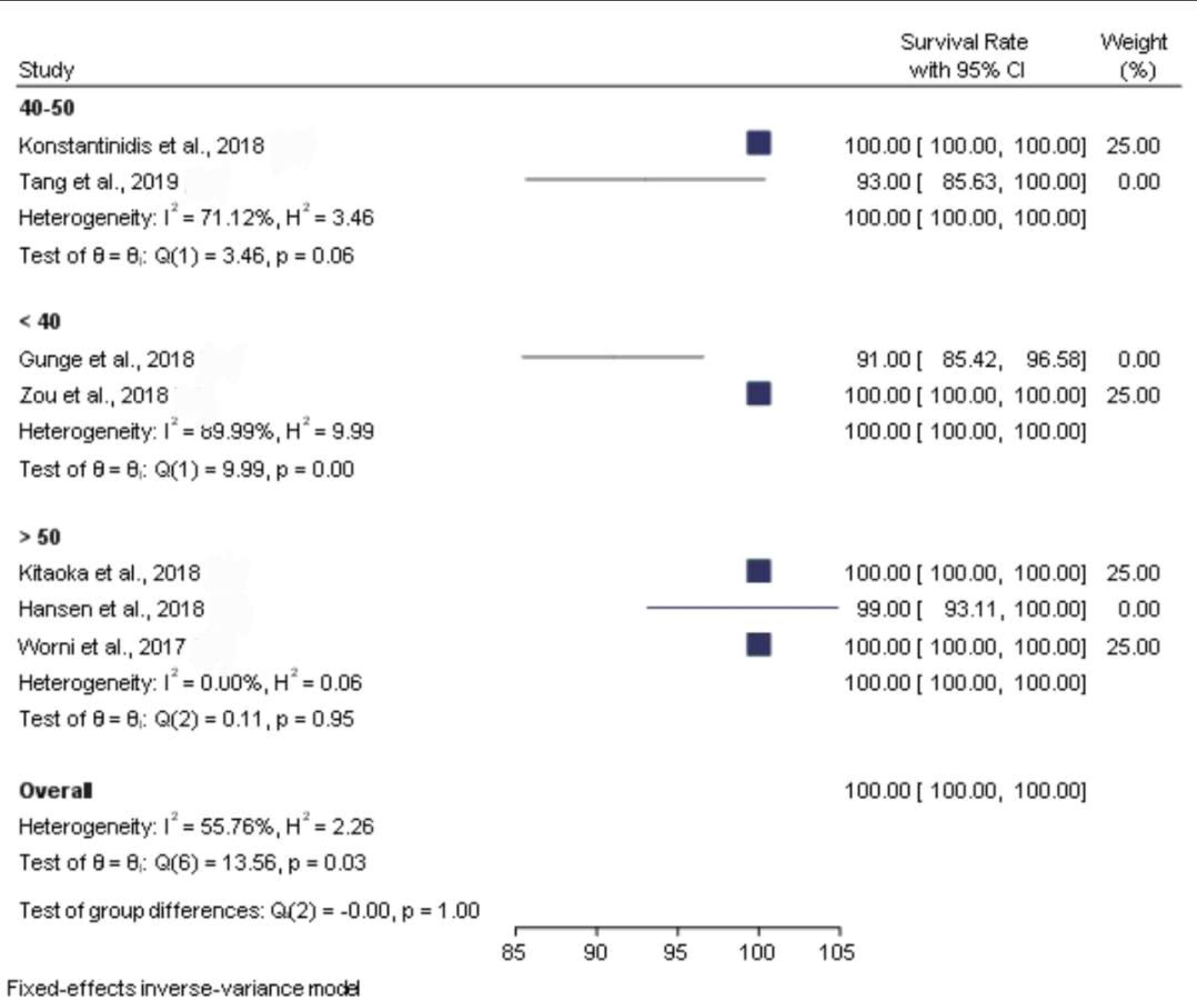
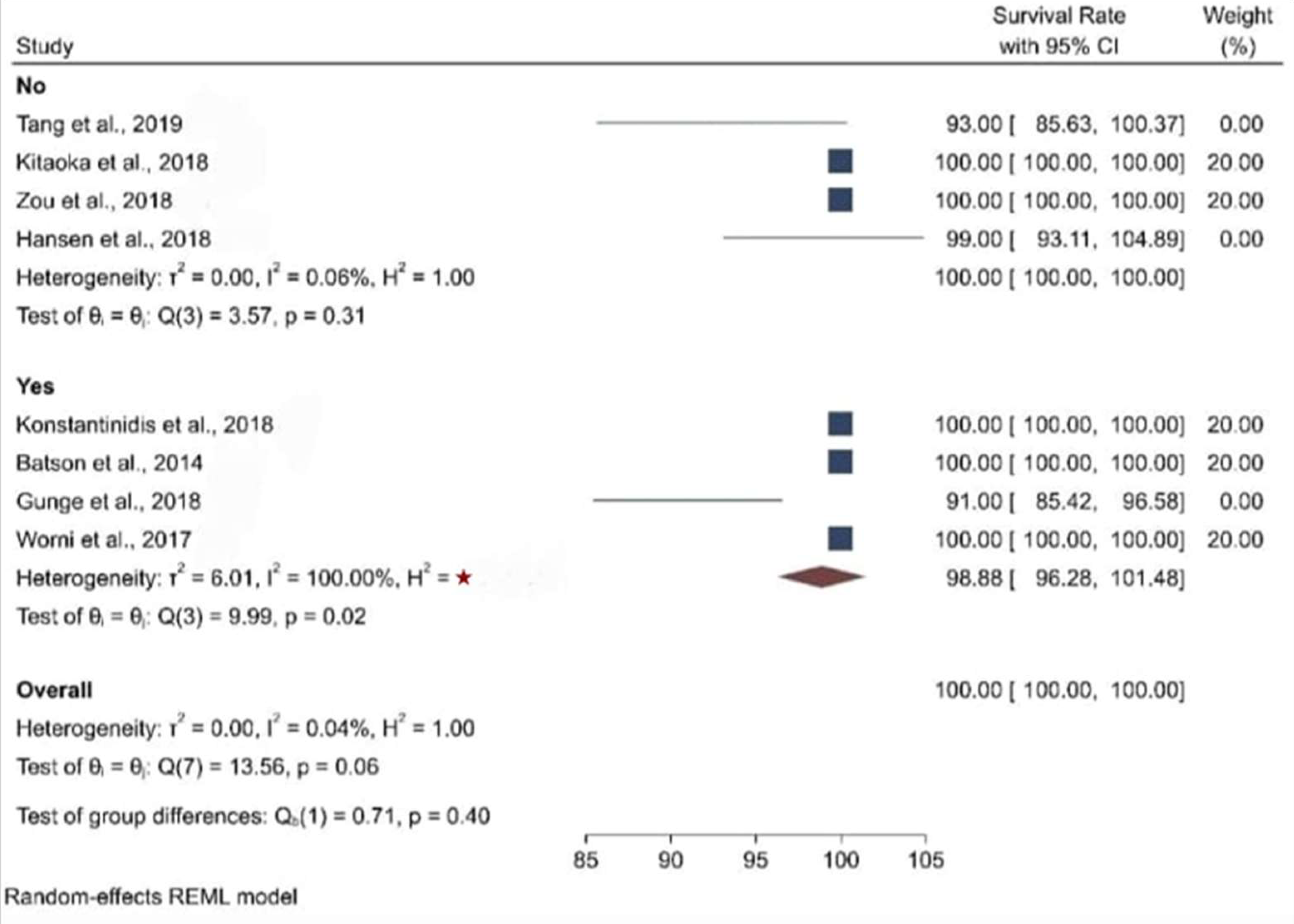
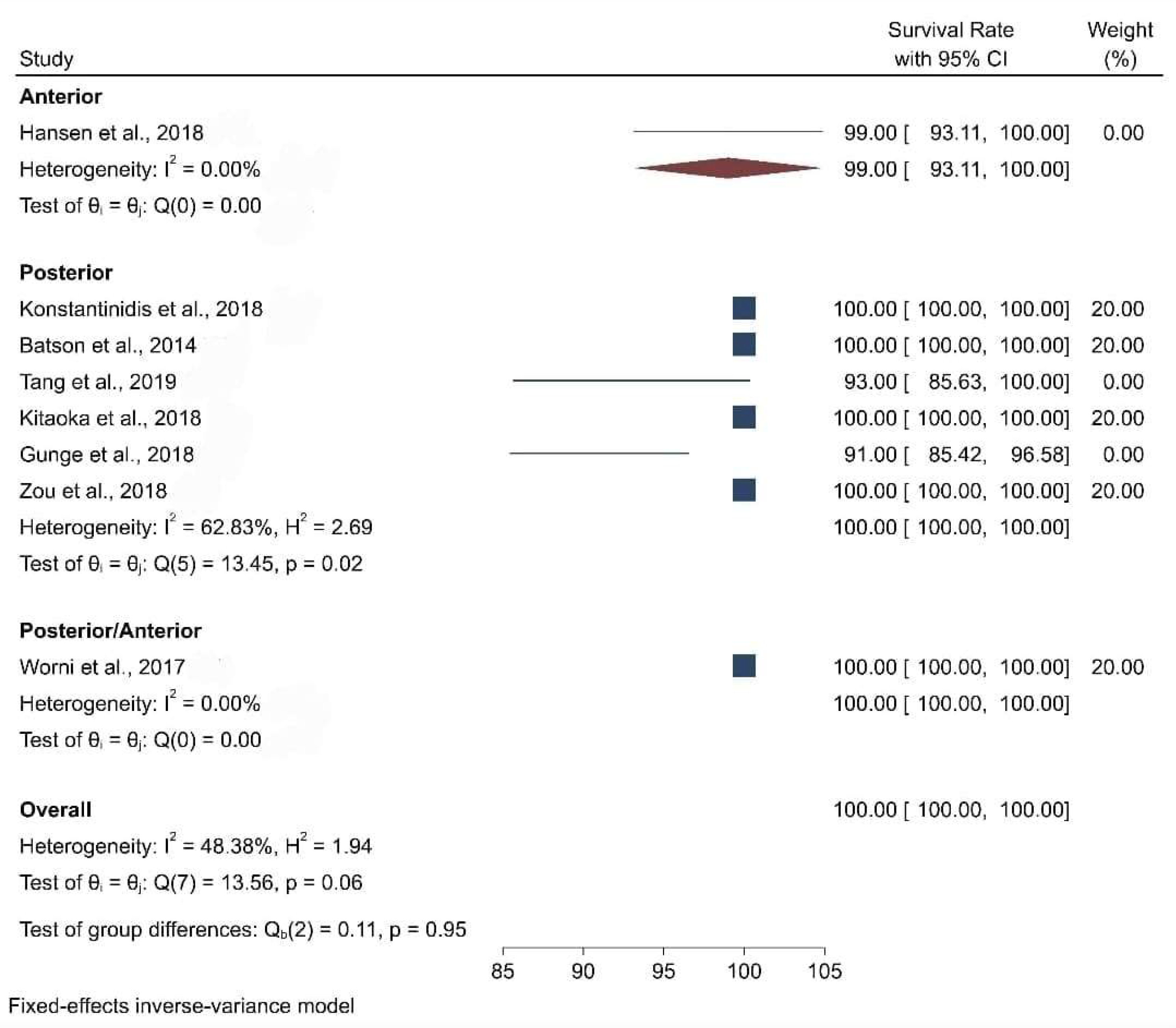
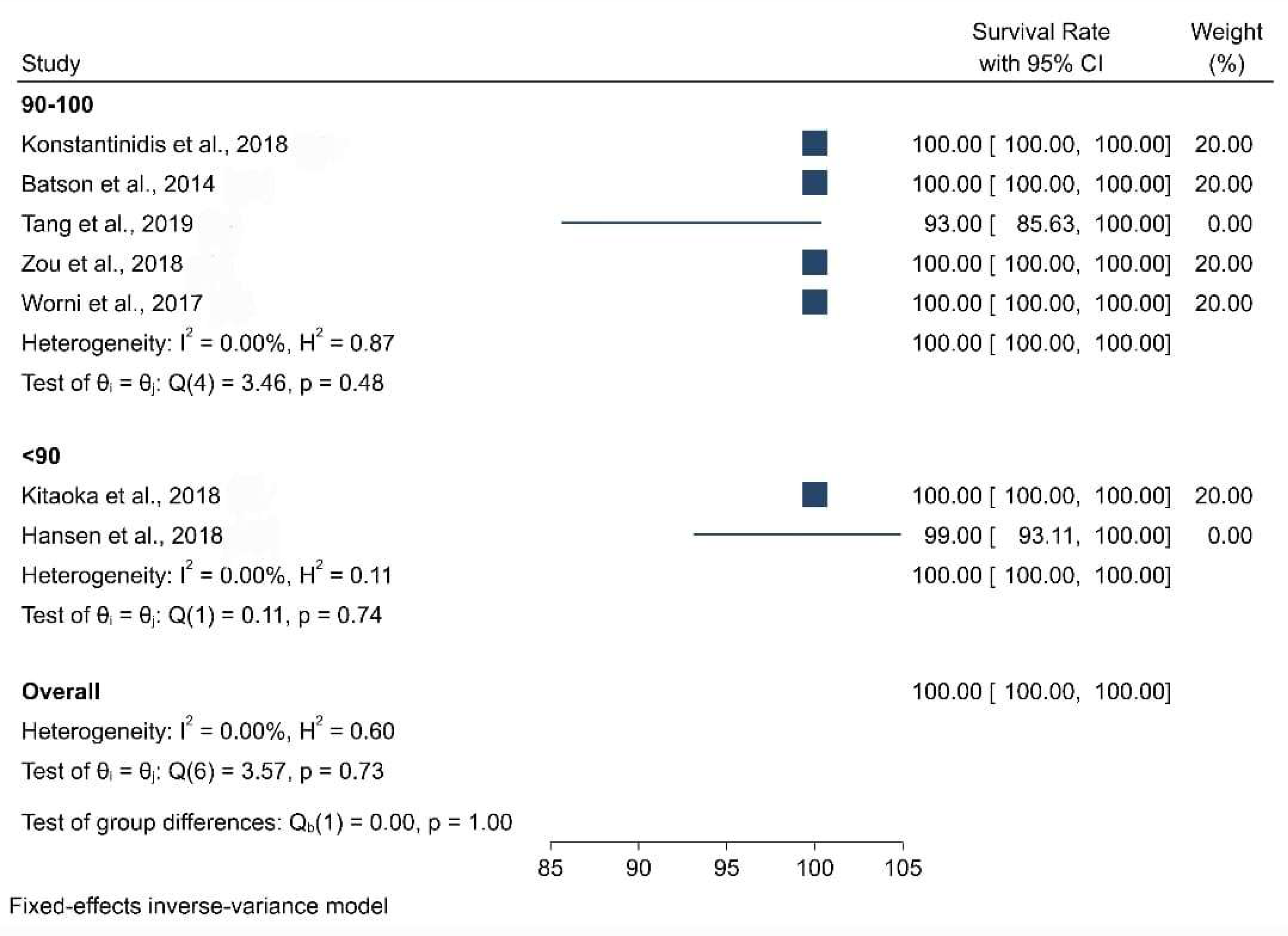
3.3. Clinical Studies
3.4. In Vitro Studies That Included the 3D Printing of Zirconium Crowns
4. Discussion
5. Conclusions
- Milled and 3D-printed zirconium crowns have shown superior biological characteristics.
- Zirconia crowns made using additive and subtractive manufacturing methods both had a similar internal fit and marginal adaptation.
- Zirconia crowns that are 3D-printed or milled can be used as alternatives to traditional prosthetics.
- Additional in vitro and in vivo investigations are required to assess the mechanical and optical qualities of 3D-printed zirconia crowns, among other factors.
