Abstract
Nanozymes represent a category of nano-biomaterial artificial enzymes distinguished by their remarkable catalytic potency, stability, cost-effectiveness, biocompatibility, and degradability. These attributes position them as premier biomaterials with extensive applicability across medical, industrial, technological, and biological domains. Following the discovery of ferromagnetic nanoparticles with peroxidase-mimicking capabilities, extensive research endeavors have been dedicated to advancing nanozyme utilization. Their capacity to emulate the functions of natural enzymes has captivated researchers, prompting in-depth investigations into their attributes and potential applications. This exploration has yielded insights and innovations in various areas, including detection mechanisms, biosensing techniques, and device development. Nanozymes exhibit diverse compositions, sizes, and forms, resembling molecular entities such as proteins and tissue-based glucose. Their rapid impact on the body necessitates a comprehensive understanding of their intricate interplay. As each day witnesses the emergence of novel methodologies and technologies, the integration of nanozymes continues to surge, promising enhanced comprehension in the times ahead. This review centers on the expansive deployment and advancement of nanozyme materials, encompassing biomedical, biotechnological, and environmental contexts.
1. Introduction
Biomimetics is a term that is used to imply having a duplicate function or mechanism in the field of biological science and technology. Biomimetics are implemented following the principles of various fields, such as biology, physics, architecture, etc., and the biomaterials that are synthesized following these principles possess the functions of the chosen interdisciplinary field, copying and imitating their biological functions [1]. They are made to imitate the principle’s function, be it equipment, machines, systems, or human body parts or tissues.
Biomaterials are materials that are used to make a device or a part of the body, which imitate the biological and physiological functions of the body which are applicable in many fields [2]. Biomaterials need to meet certain guidelines and criteria, such as that they must be biodegradable and biocompatible. They must possess the functions of attachment and cell growth so that the host does not reject the biomaterial through the induction of immunological actions [3]. Biomaterials are classified depending on the origin of the materials, which can be synthetic or natural, and how they are used in medicine (Figure 1).
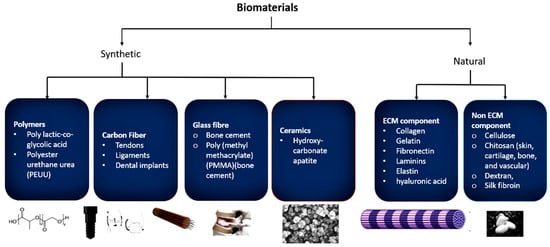
Figure 1.
General classification of biomaterial types and some examples: synthetic and natural.
Synthetic biomaterials are artificial materials used as extracellular microenvironments to mimic the function of, as well as be compatible with, the human body. Some synthetic biomaterials are polymers such as poly lactic-co-glycolic acid, polyester urethane urea (PEUU), peptidomimetic lysine-based poly(ester urethane)urea, chitosan, polyvinylchloride (blood bags), polytetrafluoroethylene (PTFE) (endoscopy, synthetic blood vessels), polyethersulfone (PES) (catheters), carbon fiber (tendons, ligaments, and dental implants), glass fiber (bone cement), poly (methyl methacrylate) (PMMA) (bone cement), polyetheretherketone (PEEK) (dentistry products), ceramics (such as hydroxycarbonate apatite, dicalcium phosphate anhydrous, dicalcium phosphate dihydrate, and tetra calcium phosphate monoxide), metals (316L SS, Ti-based, Co-based, and Mg-based alloys, NiTi, CoCr, and BMG), composites (AI2O3 and alginate), etc. [4].
Natural biomaterials are biologically derived biomaterials that can be classified into two types, namely non-ECM component mimics, which include cellulose, chitin, chitosan (skin, cartilage, bone, and vascular), alginate, dextran, and silk fibroin; and ECM component mimics such as collagen, gelatin, fibronectin, laminins, elastin, glycosaminoglycan, hyaluronic acid, etc. [5,6,7,8,9,10].
These materials are highly biocompatible and biodegradable, mimicking the functions of drug delivery, antibacterial activity, scaffolds for tissue engineering, tissue regeneration, and a wide variety of functions [11]. Unlike synthetic biomaterials, natural biomaterials are taken from an organism be it plant or animal and are used for repairing the body tissues or to act as a substitute for an organ. They show bioactivity due to their natural extracellular matrix [12]. Natural biomaterials have higher biocompatibility than synthetic biomaterials. Some of the natural biomaterials are collagen, cellulose, alginate, chitosan, etc. [12]. Some of the biomaterials’ applications and functions are dental implants, devices for nerve invigoration, drug delivery, artificial enzymes, protein engineering, mineralization, wound healing, orthopedic applications, endovascular and tissue regeneration, genome technology, and nanotechnology [13].
Nanoenzymes are nano-biomaterial-based synthetic enzymes 1–100 nm in size, with similar structures and functions, such as metal complexes, nucleic acids, glucose, and some other biomolecules. They are classified into two types: (1) enzymes that are changed into nanomaterials called hybrid nanomaterial enzymes, and (2) nanomaterials that imitate enzymatic properties and minimize biocatalytic activity [14]. Since 2007, plenty of studies were carried out on nanozymes after the discovery of ferromagnetic (NPs) nanoparticles with activities like peroxidase. It has been reported that hundreds of nanomaterials possess and mimic the catalytic activity of naturally produced enzymes. Nanozymes are low cost, with high efficiency and high dependability, unlike natural enzymes, which are high cost. The study of nanozymes is called “Nanozymology,” which connects nanotechnology, biology, and science [15].
2. Structure and Properties
Even though nanozymes have been an excellent material for implementation based on their low cost, stability, and activity, it has been a difficult task to come up with an ideal design to increase their function and activity. When compared to natural enzymes, nanozymes have a heterogenous surface with a difference in composition and surface structure as they are made of nanomaterials with lower activity. Stating that nanozymes have enzyme-like activity is not accurate. Their function is modified depending on their structure, composition, size, and various other environmental factors [16]. Their activity depends on their structure, which leads to their mechanism being either complex or simple [17]. It is very important to understand and study their structure very precisely so that it can be modified to have higher and more efficient nanozyme activity.
In recent years, there has been a huge number of studies carried out by modifying the nanozyme’s structure to enhance its efficiency. Incorporating and modifying the structure leads to an increase in efficacy of the targeted mechanism. It is important to prioritize the engineering and regulation of the structure and its phytochemical properties [18]. The factors that affect the nanozyme’s activity are its composition, size, structure, position, morphology, valence, and surface modifications, and even other environmental factors based on the applications of such nanozymes [19].
