Biomimetic Carbon Sequestration and Cyanate Detoxification Using Heat-Purified Carbonic Anhydrase from Sulfurihydrogenibium yellowstonense
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Vector Construction
2.2. Expression and Purification of SspCA
2.3. Expression and Purification of TlCyn
2.4. Activity Assays
2.5. CO2 Sequestration
2.6. Cyanate Degradation by TlCyn and SspCA
2.7. Statistical Analysis
3. Results and Discussion
3.1. Purification of SspCA by Heating
3.2. CO2 Sequestration in CaCO3
3.3. Cyanate Degradation Using TlCyn and SspCA
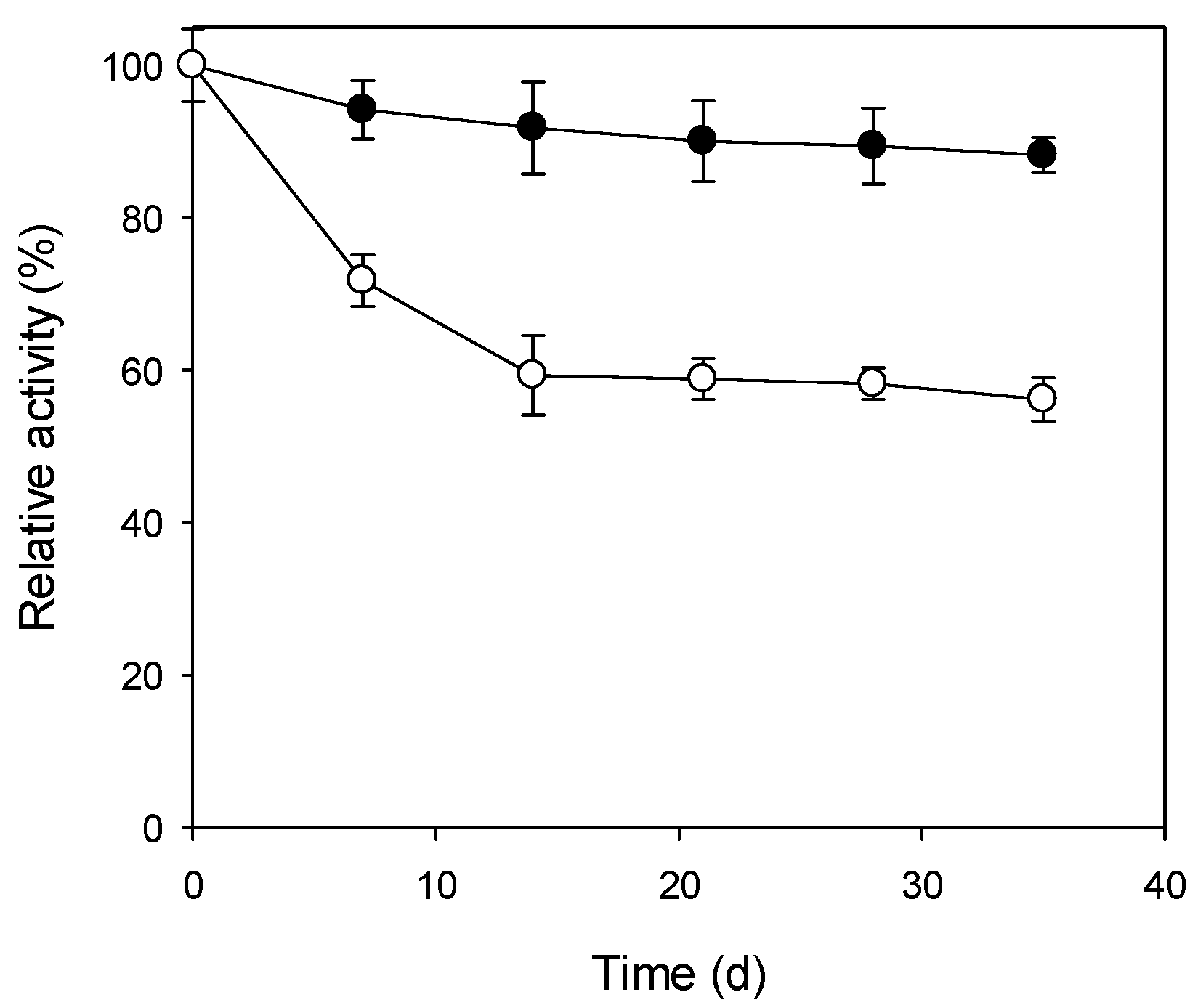
3.4. Storage Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Di Fiore, A.; Alterio, V.; Monti, S.M.; De Simone, G.; D’Ambrosio, K. Thermostable carbonic anhydrases in biotechnological applications. Int. J. Mol. Sci. 2015, 16, 15456–15480. [Google Scholar] [CrossRef] [PubMed]
- Talekar, S.; Jo, B.H.; Dordick, J.S.; Kim, J. Carbonic anhydrase for CO2 capture, conversion and utilization. Curr. Opin. Biotechnol. 2022, 74, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Alvizo, O.; Nguyen, L.J.; Savile, C.K.; Bresson, J.A.; Lakhapatri, S.L.; Solis, E.O.P.; Fox, R.J.; Broering, J.M.; Benoit, M.R.; Zimmerman, S.A.; et al. Directed evolution of an ultrastable carbonic anhydrase for highly efficient carbon capture from flue gas. Proc. Natl. Acad. Sci. USA 2014, 111, 16436–16441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lu, H.; Lu, Y. Enhanced stability and chemical resistance of a new nanoscale biocatalyst for accelerating CO2 absorption into a carbonate solution. Environ. Sci. Technol. 2013, 47, 13882–13888. [Google Scholar] [CrossRef] [PubMed]
- Bose, H.; Satyanarayana, T. Microbial carbonic anhydrases in biomimetic carbon sequestration for mitigating global warming: Prospects and perspectives. Front. Microbiol. 2017, 8, 1615. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Akhter, Y.; Chatterjee, S. A review on remediation of cyanide containing industrial wastes using biological systems with special reference to enzymatic degradation. World J. Microbiol. Biotechnol. 2019, 35, 70. [Google Scholar] [CrossRef] [PubMed]