- Long-term studies with a greater sample size utilizing diverse production procedures are needed to thoroughly establish the potential benefits of zirconia and to assert its superiority over other treatment options.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schweiger, J.; Edelhoff, D.; Güth, J.-F. 3D Printing in Digital Prosthetic Dentistry: An Overview of Recent Developments in Additive Manufacturing. J. Clin. Med. 2021, 10, 2010. [Google Scholar] [CrossRef]
- Schweiger, J.; Bomze, D.; Schwentenwein, M. 3D Printing of Zirconia–What is the Future? Curr. Oral Health Rep. 2019, 6, 339–343. [Google Scholar] [CrossRef]
- Denry, I.; Kelly, J. State of the art of zirconia for dental applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef]
- Khanlar, L.N.; Salazar Rios, A.; Tahmaseb, A.; Zandinejad, A. Additive Manufacturing of Zirconia Ceramic and Its Application in Clinical Dentistry: A Review. Dent. J. 2021, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Lerner, H.; Nagy, K.; Pranno, N.; Zarone, F.; Admakin, O.; Mangano, F. Trueness and precision of 3D-printed versus milled monolithic zirconia crowns: An in vitro study. J. Dent. 2021, 113, 103792. [Google Scholar] [CrossRef]
- Wang, W.; Sun, J. Dimensional accuracy and clinical adaptation of ceramic crowns fabricated with the stereolithography technique. J. Prosthet. Dent. 2021, 125, 657–663. [Google Scholar] [CrossRef]
- Wang, W.; Yu, H.; Liu, Y.; Jiang, X.; Gao, B. Trueness analysis of zirconia crowns fabricated with 3-dimensional printing. J. Prosthet. Dent. 2019, 121, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Hong, M.-H.; Min, B.-K.; Kim, Y.-K.; Shin, H.-J.; Kwon, T.-Y. Microstructure, Flexural Strength, and Fracture Toughness Comparison between CAD/CAM Milled and 3D-Printed Zirconia Ceramics. Appl. Sci. 2022, 12, 9088. [Google Scholar] [CrossRef]
- Abualsaud, R.; Alalawi, H. Fit, Precision, and Trueness of 3D-Printed Zirconia Crowns Compared to Milled Counterparts. Dent. J. 2022, 10, 215. [Google Scholar] [CrossRef]
- Stawarczyk, B.; Keul, C.; Eichberger, M.; Figge, D.; Edelhoff, D.; Lümkemann, N. Three generations of zirconia: From veneered to monolithic. Part I. Quintessence Int. 2017, 48, 369–380. [Google Scholar]
- Abualsaud, R.; Abussaud, M.; Assudmi, Y.; Aljoaib, G.; Khaled, A.; Alalawi, H.; Akhtar, S.; Matin, A.; Gad, M.M. Physiomechanical and Surface Characteristics of 3D-Printed Zirconia: An In Vitro Study. Materials 2022, 15, 6988. [Google Scholar] [CrossRef]
- Osman, R.; van der Veen, A.J.; Huiberts, D.; Wismeijer, D.; Alharbi, N. 3D-printing zirconia implants; a dream or a reality? An in-vitro study evaluating the dimensional accuracy, surface topography and mechanical properties of printed zirconia implant and discs. J. Mech. Behav. Biomed. Mater. 2017, 75, 521–528. [Google Scholar] [CrossRef]
- Methani, M.M.; Revilla-Leon, M.; Zandinejad, A. The potential of additive manufacturing technologies and their processing parameters for the fabrication of all-ceramic crowns: A review. J. Esthet. Restor. Dent. 2020, 32, 182–192. [Google Scholar] [CrossRef]
- Nakai, H.; Inokoshi, M.; Nozaki, K.; Komatsu, K.; Kamijo, S.; Liu, H.; Shimizubata, M.; Minakuchi, S.; Van Meerbeek, B.; Vleugels, J.; et al. Additively manufactured zirconia for dental applications. Materials 2021, 14, 3694. [Google Scholar] [CrossRef]
- Ramos, G.; Monteiro, E.; Bottino, M.; Zhang, Y.; Marques de Melo, R. Failure Probability of Three Designs of Zirconia Crowns. Int. J. Periodontics Restor. Dent. 2015, 35, 843–849. [Google Scholar] [CrossRef]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Revilla-León, M.; Methani, M.M.; Morton, D.; Zandinejad, A. Internal and marginal discrepancies associated with stereolithography (SLA) additively manufactured zirconia crowns. J. Prosthet. Dent. 2020, 124, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, R.; Triulzio, C.; Tricarico, M.G.; Bonadeo, G.; Gherlone, E.F.; Ferrari, M. In vitro analysis of the fracture resistance of CAD–CAM monolithic zirconia molar crowns with different occlusal thickness. J. Mech. Behav. Biomed. Mater. 2016, 61, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Kontonasaki, E.; Rigos, A.E.; Ilia, C.; Istantsos, T. Monolithic Zirconia: An Update to Current Knowledge. Optical Properties, Wear, and Clinical Performance. Dent. J. 2019, 7, 90. [Google Scholar] [CrossRef]
