Remineralization Induced by Biomimetic Hydroxyapatite Toothpastes on Human Enamel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Four HAPs Used in Toothpastes
2.3. Preparation of Toothpastes
2.4. Study Protocol for Obtaining Enamel Slices
2.5. Enamel Treatment with Toothpaste
2.6. Methods
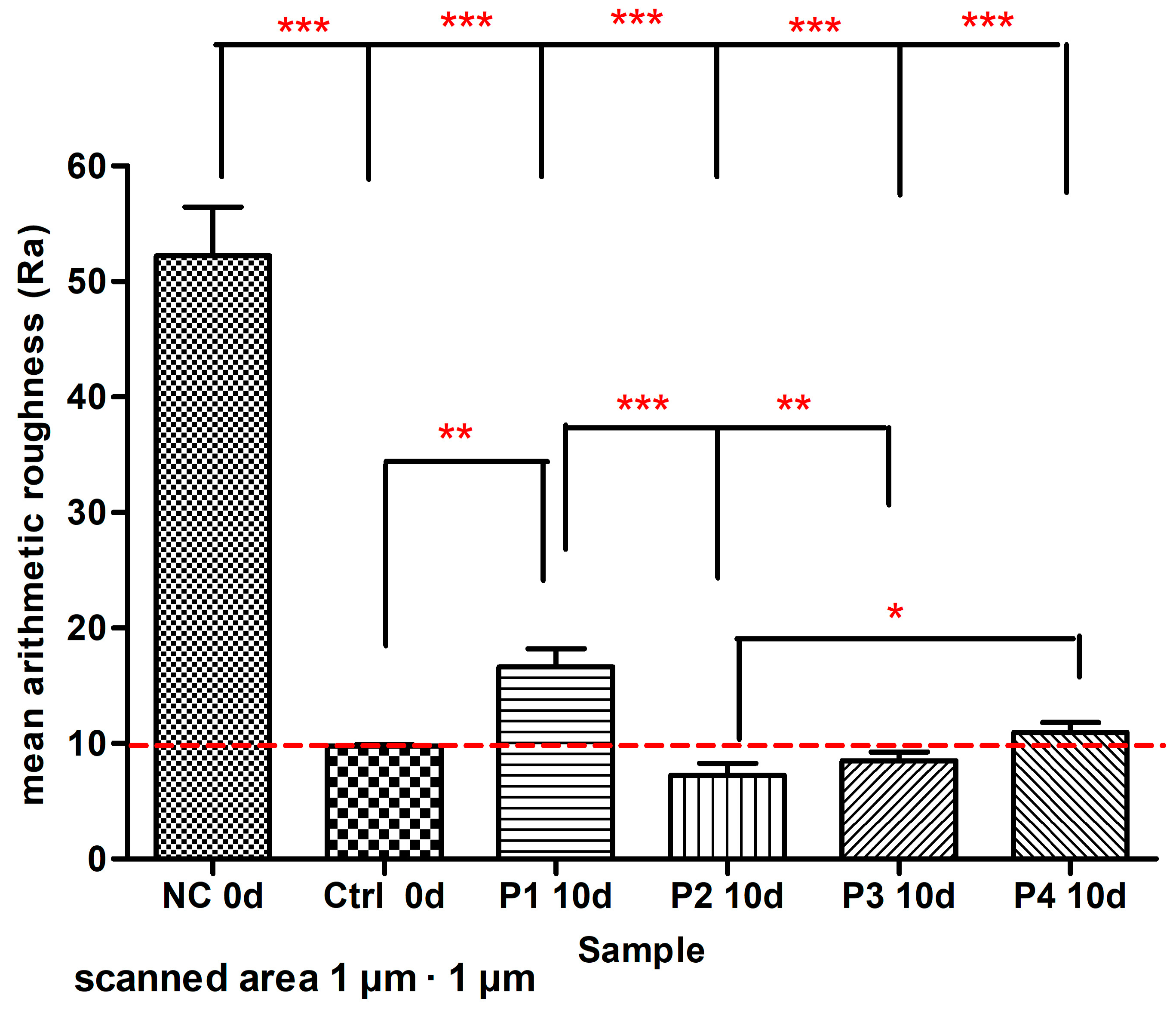
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruby, J.D.; Cox, C.F.; Akimoto, N.; Meada, N.; Momoi, Y. The caries phenomenon: A timeline from witchcraft and superstition to opinions of the 1500 s to today’s science. Int. J. Dent. 2010, 2010, 32767. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Torres Pino, E.C.; Orellana González, E. First evidence of pre-Hispanic dentistry in South America—Insights from Cusco, Peru. Homo 2016, 67, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Oxilia, G.; Peresani, M.; Romandini, M.; Matteucci, C.; Debono Spiteri, C.; Henry, A.G.; Schulz, D.; Archer, W.; Crezzini, J.; Boschin, F.; et al. Earliest evidence of dental caries manipulation in the Late Upper Palaeolithic. Sci. Rep. 2015, 5, 12150. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.C.; Chu, C.H.; Yu, O.Y. A concise review of dental sealants in caries management. Front. Oral Health 2023, 4, 1180405. [Google Scholar] [CrossRef] [PubMed]
- Warreth, A. Dental caries and its management. Int. J. Dent. 2023, 2023, 9365845. [Google Scholar] [CrossRef]
- Zhang, O.L.; Niu, J.Y.; Yin, I.X.; Yu, O.Y.; Mei, M.L.; Chu, C.H. Bioactive materials for caries management: A literature review. Dent. J. 2023, 11, 59. [Google Scholar] [CrossRef]
- Verhulst, M.J.L.; Loos, B.G.; Gerdes, V.E.A.; Teeuw, W.J. Evaluating all potential oral complications of diabetes mellitus. Front. Endocrinol. 2019, 10, 56. [Google Scholar] [CrossRef]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Chen, X.; Daliri, E.B.; Kim, N.; Kim, J.R.; Yoo, D.; Oh, D.H. Microbial etiology and prevention of dental caries: Exploiting natural products to inhibit cariogenic biofilms. Pathogens 2020, 9, 569. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Montasser, M.A.; Abd El Latief, M.H.; Modica, G.G.; Scribante, A. Home oral care with biomimetic hydroxyapatite vs. conventional fluoridated toothpaste for the remineralization and desensitizing of white spot lesions: Randomized clinical trial. Int. J. Environ. Res. Public Health 2022, 19, 8676. [Google Scholar] [CrossRef]
- Bossù, M.; Saccucci, M.; Salucci, A.; Di Giorgio, G.; Bruni, E.; Uccelletti, D.; Sarto, M.S.; Familiari, G.; Relucenti, M.; Polimeni, A. Enamel remineralization and repair results of Biomimetic Hydroxyapatite toothpaste on deciduous teeth: An effective option to fluoride toothpaste. J. Nanobiotechnol. 2019, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Limeback, H.; Enax, J.; Meyer, F. Biomimetic hydroxyapatite and caries prevention: A systematic review and meta-analysis. Can. J. Dent. Hyg. 2021, 55, 148–159. [Google Scholar]
