New Glycosalen–Manganese(III) Complexes and RCA120 Hybrid Systems as Superoxide Dismutase/Catalase Mimetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Synthesis of Salen Ligands and MnIII Complexes
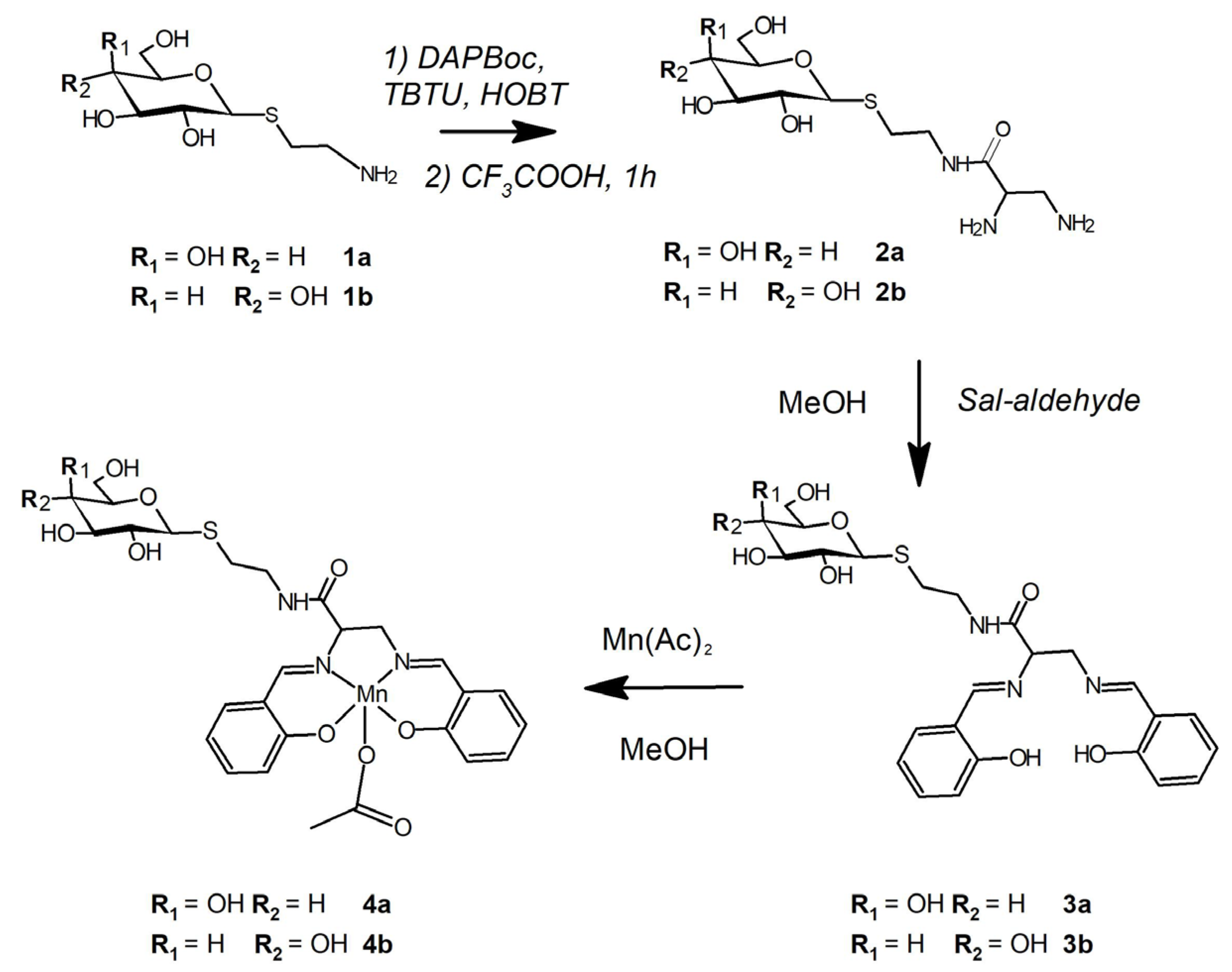
2.2.1. Synthesis of 1-deoxy-1[(S-cysteamine)]-ß-galactose (1a) and of 1-deoxy-1[(S-cysteamine)]-ß-glucose (1b)
2.2.2. Synthesis of 1-deoxy-1[(S-cysteamidopropyl(1,2-diamino)]-ß-galactose] 2a and 1-deoxy-1[(S-cysteamidopropyl(1,2-diamino)]-ß-glucose] 2b
2.2.3. Synthesis of 1-deoxy-1[(S-cysteamidopropyl(1,2-diamino)N,N′-bis(salicylidene))]-ß-galactose] 3a
2.2.4. Synthesis of Manganese(III) Complexes of 3a and 3b
2.2.5. Preparation of Hybrid MnIII and RCA120 System
2.3. Instrumentation
2.4. Superoxide Dismutase Assay
2.5. Catalase Activity Assay
2.6. Peroxidase Activity Assay
3. Results and Discussion
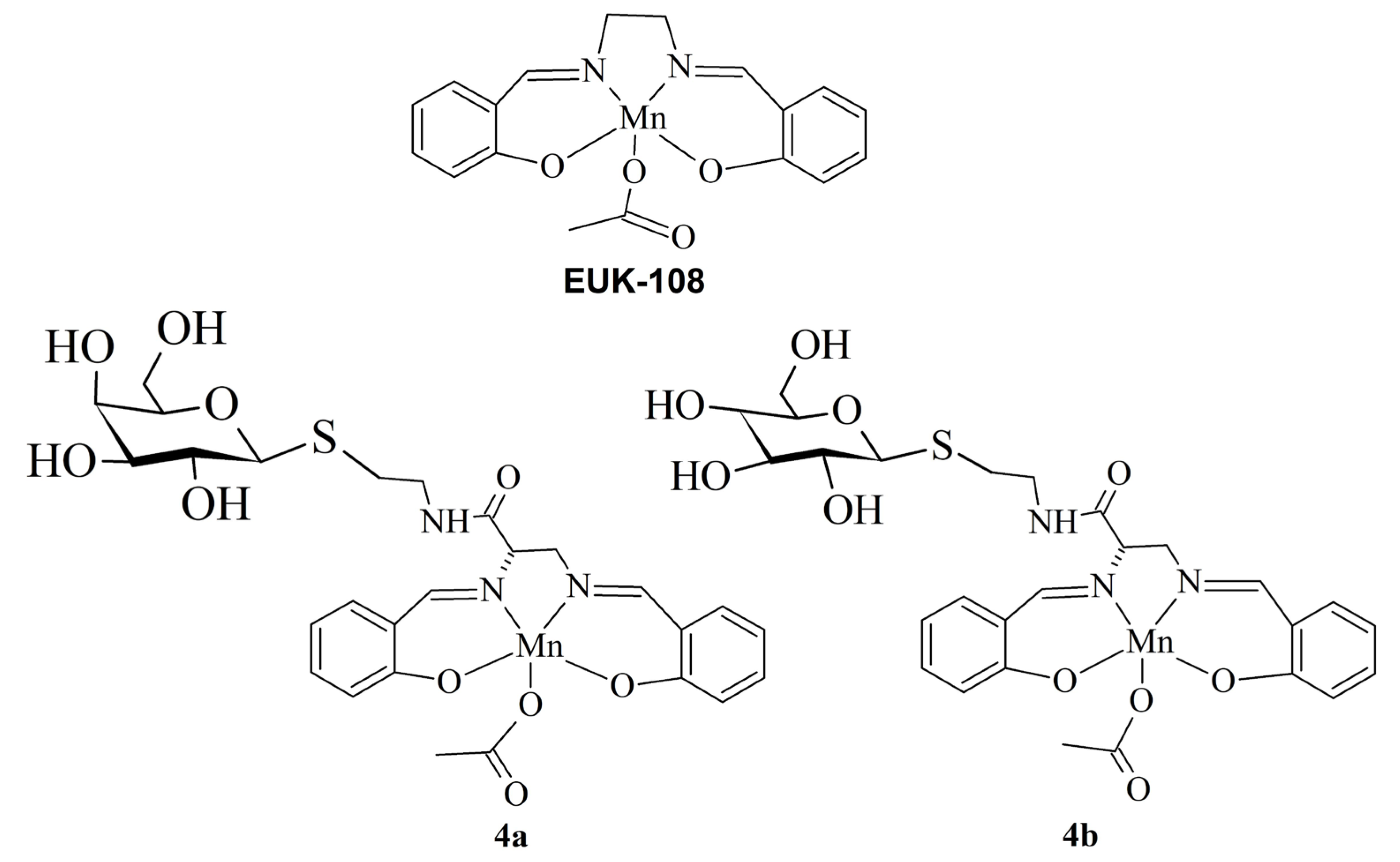
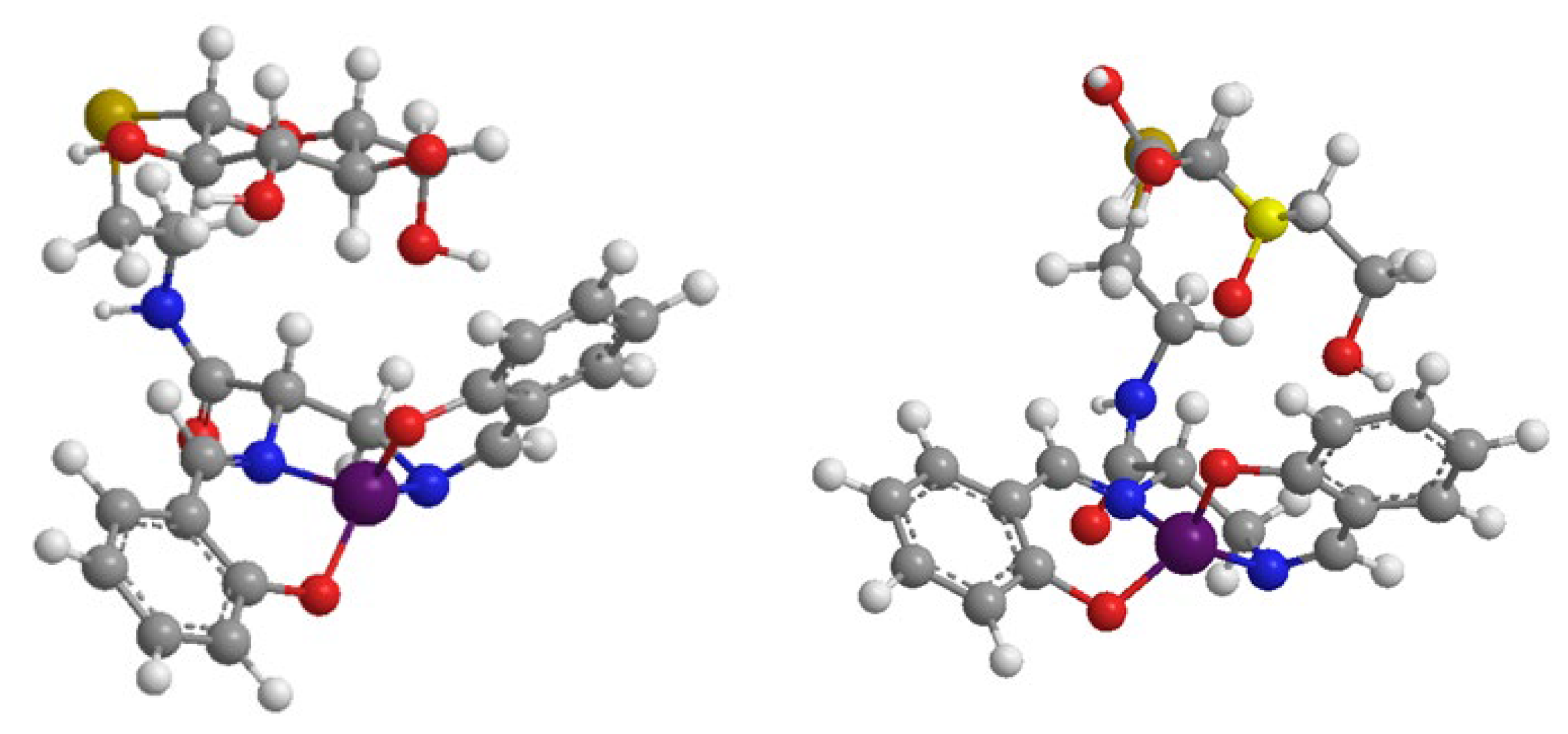
3.1. The Ligands
3.2. The Manganese(III) Complexes
3.3. SOD-like Activity
3.4. Catalase Activity
3.5. Peroxidase Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chatterjee, S. Chapter two–Oxidative stress, inflammation, and disease. In Oxidative Stress and Biomaterials; Dziubla, T., Butterfield, D.A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 35–58. ISBN 978-0-12-803269-5. [Google Scholar]