- Batson, E.R.; Cooper, L.F.; Duqum, I.; Mendonça, G. Clinical outcomes of three different crown systems with CAD/CAM technology. J. Prosthet. Dent. 2014, 112, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, I.; Trikka, D.; Gasparatos, S.; Mitsias, M. Clinical Outcomes of Monolithic Zirconia Crowns with CAD/CAM Technology. A 1-Year Follow-Up Prospective Clinical Study of 65 Patients. Int. J. Environ. Res. Public Health 2018, 15, 2523. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhao, X.; Wang, H.; Liu, B. Clinical evaluation of monolithic zirconia crowns for posterior teeth restorations. Medicine 2019, 98, e17385. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, A.; Akatsuka, R.; Kato, H.; Yoda, N.; Sasaki, K. Clinical Evaluation of Monolithic Zirconia Crowns: A Short-Term Pilot Report. Int. J. Prosthodont. 2018, 31, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.L.; Schriwer, C.; Øilo, M.; Gjengedal, H. Monolithic zirconia crowns in the aesthetic zone in heavy grinders with severe tooth wear—An observational case-series. J. Dent. 2018, 72, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Worni, A.; Katsoulis, J.; Kolgeci, L.; Worni, M.; Mericske-Stern, R. Monolithic zirconia reconstructions supported by teeth and implants: 1- to 3-year results of a case series. Quintessence Int. 2017, 48, 459–467. [Google Scholar]
- Gunge, H.; Ogino, Y.; Kihara, M.; Tsukiyama, Y.; Koyano, K. Retrospective clinical evaluation of posterior monolithic zirconia restorations after 1 to 3.5 years of clinical service. J. Oral Sci. 2018, 60, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Bai, J.; Xiang, J. Clinical performance of CAD/CAM-fabricated monolithic zirconia endocrowns on molars with extensive coronal loss of substance. Int. J. Comput. Dent. 2018, 21, 225–232. [Google Scholar] [PubMed]
- Belli, R.; Petschelt, A.; Hofner, B.; Hajtó, J.; Scherrer, S.S.; Lohbauer, U. Fracture Rates and Lifetime Estimations of CAD/CAM All-ceramic Restorations. J. Dent. Res. 2016, 95, 67–73. [Google Scholar] [CrossRef]
- Shillingburg, H.T., Jr.; Sather, D.A.; Wilson, E.L., Jr.; Cain, J.R.; Mitchell, D.L.; Blanco, L.J.; Kessler, J.C. Fundamentals of Fixed Prosthodontics, 4th ed.; Quintessence Publishing Co., Ltd.: Batavia, IL, USA, 2012; 574p. [Google Scholar]
- Pjetursson, B.E.; Lang, N.P. Prosthetic treatment planning on the basis of scientific evidence. J. Oral Rehabil. 2008, 35 (Suppl. 1), 72–79. [Google Scholar] [CrossRef]
- Stawarczyk, B.; Özcan, M.; Schmutz, F.; Trottmann, A.; Roos, M.; Hämmerle, C.H.F. Two-body wear of monolithic, veneered and glazed zirconia and their corresponding enamel antagonists. Acta Odontol. Scand. 2013, 71, 102–112. [Google Scholar] [CrossRef]
- Rosentritt, M.; Preis, V.; Behr, M.; Hahnel, S.; Handel, G.; Kolbeck, C. Two-body wear of dental porcelain and substructure oxide ceramics. Clin. Oral Investig. 2012, 16, 935–943. [Google Scholar] [CrossRef]
- Borba, M.; de Araújo, M.D.; Fukushima, K.A.; Yoshimura, H.N.; Cesar, P.F.; Griggs, J.A.; Bona, Á.D. Effect of the microstructure on the lifetime of dental ceramics. Dent. Mater. 2011, 27, 710–721. [Google Scholar] [CrossRef]
- Gonzaga, C.C.; Cesar, P.F.; Miranda, W.G., Jr.; Yoshimura, H.N. Slow crack growth and reliability of dental ceramics. Dent. Mater. 2011, 27, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Lohbauer, U.; Petschelt, A.; Greil, P. Lifetime prediction of CAD/CAM dental ceramics. J. Biomed. Mater. Res. 2002, 63, 780–785. [Google Scholar] [CrossRef]
- Sayed, M.E.; Dewan, H.; Kharaf, R.; Athlawi, M.; Alfaifi, M.; Mugri, M.H.; Bosly, R.A.-A.; Fageehi, N.Y.; Hadi, M.; Zurbtan, B.J.; et al. Efficacy of Three Commercially Available Desensitizers in Reducing Post-Operative Sensitivity Following Composite Restorations: A Randomized Controlled Clinical Trial. Polymers 2022, 14, 1417. [Google Scholar] [CrossRef] [PubMed]
