New PMMA-Based Hydroxyapatite/ZnFe2O4/ZnO Composite with Antibacterial Performance and Low Toxicity
Abstract
:1. Introduction
2. Materials and Methods
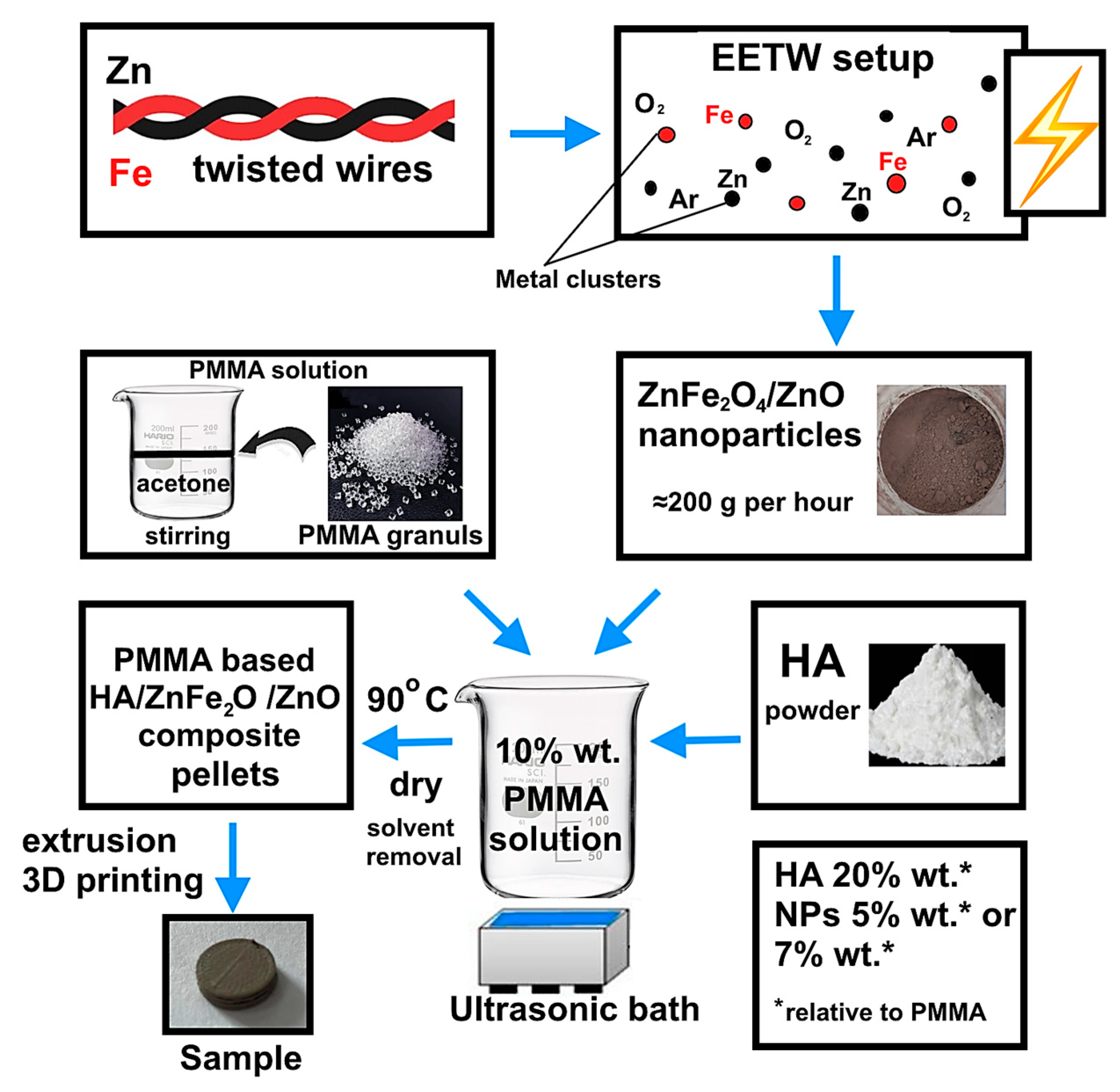
2.1. Synthesis of ZnFe2O4/ZnO Heterophase Nanoparticles
2.2. Fabrication of PMMA-Based HA/ZnFe2O4/ZnO Composite
2.3. Characterization
2.4. Antibacterial and Antifungal Activity of PMMA-Based HA/ZnFe2O4/ZnO Composite
2.5. Biocompatibility of PMMA-Based HA/ZnFe2O4/ZnO Composite
3. Results and Discussion
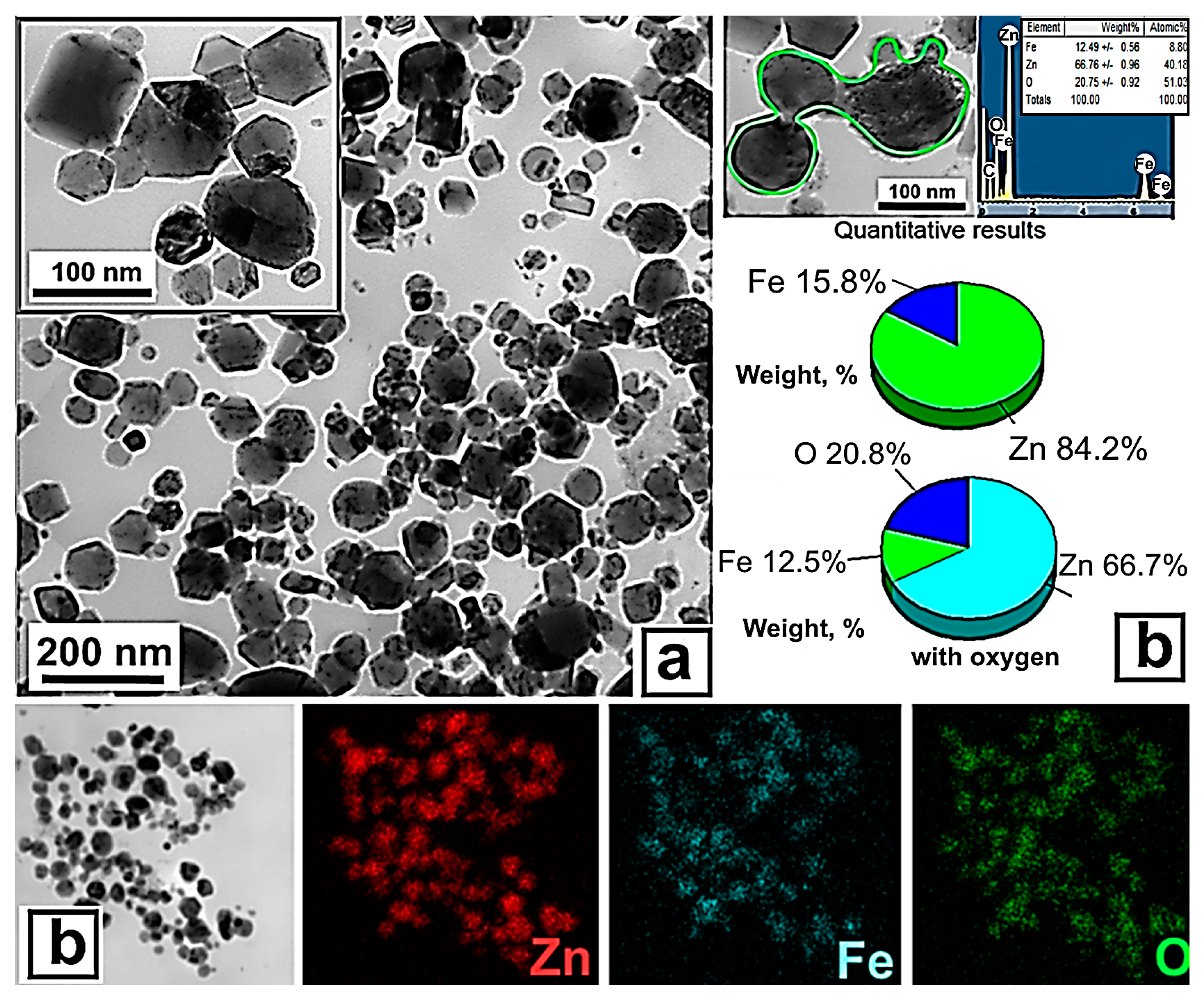
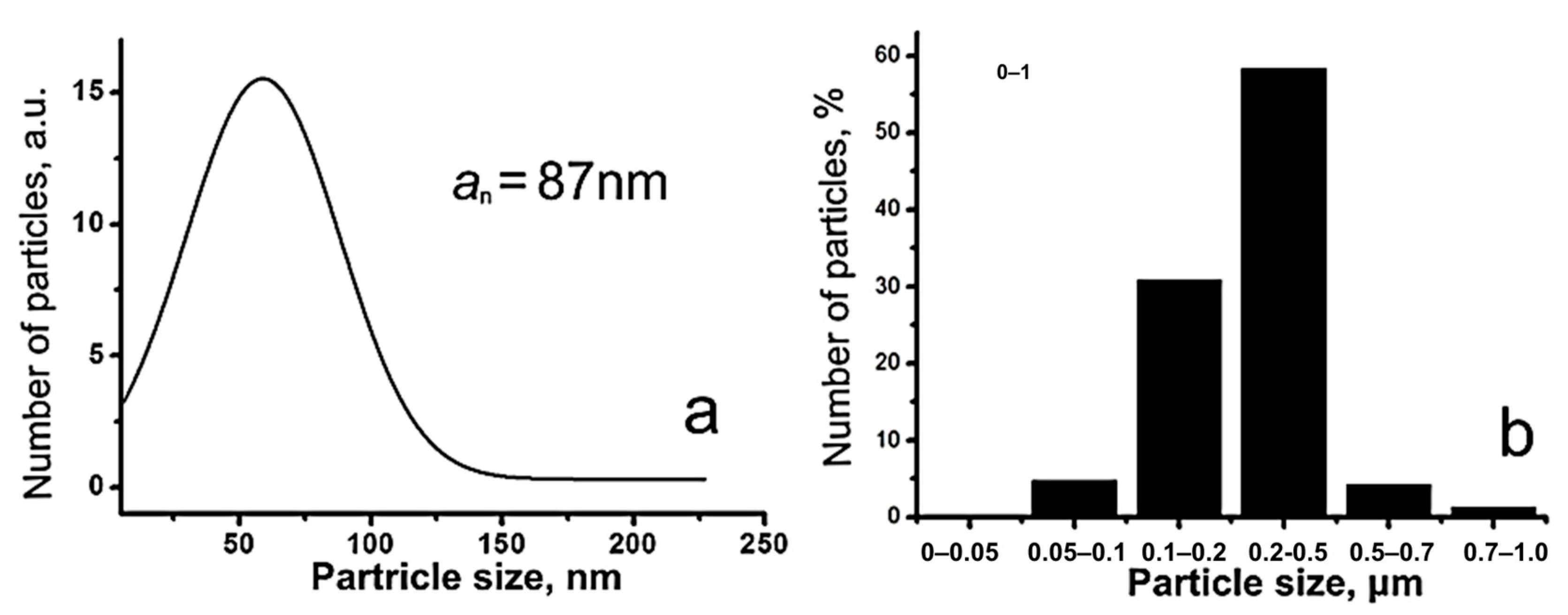
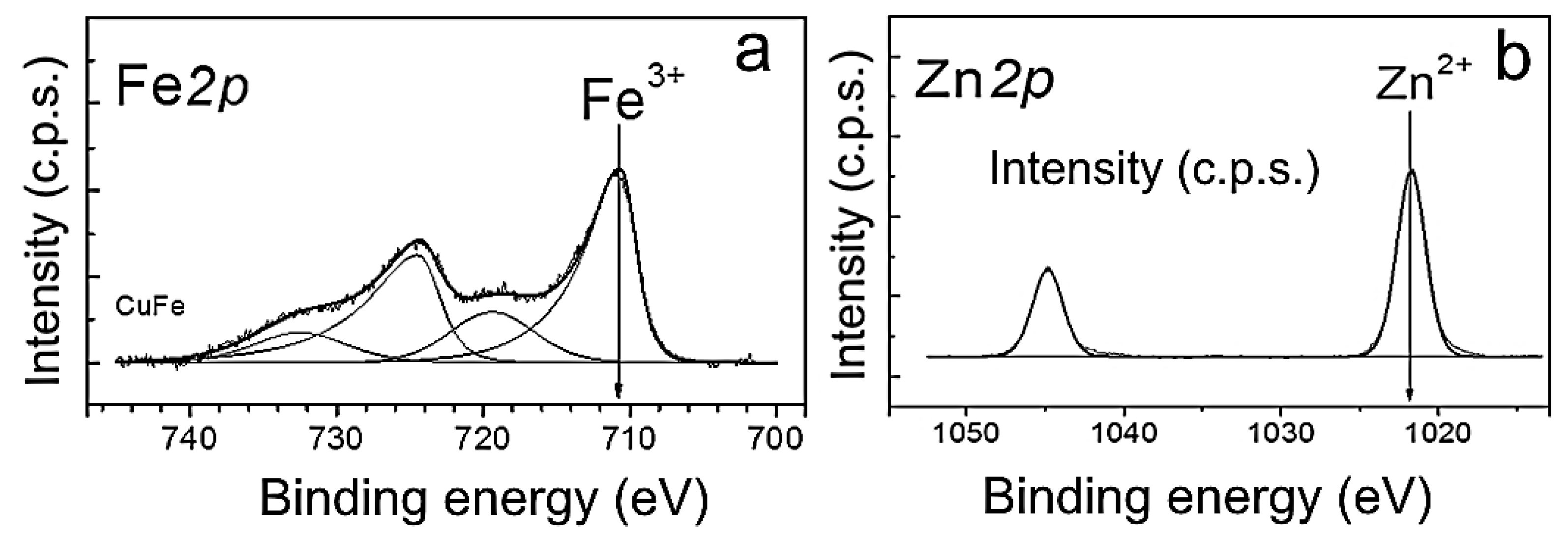
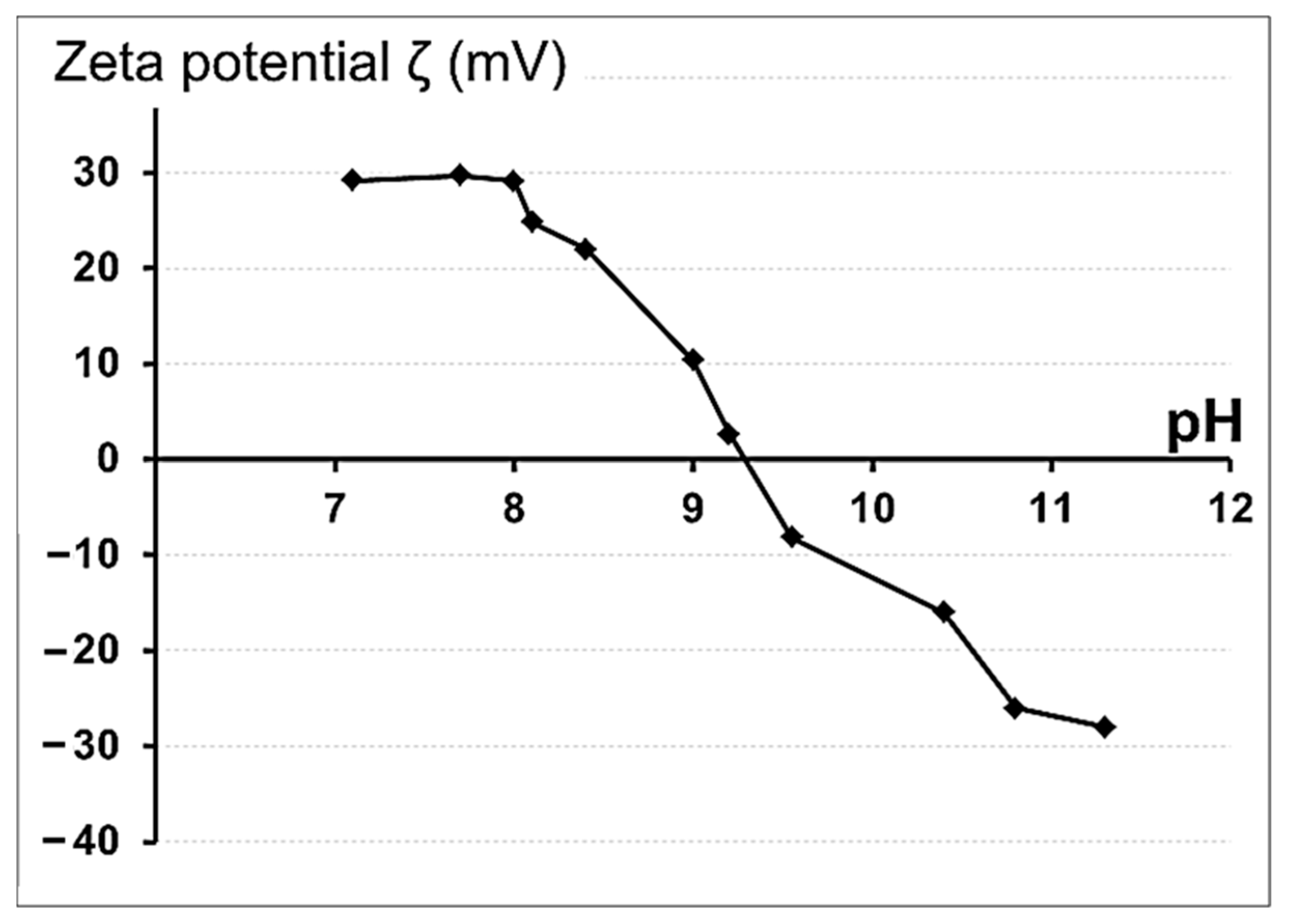
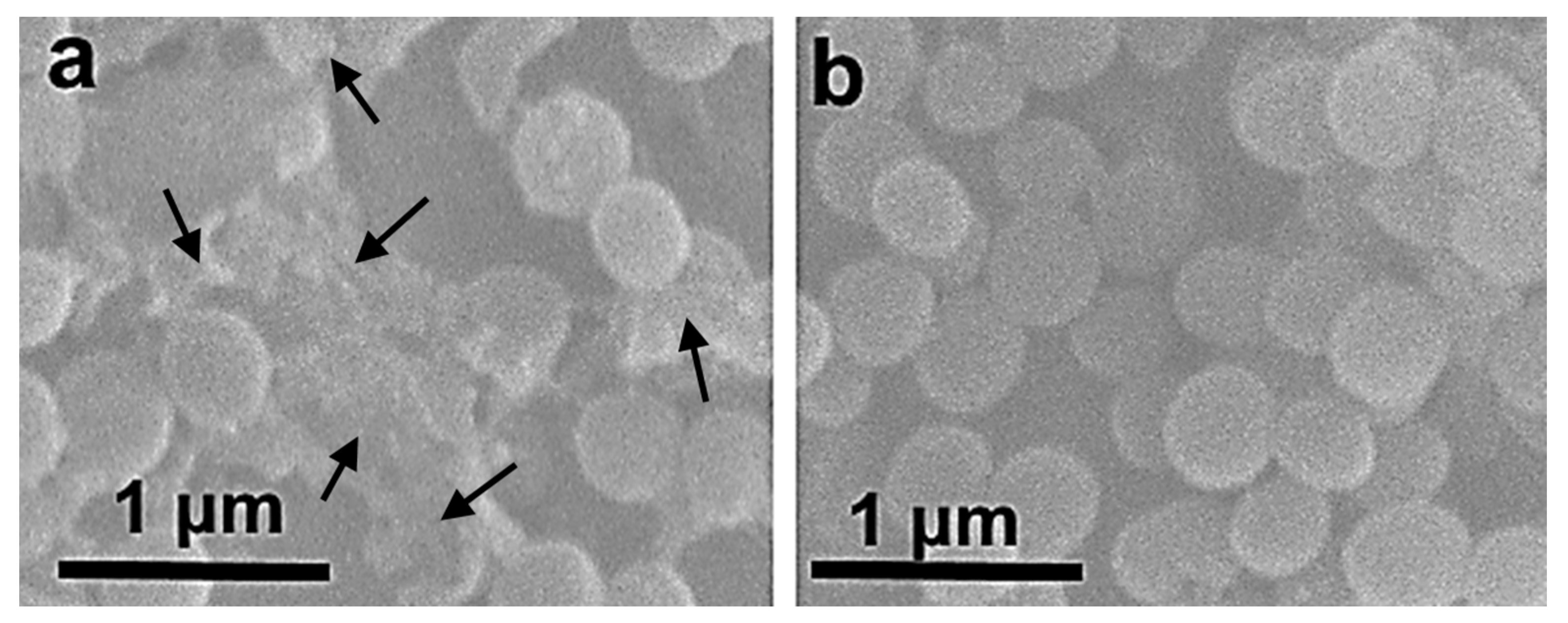
3.1. Preparation and Physicochemical Characteristics of ZnFe2O4/ZnO NPs
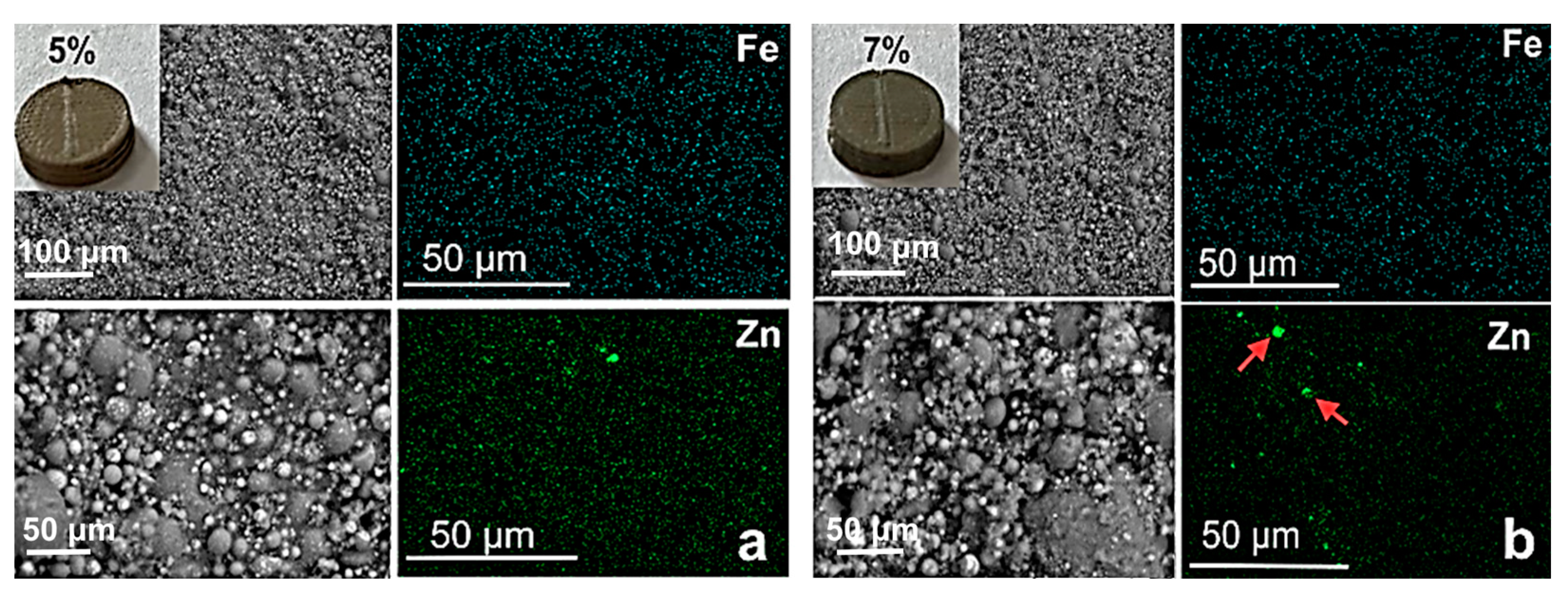
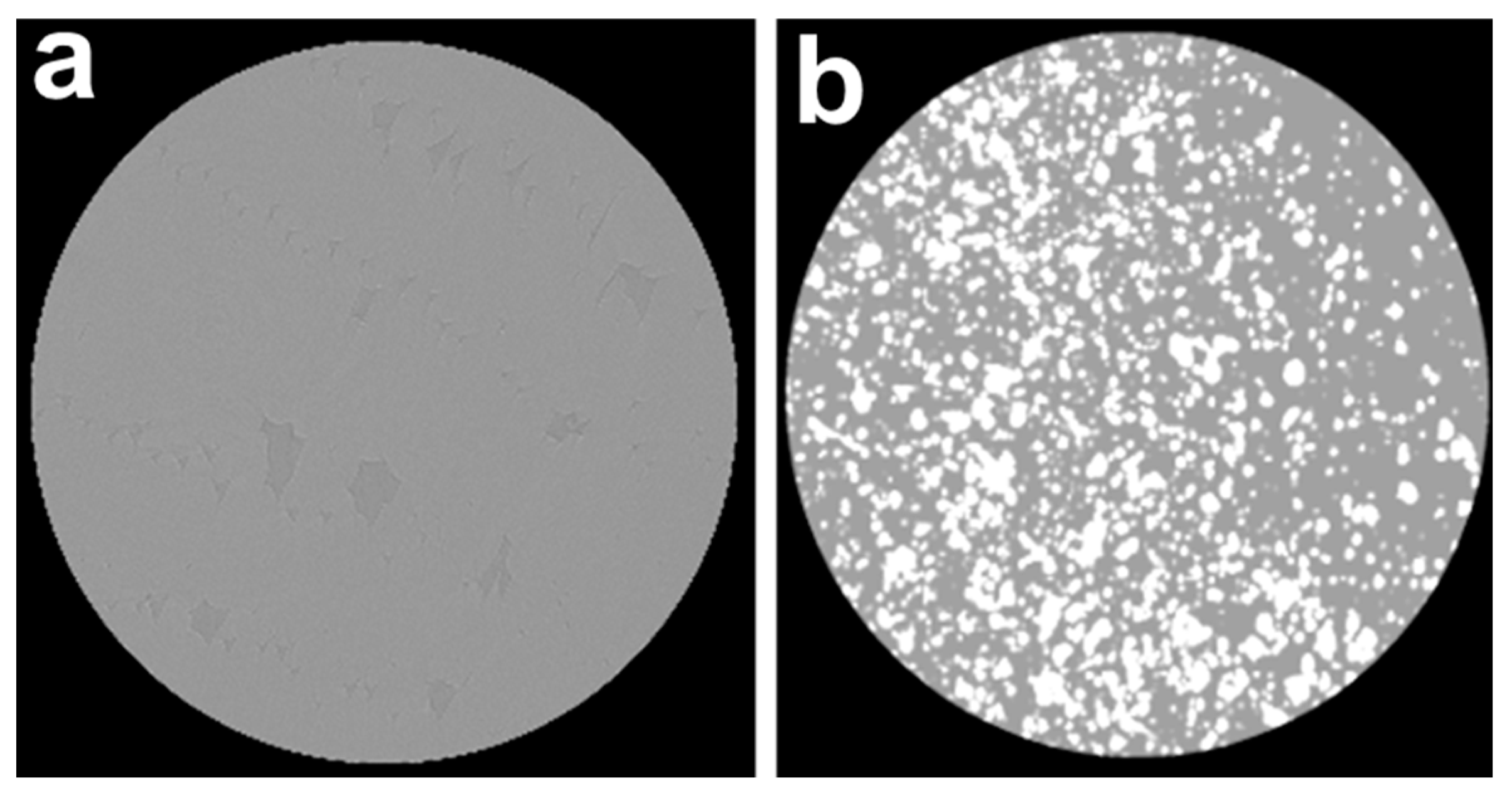
3.2. Characterization of PMMA-Based HA/ZnFe2O4/ZnO Composite
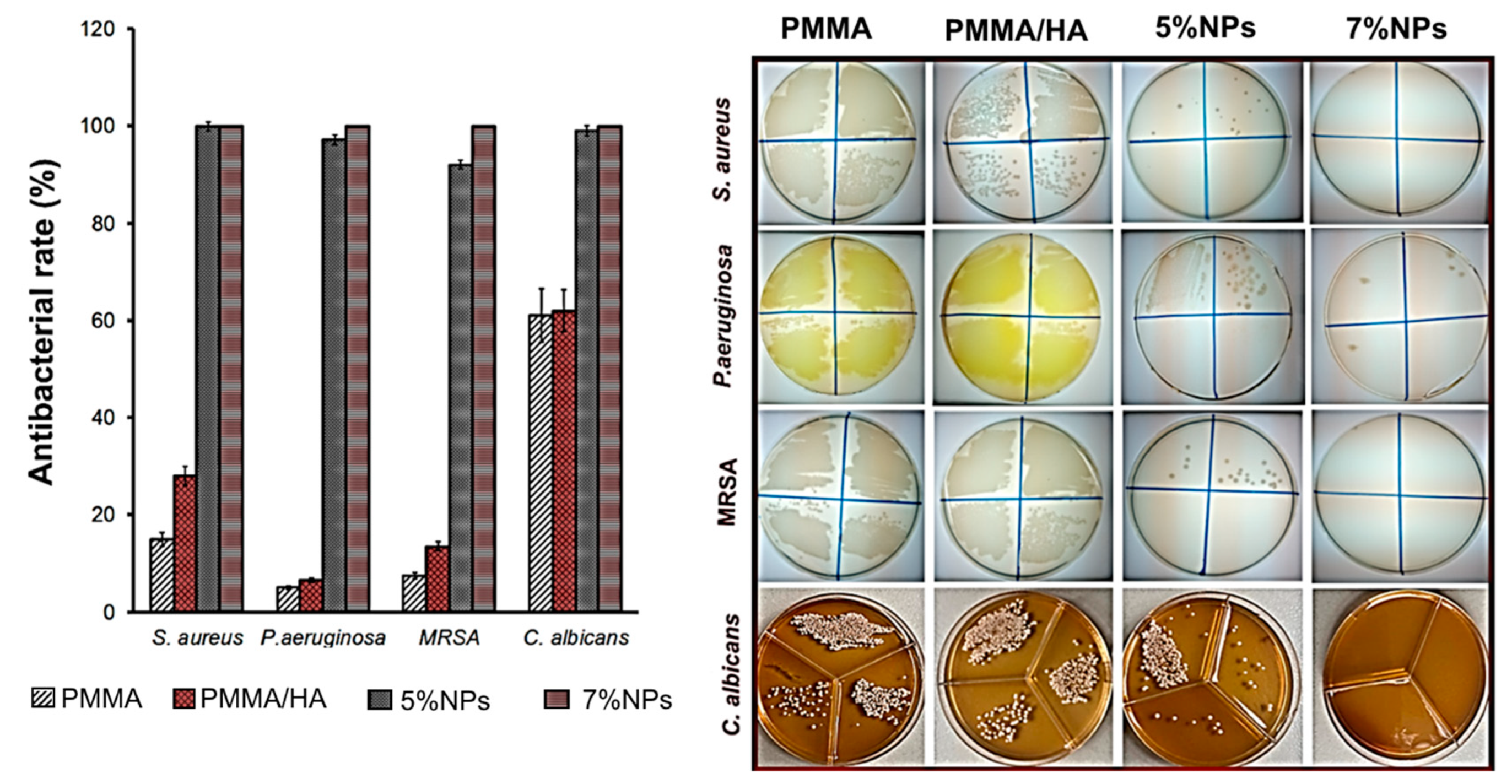
3.3. Antimicrobial Activity
- (1)
- (2)
- (3)
- Teicoic and lipoteichoic acids act as a chelating agent on Zn2+ ions, which are then carried by passive diffusion across membrane proteins [41].
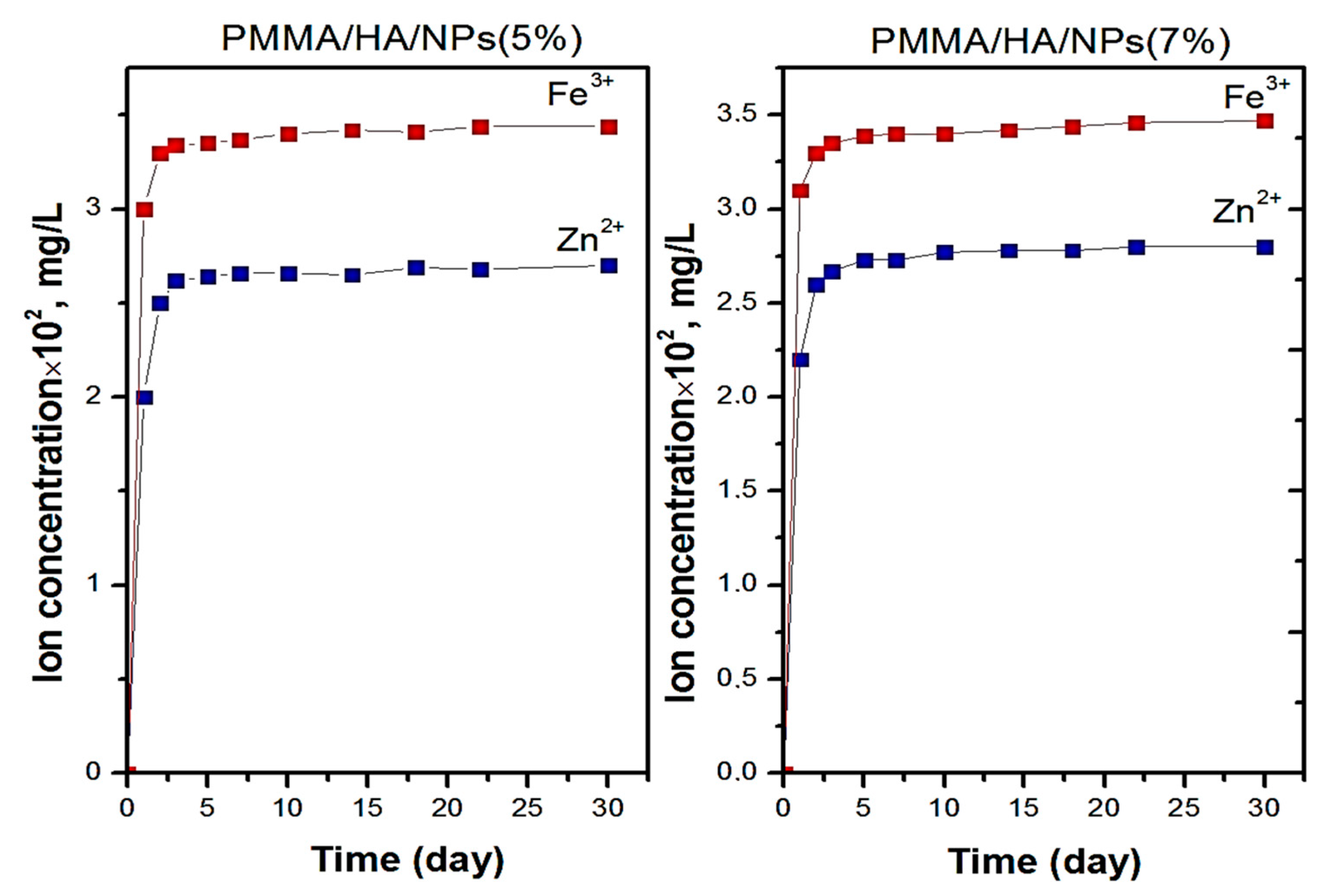
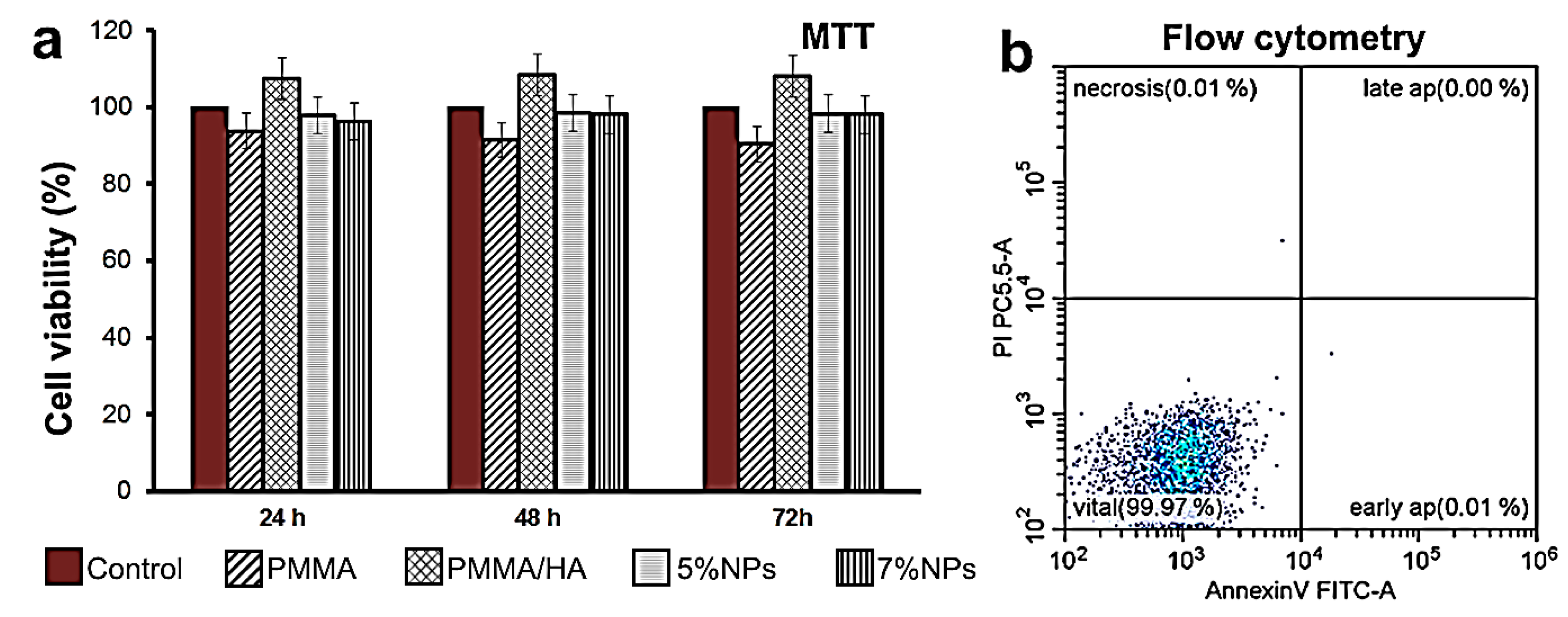
3.4. Biocompatibility of PMMA-Based HA/ZnFe2O4/ZnO Composite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Webb, J.C.J.; Spencer, R.F. The role of polymethylmethacrylate bone cement in modern orthopaedic surgery of the article. J. Bone Jt. Surg. Br. Vol. 2007, 89, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Komang-Agung, I.S.; Hydravianto, L.; Sindrawati, O.; William, P.S. Effect of polymethylmethacrylate-hydroxyapatite composites on callus formation and compressive strength in goat vertebral body. Malays. Orthop. J. 2018, 12, 6. [Google Scholar] [PubMed]