- Ramos-González, E.J.; Bitzer-Quintero, O.K.; Ortiz, G.; Hernández-Cruz, J.J.; Ramírez-Jirano, L.J. Relationship between inflammation and oxidative stress and its effect on multiple sclerosis. Neurología 2021, in press. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, H.; Silverfield, J.C.; Cheatum, D.E.; Poiley, J.; Taborn, J.; Ignaczak, T.; Multz, C.V. Intra-Articular Orgotein in Osteoarthritis of the Knee: A Placebo-Controlled Efficacy, Safety, and Dosage Comparison. Am. J. Med. 1989, 87, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Bonetta, R. Potential Therapeutic Applications of MnSODs and SOD-Mimetics. Chem. Eur. J. 2018, 24, 5032–5041. [Google Scholar] [CrossRef] [PubMed]
- Batinic-Haberle, I.; Tovmasyan, A.; Roberts, E.R.H.; Vujaskovic, Z.; Leong, K.W.; Spasojevic, I. SOD Therapeutics: Latest Insights into Their Structure-Activity Relationships and Impact on the Cellular Redox-Based Signaling Pathways. Antioxid. Redox Signal. 2014, 20, 2372–2415. [Google Scholar] [CrossRef]
- Bigham, N.P.; Wilson, J.J. Metal Coordination Complexes as Therapeutic Agents for Ischemia-Reperfusion Injury. J. Am. Chem. Soc. 2023, 145, 9389–9409. [Google Scholar] [CrossRef]