- Scribante, A.; Dermenaki Farahani, M.R.; Marino, G.; Matera, C.; Rodriguez y Baena, R.; Lanteri, V.; Butera, A. Biomimetic effect of nano-hydroxyapatite in demineralized enamel before orthodontic bonding of brackets and attachments: Visual, adhesion strength, and hardness in In Vitro tests. Biomed. Res. Int. 2020, 2020, 6747498. [Google Scholar] [CrossRef]
- Bossù, M.; Matassa, R.; Relucenti, M.; Iaculli, F.; Salucci, A.; Di Giorgio, G.; Familiari, G.; Polimeni, A.; Di Carlo, S. Morpho-chemical observations of human deciduous teeth enamel in response to biomimetic toothpastes treatment. Materials 2020, 13, 1803. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S.; Fong, H.; Yucesoy, D.T.; Cousin, T.; Gresswell, C.; Dag, S.; Huang, G.; Sarikaya, M. Biomimetic tooth repair: Amelogenin-derived peptide enables inVitro remineralization of human enamel. ACS Biomater. Sci. Eng. 2018, 4, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Imran, E.; Cooper, P.R.; Ratnayake, J.; Ekambaram, M.; Mei, M.L. Potential beneficial effects of hydroxyapatite nanoparticles on caries lesions In Vitro—A Review of the literature. Dent. J. 2023, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Hiller, K.A.; Buchalla, W.; Grillmeier, I.; Neubauer, C.; Schmalz, G. In vitro effects of hydroxyapatite containing toothpastes on dentin permeability after multiple applications and ageing. Sci. Rep. 2018, 8, 4888. [Google Scholar] [CrossRef]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite—Past, present, and future in bone regeneration. Bone Tissue Regen. Insights 2016, 7, BTRI-S36138. [Google Scholar] [CrossRef]
- Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. Hydroxyapatite for biomedical applications: A short Overview. Ceramics 2021, 4, 542–563. [Google Scholar] [CrossRef]
- Tomoaia, G.; Mocanu, A.; Vida-Simiti, I.; Jumate, N.; Bobos, L.D.; Soritau, O.; Tomoaia-Cotisel, M. Silicon effect on the composition and structure of nanocalcium phosphates: In vitro biocompatibility to human osteoblasts. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 37, 37–47. [Google Scholar] [CrossRef]
- Mocanu, A.; Furtos, G.; Răpuntean, S.; Horovitz, O.; Flore, C.; Garbo, C.; Danisteanu, A.; Răpuntean, G.; Prejmerean, C.; Tomoaia-Cotisel, M. Synthesis; characterization and antimicrobial effects of composites based on multi-substituted hydroxyapatite and silver nanoparticles. Appl. Surf. Sci. 2014, 298, 225–235. [Google Scholar] [CrossRef]
- Limeback, H.; Enax, J.; Meyer, F. Clinical evidence of biomimetic hydroxyapatite in oral care products for reducing dentin hypersensitivity: An updated systematic Review and Meta-Analysis. Biomimetics 2023, 8, 23. [Google Scholar] [CrossRef]