- Taskonak, B.; Griggs, J.A.; Mecholsky, J.J., Jr.; Yan, J.H. Analysis of subcritical crack growth in dental ceramics using fracture mechanics and fractography. Dent. Mater. 2008, 24, 700–707. [Google Scholar] [CrossRef]
- Cattani-Lorente, M.; Scherrer, S.S.; Ammann, P.; Jobin, M.; Wiskott, H.W.A. Low temperature degradation of a Y-TZP dental ceramic. Acta Biomater. 2011, 7, 858–865. [Google Scholar] [CrossRef]
- Nakamura, K.; Harada, A.; Kanno, T.; Inagaki, R.; Niwano, Y.; Milleding, P.; Örtengren, U. The influence of low-temperature degradation and cyclic loading on the fracture resistance of monolithic zirconia molar crowns. J. Mech. Behav. Biomed. Mater. 2015, 47, 49–56. [Google Scholar] [CrossRef]
- Boitelle, P.; Mawussi, B.; Tapie, L.; Fromentin, O. A systematic review of CAD/CAM fit restoration evaluations. J. Oral Rehabil. 2014, 41, 853–874. [Google Scholar] [CrossRef]
- Håff, A.; Löf, H.; Gunne, J.; Sjögren, G. A retrospective evaluation of zirconia-fixed partial dentures in general practices: An up to 13-year study. Dent. Mater. Off. Publ. Acad. Dent. Mat. 2015, 31, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Nakamura, T.; Matsumura, H.; Ban, S.; Kobayashi, T. Current status of zirconia restoration. J. Prosthodont. Res. 2013, 57, 236–261. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.; Marques, T.R.; Araujo, F.; Azevedo, L.F.; Donato, H.; Correia, A. Clinical Performance of CAD/CAM Tooth-Supported Ceramic Restorations: A Systematic Review. Int. J. Periodontics Restor. Dent. 2018, 38, 68–78. [Google Scholar] [CrossRef]
- Nedelcu, R.; Olsson, P.; Nyström, I.; Thor, A. Finish line distinctness and accuracy in 7 intraoral scanners versus conventional impression: An in vitro descriptive comparison. BMC Oral Health 2018, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Mangano, F.; Veronesi, G. Digital versus Analog Procedures for the Prosthetic Restoration of Single Implants: A Randomized Controlled Trial with 1 Year of Follow-Up. BioMed Res. Int. 2018, 2018, 5325032. [Google Scholar] [CrossRef]
- Limones, A.; Molinero-Mourelle, P.; Azevedo, L.; Romeo-Rubio, M.; Correia, A.; Gómez-Polo, M. Zirconia-ceramic versus metal-ceramic posterior multiunit tooth-supported fixed dental prostheses: A systematic review and meta analysis of randomized controlled trials. J. Am. Dent. Assoc. 2020, 151, 230–238.e7. [Google Scholar] [CrossRef]
- Sailer, I.; Makarov, N.A.; Thoma, D.S.; Zwahlen, M.; Pjetursson, B.E. All-ceramic or metal-ceramic tooth-supported fixed dental prostheses (FDPs)? A systematic review of the survival and complication rates. Part I: Single crowns (SCs). Dent. Mater. 2015, 3, 603–623. [Google Scholar] [CrossRef]
- Kontos, L.; Schille, C.; Schweizer, E.; Geis-Gerstorfer, J. Influence of surface treatment on the wear of solid zirconia. Acta Odontol. Scand. 2013, 71, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Mörmann, W.H. Clinical performance of chairside CAD/CAM feldspathic ceramic posterior shoulder crowns and endocrowns up to 12 years. Int. J. Comput. Dent. 2015, 18, 147–161. [Google Scholar] [PubMed]
| Inclusion Criteria | Exclusion criteria |
|---|---|
| All published studies that reported the 3D milling and 3D printing of zirconia crowns performed in human subjects | Studies performed in animal subjects |
| In vitro studies (since there were no clinical studies for 3D-printed zirconium) performed using 3D-printed zirconia | Gray literature |
| Studies published in English | Meta-analysis articles, Narrative reviews, Systematic reviews |
| Cross-sectional studies | Letters to the editors |
| Longitudinal studies | Studies whose full text was unavailable, Abstracts only |
| Randomized and non-randomized control trials | Commentaries |