- Policar, C.; Bouvet, J.; Bertrand, H.C.; Delsuc, N. SOD Mimics: From the Tool Box of the Chemists to Cellular Studies. Curr. Opin. Chem. Biol. 2022, 67, 102109. [Google Scholar] [CrossRef]
- Rosenthal, R.; Fish, B.; Hill, R.P.; Huffman, K.; Lazarova, Z.; Mahmood, J.; Medhora, M.; Molthen, R.; Moulder, J.E.; Sonis, S.; et al. Salen Mn Complexes Mitigate Radiation Injury in Normal Tissues. Anticancer Agents Med. Chem. 2011, 11, 359–372. [Google Scholar] [CrossRef]
- Karlsson, J.O.G.; Jynge, P.; Ignarro, L.J. May Mangafodipir or Other SOD Mimetics Contribute to Better Care in COVID-19 Patients? Antioxid. Basel Switz. 2020, 9, 971. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sun, X.; Chen, B.; Dai, R.; Xi, Z.; Xu, H. Insights into Manganese Superoxide Dismutase and Human Diseases. Int. J. Mol. Sci. 2022, 23, 15893. [Google Scholar] [CrossRef] [PubMed]
- Miriyala, S.; Spasojevic, I.; Tovmasyan, A.; Salvemini, D.; Vujaskovic, Z.; Clair, D.S.; Batinic-Haberle, I. Manganese Superoxide Dismutase, MnSOD and Its Mimics. Biochim. Biophys. Acta 2012, 1822, 794–814. [Google Scholar] [CrossRef]