Nanomaterial properties are dependent on size as nanozymes are small and can be exposed more to the active sites of the structure. The catalytic activity is dependent on the interaction with the substrate when it binds [20]. Metal-based nanomaterials such as gold (Au) have higher catalytic activity because they have more interactions with the substrate due to their smaller size, whereas silver (Ag) has a catalytic activity dependent on the pH, the same as Au [20]. A change in pH has a significant effect on the state of H2O2, and platinum (Pt), being larger, has less catalytic activity. Pt nanozymes 1 nm or smaller show the highest scavenging ability, and those 3 nm in size have increased H2O2 decomposition [21].
Metal oxide-based nanozyme activity was first reported for Fe3O4, and further studies on its size and activity were carried out where it was shown that the activity of Fe3O4 increased when it was smaller in size and with higher POD. CeO2 also showed higher SOD-like activity with a smaller size, and considering all of the studies that were carried out, it shows that a smaller nanomaterial leads to higher contact with the substrate and increased catalytic-like activity [22].
Depending on the morphology, the catalytic-like activity differs just like the size factor. Nanozymes show POD-like activity at a low pH and CAT-like activity with a high pH. The decomposition and adsorption depend on the pH. The catalytic-like activity depends on the metal, and in the following order of Pd, Pt, Au, and Ag, the activity rate decreases. The surface activity mechanism is important for many nanozymes, such as iron oxide, etc. [16].
Surface modification is one of the factors that affect the activity of nanozymes with changes in the surface and microenvironment. Surface modifications by small molecules, polymers, covalent, etc. act as a coupling for further attachment of functional groups after acting as a stabilizer for the production and synthesis of nanomaterials. Therefore, it can be adjusted for an increased catalytic effect. For example, Au nanozyme synthesized by using CDs and PAA (sodium polyacrylate) and carbon dots as a soft template, stabilizer and reducing agent, respectively, showed POD-like activity that generated OH, unlike other the activity of other metals or nanomaterials [23].
3. Classification of Nanozymes
Nanozymes have been developing alarmingly fast. They can be classified depending on their activity or based on compounds, like iron, selenium, vanadium, carbon, etc., or their catalytic enzyme-like function, such as glutathione peroxidase, oxidases, catalase, superoxide dismutase, hydroperoxide lyase, peroxidase (POD), Au nanoparticles, polypyrrole nanoparticles, and graphene oxide (GO) nanosheets, or the action of the nanozyme biomaterial [24]. Enzymes have the function of catalyzing reactions and are involved in removing toxins, respiratory functions, muscle growth, etc. Nanozymes mimic multiple functions in the human body with a single nanomaterial compound. A compound with an artificial enzyme sometimes plays multi-enzyme roles and functions in the body [25].
Nanozymes also play a major role in technology with the introduction and development of detection devices and equipment with multiple uses in both biology and technology. This has become an area of research interest. These materials contribute a lot to technology, biomedical treatments, detection, and the diagnosis of disease (Table 1). Nanozymes help in sensing and detecting proteins, tumors, molecules, antimicrobial activity, immunoassays, cancer therapy, phenol degradation, etc. [26].

Table 1.
Nanomaterials and their biomedical applications with enzymatic activities.
4. Biomedical Applications
Research on nanozymes in recent years has made a huge number of developments in sensing, antibacterial activity, cancer treatment, antioxidant activity, and environmental treatment. Nanoparticles with inherent catalytic activity have plenty of biomedical applications, including detection, diagnosis, treatment, and therapy. They are controlled by factors such as hydrogen peroxidase, metal ions, etc. Nanozyme studies proved that many nanomaterials imitate enzyme-like activity, such as oxidase (OXD), glucose oxidase (GOD), peroxidase (POD), catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx). The advancement of nanozymes has proceeded to the point where they can be used in the development of advanced variations in different fields.
4.1. Antioxidant Activity
Most nanozymes possess antioxidant characteristics, such as eliminating oxidative reactions by eliminating the chain reaction which releases byproduct compounds, causing severe irreversible damage to the human body, such as those occurring in neurodegenerative diseases, renal diseases, tumors, inflammation, Alzheimer’s disease, Parkinson’s disease, etc. ROS cause the death of cells, which affects health. Nanozymes maintain the ROS levels in the body by eliminating excess ROS activity by an antioxidant mechanism.
Nanozymes can act in cytoprotection, where recently, Yang et al., 2023 [31], further studied Pt/Co-SA-NSG. They found that normal mitochondrial function increased with increased antioxidant levels, and the nanozyme decreased inflammation by having superoxide dismutase- and catalase-like activity (Figure 2). Fu et al., 2023 [59], showed and revealed that palladium clusters incubated with insulin (Pd In) reduced the severity of Alzheimer’s disease. Tri-element nanozyme (PtCuSe nanozyme) has potential in treating neurodegenerative disease and Parkinson’s disease by showing catalase and superoxide dismutase activity [60,63].
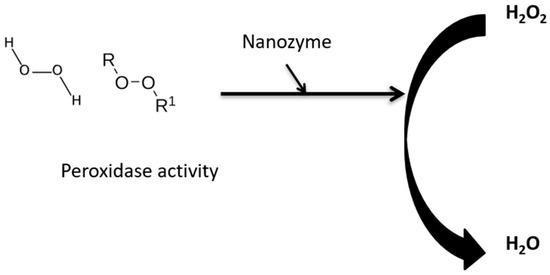
Figure 2.
Role of nanozymes in peroxidase catalytic activity.
Two-dimensional cobalt hydroxide oxide nanosheets (Co NSs) with a multienzyme activity of SOD, CAT, and POD protect cells from oxidative damage and scavenge ROS that induce NLRP3 inflammasome and DSS- induced colitis. Co-NSs have increased anti-inflammatory action with therapeutic effects and treatment potential against inflammatory disease [61]. A carbon dot superoxide dismutase (C-dot SOD) nanozyme showed increased potential in healing acute lung injury and protected cells against oxidative damage due to lack of oxygen, and these C-dot SODs scavenge ROS by inhibiting inflammasomes to manage ROS-induced diseases (Figure 3) [62,64].
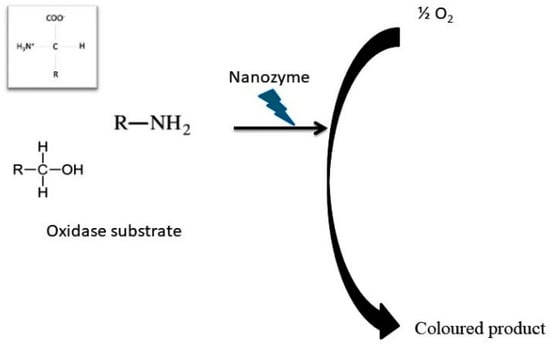
Figure 3.
Role of nanozymes in oxidation catalytic activity.