- Chen, L.; Al-Bayatee, S.; Khurshid, Z.; Shavandi, A.; Brunton, P.; Ratnayake, J. Hydroxyapatite in oral care products-A review. Materials 2021, 14, 4865. [Google Scholar] [CrossRef]
- Limeback, H.; Meyer, F.; Enax, J. Tooth whitening with hydroxyapatite: A systematic review. Dent. J. 2023, 11, 50. [Google Scholar] [CrossRef]
- O’Hagan-Wong, K.; Enax, J.; Meyer, F.; Ganss, B. The use of hydroxyapatite toothpaste to prevent dental caries. Odontology 2022, 110, 223–230. [Google Scholar] [CrossRef]
- Amaechi, B.T.; AbdulAzees, P.A.; Alshareif, D.O.; Shehata, M.A.; Lima, P.P.C.S.; Abdollahi, A.; Kalkhorani, P.S.; Evans, V. Comparative efficacy of a hydroxyapatite and a fluoride toothpaste for prevention and remineralization of dental caries in children. BDJ Open. 2019, 5, 18. [Google Scholar] [CrossRef]
- Degli Esposti, L.; Ionescu, A.C.; Brambilla, E.; Tampieri, A.; Iafisco, M. Characterization of a toothpaste containing bioactive hydroxyapatites and In Vitro evaluation of its efficacy to remineralize enamel and to occlude dentinal tubules. Materials 2020, 13, 2928. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, J.T.B.; Mucalo, M.; Dias, G.J. Substituted hydroxyapatites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1285–1299. [Google Scholar] [CrossRef]
- Zastulka, A.; Clichici, S.; Tomoaia-Cotisel, M.; Mocanu, A.; Roman, C.; Olteanu, C.-D.; Culic, B.; Mocan, T. Recent trends in hydroxyapatite supplementation for osteoregenerative purposes. Materials 2023, 16, 1303. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Gallo, S.; Pascadopoli, M.; Quintini, M.; Lelli, M.; Tarterini, F.; Foltran, I.; Scribante, A. Biomimetic action of Zinc Hydroxyapatite on remineralization of enamel and dentin: A Review. Biomimetics 2023, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Kranz, S.; Heyder, M.; Mueller, S.; Guellmar, A.; Krafft, C.; Nietzsche, S.; Tschirpke, C.; Herold, V.; Sigusch, B.; Reise, M. Remineralization of artificially demineralized human enamel and dentin samples by zinc-carbonate hydroxyapatite nanocrystals. Materials 2022, 15, 7173. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Bhatia, A.; Varma, H. Development, characterization and comparison of two strontium doped nano hydroxyapatite molecules for enamel repair/regeneration. Dent. Mater. 2016, 32, 646–659. [Google Scholar] [CrossRef]
- Rapuntean, S.; Frangopol, P.T.; Hodisan, I.; Tomoaia, G.; Oltean-Dan, D.; Mocanu, A.; Prejmerean, C.; Soritau, O.; Racz, L.Z.; Tomoaia-Cotisel, M. In vitro response of human osteoblasts cultured on strontium substituted hydroxyapatites. Rev. Chim. 2018, 69, 3537-3444. [Google Scholar] [CrossRef]