- Vincent, A.; Thauvin, M.; Quévrain, E.; Mathieu, E.; Layani, S.; Seksik, P.; Batinic-Haberle, I.; Vriz, S.; Policar, C.; Delsuc, N. Evaluation of the Compounds Commonly Known as Superoxide Dismutase and Catalase Mimics in Cellular Models. J. Inorg. Biochem. 2021, 219, 111431. [Google Scholar] [CrossRef] [PubMed]
- Mapuskar, K.A.; Vasquez Martinez, G.; Pulliam, C.F.; Petronek, M.S.; Steinbach, E.J.; Monga, V.; Furqan, M.; Jetton, J.G.; Saunders, D.P.; Pearce, A.; et al. Avasopasem Manganese (GC4419) Protects against Cisplatin-Induced Chronic Kidney Disease: An Exploratory Analysis of Renal Metrics from a Randomized Phase 2b Clinical Trial in Head and Neck Cancer Patients. Redox Biol. 2023, 60, 102599. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.M.; Lee, C.M.; Saunders, D.P.; Curtis, A.; Dunlap, N.; Nangia, C.; Lee, A.S.; Gordon, S.M.; Kovoor, P.; Arevalo-Araujo, R.; et al. Phase IIb, Randomized, Double-Blind Trial of GC4419 Versus Placebo to Reduce Severe Oral Mucositis Due to Concurrent Radiotherapy and Cisplatin For Head and Neck Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 3256–3265. [Google Scholar] [CrossRef] [PubMed]
- Galera Therapeutics, Inc. A Pilot, Randomized, Placebo-Controlled Trial of GC4419 (Avasopasem Manganese) in Patients with Critical Illness Due to SARS-CoV-2 Infection (COVID-19). 2022. Available online: Clinicaltrials.gov (accessed on 7 July 2023).