| PubMed | ((Monolithic Zirconia Crowns) OR (Crowns)) OR (Dental Porcelain) OR (Dental Ceramic) OR (Zirconia) OR (Dental Prosthesis) OR (Dental Crown) OR (Ceramics) AND (CAD CAM) OR (Subtractive Manufacturing) OR (3D milling) OR (Digital one-piece casting) AND (3D printing) OR (Printing three dimensional) OR (3D printing using zirconia) OR (Direct inkjet printing) OR (Stereolithography) OR (Additive manufacturing technologies) OR (Additive technology) AND (Marginal adaptation) OR (Biaxial flexural strength) OR (Colour infiltration) OR (mechanical properties) OR (Strength) OR (Marginal fit) OR (Trueness) OR (Clinical precision) | 564 |
| Google scholar | Monolithic Zirconia Crowns, Crowns, Dental Porcelain, Dental Ceramic, Zirconia, Dental Prosthesis, Dental Crown, Ceramics, CAD CAM, Subtractive Manufacturing, 3D milling, Digital one-piece casting, 3D printing, Printing three dimensional, 3D printing using zirconia, Direct inkjet printing, Stereolithography, Additive manufacturing technologies, Additive technology, Marginal adaptation, Biaxial flexural strength, Colour infiltration, mechanical properties, Strength, Marginal fit, Trueness, Clinical precision | 37 |
| Cochrane | ID Search Hits #1 MeSH descriptor: [Crowns] explode all trees 864 #2 MeSH descriptor: [Dental Porcelain] explode all trees 380 #3 MeSH descriptor: [Ceramics] explode all trees 684 #4 MeSH descriptor: [Computer-Aided Design] explode all trees 360 #5 MeSH descriptor: [Printing, Three-Dimensional] explode all trees 114 #6 MeSH descriptor: [Stereolithography] explode all trees 2 #7 MeSH descriptor: [Dental Marginal Adaptation] explode all trees 535 #8 (Strength): ti, ab, kw (Word variations have been searched) 46545 #9 Monolithic zirconia crowns 67 #10 3D printing 463 #11 Milling 191 #12 CAD CAM 513 #13 Zirconia 627 #14 Dental Ceramic 945 #15 Zirconia 627 #16 Dental Prosthesis 2182 #17 Digital one-piece casting 1 #18 3D printing using zirconia 1 #19 Biaxial flexural strength 13 #20 Colour infiltration 113 #21 Partial sintering 8 #22 Additive technology 196 #23 Dental crown 1366 #24 Direct inkjet printing 0 #25 Mechanical properties 2035 #26 Biomedical applications 306 #27 Marginal fit 338 #28 Digital impression 374 #29 Intraoral scanner 96 | 549 |
| Authors | Selection (up to 4 *) | Comparability (up to 2 *) | Outcome (up to 3 *) | Total | Interpretation |
|---|---|---|---|---|---|
| Batson et al. [20] | ** | ** | 4/9 | Moderate | |
| Konstantinidis et al. [21] | *** | *** | 6/9 | Moderate | |
| Tang et al. [22] | ** | ** | 4/9 | Moderate | |
| Kitaoka et al. [23] | ** | ** | 4/9 | Moderate | |
| Hansen et al. [24] | ** | ** | ** | 6/9 | Moderate |
| Worni et al. [25] | ** | *** | 5/9 | Moderate | |
| Gunge et al. [26] | ** | ** | ** | 6/9 | Moderate |
| Zou et al. [27] | ** | *** | 5/9 | Moderate | |
| Belli et al. [28] | ** | ** | 4/9 | Moderate |
| Article (Authors/Year of Publication) | Batson et al., 2014 [20] | Konstantinidis et al., 2018 [21] | Tang et al., 2019 [22] | Kitaoka et al., 2018 [23] | Hansen et al., 2018 [24] | Worni et al., 2017 [25] | Gunge et al., 2018 [26] | Zou et al., 2018 [27] | Belli et al., 2015 [28] |
|---|---|---|---|---|---|---|---|---|---|
| Age | Data not found | 49.52 | 41.3 | 54 | 56.3 | 59.1 | >20 | 37 | Data not found |
| Individuals (n) | 22 | 65 | 46 | 18 | 13 | 40 | 101 | 289 | Data not found |
| Zirconia Restorations (n) | 10 | 65 | 49 | 26 | 84 | 238 | 148 | 321 | 716 |
| Glaze/Stain (Yes or No) | Yes | Yes | No | No | No | Yes | Yes | No | Data not found |
| Location (Anterior/Posterior) | Posterior | Posterior | Posterior | Posterior | Anterior | Posterior/Anterior | Posterior | Posterior | Posterior |
| Presence/Absence of Plaque | Data not found | Absence | Presence | Presence | Presence | Presence | Data not found | Data not found | Data not found |
| Surface Treatment | Data not found | Data not found | Final polishing | Data not found | Data not found | Data not found | Final polishing | Data not found | Data not found |
| Marginal Integrity | 90% | 93.80% | 100% | 88.46% | 31.60% | 100% | Data not found | 98.80% | Data not found |
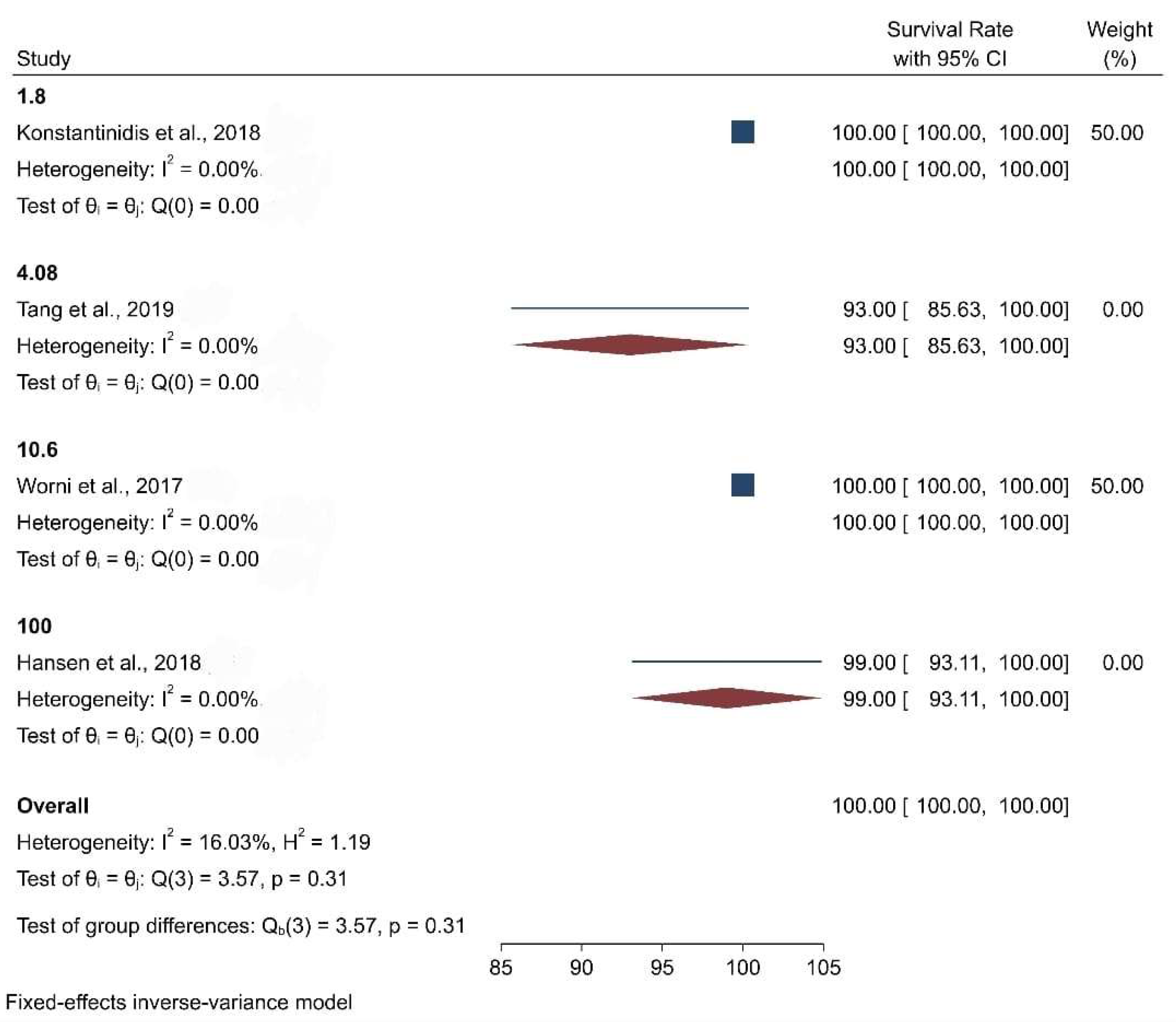
| Bleeding on Probing (BOP) | No alteration | 1.80% | 4.08% | No alteration | 100% | 10.60% | Data not found | Data not found | Data not found |