- Ullah, I.; Siddiqui, M.A.; Kolawole, S.K.; Liu, H.; Zhang, J.; Ren, L.; Yang, K. Synthesis, characterization and in vitro evaluation of zinc and strontium binary doped hydroxyapatite for biomedical application. Ceram. Int. 2020, 46, 14448–14459. [Google Scholar] [CrossRef]
- Stanić, V.; Dimitrijević, S.; Antić-Stanković, J.; Mitrić, M.; Jokić, B.; Plećaš, I.B.; Raičević, S. Synthesis, characterization and antimicrobial activity of copper and zinc-doped hydroxyapatite nanopowders. Appl. Surf. Sci. 2010, 256, 6083–6089. [Google Scholar] [CrossRef]
- Uysal, I.; Severcan, F.; Tezcaner, A.; Evis, Z. Co-doping of hydroxyapatite with zinc and fluoride improves mechanical and biological properties of hydroxyapatite. Prog. Nat. Sci. Mater. Int. 2014, 24, 340–349. [Google Scholar] [CrossRef]
- Erdem, U.; Dogan, D.; Bozer, B.M.; Karaboga, S.; Turkoz, M.B.; Metin, A.Ü.; Yıldırım, G. Evolution of dynamics of physico-chemical and mechanical properties of hydroxyapatite with fluorine addition and degradation stability of new matrices. J. Mech. Behav. Biomed. Mater. 2022, 135, 105454. [Google Scholar] [CrossRef]
- Turkoz, M.; Atilla, A.O.; Evis, Z. Silver and fluoride doped hydroxyapatites: Investigation by microstructure, mechanical and antibacterial properties. Ceram. Int. 2013, 39, 8925–8931. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Degli Esposti, L.; Iafisco, M.; Brambilla, E. Dental tissue remineralization by bioactive calcium phosphate nanoparticles formulations. Sci. Rep. 2022, 12, 5994. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, S.; Yang, Z.; Yang, P.; Liu, A. High-strength and tough bioactive Mg-doped hydroxyapatite bioceramics with oriented microchannels. Ceram. Int. 2022, 48, 13494–13507. [Google Scholar] [CrossRef]
- Dasgupta, S.; Banerjee, S.S.; Bandyopadhyay, A.; Bose, S. Zn- and Mg-doped hydroxyapatite nanoparticles for controlled release of protein. Langmuir. 2010, 26, 4958–4964. [Google Scholar] [CrossRef] [PubMed]
- Vladescu, A.; Cotrut, C.M.; Ak Azem, F.; Bramowicz, M.; Pana, I.; Braic, V.; Birlik, I.; Kiss, A.; Braic, M.; Abdulgader, R.; et al. Sputtered Si and Mg doped hydroxyapatite for biomedical applications. Biomed. Mater. 2018, 13, 025011. [Google Scholar] [CrossRef] [PubMed]
- Iconaru, S.L.; Predoi, D.; Ciobanu, C.S.; Motelica-Heino, M.; Guegan, R.; Bleotu, C. Development of Silver doped hydroxyapatite thin films for biomedical applications. Coatings 2022, 12, 341. [Google Scholar] [CrossRef]
- Khonina, T.G.; Chupakhin, O.N.; Shur, V.Y.; Turygin, A.P.; Sadovsky, V.V.; Mandra, Y.V.; Sementsova, E.A.; Kotikova, A.Y.; Legkikh, A.V.; Nikitina, E.Y.; et al. Silicon-hydroxyapatite-glycerohydrogel as a promising biomaterial for dental applications. Colloids Surf. B 2020, 189, 110851. [Google Scholar] [CrossRef] [PubMed]