- Mathieu, E.; Bernard, A.-S.; Ching, H.Y.V.; Somogyi, A.; Medjoubi, K.; Fores, J.R.; Bertrand, H.C.; Vincent, A.; Trépout, S.; Guerquin-Kern, J.-L.; et al. Anti-Inflammatory Activity of Superoxide Dismutase Mimics Functionalized with Cell-Penetrating Peptides. Dalton Trans. 2020, 49, 2323–2330. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tovmasyan, A.; Spasojevic, I. An Educational Overview of the Chemistry, Biochemistry and Therapeutic Aspects of Mn Porphyrins—From Superoxide Dismutation to H2O2-Driven Pathways. Redox Biol. 2015, 5, 43–65. [Google Scholar] [CrossRef]
- Signorella, S.; Palopoli, C.; Ledesma, G. Rationally Designed Mimics of Antioxidant Manganoenzymes: Role of Structural Features in the Quest for Catalysts with Catalase and Superoxide Dismutase Activity. Coord. Chem. Rev. 2018, 365, 75–102. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tovmasyan, A.; Huang, Z.; Duan, W.; Du, L.; Siamakpour-Reihani, S.; Cao, Z.; Sheng, H.; Spasojevic, I.; Alvarez Secord, A. H2O2-Driven Anticancer Activity of Mn Porphyrins and the Underlying Molecular Pathways. Oxid. Med. Cell. Longev. 2021, 2021, 6653790. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; Arif, F.; Patel, S.; Yuan, X.; Mannam, V.; Howard, S.; Batinic-Haberle, I.; Fukuto, J.; Minnion, M.; et al. Manganese Porphyrin-Based SOD Mimetics Produce Polysulfides from Hydrogen Sulfide. Antioxidants 2019, 8, 639. [Google Scholar] [CrossRef] [PubMed]
- Doctrow, S.R.; Fish, B.; Huffman, K.D.; Lazarova, Z.; Medhora, M.; Williams, J.P.; Moulder, J.E. Salen Manganese Complexes Mitigate Radiation Injury in Normal Tissues Through Modulation of Tissue Environment, Including Through Redox Mechanisms. In Redox-Active Therapeutics; Batinić-Haberle, I., Rebouças, J.S., Spasojević, I., Eds.; Oxidative Stress in Applied Basic Research and Clinical Practice; Springer International Publishing: Cham, Switzerland, 2016; pp. 265–285. ISBN 978-3-319-30705-3. [Google Scholar]
- Rouco, L.; González-Noya, A.M.; Pedrido, R.; Maneiro, M. Pursuing the Elixir of Life: In Vivo Antioxidative Effects of Manganosalen Complexes. Antioxidants 2020, 9, 727. [Google Scholar] [CrossRef] [PubMed]
- Noritake, Y.; Umezawa, N.; Kato, N.; Higuchi, T. Manganese Salen Complexes with Acid–Base Catalytic Auxiliary: Functional Mimetics of Catalase. Inorg. Chem. 2013, 52, 3653–3662. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.C.; d’Emmanuele di Villa Bianca, R.; Wayman, N.S.; Pinto, A.; Sharpe, M.A.; Cuzzocrea, S.; Chatterjee, P.K.; Thiemermann, C. A Superoxide Dismutase Mimetic with Catalase Activity (EUK-8) Reduces the Organ Injury in Endotoxic Shock. Eur. J. Pharmacol. 2003, 466, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Himori, K.; Abe, M.; Tatebayashi, D.; Lee, J.; Westerblad, H.; Lanner, J.T.; Yamada, T. Superoxide Dismutase/Catalase Mimetic EUK-134 Prevents Diaphragm Muscle Weakness in Monocrotalin-Induced Pulmonary Hypertension. PLoS ONE 2017, 12, e0169146. [Google Scholar] [CrossRef] [PubMed]