4.2. Cancer Therapy
Nanozymes with catalytic-like activity are used in cancer therapy to induce a hypoxic microenvironment for the tumor cells, limiting the growth of cancer cells, eliminating the cell structure, and inhibiting ROS species. RNA interference is a basic system for gene regulation that is mediated by the RNA-induced silencing complex (RISC). PtFe@Fe3O4 with dual enzyme activity has a photothermal effect that eliminates tumor cells, causing a hypoxic microenvironment around the tumor cells using peroxidase- and catalase-like activities in the acidic tumor microenvironment (Figure 4) [65].
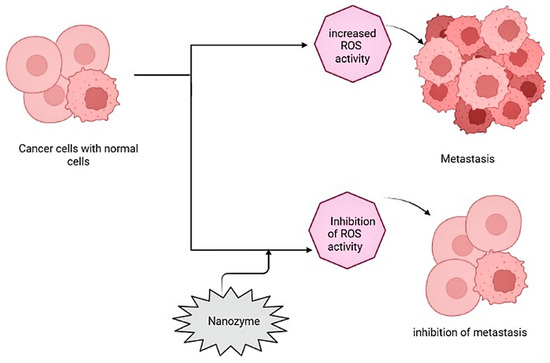
Figure 4.
Illustration of antitumor mechanism/activity by nanozymes against cancer cells.
Shen et al., 2020 [66] carried out research and determined that Ir@MnFe2O4 NPs have glutathione activity with huge implications for cancer therapy. CoPc-Mn/Ti3C2Tx showed highly precise anticancer activity through a multimodal strategy (photoacoustic imaging) for photothermal therapy [67].
Platinum-doped plasmonic gold nanostar–glucose oxidase (Pt-AuNS-GOx) increases the efficacy of hypoxia alleviation and cancer treatment, induces an energy barrier reduction for oxygen production in acidic and aerobic environments, inhibits the hypoxic microenvironment of tumor tissues, increases apoptosis, and inhibits metastasis of cancer with therapeutic efficacy [68].
4.3. Antibacterial Activity
Millions of people each year have been affected by infectious illnesses caused by bacteria up to this point, ranking as one of the largest global health issues. Recently, Shi et al., 2022 [69] designed porous graphitic carbon nitride C3N5 nanosheets (denoted as PtRu/C3N5) that are piezo-augmented and photocatalytic nanozyme-integrated microneedles, which imitate oxidase activity and are nanotherapeutics with multiple activities. They highlighted their use in therapeutic strategies based on their antibacterial and anti-inflammatory actions. NiCo2O4 exhibits a high antibacterial effect without any worry about antimicrobial resistance, imitating the biological mechanism of antibodies [70]. N-CNDs inhibit bacterial growth by exhibiting antibacterial action against both Gram-positive and Gram-negative bacteria, as carbon-based nanozymes have multiple activities and are used in food safety from bacterial contamination [71]. Most of the helper cells are seriously changed as the tributer norms are reached.
4.4. Neurodegenerative Disease Therapy
Therapies aimed at neurodegenerative diseases necessitate the precise regulation of intercellular levels of reactive oxygen species (ROS), given their close association with the pathogenesis of nervous system disorders. Consequently, nanozymes exhibiting ROS scavenging capabilities hold immense potential for effective neuroprotection. Drawing inspiration from the pivotal role of manganese (Mn) in the catalytic processes of natural superoxide dismutase (SOD) enzymes, Mn-based nanozymes have garnered significant attention as antioxidants. In pursuit of nanozymes with potent and broad-spectrum antioxidant prowess, Singh et al. engineered the morphology of Mn3O4 nanoparticles (NPs) and introduced a biocompatible flower-like Mn3O4 nanostructure (referred to as Mnf), which efficiently combats elevated ROS levels under pathophysiological conditions. The Mnf exhibits multiple enzyme-like activities, encompassing SOD-, catalase (CAT)-, and glutathione peroxidase (GPx)-like functions, surpassing those of Mn3O4 NPs with alternative morphologies. In vitro studies substantiate the robust cytoprotective effects of Mnf against neurotoxin-induced cell death in SHSY-5Y cells, thereby holding promise for mitigating ROS-mediated neurodegenerative diseases like Parkinson’s disease. Furthermore, it has been reported that cerium nanoparticles (CeNPs) smaller than 5 nm demonstrate exceptional catalytic properties akin to CAT and SOD [71]. Building upon this discovery, our research group engineered a nanocomposite loaded with methylene blue (MB) and adorned with CeNPs on its surface, presenting a notable therapeutic avenue for Alzheimer’s disease (AD) treatment. The CeNPs effectively scavenge intracellular ROS, mitigating mitochondrial oxidative stress and curtailing tau hyperphosphorylation when combined with MB. This comprehensive approach alleviates AD symptoms both in vitro and in vivo, underscoring the potential of addressing mitochondrial dysfunction in neuroinflammation for the treatment of various neurodegenerative diseases.
4.5. Injury Therapy
Excessive levels of reactive oxygen species (ROS) and inflammation represent significant challenges that hinder the recovery and treatment of injuries. This is because the resulting oxidative stress triggers the opening of inter-endothelial junctions and facilitates the migration of inflammatory cells across the endothelial barrier. Hence, it becomes imperative to counteract the surplus ROS and promote oxygen (O2) generation within damaged cells and injury sites. This can be achieved through the utilization of nanozymes endowed with superoxide dismutase (SOD)- and catalase (CAT)-like activities. For instance, Zhang et al. devised micelle-like nanoparticles by conjugating polyethylene glycol (PEG) with manganese protoporphyrin (PEG-MnPP) for the treatment of acute liver failure. These PEG-MnPP nanoparticles, with extended circulation in the bloodstream, exhibit CAT- and SOD-like activities, effectively neutralizing hydrogen peroxide (H2O2) for enhanced management of acetaminophen (APAP)-induced acute liver failure. Singh et al. engineered cerium nanoparticles (CeNPs) with CAT-like activity, successfully safeguarding human hepatic cells from a catalasemia induced by 3-AT. Moreover, our research group developed PEG-coated CeNPs with CAT- and SOD-like activities, offering a highly efficient approach for treating drug-induced liver injury (DILI). CeNPs can directly scavenge ROS, detoxifying DILI. Additionally, the O2 produced during CeNPs’ ROS-scavenging process effectively inhibits pro-inflammatory macrophages, mitigating inflammation-induced damage to liver tissue. Consequently, the dual roles of CeNPs in detoxification and inflammation regulation significantly extend the therapeutic window for DILI treatment compared to conventional N-acetylcysteine.