- Silva, E.D.S.; Ribeiro, L.A.; Nascimento, M.C.B.C.D.; Ito, E.N. Rheological and mechanical characterization of poly (methyl methacrylate)/silica (PMMA/SiO2) composites. Mater. Res. 2014, 17, 926–932. [Google Scholar] [CrossRef]
- Wijesinghe, W.L.; Mantilaka, M.M.M.G.P.G.; Karunarathne, T.S.E.F.; Rajapakse, R.M.G. Synthesis of a hydroxyapatite/poly (methyl methacrylate) nanocomposite using dolomite. Nanoscale Adv. 2019, 1, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.; Chauhan, M.; Vaish, A. Bone cement. J. Clin. Orthop. Trauma 2013, 4, 157–163. [Google Scholar] [CrossRef]
- Li, M.; Wang, S.; Li, R.; Wang, Y.; Fan, X.; Gong, W.; Ma, Y. The mechanical and antibacterial properties of boron nitride/silver nanocomposite enhanced polymethyl methacrylate resin for application in oral denture bases. Biomimetics 2022, 7, 138. [Google Scholar] [CrossRef]
- Teotia, A.K.; Raina, D.B.; Singh, C.; Sinha, N.; Isaksson, H.; Tägil, M. Nano-hydroxyapatite bone substitute functionalized with bone active molecules for enhanced cranial bone regeneration. ACS Appl. Mater. Interfaces 2017, 9, 6816–6828. [Google Scholar] [CrossRef]
- Pu’ad, N.M.; Haq, R.A.; Noh, H.M.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Synthesis method of hydroxyapatite: A review. Mater. Today Proc. 2020, 29, 233–239. [Google Scholar] [CrossRef]
- Kobayashi, M.; Nihonmatsu, S.; Okawara, T.; Onuki, H.; Sakagami, H.; Nakajima, H.; Shimada, J. Adhesion and proliferation of osteoblastic cells on hydroxyapatite-dispersed Ti-based composite plate. In Vivo 2019, 33, 1067–1079. [Google Scholar] [CrossRef]
- Chamrad, J.; Marcián, P.; Cizek, J. Beneficial osseointegration effect of hydroxyapatite coating on cranial implant–FEM investigation. PLoS ONE 2021, 16, e0254837. [Google Scholar] [CrossRef]