- Menezes, L.B.; Segat, B.B.; Tolentino, H.; Pires, D.C.; Mattos, L.M.D.M.; Hottum, H.M.; Pereira, M.D.; Latini, A.; Horn, A.J.; Fernandes, C. ROS Scavenging of SOD/CAT Mimics Probed by EPR and Reduction of Lipid Peroxidation in S. Cerevisiae and Mouse Liver, under Severe Hydroxyl Radical Stress Condition. J. Inorg. Biochem. 2023, 239, 112062. [Google Scholar] [CrossRef] [PubMed]
- Segat, B.B.; Menezes, L.B.; Cervo, R.; Cargnelutti, R.; Tolentino, H.; Latini, A.; Horn, A.J.; Fernandes, C. Scavenging of Reactive Species Probed by EPR and Ex-Vivo Nanomolar Reduction of Lipid Peroxidation of Manganese Complexes. J. Inorg. Biochem. 2023, 239, 112060. [Google Scholar] [CrossRef]
- Ning, Y.; Huo, Y.; Xue, H.; Du, Y.; Yao, Y.; Sedgwick, A.C.; Lin, H.; Li, C.; Jiang, S.-D.; Wang, B.-W.; et al. Tri-Manganese(III) Salen-Based Cryptands: A Metal Cooperative Antioxidant Strategy That Overcomes Ischemic Stroke Damage in Vivo. J. Am. Chem. Soc. 2020, 142, 10219–10227. [Google Scholar] [CrossRef]
- Rouco, L.; Alvariño, R.; Alfonso, A.; Romero, M.J.; Pedrido, R.; Maneiro, M. Neuroprotective Effects of Fluorophore-Labelled Manganese Complexes: Determination of ROS Production, Mitochondrial Membrane Potential and Confocal Fluorescence Microscopy Studies in Neuroblastoma Cells. J. Inorg. Biochem. 2022, 227, 111670. [Google Scholar] [CrossRef]
- Doctrow, S.R.; Huffman, K.; Marcus, C.B.; Musleh, W.; Bruce, A.; Baudry, M.; Malfroy, B. Salen-Manganese Complexes: Combined Superoxide Dismutase/Catalase Mimics with Broad Pharmacological Efficacy. Adv. Pharmacol. 1996, 38, 247–269. [Google Scholar]
- Puglisi, A.; Tabbì, G.; Vecchio, G. Bioconjugates of Cyclodextrins of Manganese Salen-Type Ligand with Superoxide Dismutase Activity. J. Inorg. Biochem. 2004, 98, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Lanza, V.; D’Agata, R.; Iacono, G.; Bellia, F.; Spoto, G.; Vecchio, G. Cyclam Glycoconjugates as Lectin Ligands and Protective Agents of Metal-Induced Amyloid Aggregation. J. Inorg. Biochem. 2015, 153, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Maljaars, C.E.P.; Halkes, K.M.; De Oude, W.L.; Haseley, S.R.; Upton, P.J.; McDonnell, M.B.; Kamerling, J.P. Affinity Determination of Ricinus communis Agglutinin Ligands Identified from Combinatorial O- and S-, N-Glycopeptide Libraries. J. Comb. Chem. 2006, 8, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Hanreich, S.; Bonandi, E.; Drienovská, I. Design of Artificial Enzymes: Insights into Protein Scaffolds. ChemBioChem 2023, 24, e202200566. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.J.; Ward, T.R. Artificial Metalloenzymes: Challenges and Opportunities. ACS Cent. Sci. 2019, 5, 1120–1136. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, W.; Chen, X.; Wu, F.; Fan, S.; Yu, P.; Mao, L. A Versatile Artificial Metalloenzyme Scaffold Enabling Direct Bioelectrocatalysis in Solution. Sci. Adv. 2022, 8, eabo3315. [Google Scholar] [CrossRef] [PubMed]
- DiPrimio, D.J.; Holland, P.L. Repurposing Metalloproteins as Mimics of Natural Metalloenzymes for Small-Molecule Activation. J. Inorg. Biochem. 2021, 219, 111430. [Google Scholar] [CrossRef]