Furthermore, Huang et al. harnessed citric acid-modified CeNPs to alleviate rhabdomyolysis-induced acute kidney injury by providing robust protection to renal cells against ROS. In a separate study, Mugesh and colleagues demonstrated that cerium vanadate (CeVO4) nanorods exhibit SOD-like activity within cells, even when the natural enzyme is down-regulated due to specific gene silencing. CeVO4 effectively shielded SOD-depleted cells from mitochondrial damage by simultaneously regulating superoxide levels and restoring the function of antiapoptotic Bcl-2 family proteins. This preservation of mitochondrial integrity enabled the efficient regulation of ATP levels in neuronal cells under oxidative stress, showcasing CeVO4′s immense potential for treating diseases associated with mitochondrial dysfunction. Overall, chemically designed nanozymes with antioxidant activities have shown remarkable efficacy in managing diseases related to oxidative damage, particularly in the context of injury therapy. Moreover, these nanozymes can compensate for functional deficits resulting from genetic defects, underscoring their versatile therapeutic potential.
4.6. Biosensor
Biosensors have been used for hundreds of years for the detection of various compounds, such as molecules, ions, metals, proteins, cancer cells, nuclei acids, pesticides, etc. Many biosensor strategies have been built upon by researchers, such as in the form of electrochemical biosensors, colorimetric sensors, surface-enhanced Raman spectroscopy (SERS), and florescent meters. These were developed after clear knowledge was achieved on nanomaterials as well as metal oxides and frameworks [72]. Natural biomaterials are biologically derived biomaterials and are classified into two types, non-ECM component mimics, such as cellulose, chitin, chitosan (skin, cartilage, bone, and vascular tissue), alginate, dextran, and silk fibroin, and ECM component mimics, such as collagen, gelatin, fibronectin, laminins, elastin, glycosaminoglycan, hyaluronic acid, etc. [5].
Electrochemical biosensor-integrated devices are widely used in the medical field for the detection of breast cancer [73], infectious viral disease [74], food-borne pathogens [75], glucose and pH [76], etc. The researchers built upon new and recent findings on incorporating nanomaterials and developing methods and devices. Ag-Fe3O4 nanozymes were developed with a peroxidase-like activity for the identification of sulfur ions colorimetrically. They have a dual activity, where they catalyze the degradation of triarylmethane dye at a rate of 99%, and can be reused ten times or more [77]. Single-atom nanozymes (SANs) are used as electrochemical sensors due to their stability, selectivity, and increased sensitivity to detect molecules such as hydrogen peroxide, glucose, oxygen, and uric acid for monitoring food safety and human health. It is accurate and has high quantitative sensitivity to eliminate the negative impacts on food safety [78].
5. Nanozymes in Biodiversity and Environmental Treatment
With the development of industries, there is increased environmental pollution caused by humans, which disturbs ecological systems and disrupts the life cycle of human beings. The pollution caused by humans, such as the disposal of wastes such as oil, drainage, heavy metals, and other waste into water, is an increasing problem. By applying bio-technology, the factors that contribute to pollution can be solved, and treatment can be strategized. Catalase, superoxide dismutase, and other peroxidase activities are often applied in the remediation of the environment (Figure 5) [63,79].
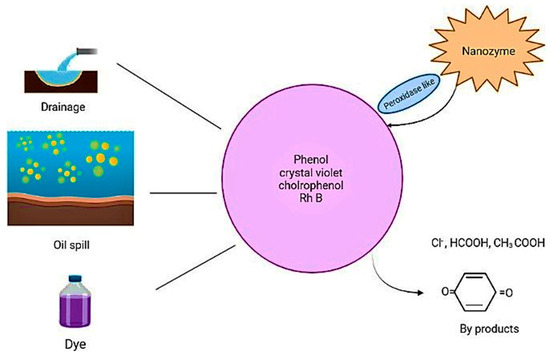
Figure 5.
Mechanisms behind the use of nanozymes in degrading environmental pollutants.
Nanozymes can sense fertilizers, heavy metals, pesticides, and other biological factors, which can be used in implementing treatments for pollutant degradation, and bio-fouling processes. The detection of molecules and organic compounds quantitatively and qualitatively can be carried out with nanozymes. They can degrade dyes, phenols, persistent pollutants, toxic pollutants, and chemical agents [80]. Portable and recyclable agarose gel films loaded with CeO2 @ZIF-8 nanozymes were used to study the degradation of dye in wastewater and showed an 80% efficacy after 5 uses. A non-photodegradation catalytic system with even better development can be implemented for environmental remediation and eliminating dye pollution [81]. Co3O4/CoFe2O4 hollow nanocubes (HNCs) have a multifunction and multienzyme activity for the detection of L-cysteine in food and degradation of rhodamine B by 99.24%, and can be reused more than 20 times for the detection of norfloxacin in drugs [9,82]. CH-Cu nanozymes were reported to have a higher efficiency than laccase in the degradation of phenolic pollutants, such as chlorophenols and bisphenols, and based on these nanozymes, a detection method for epinephrine was developed where a smartphone can perform the quantitative analysis [83,84].
6. Conclusions
These new-generation nanozymes with high catalytic activity and low cost make excellent materials for carrying out further studies and development. They exhibit a great capability for disease therapy, detection, biosensing etc. In this review, we have briefly summarized the recent developments and findings on nanozyme functions and applications. Nanozymes with enzyme-like activity can be utilized tremendously to our advantage. Nanozymes with high biocompatibility and stability received attention and made it possible to develop and apply nanomaterials for sensing, antibacterial activity, antioxidant activity, and tumor therapy. Most nanozymes showing dual enzymatic activity and functions can bring greater results. The selectivity of these nanozymes for development can be further studied, and research can be carried out. With the future in consideration, a lot of developments and implementations can be made with nanozymes in the biomedical field.