- Yang, B.; Li, M.; Wu, Y.; Huang, J.; Zhang, H.; Deng, C. Preparation and characterization of bone-like hydroxyapatite/poly (methyl methacrylate) composite biomaterials. Sci. Eng. Compos. Mater. 2013, 20, 147–153. [Google Scholar] [CrossRef]
- Aldabib, J.M. Effect of silane coupling agent content on mechanical properties of hydroxyapatite/poly (methyl methacrylate) denture base composite. J. Sci. Perspect. 2021, 5, 37–45. [Google Scholar] [CrossRef]
- Lamkhao, S.; Phaya, M.; Jansakun, C.; Chandet, N.; Thongkorn, K.; Rujijanagul, G.; Randorn, C. Synthesis of hydroxyapatite with antibacterial properties using a microwave-assisted combustion method. Sci. Rep. 2019, 9, 4015. [Google Scholar] [CrossRef] [PubMed]
- Akshaya, S.; Rowlo, P.K.; Dukle, A.; Nathanael, A.J. Antibacterial coatings for titanium implants: Recent trends and future perspectives. Antibiotics 2022, 11, 1719. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Kadir, M.R.A.; Mahmood, N.H.; Salim, N.; Froemming, G.R.; Balaji, H.R.; Kamarul, T. Characterization, antibacterial and in vitro compatibility of zinc-silver doped hydroxyapatite nanoparticles prepared through microwave synthesis. Ceram. Int. 2014, 40, 4507–4513. [Google Scholar] [CrossRef]
- Bee, S.L.; Bustami, Y.; Ul-Hamid, A.; Lim, K.; Abdul Hamid, Z.A. Synthesis of silver nanoparticle-decorated hydroxyapatite nanocomposite with combined bioactivity and antibacterial properties. J. Mater. Sci. Mater. Med. 2021, 32, 106. [Google Scholar] [CrossRef]
- Elbasuney, S.; El-Sayyad, G.S.; Radwan, S.M.; Correa-Duarte, M.A. Antimicrobial, and antibiofilm activities of silver doped hydroxyapatite: A novel bioceramic material for dental filling. J. Inorg. Organomet. Polym. Mater. 2022, 32, 4559–4575. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and cytotoxic properties of silver nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Massuyeau, F.; Constantin, L.V.; Predoi, D. Structural and physical properties of antibacterial Ag-doped nano-hydroxyapatite synthesized at 100 °C. Nanoscale Res. Lett. 2011, 6, 613. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Souto, E.B. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Hyder, F.; Manjura Hoque, S. Brain tumor diagnostics and therapeutics with superparamagnetic ferrite nanoparticles. Contrast Media Mol. Imaging 2017, 11, 6387217. [Google Scholar] [CrossRef] [PubMed]
- Kmita, A.; Lachowicz, D.; Żukrowski, J.; Gajewska, M.; Szczerba, W.; Kuciakowski, J.; Sikora, M. One-step synthesis of long term stable superparamagnetic colloid of zinc ferrite nanorods in water. Materials 2019, 12, 1048. [Google Scholar] [CrossRef] [PubMed]
- Haghniaz, R.; Rabbani, A.; Vajhadin, F.; Khan, T.; Kousar, R.; Khan, A.R.; Wahid, F. Anti-bacterial and wound healing-promoting effects of zinc ferrite nanoparticles. J. Nanobiotechnol. 2021, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Teng, G.; Jin, X.; Wang, L.; Qiang, Z.; Ma, W.; Zhang, C. Shape-controlled synthesis of coral-like ZnO/C-ZnFe2O4 hierarchical structures and their improved photocatalytic antibacterial efficiency under visible light illumination. Ind. Eng. Chem. Res. 2020, 59, 11219–11231. [Google Scholar] [CrossRef]
- AlSalhi, M.S.; Devanesan, S.; Asemi, N.; Ahamed, A. Concurrent fabrication of ZnO–ZnFe2O4 hybrid nanocomposite for enhancing photocatalytic degradation of organic pollutants and its bacterial inactivation. Chemosphere 2023, 318, 137928. [Google Scholar] [CrossRef] [PubMed]
- Bakina, O.; Glazkova, E.; Rodkevich, N.; Mosunov, A.; Chzhou, V.; Lerner, M. Electroexplosive synthesis of composite ZnO/ZnFe2O4/Zn nanoparticles with photocatalytic and antibacterial activity. Mater. Sci. Semicond. Process. 2022, 152, 107076. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, T.; Zheng, Y.F. Microstructure, mechanical property, biodegradation behavior, and biocompatibility of biodegradable Fe–Fe2O3 composites. J. Biomed. Mater. Res. Part A 2014, 102, 2277–2287. [Google Scholar] [CrossRef]