- Basiri, H.; Abouei Mehrizi, A.; Bakhshi, F. Synthesis and characterization of Magnesium hydroxyapatite nanopowders for enamel remineralization of initial caries lesions. Adv. Mater. Technol. 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Rajendran, R.; Nair, K.R.; Sandhya, R.; Ashik, P.M.; Veedu, R.P.; Saleem, S. Evaluation of remineralization potential and cytotoxicity of a novel strontium-doped nanohydroxyapatite paste: An in vitro study. J Conserv. Dent. 2020, 23, 330–336. [Google Scholar] [CrossRef]
- Xu, J.; Shi, H.; Luo, J.; Yao, H.; Wang, P.; Li, Z.; Wei, J. Advanced materials for enamel remineralization. Front. Bioeng. Biotechnol. 2022, 10, 985881. [Google Scholar] [CrossRef]
- Valkenburg, C.; Else Slot, D.; Van der Weijden, G.F. What is the effect of active ingredients in dentifrice on inhibiting the regrowth of overnight plaque? A systematic review. Int. J. Dent. Hyg. 2020, 18, 128–141. [Google Scholar] [CrossRef]
- Camargo, S.E.A.; Milhan, N.V.M.; Saraiva, F.O.; Oliveira, J.R.; Oliveira, L.D.; Camargo, C.H.R. Are desensitizing toothpastes equally biocompatible and effective against microorganisms? Braz. Dent. J. 2017, 28, 604–611. [Google Scholar] [CrossRef]
- Vranić, E.; Lacević, A.; Mehmedagić, A.; Uzunović, A. Formulation ingredients for toothpastes and mouthwashes. Bosn. J. Basic Med. Sci. 2004, 4, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Kanouté, A.; Dieng, S.N.; Diop, M.; Dieng, A.; Sene, A.K.; Diouf, M.; Lo, C.M.; Faye, D.; Carrouel, F. Chemical vs. natural toothpaste: Which formulas for which properties? A scoping review. J. Public Health Afr. 2022, 13, 1945. [Google Scholar] [CrossRef] [PubMed]
- Garbo, C.; Sindilaru, M.; Carlea, A.; Tomoaia, G.; Almasan, V.; Petean, I.; Mocanu, A.; Horovitz, O.; Tomoaia-Cotisel, M. Synthesis and structural characterization of novel porous zinc substituted nanohydroxyapatite powders. Part. Sci. Technol. 2017, 35, 29–37. [Google Scholar] [CrossRef]
- Oltean-Dan, D.; Dogaru, G.B.; Tomoaia-Cotisel, M.; Apostu, D.; Mester, A.; Benea, H.R.C.; Paiusan, M.G.; Jianu, E.M.; Mocanu, A.; Balint, R.; et al. Enhancement of bone consolidation using high-frequency pulsed electromagnetic short-waves and titanium implants coated with biomimetic composite embedded into PLA matrix: In vivo evaluation. Int. J. Nanomed. 2019, 14, 5799–5816. [Google Scholar] [CrossRef] [PubMed]
- Florea, D.A.; Mocanu, A.; Pop, L.C.; Tomoaia, G.; Dobrota, C.-T.; Varhelyi, C., Jr.; Tomoaia-Cotisel, M. Remineralization of tooth enamel with hydroxyapatite nanoparticles: An in vitro study. Stud. Univ. Babes Bolyai Chem. 2023, 68, 99–113. [Google Scholar] [CrossRef]