Author Contributions
Conceptualization, S.J., R.S. and E.S.; software, A.P.; resources, S.J. and A.P., data curation, S.J. and E.S., writing—original draft preparation, S.J., writing—review and editing, R.S., T.R. and J.L.; visualization, S.J., T.R. and J.L.; supervision, S.J. and E.S., project administration, S.J. and T.R. All authors contributed to conceptualizing, drafting, and revising the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tamerler, C.; Sarikaya, M. Molecular Biomimetics: Nanotechnology and Bionanotechnology Using Genetically Engineered Peptides. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 1705–1726. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Hunt, J.A. Biomimetic Materials Processing for Tissue-Engineering Processes. J. Mater. Chem. 2007, 17, 3974. [Google Scholar] [CrossRef]
- Huzum, B.; Puha, B.; Necoara, R.M.; Gheorghevici, S.; Puha, G.; Filip, A.; Sirbu, P.D.; Alexa, O. Biocompatibility Assessment of Biomaterials Used in Orthopedic Devices: An Overview (Review). Exp. Ther. Med. 2021, 22, 1315. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhayay, M.; Chatterjee, A. Classification of Foods, Biomaterials, and Microorganisms. In Sterilization and Preservation: Applications of Supercritical Carbon Dioxide; Mukhopadhayay, M., Chatterjee, A., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 9–42. ISBN 978-3-031-17370-7. [Google Scholar]
- Liu, S.; Yu, J.-M.; Gan, Y.-C.; Qiu, X.-Z.; Gao, Z.-C.; Wang, H.; Chen, S.-X.; Xiong, Y.; Liu, G.-H.; Lin, S.-E.; et al. Biomimetic Natural Biomaterials for Tissue Engineering and Regenerative Medicine: New Biosynthesis Methods, Recent Advances, and Emerging Applications. Mil. Med. Res. 2023, 10, 16. [Google Scholar] [CrossRef]
- Khanna, A.; Zamani, M.; Huang, N.F. Extracellular matrix-based biomaterials for cardiovascular tissue engineering. J. Cardiovasc. Dev. Dis. 2021, 8, 137. [Google Scholar] [CrossRef]
- Xing, H.; Lee, H.; Luo, L.; Kyriakides, T.R. Extracellular matrix-derived biomaterials in engineering cell function. Biotechnol. Adv. 2020, 42, 107421. [Google Scholar] [CrossRef]
- Xu, M.; Su, T.; Jin, X.; Li, Y.; Yao, Y.; Liu, K.; He, Y. Inflammation-mediated matrix remodeling of extracellular matrix-mimicking biomaterials in tissue engineering and regenerative medicine. Acta Biomater. 2022, 151, 106–117. [Google Scholar] [CrossRef]
- Tonti, O.R.; Larson, H.; Lipp, S.N.; Luetkemeyer, C.M.; Makam, M.; Vargas, D.; Calve, S. Tissue-specific parameters for the design of ECM-mimetic biomaterials. Acta Biomater. 2021, 132, 83–102. [Google Scholar] [CrossRef]
- Li, H.; Bao, M.; Nie, Y. Extracellular matrix–based biomaterials for cardiac regeneration and repair. Heart Fail. Rev. 2021, 26, 1231–1248. [Google Scholar] [CrossRef]
- Agarwal, T.; Chiesa, I.; Costantini, M.; Lopamarda, A.; Tirelli, M.C.; Borra, O.P.; Varshapally, S.V.S.; Kumar, Y.A.V.; Koteswara Reddy, G.; De Maria, C.; et al. Chitosan and Its Derivatives in 3D/4D (Bio) Printing for Tissue Engineering and Drug Delivery Applications. Int. J. Biol. Macromol. 2023, 246, 125669. [Google Scholar] [CrossRef]
- Prasathkumar, M.; Sadhasivam, S. Chitosan/Hyaluronic Acid/Alginate and an Assorted Polymers Loaded with Honey, Plant, and Marine Compounds for Progressive Wound Healing—Know-How. Int. J. Biol. Macromol. 2021, 186, 656–685. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, N.; García, R.G.; Martínez, J.D.H.; Briones, C.M.; Martínez Ramos, A.M.; Tamez, M.F.L.; Del Valle, B.G.; Segura, F.J.M. Recent Advances in Designing Fibrous Biomaterials for the Domain of Biomedical, Clinical, and Environmental Applications. ACS Biomater. Sci. Eng. 2022, 8, 3690–3716. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Su, G.; Wu, J.; Song, C.; Lu, Z.; Wu, C.; Wang, Y.; Wang, P.; He, M.; Zhao, Y.; et al. Co3O4/CoFe2O4 Hollow Nanocube Multifunctional Nanozyme with Oxygen Vacancies for Deep-Learning-Assisted Smartphone Biosensing and Organic Pollutant Degradation. ACS Appl. Mater. Interfaces 2023, 15, 11787–11801. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Gao, L. Nanozymology: An Overview. In Nanozymology: Connecting Biology and Nanotechnology; Yan, X., Ed.; Nanostructure Science and Technology; Springer: Singapore, 2020; pp. 3–16. ISBN 9789811514906. [Google Scholar]
- Wang, M.; Zhu, P.; Liu, S.; Chen, Y.; Liang, D.; Liu, Y.; Chen, W.; Du, L.; Wu, C. Application of Nanozymes in Environmental Monitoring, Management, and Protection. Biosensors 2023, 13, 314. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Roy, P.; Karmodak, N.; Jemmis, E.D.; Mugesh, G. Nanoisozymes: Crystal-Facet-Dependent Enzyme-Mimetic Activity of V2O5 Nanomaterials. Angew. Chem. Int. Ed. 2018, 57, 4510–4515. [Google Scholar] [CrossRef]
- Gugleva, V.; Ivanova, N.; Sotirova, Y.; Andonova, V. Dermal Drug Delivery of Phytochemicals with Phenolic Structure via Lipid-Based Nanotechnologies. Pharmaceuticals 2021, 14, 837. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, H.; Fan, K. Structure-Activity Mechanism of Iron Oxide Nanozymes. In ACS Symposium Series; Wang, X., Ed.; American Chemical Society: Washington, DC, USA, 2022; Volume 1422, pp. 1–35. ISBN 978-0-8412-9751-7. [Google Scholar]
- Liu, Q.; Zhang, A.; Wang, R.; Zhang, Q.; Cui, D. A Review on Metal- and Metal Oxide-Based Nanozymes: Properties, Mechanisms, and Applications. Nano-Micro Lett. 2021, 13, 154. [Google Scholar] [CrossRef]