- Lozhkomoev, A.S.; Pervikov, A.V.; Kazantsev, S.O.; Sharipova, A.F.; Rodkevich, N.G.; Toropkov, N.E.; Lerner, M.I. Synthesis of Fe/Fe3O4 core-shell nanoparticles by electrical explosion of the iron wire in an oxygen-containing atmosphere. J. Nanoparticle Res. 2021, 23, 73. [Google Scholar] [CrossRef]
- Lozhkomoev, A.S.; Kazantsev, S.O.; Kondranova, A.M.; Fomenko, A.N.; Pervikov, A.V.; Rodkevich, N.G.; Bakina, O.V. Design of antimicrobial composite nanoparticles ZnxMe (100-x)/O by electrical explosion of two wires in the oxygen-containing atmosphere. Mater. Des. 2019, 183, 108099. [Google Scholar] [CrossRef]
- Metwally, S.; Ferraris, S.; Spriano, S.; Krysiak, Z.J.; Kaniuk, Ł.; Marzec, M.M.; Stachewicz, U. Surface potential and roughness controlled cell adhesion and collagen formation in electrospun PCL fibers for bone regeneration. Mater. Des. 2020, 194, 108915. [Google Scholar] [CrossRef]
- Zhang, F.; Lan, J.; Yang, Y.; Wei, T.; Tan, R.; Song, W. Adsorption behavior and mechanism of methyl blue on zinc oxide nanoparticles. J. Nanoparticle Res. 2013, 15, 1–10. [Google Scholar] [CrossRef]
- Cai, K.; Frant, M.; Bossert, J.; Hildebrand, G.; Liefeith, K.; Jandt, K.D. Surface functionalized titanium thin films: Zeta-potential, protein adsorption and cell proliferation. Colloids Surf. B: Biointerfaces 2006, 50, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Liang, E.; Wang, J. Fabrication of magnetic ZnO/ZnFe2O4/diatomite composites: Improved photocatalytic efficiency under visible light irradiation. J. Mater. Sci. Mater. Electron. 2022, 33, 1405–1424. [Google Scholar] [CrossRef]