- Oltean-Dan, D.; Dogaru, G.-B.; Jianu, E.-M.; Riga, S.; Tomoaia-Cotisel, M.; Mocanu, A.; Barbu-Tudoran, L.; Tomoaia, G. Biomimetic composite coatings for activation of titanium implant surfaces: Methodological approach and in vivo enhanced osseointegration. Micromachines 2021, 12, 1352. [Google Scholar] [CrossRef]
- Garbo, C.; Locs, J.; D’Este, M.; Demazeau, G.; Mocanu, A.; Roman, C.; Horovitz, O.; Tomoaia-Cotisel, M. Advanced Mg, Zn, Sr, Si Multi-Substituted hydroxyapatites for bone regeneration. Int. J. Nanomed. 2020, 15, 1037–1058. [Google Scholar] [CrossRef]
- Mocanu, A.; Cadar, O.; Frangopol, P.T.; Petean, I.; Tomoaia, G.; Paltinean, G.A.; Racz, C.P.; Horovitz, O.; Tomoaia-Cotisel, M. Ion release from hydroxyapatite and substituted hydroxyapatites in different immersion liquids: In vitro experiments and theoretical modelling study. R. Soc. Open Sci. 2021, 8, 201785. [Google Scholar] [CrossRef]
- Mocanu, A.; Balint, R.; Garbo, C.; Timis, L.; Petean, I.; Horovitz, O.; Tomoaia-Cotisel, M. Low crystallinity nanohydroxyapatite prepared at room temperature. Stud. Univ. Babes Bolyai Chem. 2017, 62, 95–103. [Google Scholar] [CrossRef]
- Medeiros, M.I.; Carlo, H.L.; Lacerda-Santos, R.; Lima, B.A.G.; Sousa, F.B.; Rodrigues, J.A.; Carvalho, F.G. Thickness and nanomechanical properties of protective layer formed by TiF4 varnish on enamel after erosion. Braz. Oral Res. 2016, 30, S1806-83242016000100264. [Google Scholar] [CrossRef]
- Poggio, C.; Ceci, M.; Beltrami, R.; Lombardini, M.; Colombo, M. Atomic force microscopy study of enamel remineralization. Ann. Stomatol. 2014, 5, 98–102. [Google Scholar]
- Agrawal, N.; Shashikiran, N.D.; Singla, S.; Ravi, K.S.; Kulkarni, V.K. Atomic force microscopic comparison of remineralization with casein-phosphopeptide amorphous calcium phosphate paste, acidulated phosphate fluoride gel and iron supplement in primary and permanent teeth: An in-vitro study. Contemp. Clin. Dent. 2014, 5, 75–80. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Hillsdale; Lawrence Erlbaum Associates: New York, NY, USA, 1988; pp. 273–403. [Google Scholar]
- Racz, C.P.; Racz, L.Z.; Floare, C.G.; Tomoaia, G.; Horovitz, O.; Riga, S.; Kacso, I.; Borodi, G.; Sarkozi, M.; Mocanu, A.; et al. Curcumin and whey protein concentrate binding: Thermodynamic and structural approach. Food Hydrocoll. 2023, 139, 108547. [Google Scholar] [CrossRef]
- Zdrenghea, U.V.; Tomoaia, G.; Pop-Toader, D.-V.; Mocanu, A.; Horovitz, O.; Tomoaia-Cotisel, M. Procaine effect on human erythrocyte membrane explored by atomic force microscopy. Comb. Chem. High Throughput Screen. 2011, 14, 237–247. [Google Scholar] [CrossRef]
- Frangopol, P.T.; Mocanu, A.; Almasan, V.; Garbo, C.; Balint, R.; Borodi, G.; Bratu, I.; Horovitz, O.; Tomoaia-Cotisel, M. Synthesis and structural characterization of strontium substituted hydroxyapatites. Rev. Roum. Chim. 2016, 61, 337–344. [Google Scholar]