- Jeschek, M.; Reuter, R.; Heinisch, T.; Trindler, C.; Klehr, J.; Panke, S.; Ward, T.R. Directed Evolution of Artificial Metalloenzymes for in Vivo Metathesis. Nature 2016, 537, 661–665. [Google Scholar] [CrossRef]
- Haikarainen, A.; Sipilä, J.; Pietikäinen, P.; Pajunen, A.; Mutikainen, I. Synthesis and Characterization of Bulky Salen-Type Complexes of Co, Cu, Fe, Mn and Ni with Amphiphilic Solubility Properties. J. Chem. Soc. Dalton Trans. 2001, 7, 991–995. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Lanza, V.; Vecchio, G. New Conjugates of Superoxide Dismutase/Catalase Mimetics with Cyclodestrins. J. Inorg. Biochem. 2009, 103, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Doctrow, S.R.; Huffman, K.; Marcus, C.B.; Tocco, G.; Malfroy, E.; Adinolfi, C.A.; Kruk, H.; Baker, K.; Lazarowych, N.; Mascarenhas, J.; et al. Salen−Manganese Complexes as Catalytic Scavengers of Hydrogen Peroxide and Cytoprotective Agents: Structure−Activity Relationship Studies. J. Med. Chem. 2002, 45, 4549–4558. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.K.; Zhuang, J.; Doctrow, S.R.; Malfroy, B.; Benson, P.F.; Menconi, M.J.; Fink, M.P. EUK-8, a Synthetic Superoxide Dismutase and Catalase Mimetic, Ameliorates Acute Lung Injury in Endotoxemic Swine. J. Pharmacol. Exp. Ther. 1995, 275, 798–806. [Google Scholar] [PubMed]
- Zhao, H.; Zhang, R.; Yan, X.; Fan, K. Superoxide Dismutase Nanozymes: An Emerging Star for Anti-Oxidation. J. Mater. Chem. B 2021, 9, 6939–6957. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. (Eds.) Estimation of Superoxide Dismutase (SOD). In Plant-Microbe Interactions: Laboratory Techniques; Springer Protocols Handbooks; Springer US: New York, NY, USA, 2021; pp. 117–118. ISBN 978-1-07-161080-0. [Google Scholar]
- Durot, S.; Policar, C.; Cisnetti, F.; Lambert, F.; Renault, J.-P.; Pelosi, G.; Blain, G.; Korri-Youssoufi, H.; Mahy, J.-P. Series of Mn Complexes Based on N-Centered Ligands and Superoxide—Reactivity in an Anhydrous Medium and SOD-Like Activity in an Aqueous Medium Correlated to MnII/MnIII Redox Potentials. Eur. J. Inorg. Chem. 2005, 2005, 3513–3523. [Google Scholar] [CrossRef]
- Bielski, B.H.J.; Shiue, G.G.; Bajuk, S. Reduction of Nitro Blue Tetrazolium by CO2- and O2- Radicals. J. Phys. Chem. 1980, 84, 830–833. [Google Scholar] [CrossRef]
- Shaw, S.; White, J.D. Asymmetric Catalysis Using Chiral Salen–Metal Complexes: Recent Advances. Chem. Rev. 2019, 119, 9381–9426. [Google Scholar] [CrossRef]
- Bonomo, R.P.; Bruno, V.; Conte, E.; De Guidi, G.; La Mendola, D.; Maccarrone, G.; Nicoletti, F.; Rizzarelli, E.; Sortino, S.; Vecchio, G. Potentiometric, Spectroscopic and Antioxidant Activity Studies of SOD Mimics Containing Carnosine. J. Chem. Soc. Dalton Trans. 2003, 3, 4406–4415. [Google Scholar] [CrossRef]
- Shimazaki, K.; Walborg, E.F.; Neri, G.; Jirgensons, B. Circular Dichroism and Saccharide-Induced Conformational Transitions of Lectins from Ricinus Communis. Arch. Biochem. Biophys. 1975, 169, 731–736. [Google Scholar] [CrossRef]
- Xu, Z.; Pan, S.; Li, G.; He, Y.-F.; Wang, R. Albumin Conjugating Amino Acid Schiff-Base Metal Complexes for Scavenging Superoxide Anion Radical. J. Inorg. Organomet. Polym. Mater. 2015, 25, 1313–1319. [Google Scholar] [CrossRef]