- Tu, Y.J.; Chan, T.S.; Tu, H.W.; Wang, S.L.; You, C.F.; Chang, C.K. Rapid and efficient removal/recovery of molybdenum onto ZnFe2O4 nanoparticles. Chemosphere 2016, 148, 452–458. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Raeisi Shahraki, R.; Seyyed Ebrahimi, S.A.; Masoudpanah, S.M. Magnetic properties of zinc ferrite nanoparticles synthesized by coprecipitation method. J. Supercond. Nov. Magn. 2014, 27, 1587–1592. [Google Scholar] [CrossRef]
- Falak, P.; Hassanzadeh-Tabrizi, S.A.; Saffar-Teluri, A. Synthesis, characterization, and magnetic properties of ZnO-ZnFe2O4 nanoparticles with high photocatalytic activity. J. Magn. Magn. Mater. 2017, 441, 98–104. [Google Scholar] [CrossRef]
- Ning, C.; Wang, X.; Li, L.; Zhu, Y.; Li, M.; Yu, P.; Mao, C. Concentration ranges of antibacterial cations for showing the highest antibacterial efficacy but the least cytotoxicity against mammalian cells: Implications for a new antibacterial mechanism. Chem. Res. Toxicol. 2015, 28, 1815–1822. [Google Scholar] [CrossRef]
- Sun, H.Q.; Lu, X.M.; Gao, P.J. The exploration of the antibacterial mechanism of Fe3+ against bacteria. Braz. J. Microbiol. 2011, 42, 410–414. [Google Scholar] [CrossRef]
- Glazkova, E.; Bakina, O.; Rodkevich, N.; Mosunov, A.; Evstigneev, M.; Evstigneev, V.; Lerner, M. Antibacterial Properties of PMMA Functionalized with CuFe2O4/Cu2O/CuO Nanoparticles. Coatings 2022, 12, 957. [Google Scholar] [CrossRef]
- Tong, G.; Du, F.; Wu, W.; Wu, R.; Liu, F.; Liang, Y. Enhanced reactive oxygen species (ROS) yields and antibacterial activity of spongy ZnO/ZnFe2O4 hybrid micro-hexahedra selectively synthesized through a versatile glucose-engineered co-precipitation/annealing process. J. Mater. Chem. B 2013, 1, 2647–2657. [Google Scholar] [CrossRef]