- Wang, J.; Liu, Z.; Ren, B.; Wang, Q.; Wu, J.; Yang, N.; Sui, X.; Li, L.; Li, M.; Zhang, X.; et al. Biomimetic mineralisation systems for in situ enamel restoration inspired by amelogenesis. J. Mater. Sci. Mater. Med. 2021, 32, 115. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xiao, Z.; Yang, J.; Lu, D.; Kishen, A.; Li, Y.; Chen, Z.; Que, K.; Zhang, Q.; Deng, X.; et al. Oriented and ordered biomimetic remineralization of the surface of demineralized dental enamel using HAP@ACP nanoparticles guided by glycine. Sci. Rep. 2017, 7, 40701. [Google Scholar] [CrossRef]
- Rodemer, T.; Pütz, N.; Hannig, M. Influence of hydroxyapatite nanoparticles on the formation of calcium fluoride surface layer on enamel and dentine in vitro. Sci. Rep. 2022, 12, 17612. [Google Scholar] [CrossRef]
- Vinoth Kumar, K.C.; Jani Subha, T.; Ahila, K.G.; Ravindran, B.; Chang, S.W.; Mahmoud, A.H.; Mohammed, O.B.; Rathi, M.A. Spectral characterization of hydroxyapatite extracted from Black Sumatra and Fighting cock bone samples: A comparative analysis. Saudi J. Biol. Sci. 2021, 28, 840–846. [Google Scholar] [CrossRef]
- Sahadat Hossain, M.; Ahmed, S. FTIR spectrum analysis to predict the crystalline and amorphous phases of hydroxyapatite: A comparison of vibrational motion to reflection. RSC Adv. 2023, 13, 14625–14630. [Google Scholar] [CrossRef]
- Bakan, F.; Laçin, O.; Sarac, H. A novel low temperature sol–gel synthesis process for thermally stable nano crystalline hydroxyapatite. Powder Technol. 2013, 233, 295–302. [Google Scholar] [CrossRef]
- Li, L.; Pan, H.; Tao, J.; Xu, X.; Mao, C.; Gub, X.; Tang, R. Repair of enamel by using hydroxyapatite nanoparticles as the building blocks. J. Mater. Chem. 2008, 18, 4079–4084. [Google Scholar] [CrossRef]
- Babayevska, N.; Woźniak-Budych, M.; Litowczenko, J.; Peplińska, B.; Jarek, M.; Florczak, P.; Bartkowiak, G.; Czarnecka, B.; Jurga, S. Novel nanosystems to enhance biological activity of hydroxyapatite against dental caries. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 124, 112062. [Google Scholar] [CrossRef] [PubMed]
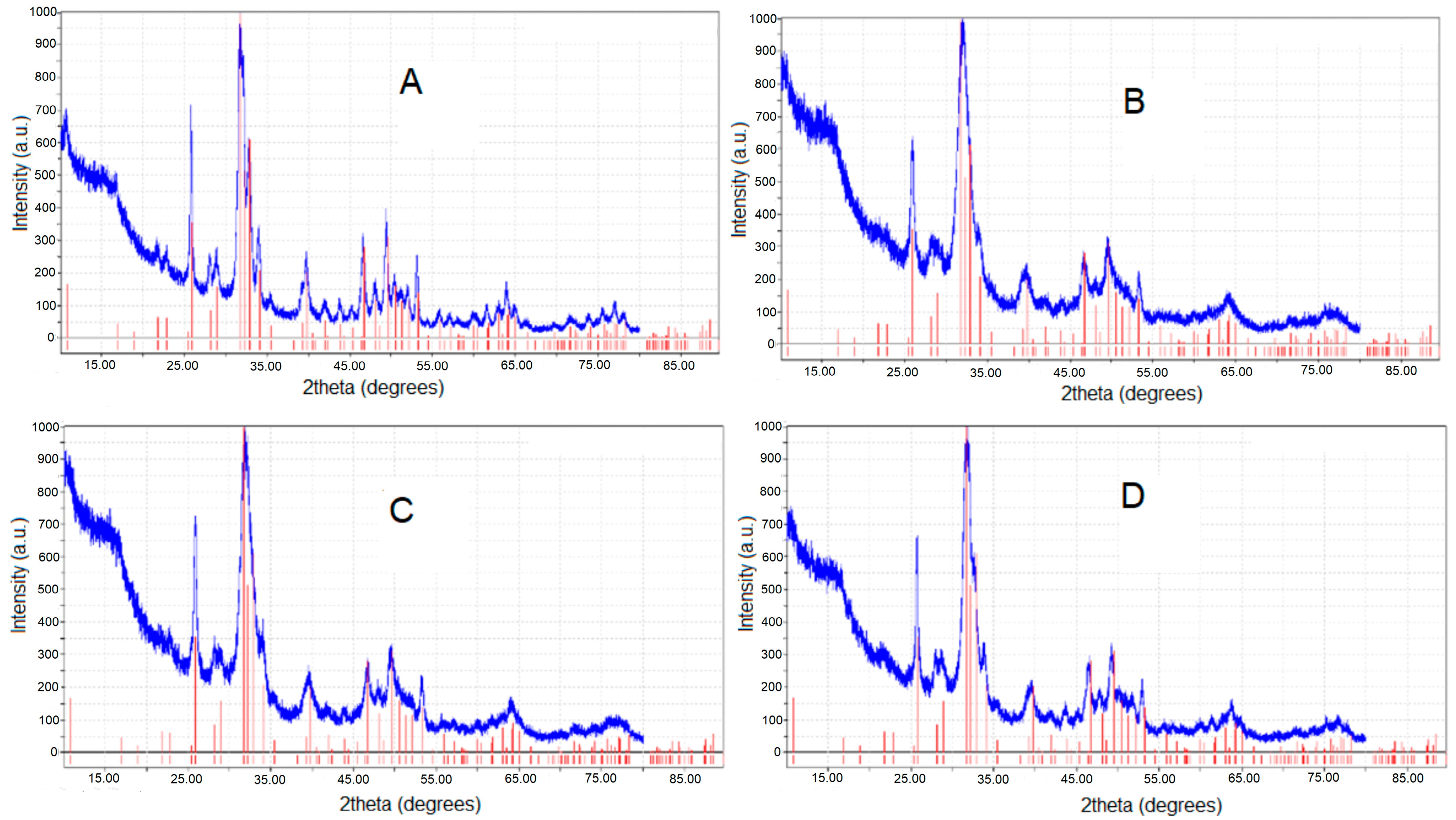
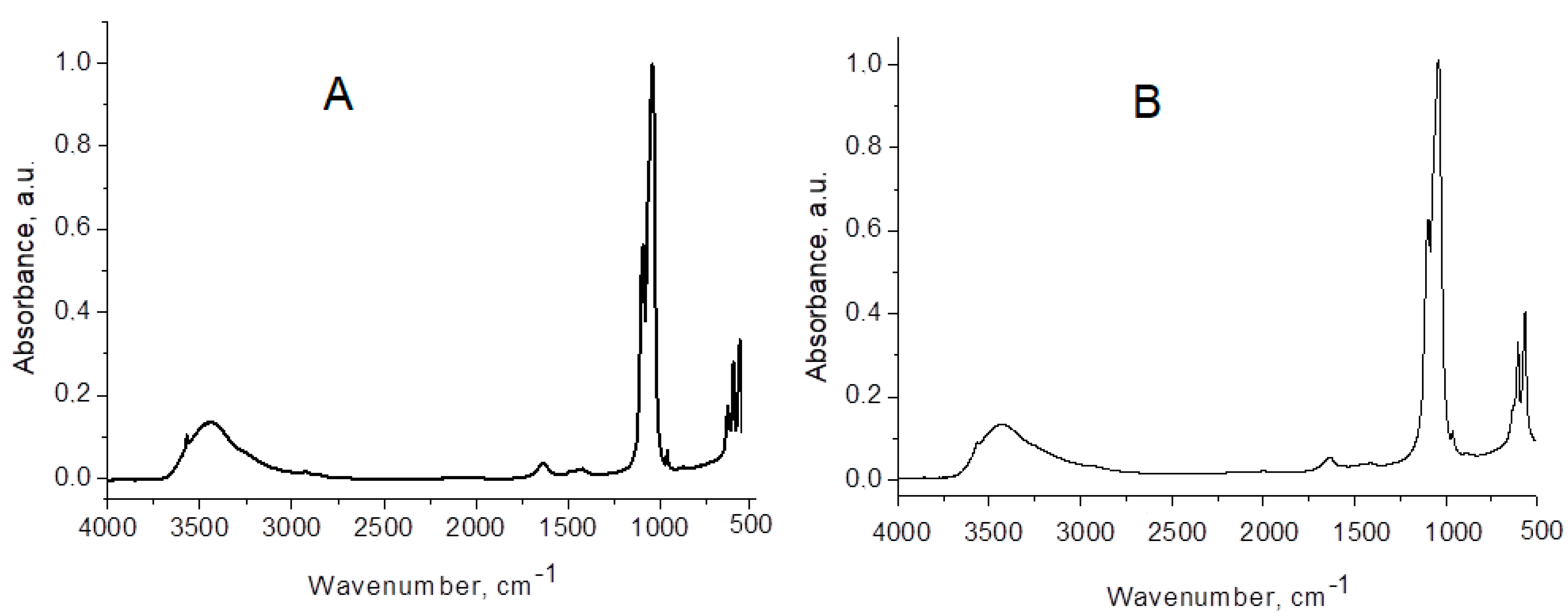
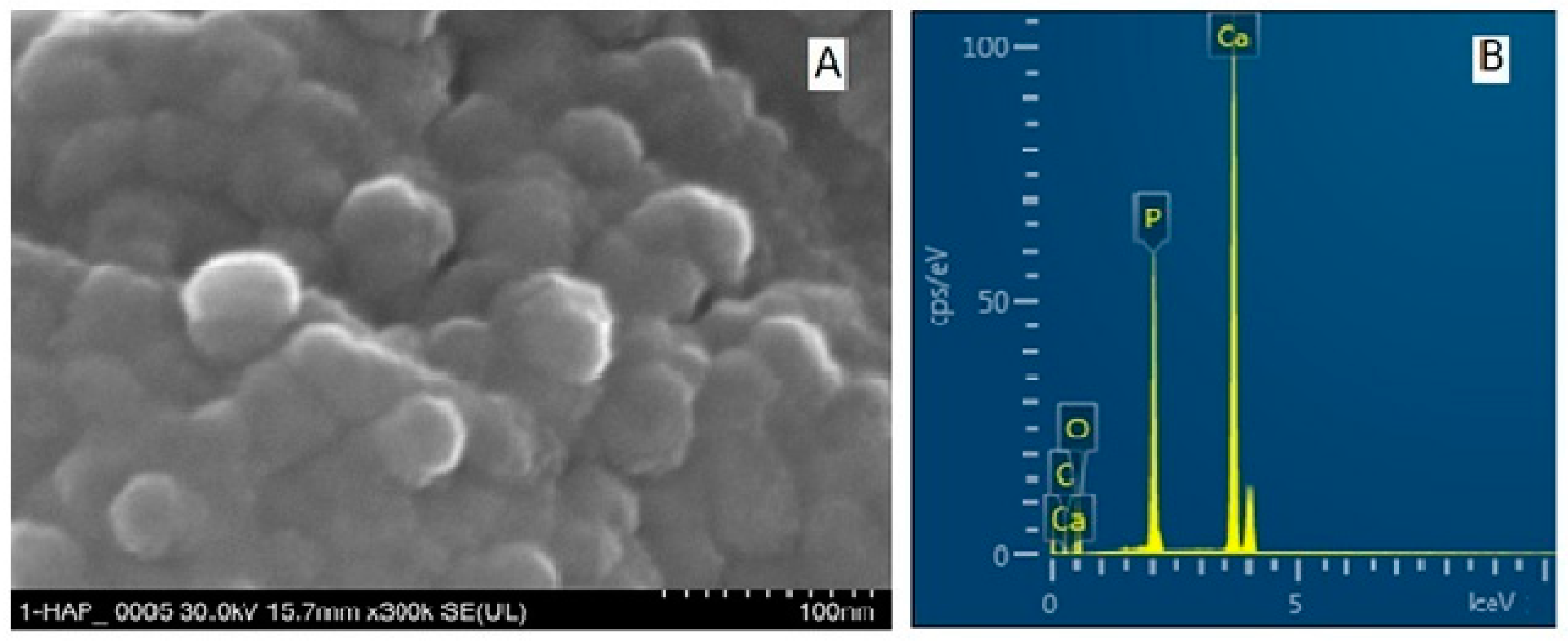
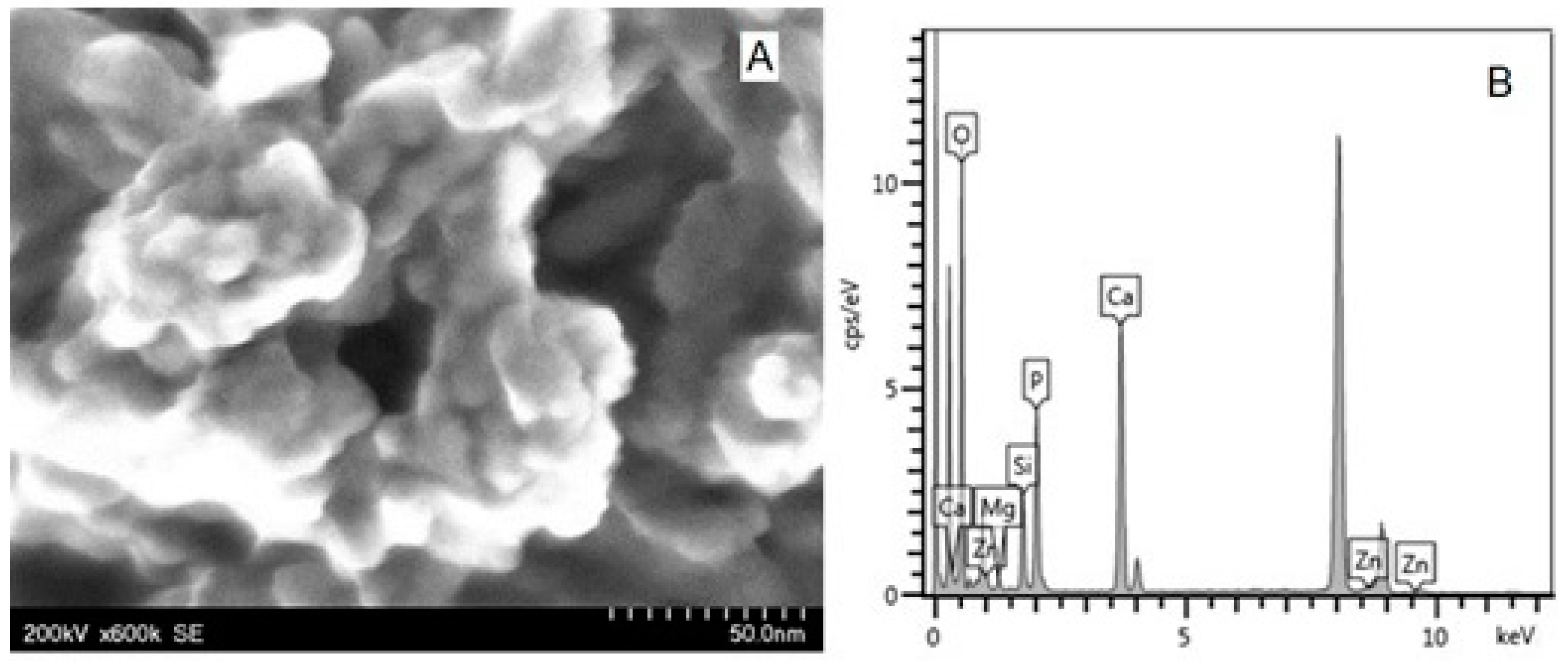
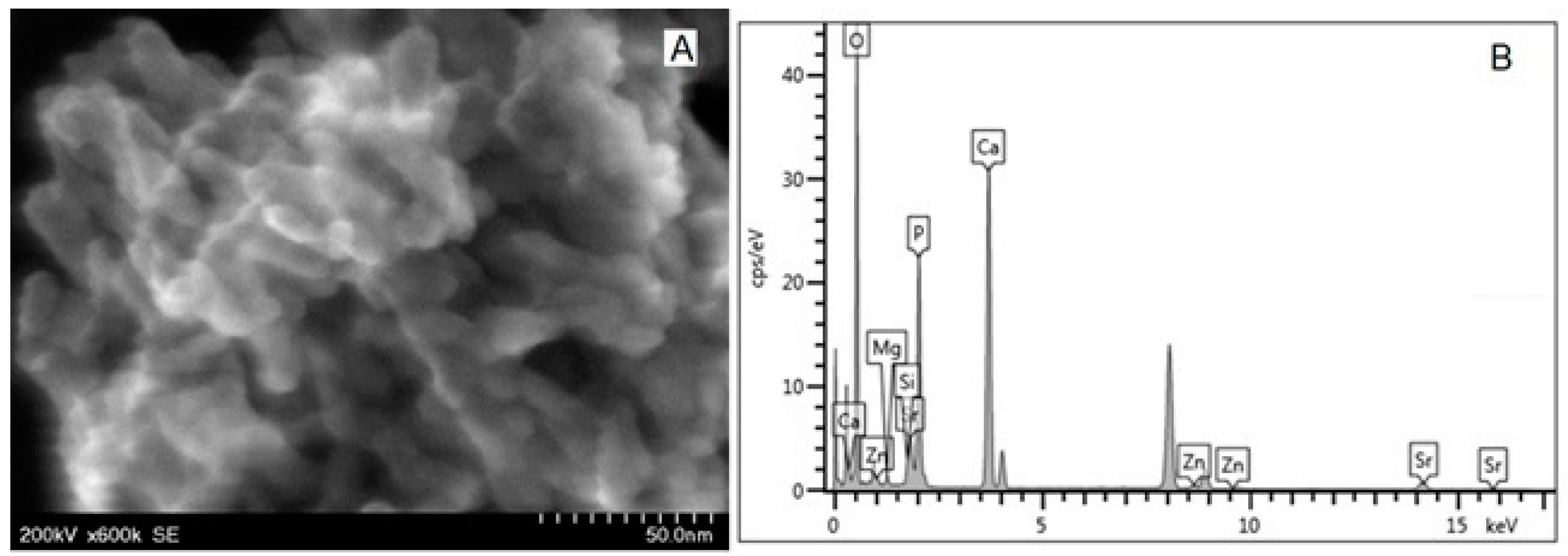
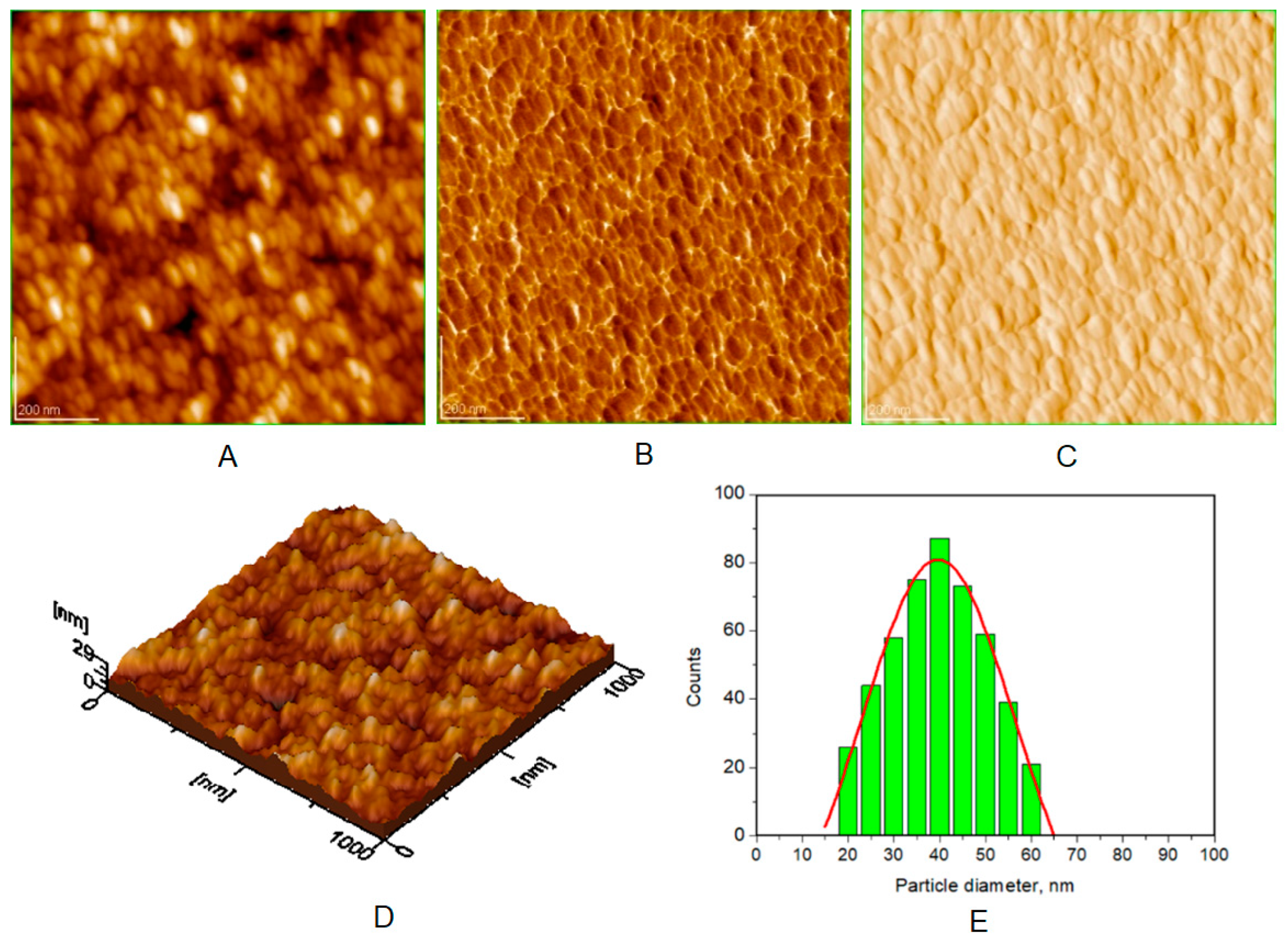
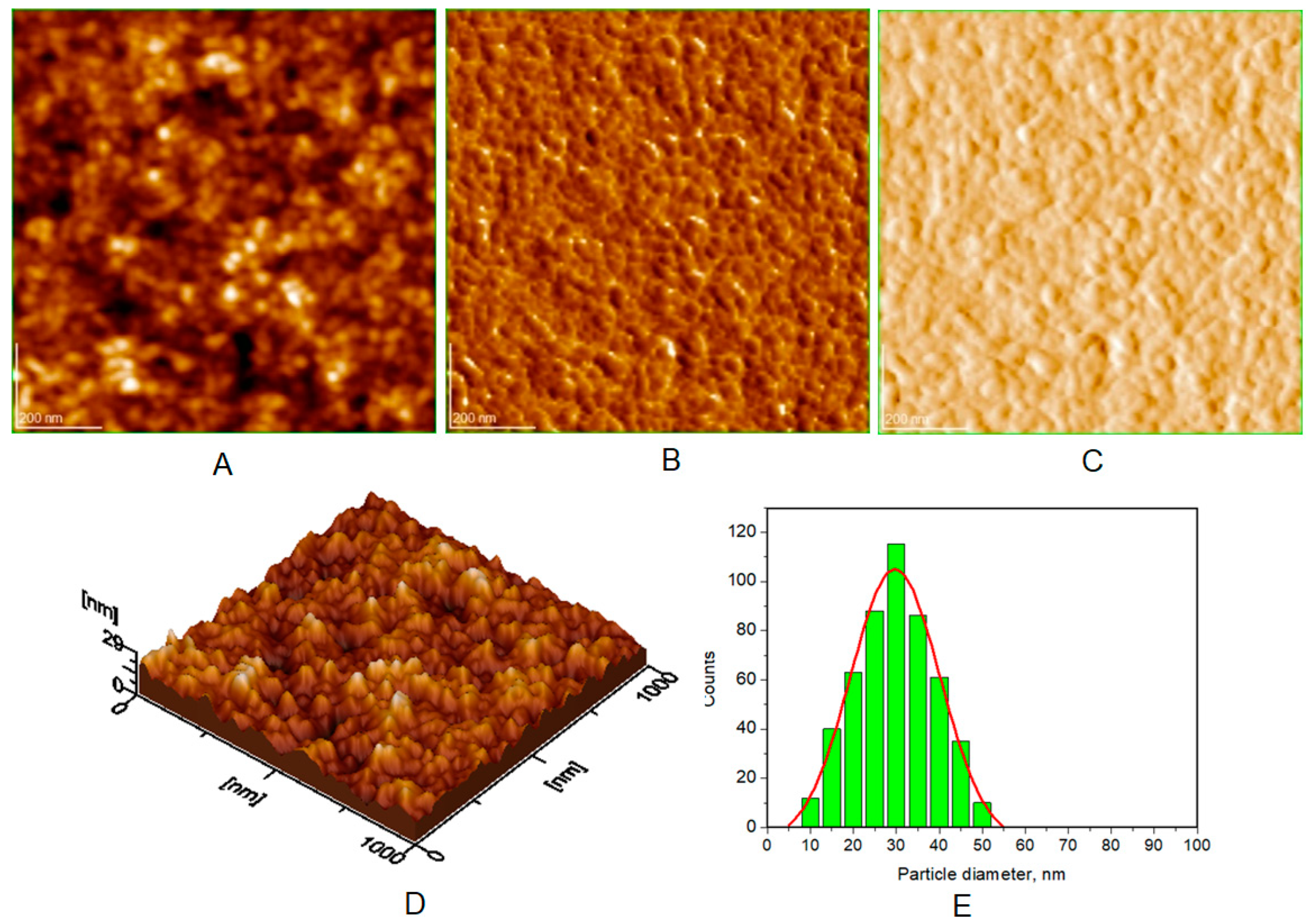
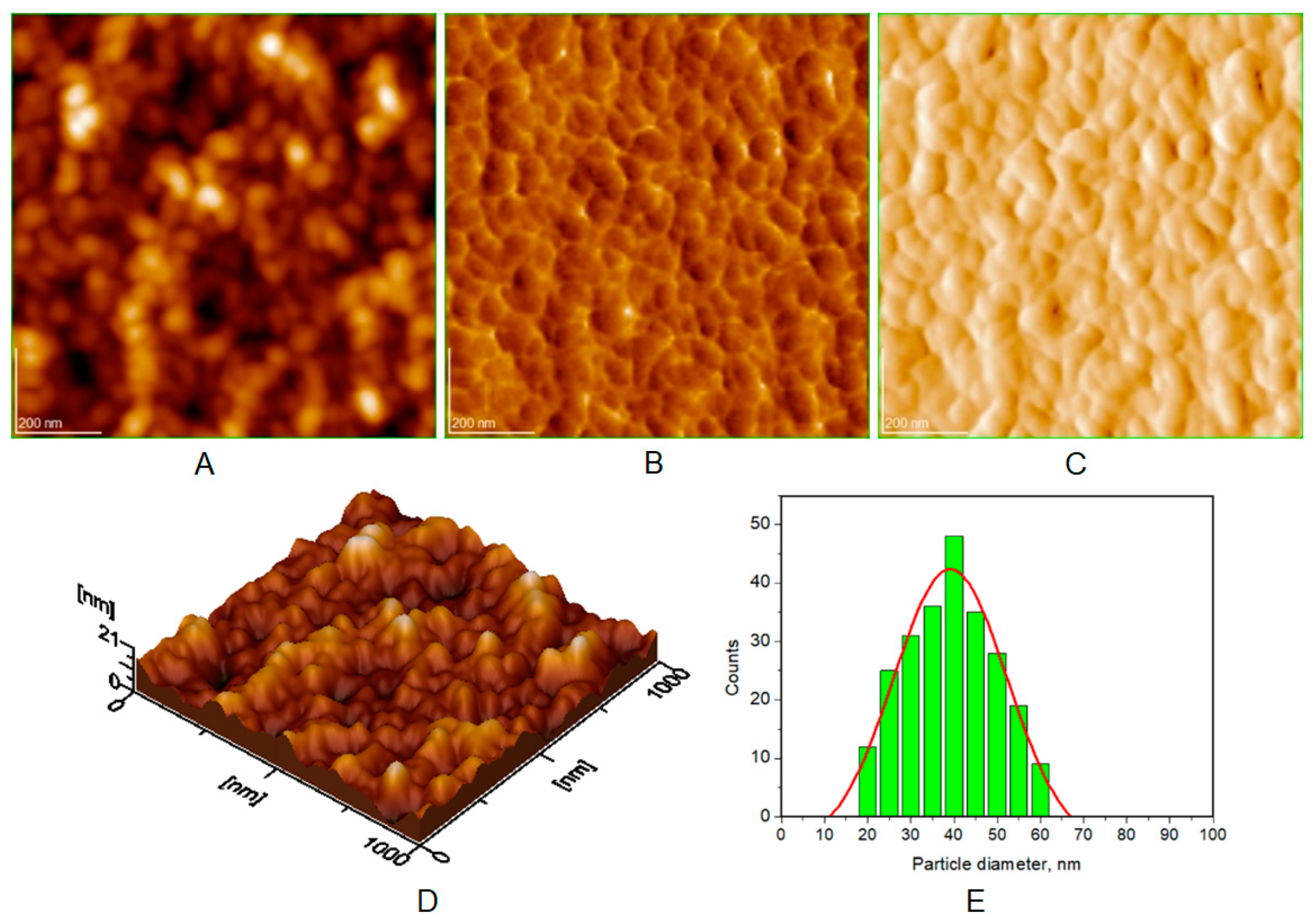
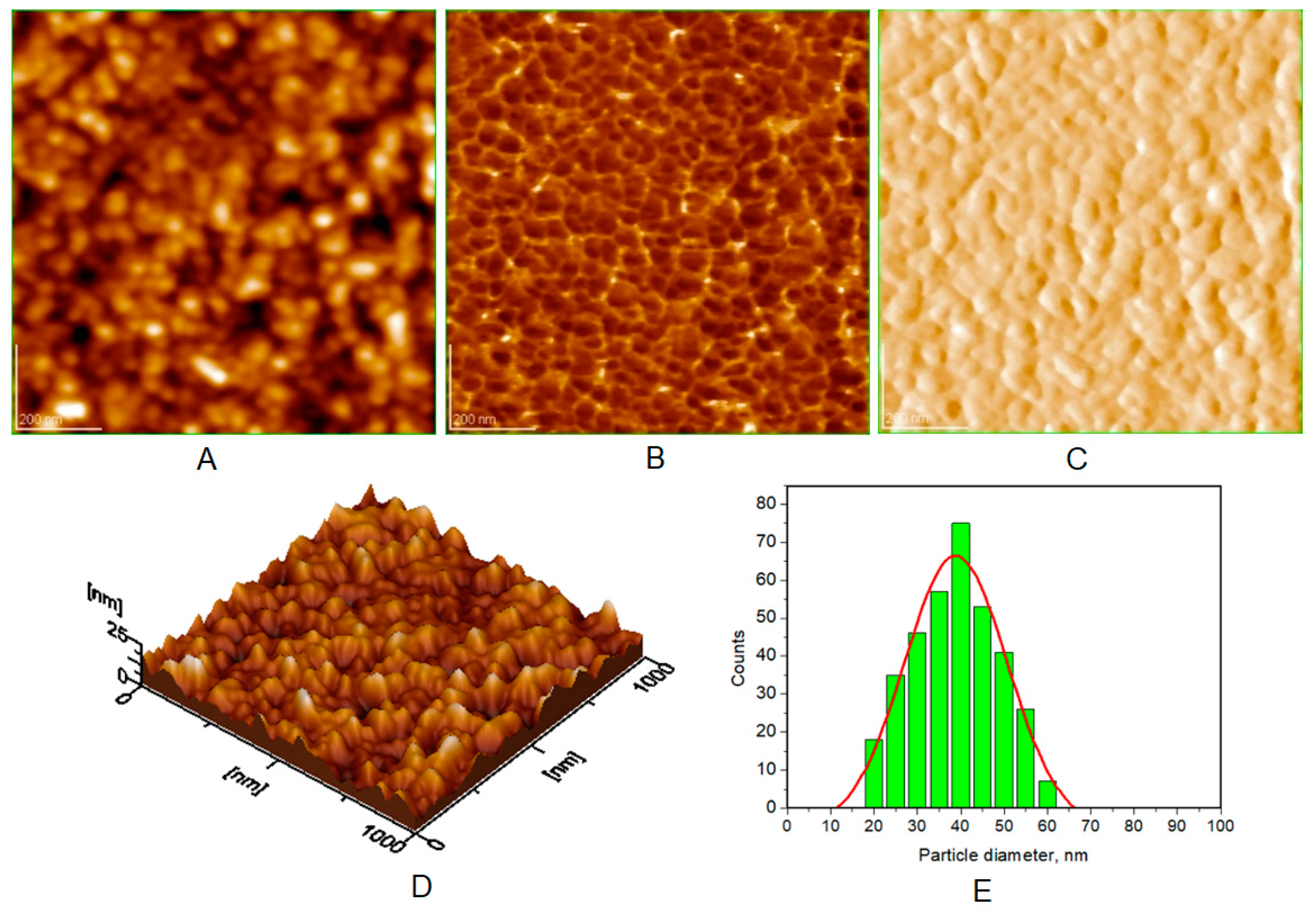
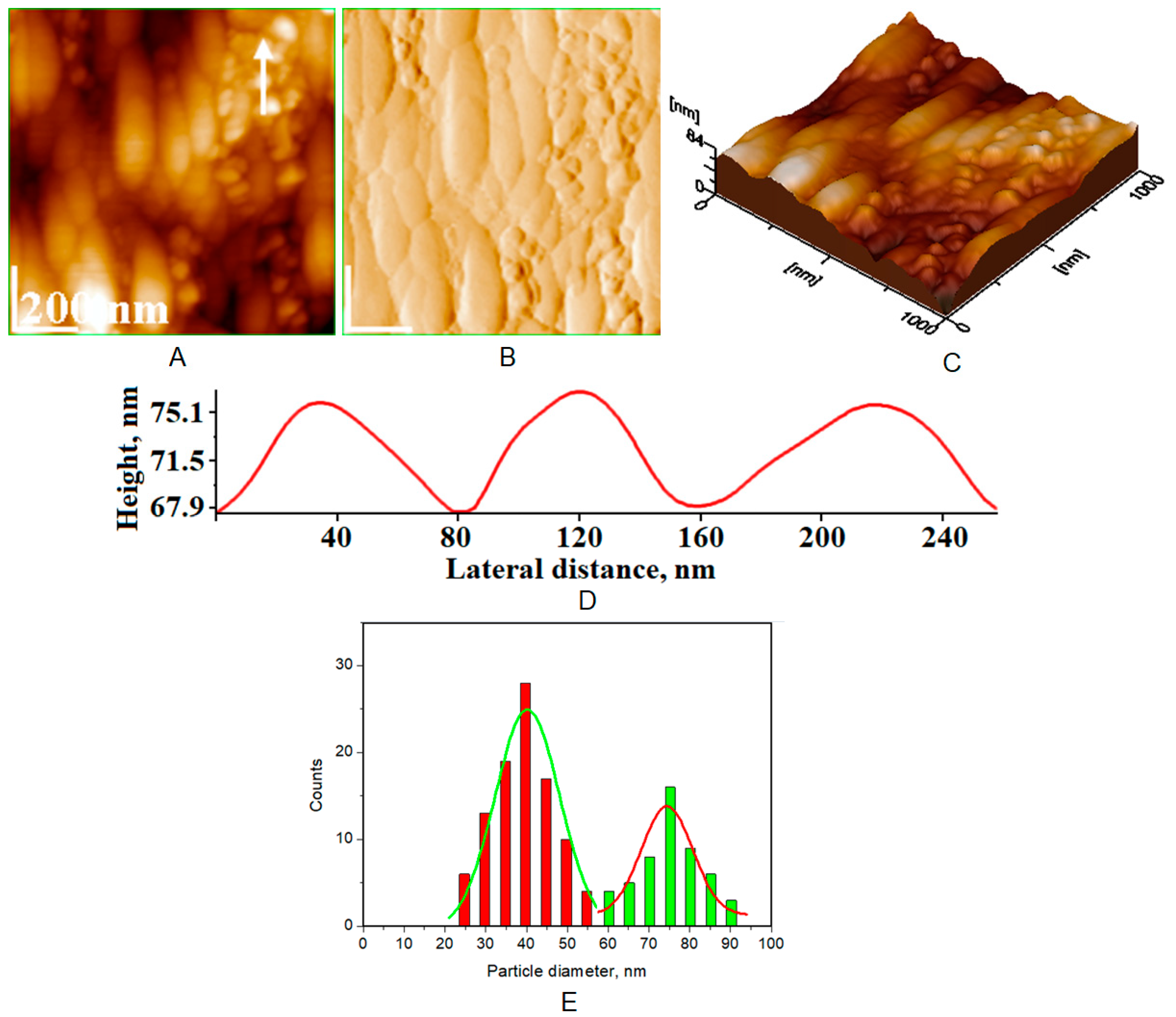
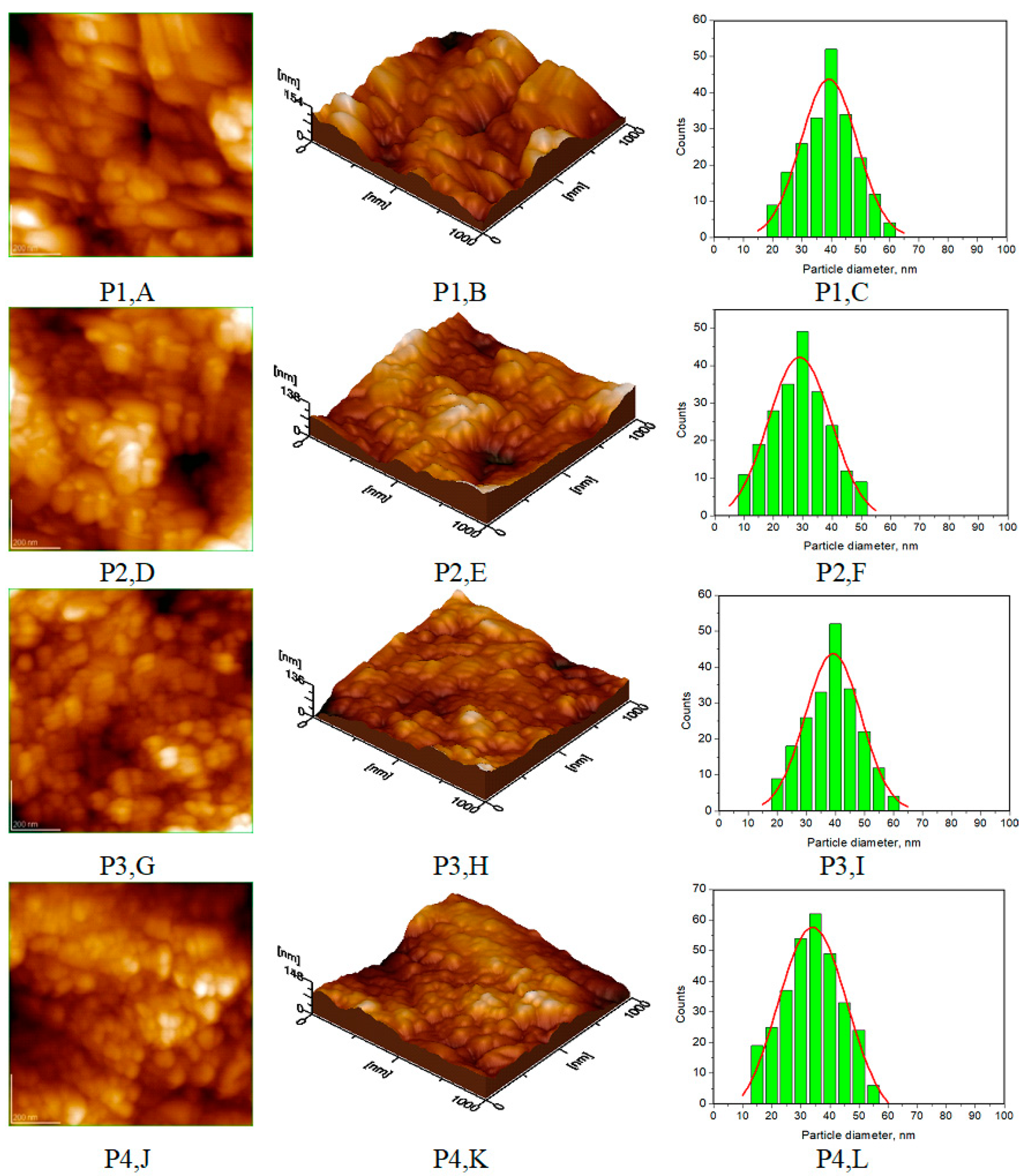
| Toothpaste Symbol | HAPs Type | Substitution Elements (wt%) | HAPs Chemical Formula |
|---|---|---|---|
| P1 | HAP-Zn | Zn 5.00 | Ca9.22Zn0.78(PO4)6(OH)2 |
| P2 | HAP | - | Ca10(PO4)6(OH)2 |
| P3 | HAP-Mg-Zn-Sr-Si | Mg 0.23 Zn 3.09 Sr 10.00 Si 2.00 | Ca8.19Mg0.10Zn0.5Sr1.21(PO4)5.25(SiO4)0.75(OH)1.25 |