- Oliveri, V.; Vecchio, G. A Novel Artificial Superoxide Dismutase: Non-Covalent Conjugation of Albumin with a MnIIIsalophen Type Complex. Eur. J. Med. Chem. 2011, 46, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Furuya, T.; Nakane, D.; Kitanishi, K.; Katsuumi, N.; Tsaturyan, A.; Shcherbakov, I.N.; Unno, M.; Akitsu, T. A Novel Hybrid Protein Composed of Superoxide-Dismutase-Active Cu(II) Complex and Lysozyme. Sci. Rep. 2023, 13, 6892. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-C.; Lee, S.-Y. Peroxidase-like Oxidative Activity of a Manganese-Coordinated Histidyl Bolaamphiphile Self-Assembly. Nanoscale 2015, 7, 17063–17070. [Google Scholar] [CrossRef]
- Bermejo, M.R.; Fernández, M.I.; González-Noya, A.M.; Maneiro, M.; Pedrido, R.; Rodríguez, M.J.; García-Monteagudo, J.C.; Donnadieu, B. Novel Peroxidase Mimics: μ-Aqua Manganese–Schiff Base Dimers. J. Inorg. Biochem. 2006, 100, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Castilla, C.; Naranjo, Á.; Victoria López-Ramón, M.; Siles, E.; López-Peñalver, J.J.; de Almodóvar, J.M.R. Influence of the Hydrodynamic Size and ζ Potential of Manganese Ferrite Nanozymes as Peroxidase-Mimicking Catalysts at pH 4 in Different Buffers. J. Catal. 2022, 414, 179–185. [Google Scholar] [CrossRef]
- Liberato, A.; Fernández-Trujillo, M.J.; Máñez, Á.; Maneiro, M.; Rodríguez-Silva, L.; Basallote, M.G. Pitfalls in the ABTS Peroxidase Activity Test: Interference of Photochemical Processes. Inorg. Chem. 2018, 57, 14471–14475. [Google Scholar] [CrossRef]
| Complex | I50 (μM) | kcat (M−1 s−1) |
|---|---|---|
| EUK-108 | 2.05 (±0.03) | (7.2 ± 0.9) × 106 |
| 4a | 0.20 (±0.04) | (7.4 ± 0.7) × 107 |
| 4b | 0.25 (±0.05) | (5.9 ± 0.4) × 107 |
| 4a/RCA120 | 0.05 (±0.01) | (2.9 ± 0.3) × 108 |
| 4b/RCA120 | 0.20 (±0.03) | (8.0 ± 0.4) × 107 |
| Complex | CAT Activity | Peroxidase Activity |
|---|---|---|
| µmol H2O2/min/mmol | µM ABTS/min | |
| EUK108 | 32.2 (±1.7) | 18.3 (±0.7) |
| 4a | 40.8 (±1.5) | 21.6 (±0.6) |
| 4b | 40.6 (±1.2) | 23.3 (±0.5) |
| 4a/RCA120 | 31.5 (±1.5) | 35.7 (±0.7) |
| 4b/RCA120 | 28.8 (±1.2) | 25.3 (±1.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanza, V.; Vecchio, G. New Glycosalen–Manganese(III) Complexes and RCA120 Hybrid Systems as Superoxide Dismutase/Catalase Mimetics. Biomimetics 2023, 8, 447. https://doi.org/10.3390/biomimetics8050447
Lanza V, Vecchio G. New Glycosalen–Manganese(III) Complexes and RCA120 Hybrid Systems as Superoxide Dismutase/Catalase Mimetics. Biomimetics. 2023; 8(5):447. https://doi.org/10.3390/biomimetics8050447
Chicago/Turabian StyleLanza, Valeria, and Graziella Vecchio. 2023. "New Glycosalen–Manganese(III) Complexes and RCA120 Hybrid Systems as Superoxide Dismutase/Catalase Mimetics" Biomimetics 8, no. 5: 447. https://doi.org/10.3390/biomimetics8050447
APA StyleLanza, V., & Vecchio, G. (2023). New Glycosalen–Manganese(III) Complexes and RCA120 Hybrid Systems as Superoxide Dismutase/Catalase Mimetics. Biomimetics, 8(5), 447. https://doi.org/10.3390/biomimetics8050447