- He, W.; Zhou, Y.-T.; Wamer, W.G.; Boudreau, M.D.; Yin, J.-J. Mechanisms of the PH Dependent Generation of Hydroxyl Radicals and Oxygen Induced by Ag Nanoparticles. Biomaterials 2012, 33, 7547–7555. [Google Scholar] [CrossRef]
- Filippova, A.D.; Sozarukova, M.M.; Baranchikov, A.E.; Kottsov, S.Y.; Cherednichenko, K.A.; Ivanov, V.K. Peroxidase-like Activity of CeO2 Nanozymes: Particle Size and Chemical Environment Matter. Molecules 2023, 28, 3811. [Google Scholar] [CrossRef]
- Hu, W.; Younis, M.; Zhou, Y.; Xia, X.-H. In Situ Fabrication of Ultrasmall Gold Nanoparticles/2D MOFs Hybrid as Nanozyme for Antibacterial Therapy. Small 2020, 16, 2000553. [Google Scholar] [CrossRef]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New Horizons for Responsive Biomedical Applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yu, D.; Qiu, Y.; Chu, L.; Lin, Y. The Role of Nanomaterials in Modulating the Structure and Function of Biomimetic Catalysts. Front. Chem. 2020, 8, 764. [Google Scholar] [CrossRef] [PubMed]
- Songca, S.P. Applications of Nanozymology in the Detection and Identification of Viral, Bacterial and Fungal Pathogens. Int. J. Mol. Sci. 2022, 23, 4638. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Dashtian, K.; Zare-Dorabei, R.; Ghafuri, H.; Mahdavi, M.; Amourizi, F. Photo-Responsive Oxidase-like Nanozyme Based on a Vanadium-Docked Porphyrinic Covalent Organic Framework for Colorimetric L-Arginine Sensing. Anal. Chim. Acta 2023, 1247, 340924. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhu, H.; Feng, L.; Zhu, Y.; Liu, B.; Yu, C.; Gai, S.; Yang, P. 2D Piezoelectric BiVO4 Artificial Nanozyme with Adjustable Vanadium Vacancy for Ultrasound Enhanced Piezoelectric/Sonodynamic Therapy. Small 2023, 19, 2301349. [Google Scholar] [CrossRef]
- Sun, X.; He, X.; Zhu, Y.; Obeng, E.; Zeng, B.; Deng, H.; Shen, J.; Hu, R. Valence-Switchable and Biocatalytic Vanadium-Based MXene Nanoplatform with Photothermal-Enhanced Dual Enzyme-like Activities for Anti-Infective Therapy. Chem. Eng. J. 2023, 451, 138985. [Google Scholar] [CrossRef]
- Biswas, R.; Ghosh, D.; Dasgupta, S.; Bhaduri, S.N.; Banerjee, R.; Datta, P.; Biswas, P. Vanadium-Incorporated Mesoporous Silica as Oxidase Mimic for Colorimetric Dopamine Detection and Anticancer Activity. ChemistrySelect 2023, 8, e202204989. [Google Scholar] [CrossRef]
- Sharifnezhad, A.H.; Dashtian, K.; Amourizi, F.; Zare-Dorabei, R. Development of Peptide Impregnated V/Fe Bimetal Prussian Blue Analogue as Robust Nanozyme for Colorimetric Fish Freshness Assessment. Anal. Chim. Acta 2023, 1237, 340555. [Google Scholar] [CrossRef]
- Li, H.; Zhao, S.; Wang, Z.; Li, F. Controllable Preparation of 2D V2O5 Peroxidase-Mimetic Nanozyme to Develop Portable Paper-Based Analytical Device for Intelligent Pesticide Assay. Small 2023, 19, 2206465. [Google Scholar] [CrossRef]
- Zou, L.; Li, X.; Huang, Y.; Wang, C.; Fang, Y.; Zhao, J.; Jin, Q.; Ji, J. Raspberry-like Gold Nanozyme-Hybrid Liposomes for Hypoxia-Enhanced Biofilm Eradication. Nano Today 2023, 50, 101828. [Google Scholar] [CrossRef]
- Garehbaghi, S.; Ashrafi, A.M.; Adam, V.; Richtera, L. Surface Modification Strategies and the Functional Mechanisms of Gold Nanozyme in Biosensing and Bioassay. Mater. Today Bio 2023, 20, 100656. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhou, R.; Wu, K.; Zhu, G. Colorimetric Method Transforms into Highly Sensitive Homogeneous Voltammetric Sensing Strategy for Mercury Ion Based on Mercury-Stimulated Ti3C2Tx MXene Nanoribbons@gold Nanozyme Activity. Anal. Chim. Acta 2023, 1250, 340975. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xiang, J.; Su, W.; Guo, J.; Deng, J.; Tang, L.; Li, G.; Liang, Y.; Zheng, L.; He, M.; et al. Modulating Pt Nanozyme by Using Isolated Cobalt Atoms to Enhance Catalytic Activity for Alleviating Osteoarthritis. Nano Today 2023, 49, 101809. [Google Scholar] [CrossRef]
- Gao, P.; Wei, R.; Chen, Y.; Li, X.; Pan, W.; Li, N.; Tang, B. Pt Nanozyme-Bridged Covalent Organic Framework-Aptamer Nanoplatform for Tumor Targeted Self-Strengthening Photocatalytic Therapy. Biomaterials 2023, 297, 122109. [Google Scholar] [CrossRef]
- Lee, H.B.; Son, S.E.; Gupta, P.K.; Venkatesan, J.; Hur, W.; Park, J.; Nyeon Kim, S.; Hun Seong, G. Mesoporous Platinum Nanozyme-Based Competitive Immunoassay for Sensitive Detection of 25-Hydroxyvitamin D. Mater. Lett. 2023, 330, 133286. [Google Scholar] [CrossRef]
- Zhu, D.; Wu, H.; Jiang, K.; Xu, Y.; Miao, Z.; Wang, H.; Ma, Y. Zero-Valence Selenium-Enriched Prussian Blue Nanozymes Reconstruct Intestinal Barrier against Inflammatory Bowel Disease via Inhibiting Ferroptosis and T Cells Differentiation. Adv. Healthc. Mater. 2023, 12, 2203160. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, A.; Xu, Z.; Tian, R.; Hou, C.; Luo, Q.; Sun, H.; Xu, J.; Yu, S.; Wang, T.; et al. Engineering Biomimetic ATP-Responsive Se-Containing Core-Shell Cascade Nanozyme for Efficient Tumor Combination Therapy. Chem. Eng. J. 2023, 454, 140165. [Google Scholar] [CrossRef]
- Li, W.; Bei, Y.; Pan, X.; Zhu, J.; Zhang, Z.; Zhang, T.; Liu, J.; Wu, D.; Li, M.; Wu, Y.; et al. Selenide-Linked Polydopamine-Reinforced Hybrid Hydrogels with on-Demand Degradation and Light-Triggered Nanozyme Release for Diabetic Wound Healing. Biomater. Res. 2023, 27, 49. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Tan, W.; Jin, P.; Zhang, P.; Li, K. Bioinspired Coassembly of Copper Ions and Nicotinamide Adenine Dinucleotides for Single-Site Nanozyme with Dual Catalytic Functions. Anal. Chem. 2023, 95, 2865–2873. [Google Scholar] [CrossRef]