| Color Stability (Yes or No) | No alteration | No | Yes | No | Yes | No | Data not found | Yes | Data not found |
| Dental Vitality (n) | Data not found | 19 | Data not found | 3 | Data not found | 0 | 0 | 0 | Data not found |
| Failures (n) | Data not found | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 |
| Survival Rate | Data not found | 100% | 93% | 100% | 98.81% | 100% | 91% | 100% | 100% |
| Studies | Property Measured | Number of Ceramic Crowns | Techniques/Machines | Result | Conclusion |
|---|---|---|---|---|---|
| Wang, W. 2021 [6] | Dimensional accuracy and clinical adaptation | 10 | A conventional CAD–CAM system, X-MILL500 (XM) zirconia and 2 different stereolithography systems, CeraFab7500 (CF) alumina and CSL150 (CL) zirconia | CeraFab7500 (41 ± 11 mm) had better dimensional accuracy than CSL150 (65 ± 6 mm) or X-MILL500 (72 ± 13 mm) (P.05) | Better adaptation in the marginal, corner, and occlusal areas for X-MILL500 but reduced adaptation compared to CeraFab7500 and CSL150 (P.05) in the axial area |
| Wang, W. 2019 [7] | 3D trueness | 10 | 3D-printing system (CERAMAKER 900; 3DCeram Co) 5-axis, 2-bur milling machine (DWX-50; Roland DG Corp) for processing of the ZrO2 block (Zenostar; Wieland Dental) | The trueness values for both the systems had p-value less than 0.05 | The trueness of all the surfaces of 3D-printed crowns equaled the trueness of the CAD-CAM crowns |
| Lerner, H. 2021 [5] | Marginal adaptation | 10 | (LCM) printer (Cerafab S65®, Lithoz, Vienna, Austria) 5-axis milling machine (DWX-52D®, DGShape, a Roland Company, Hamamatsu, Japan) | Median differences measured on margins and occlusal levels were 26.9 µm and 8.2 µm for printed and milled crowns, respectively | Statistically higher trueness in the milled crowns as compared to the 3D-printed ones |
| Kim, M.S. 2022 [8] | Microstructure, flexural strength, and fracture toughness | No Data | Milling machine (Zirkonzahn CAD/CAM System 5-TEC, Zirkonzahn), 3D printer (CeraMaker 900, 3DCeram) | The three-point flexural strength values of the Y-TZP ceramics produced by SM and SLA were 927 and 865 MPa, respectively | No significant changes in flexural strength (p = 0.242) or fracture toughness (p = 0.101) |
| Abualsaud, R. 2022 [9] | Internal fit, marginal adaptation, precision, and trueness | 20 | 5-axis milling machine (PrograMill PM7, Ivoclar Vivadent, Schaan, Liechtenstein) 3D-printer (CERAMAKER C900 Flex, 3DCeram Sinto, Bonnac-laCôte, France | At the occlusal (8.77 0.89 m) and intaglio (23.90 1.60 m) surfaces of 3D-printed crowns, the highest and lowest trueness values were observed | Similarities existed between the internal and marginal fits of the two production methods |
| Abulsaud, R. 2022 [11] | Physiomechanical and surface properties | 80 | stereo-lithography using a 3D-printer (CERAMAKER C900 Flex, 3DCeram Sinto, France) Dry milling using a 5-axis milling machine (PM7) | The greatest and lowest reported densities were milled (6.056 0.116 g/cm3) and tilted (5.942 0.266 g/cm3), respectively | The biaxial flexural strength of the milled group (1507.27 ± 340.10 MPa) were significantly higher than those of the 3D-printed groups (p < 0.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dewan, H. Clinical Effectiveness of 3D-Milled and 3D-Printed Zirconia Prosthesis—A Systematic Review and Meta-Analysis. Biomimetics 2023, 8, 394. https://doi.org/10.3390/biomimetics8050394
Dewan H. Clinical Effectiveness of 3D-Milled and 3D-Printed Zirconia Prosthesis—A Systematic Review and Meta-Analysis. Biomimetics. 2023; 8(5):394. https://doi.org/10.3390/biomimetics8050394
Chicago/Turabian StyleDewan, Harisha. 2023. "Clinical Effectiveness of 3D-Milled and 3D-Printed Zirconia Prosthesis—A Systematic Review and Meta-Analysis" Biomimetics 8, no. 5: 394. https://doi.org/10.3390/biomimetics8050394
APA StyleDewan, H. (2023). Clinical Effectiveness of 3D-Milled and 3D-Printed Zirconia Prosthesis—A Systematic Review and Meta-Analysis. Biomimetics, 8(5), 394. https://doi.org/10.3390/biomimetics8050394