| P4 | HAP-Mg-Zn-Si | Mg 2.50 Zn 1.34 Si 2.90 | Ca8.80Mg1.00Zn0.20(PO4)5.00(SiO4)1.00(OH)1.00 |
| Hydroxyapatites | HAP-Zn | HAP | HAP-Mg-Zn-Sr-Si | HAP-Mg-Zn-Si |
|---|---|---|---|---|
| Toothpastes | P1 | P2 | P3 | P4 |
| Crystallites size (nm), from XRD data | 30.3 | 33.1 | 28.2 | 30.6 |
| Crystallinity (%), from XRD data | 30.5 | 36.6 | 28.7 | 30.3 |
| Lattice parameters: | ||||
| a = b (nm) | 0.9421 | 0.9426 | 0.9466 | 0.9445 |
| c (nm) | 0.6862 | 0.6881 | 0.6904 | 0.6883 |
| Average diameter of NPs (nm), from AFM approach * | 40 ± 5 | 30 ± 3 | 37 ± 4 | 38 ± 5 |
| HAP | HAP-Zn | HAP-Mg-Zn-Si | HAP-Mg-Zn-Sr-Si | Assignment of HAP Vibrations |
|---|---|---|---|---|
| 3570 | sh | sh | sh | stretching: structural O-H from HAP |
| 3438 | 3437 | 3430 | 3430 | O-H…O stretching: absorbed water with H-bonding |
| 1635 | 1635 | 1632 | 1633 | absorbed water bending mode ν2 |
| - | - | 1488 | 1489 | CO32− |
| 1421 | 1407 | 1421 | 1420 | CO32− |
| 1385 | - | - | - | CO32− |
| 1094 | 1096 | 1096 | 1096 | PO4 asymmetric stretching ν3 |
| 1043 | 1039 | 1039 | 1039 | PO4 asymmetric stretching ν3 |
| 962 | 963 | 963 | 963 | PO4 symmetric stretching ν1 (forbidden in IR) |
| 875 | - | 874 | - | CO32− |
| 634 | sh | sh | sh | OH vibration |
| 603 | 604 | 605 | 605 | PO4 asymmetric bending ν4 |
| 567 | 566 | 566 | 566 | PO4 asymmetric bending ν4 |
| 472 | 474 | 473 | - | PO4 symmetric bending ν2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florea, A.-D.; Pop, L.C.; Benea, H.-R.-C.; Tomoaia, G.; Racz, C.-P.; Mocanu, A.; Dobrota, C.-T.; Balint, R.; Soritau, O.; Tomoaia-Cotisel, M. Remineralization Induced by Biomimetic Hydroxyapatite Toothpastes on Human Enamel. Biomimetics 2023, 8, 450. https://doi.org/10.3390/biomimetics8060450
Florea A-D, Pop LC, Benea H-R-C, Tomoaia G, Racz C-P, Mocanu A, Dobrota C-T, Balint R, Soritau O, Tomoaia-Cotisel M. Remineralization Induced by Biomimetic Hydroxyapatite Toothpastes on Human Enamel. Biomimetics. 2023; 8(6):450. https://doi.org/10.3390/biomimetics8060450
Chicago/Turabian StyleFlorea, Alexandra-Diana, Lucian Cristian Pop, Horea-Rares-Ciprian Benea, Gheorghe Tomoaia, Csaba-Pal Racz, Aurora Mocanu, Cristina-Teodora Dobrota, Reka Balint, Olga Soritau, and Maria Tomoaia-Cotisel. 2023. "Remineralization Induced by Biomimetic Hydroxyapatite Toothpastes on Human Enamel" Biomimetics 8, no. 6: 450. https://doi.org/10.3390/biomimetics8060450
APA StyleFlorea, A.-D., Pop, L. C., Benea, H.-R.-C., Tomoaia, G., Racz, C.-P., Mocanu, A., Dobrota, C.-T., Balint, R., Soritau, O., & Tomoaia-Cotisel, M. (2023). Remineralization Induced by Biomimetic Hydroxyapatite Toothpastes on Human Enamel. Biomimetics, 8(6), 450. https://doi.org/10.3390/biomimetics8060450