- Luo, B.; Cai, J.; Xiong, Y.; Ding, X.; Li, X.; Li, S.; Xu, C.; Vasil’kov, A.Y.; Bai, Y.; Wang, X. Quaternized Chitosan Coated Copper Sulfide Nanozyme with Peroxidase-like Activity for Synergistic Antibacteria and Promoting Infected Wound Healing. Int. J. Biol. Macromol. 2023, 246, 125651. [Google Scholar] [CrossRef]
- Yang, D.; Huo, J.; Zhang, Z.; An, Z.; Dong, H.; Wang, Y.; Duan, W.; Chen, L.; He, M.; Gao, S.; et al. Citric Acid Modified Ultrasmall Copper Peroxide Nanozyme for in Situ Remediation of Environmental Sulfonylurea Herbicide Contamination. J. Hazard. Mater. 2023, 443, 130265. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.; Zeng, W.; Liu, J.; Zhang, L.; Cao, Y.; Li, P.; Ran, H.; Wang, Z. Engineered Biomimetic Copper Sulfide Nanozyme Mediates “Don’t Eat Me” Signaling for Photothermal and Chemodynamic Precision Therapies of Breast Cancer. ACS Appl. Mater. Interfaces 2023, 15, 24071–24083. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, H.B.; Son, S.E.; Gupta, P.K.; Park, Y.; Hur, W.; Seong, G.H. Determination of Lysophosphatidylcholine Using Peroxidase-Mimic PVP/PtRu Nanozyme. Anal. Bioanal. Chem. 2023, 415, 1865–1876. [Google Scholar] [CrossRef]
- Chen, S.; LI, Q.; Cheng, C.; Su, B. #5050 Antioxidant porphyrin-based nanozyme with single ruthenium for treatment of acute kidney injury. Nephrol. Dial. Transplant. 2023, 38, gfad063c_5050. [Google Scholar] [CrossRef]
- Singh, A.K.; Bijalwan, K.; Kaushal, N.; Kumari, A.; Saha, A.; Indra, A. Oxidase-like Nanozyme Activity of Manganese Metal–Organic Framework Nanosheets for Colorimetric and Fluorescence Sensing of l-Cysteine. ACS Appl. Nano Mater. 2023, 6, 8036–8045. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Xiaohalati, X.; Su, Q.; Liu, J.; Cai, B.; Yang, W.; Wang, Z.; Wang, L. A Bioinspired Manganese-Organic Framework Ameliorates Ischemic Stroke through Its Intrinsic Nanozyme Activity and Upregulating Endogenous Antioxidant Enzymes. Adv. Sci. 2023, 10, 2206854. [Google Scholar] [CrossRef]
- Wang, Y.; Cho, A.; Jia, G.; Cui, X.; Shin, J.; Nam, I.; Noh, K.-J.; Park, B.J.; Huang, R.; Han, J.W. Tuning Local Coordination Environments of Manganese Single-Atom Nanozymes with Multi-Enzyme Properties for Selective Colorimetric Biosensing. Angew. Chem. 2023, 135, e202300119. [Google Scholar] [CrossRef]
- Sisakhtnezhad, S.; Rahimi, M.; Mohammadi, S. Biomedical Applications of MnO2 Nanomaterials as Nanozyme-Based Theranostics. Biomed. Pharmacother. 2023, 163, 114833. [Google Scholar] [CrossRef]
- Sun, Y.; Jing, X.; Xu, B.; Liu, H.; Chen, M.; Wu, Q.; Huang, Z.; Zheng, L.; Bi, X.; Nie, Y.; et al. A Single-Atom Iron Nanozyme Reactor for α-Ketoglutarate Synthesis. Chem. Eng. J. 2023, 466, 143269. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Lee, D.H.; Nguyen, P.T.; Le, P.G.; Kim, M.I. Foldable Paper Microfluidic Device Based on Single Iron Site-Containing Hydrogel Nanozyme for Efficient Glucose Biosensing. Chem. Eng. J. 2023, 454, 140541. [Google Scholar] [CrossRef]
- Tanvir, F.; Sardar, N.; Yaqub, A.; Ditta, S.A. Synthesis of Iron Oxide Nanoparticles, Characterization, Uses as Nanozyme and Future Prospects. Bioinspired Biomim. Nanobiomater. 2022, 11, 156–167. [Google Scholar] [CrossRef]
- Zhu, Q.; Huang, Y.; Zhu, X.; Peng, L.; Wang, H.; Gao, S.; Yang, Z.; Zhang, J.; Liu, X. Mannose-Coated Superparamagnetic Iron Oxide Nanozyme for Preventing Postoperative Cognitive Dysfunction. Mater. Today Bio 2023, 19, 100568. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Neal, C.J.; Sakthivel, T.S.; Fu, Y.; Omer, M.; Adhikary, A.; Ward, S.; Ta, K.M.; Moxon, S.; Molinari, M.; et al. A Novel Approach for the Prevention of Ionizing Radiation-Induced Bone Loss Using a Designer Multifunctional Cerium Oxide Nanozyme. Bioact. Mater. 2023, 21, 547–565. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Cheng, K.; Yang, M.; Deng, Z.; Ma, Y.; Yan, X.; Zhang, Y.; Jia, Z.; Wang, J.; Tu, K.; et al. Orally Administration of Cerium Oxide Nanozyme for Computed Tomography Imaging and Anti-Inflammatory/Anti-Fibrotic Therapy of Inflammatory Bowel Disease. J. Nanobiotechnol. 2023, 21, 21. [Google Scholar] [CrossRef]
- Murugan, C.; Park, S. Cerium Ferrite @ Molybdenum Disulfide Nanozyme for Intracellular ROS Generation and Photothermal-Based Cancer Therapy. J. Photochem. Photobiol. A Chem. 2023, 437, 114466. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, X.; Yang, T.; Li, Y.; Liu, X.; Zhang, P.; Feng, B.; Qing, T. Transition Metal-Doped Germanium Oxide Nanozyme with Enhanced Enzyme-like Activity for Rapid Detection of Pesticide Residues in Water Samples. Anal. Chim. Acta 2023, 1245, 340861. [Google Scholar] [CrossRef]
- Zhao, Q.; Zheng, L.; Gao, Y.; Li, J.; Wei, J.; Zhang, M.; Sun, J.; Ouyang, J.; Na, N. Dual Active Centers Linked by a Reversible Electron Station as a Multifunctional Nanozyme to Induce Synergetically Enhanced Cascade Catalysis for Tumor-Specific Therapy. J. Am. Chem. Soc. 2023, 145, 12586–12600. [Google Scholar] [CrossRef]
- Yuan, J.; Duan, H.; Wang, L.; Wang, S.; Li, Y.; Lin, J. A Three-in-One Hybrid Nanozyme for Sensitive Colorimetric Biosensing of Pathogens. Food Chem. 2023, 408, 135212. [Google Scholar] [CrossRef]
- Du, W.; Chen, W.; Wang, J.; Zhang, H.; Song, L.; Hu, Y.; Ma, X. A Dual-Nanozyme-Loaded Black Phosphorus Multifunctional Therapeutic Platform for Combined Photothermal/Photodynamic/Starvation Cancer Therapy. J. Mater. Chem. B 2023, 11, 5185–5194. [Google Scholar] [CrossRef]