- Raj, N.B.; PavithraGowda, N.T.; Pooja, O.S.; Purushotham, B.; Kumar, M.A.; Sukrutha, S.K.; Boppana, S.B. Harnessing ZnO nanoparticles for antimicrobial and photocatalytic activities. J. Photochem. Photobiol. 2021, 6, 100021. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.D.M.R.; Lopes, P.R.M.; de Moraes, P.B.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.N.; Huang, X.T.; Jiang, X.L.; Deng, T.W.; Li, Q.X.; Li, J.X.; Fu, J.J. The antibacterial effects of supermolecular nano-carriers by combination of silver and photodynamic therapy. Front. Chem. 2021, 9, 666408. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Jha, E.; Panda, P.K.; Thirumurugan, A.; Patro, S.; Parashar, S.K.S.; Suar, M. Molecular insights to alkaline based bio-fabrication of silver nanoparticles for inverse cytotoxicity and enhanced antibacterial activity. Mater. Sci. Eng. C 2018, 92, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Arun, T.; Verma, S.K.; Panda, P.K.; Joseyphus, R.J.; Jha, E.; Akbari-Fakhrabadi, A.; Satyam, P.V. Facile synthesized novel hybrid graphene oxide/cobalt ferrite magnetic nanoparticles based surface coating material inhibit bacterial secretion pathway for antibacterial effect. Mater. Sci. Eng. C 2019, 104, 109932. [Google Scholar] [CrossRef] [PubMed]
- Aldenhoff, Y.B.J.; Kruft, M.A.B.; Pijpers, A.P.; van der Veen, F.H.; Bulstra, S.K.; Kuijer, R.; Koole, L.H. Stability of radiopaque iodine-containing biomaterials. Biomaterials 2002, 23, 881–886. [Google Scholar] [CrossRef]
- Chaberska, H.; Kaczmarek, H.; Bazylak, G. Viability of murine 3T3 fibroblasts on the poly (methyl methacrylate) surface modified by constant UV irradiation. Polim. W Med. 2007, 37, 13–19. [Google Scholar]
- Mahadevan, G.; Valiyaveettil, S. Understanding the interactions of poly (methyl methacrylate) and poly (vinyl chloride) nanoparticles with BHK-21 cell line. Sci. Rep. 2021, 11, 2089. [Google Scholar] [CrossRef]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef]