- Xue, Y.; Zhong, H.; Liu, B.; Qin, S.; Chen, Z.; Li, K.; Zheng, L.; Zuo, X. Colorimetric Identification of Multiple Terpenoids Based on Bimetallic FeCu/NPCs Nanozymes. Anal. Biochem. 2023, 672, 115160. [Google Scholar] [CrossRef]
- Fu, S.; Li, C.; Yang, W.; Chen, H.; Wang, Y.; Zhu, Y.; Zhu, J.; Zhang, B.; Xia, X.; Zheng, J.C. Insulin-incubated Palladium Clusters Alleviate Alzheimer’s Disease-like Phenotypes in a Preclinical Mouse Model. MedComm 2023, 4, e272. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ding, X.; Li, L.; Li, Q.; Li, Z.; Lin, H. Tri-Element Nanozyme PtCuSe as an Ingenious Cascade Catalytic Machine for the Amelioration of Parkinson’s Disease-like Symptoms. Front. Bioeng. Biotechnol. 2023, 11, 1208693. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, P.; Zhu, Y.; Qian, J.; Huang, X.; Zhang, W.; Zhang, H.; Mo, Q.; Lu, Y.; Zhang, Y. 2D Cobalt Oxyhydroxide Nanozymes Inhibit Inflammation by Targeting the NLRP3 Inflammasome. Adv. Funct. Mater. 2023, 33, 2214693. [Google Scholar] [CrossRef]
- Liu, C.; Fan, W.; Cheng, W.-X.; Gu, Y.; Chen, Y.; Zhou, W.; Yu, X.-F.; Chen, M.; Zhu, M.; Fan, K.; et al. Red Emissive Carbon Dot Superoxide Dismutase Nanozyme for Bioimaging and Ameliorating Acute Lung Injury. Adv. Funct. Mater. 2023, 33, 2213856. [Google Scholar] [CrossRef]
- Li, S.; Shang, L.; Xu, B.; Wang, S.; Gu, K.; Wu, Q.; Sun, Y.; Zhang, Q.; Yang, H.; Zhang, F.; et al. A Nanozyme with Photo-Enhanced Dual Enzyme-Like Activities for Deep Pancreatic Cancer Therapy. Angew. Chem. 2019, 131, 12754–12761. [Google Scholar] [CrossRef]
- Shen, J.; Rees, T.W.; Zhou, Z.; Yang, S.; Ji, L.; Chao, H. A Mitochondria-Targeting Magnetothermogenic Nanozyme for Magnet-Induced Synergistic Cancer Therapy. Biomaterials 2020, 251, 120079. [Google Scholar] [CrossRef]
- Duan, F.; Jia, Q.; Liang, G.; Wang, M.; Zhu, L.; McHugh, K.J.; Jing, L.; Du, M.; Zhang, Z. Schottky Junction Nanozyme Based on Mn-Bridged Co-Phthalocyanines and Ti3C2Tx Nanosheets Boosts Integrative Type I and II Photosensitization for Multimodal Cancer Therapy. ACS Nano 2023, 17, 11290–11308. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Xu, G.; Zhang, Z.; Wang, W.; Zhang, C.; Zhao, M.; Qu, Y.; Li, W.; Ji, M.; Liu, Y.; et al. Coupling Doping and Localized Surface Plasmon Resonance toward Acidic PH-Preferential Catalase-like Nanozyme for Oxygen-Dominated Synergistic Cancer Therapy. Chem. Eng. J. 2023, 465, 142961. [Google Scholar] [CrossRef]
- Shi, S.; Jiang, Y.; Yu, Y.; Liang, M.; Bai, Q.; Wang, L.; Yang, D.; Sui, N.; Zhu, Z. Piezo-Augmented and Photocatalytic Nanozyme Integrated Microneedles for Antibacterial and Anti-Inflammatory Combination Therapy. Adv. Funct. Mater. 2023, 33, 2210850. [Google Scholar] [CrossRef]
- Song, N.; Yu, Y.; Zhang, Y.; Wang, Z.; Guo, Z.; Zhang, J.; Zhang, C.; Liang, M. Bioinspired Hierarchical Self-Assembled Nanozyme for Efficient Antibacterial Treatment. Adv. Mater. 2023, 2210455. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Q.; Wang, Q.; Dong, W.; Liu, Y.; Hu, Q.; Song, X.; Shuang, S.; Dong, C.; Gong, X. Metal-Free Nitrogen-Doped Carbon Nanodots as an Artificial Nanozyme for Enhanced Antibacterial Activity. J. Clean. Prod. 2023, 411, 137337. [Google Scholar] [CrossRef]
- Kurup, C.P.; Ahmed, M.U. Nanozymes towards Personalized Diagnostics: A Recent Progress in Biosensing. Biosensors 2023, 13, 461. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour-Haratbar, A.; Boraei, S.B.A.; Zare, Y.; Rhee, K.Y.; Park, S.-J. Graphene-Based Electrochemical Biosensors for Breast Cancer Detection. Biosensors 2023, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Curulli, A. Functional Nanomaterials Enhancing Electrochemical Biosensors as Smart Tools for Detecting Infectious Viral Diseases. Molecules 2023, 28, 3777. [Google Scholar] [CrossRef]
- Zolti, O.; Suganthan, B.; Ramasamy, R.P. Lab-on-a-Chip Electrochemical Biosensors for Foodborne Pathogen Detection: A Review of Common Standards and Recent Progress. Biosensors 2023, 13, 215. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, H.; Yuan, M.; Yu, J.; Wang, Z.; Chen, X. One-Step Laser Synthesis Platinum Nanostructured 3D Porous Graphene: A Flexible Dual-Functional Electrochemical Biosensor for Glucose and PH Detection in Human Perspiration. Talanta 2023, 257, 124362. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Tan, Y.; Liu, X.; Fu, L.; Qing, W. Ag-Fe3O4 Nanozyme with Peroxidase-like Activity for Colorimetric Detection of Sulfide Ions and Dye Degradation. J. Environ. Chem. Eng. 2023, 11, 109150. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Z.; Guan, J. Single-Atom Nanozyme-Based Electrochemical Sensors for Health and Food Safety Monitoring. Food Chem. 2023, 425, 136518. [Google Scholar] [CrossRef]
- Singh, R.; Umapathi, A.; Patel, G.; Patra, C.; Malik, U.; Bhargava, S.K.; Daima, H.K. Nanozyme-Based Pollutant Sensing and Environmental Treatment: Trends, Challenges, and Perspectives. Sci. Total Environ. 2023, 854, 158771. [Google Scholar] [CrossRef]
- Yang, T.; Liu, X.; Zeng, Z.; Wang, X.; Zhang, P.; Feng, B.; Tian, K.; Qing, T. Efficient and Recyclable Degradation of Organic Dye Pollutants by CeO2 @ZIF-8 Nanozyme-Based Non-Photocatalytic System. Environ. Pollut. 2023, 316, 120643. [Google Scholar] [CrossRef]
- Wang, J.; Huang, R.; Qi, W.; Su, R.; Binks, B.P.; He, Z. Construction of a Bioinspired Laccase-Mimicking Nanozyme for the Degradation and Detection of Phenolic Pollutants. Appl. Catal. B Environ. 2019, 254, 452–462. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).