- Harb, S.V.; Bassous, N.J.; de Souza, T.A.; Trentin, A.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Hydroxyapatite and β-TCP modified PMMA-TiO2 and PMMA-ZrO2 coatings for bioactive corrosion protection of Ti6Al4V implants. Mater. Sci. Eng. C 2020, 116, 111149. [Google Scholar] [CrossRef]


| Sample Designation | Component Mass Ratio, % | ||
|---|---|---|---|
| PMMA | HA | ZnFe2O4/ZnO NPs | |
| PMMA | 100 | 0 | 0 |
| PMMA/HA | 80 | 20 | 0 |
| PMMA/HA/NPs (5%) | 75 | 20 | 5 |
| PMMA/HA/NPs (7%) | 73 | 20 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakina, O.; Svarovskaya, N.; Ivanova, L.; Glazkova, E.; Rodkevich, N.; Evstigneev, V.; Evstigneev, M.; Mosunov, A.; Lerner, M. New PMMA-Based Hydroxyapatite/ZnFe2O4/ZnO Composite with Antibacterial Performance and Low Toxicity. Biomimetics 2023, 8, 488. https://doi.org/10.3390/biomimetics8060488
Bakina O, Svarovskaya N, Ivanova L, Glazkova E, Rodkevich N, Evstigneev V, Evstigneev M, Mosunov A, Lerner M. New PMMA-Based Hydroxyapatite/ZnFe2O4/ZnO Composite with Antibacterial Performance and Low Toxicity. Biomimetics. 2023; 8(6):488. https://doi.org/10.3390/biomimetics8060488
Chicago/Turabian StyleBakina, Olga, Natalia Svarovskaya, Ludmila Ivanova, Elena Glazkova, Nikolay Rodkevich, Vladyslav Evstigneev, Maxim Evstigneev, Andrey Mosunov, and Marat Lerner. 2023. "New PMMA-Based Hydroxyapatite/ZnFe2O4/ZnO Composite with Antibacterial Performance and Low Toxicity" Biomimetics 8, no. 6: 488. https://doi.org/10.3390/biomimetics8060488
APA StyleBakina, O., Svarovskaya, N., Ivanova, L., Glazkova, E., Rodkevich, N., Evstigneev, V., Evstigneev, M., Mosunov, A., & Lerner, M. (2023). New PMMA-Based Hydroxyapatite/ZnFe2O4/ZnO Composite with Antibacterial Performance and Low Toxicity. Biomimetics, 8(6), 488. https://doi.org/10.3390/biomimetics8060488