- Elmore, M.H.; McGary, K.L.; Wisecaver, J.H.; Slot, J.C.; Geiser, D.M.; Sink, S.; O’Donnell, K.; Rokas, A. Clustering of two genes putatively involved in cyanate detoxification evolved recently and independently in multiple fungal lineages. Genome Biol. Evol. 2015, 7, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, B.; Pillai, S.; Permaul, K.; Singh, S. A novel strategy for the efficient removal of toxic cyanate by the combinatorial use of recombinant enzymes immobilized on aminosilane modified magnetic nanoparticles. Bioresour. Technol. 2018, 253, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; De Luca, V.; Carginale, V.; Cannio, R.; Rossi, M. Biochemical properties of a novel and highly thermostable bacterial alpha-carbonic anhydrase from Sulfurihydrogenibium yellowstonense YO3AOP1. J. Enzym. Inhib. Med. Chem. 2012, 27, 892–897. [Google Scholar]
- Luca, V.D.; Vullo, D.; Scozzafava, A.; Carginale, V.; Rossi, M.; Supuran, C.T.; Capasso, C. An α-carbonic anhydrase from the thermophilic bacterium Sulphurihydrogenibium azorense is the fastest enzyme known for the CO2 hydration reaction. Bioorg. Med. Chem. 2013, 21, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, B.; Pillai, S.; Permaul, K.; Singh, S. Expression of a novel recombinant cyanate hydratase (rTl-Cyn) in Pichia pastoris, characteristics and applicability in the detoxification of cyanate. Bioresour. Technol. 2017, 238, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Jo, B.H.; Seo, J.H.; Yang, Y.J.; Baek, K.; Choi, Y.S.; Pack, S.P.; Oh, S.H.; Cha, H.J. Bioinspired silica nanocomposite with autoencapsulated carbonic anhydrase as a robust biocatalyst for CO2 sequestration. ACS Catal. 2014, 4, 4332–4340. [Google Scholar] [CrossRef]
- Jo, B.H.; Seo, J.H.; Cha, H.J. Bacterial extremo-α-carbonic anhydrases from deep-sea hydrothermal vents as potential biocatalysts for CO2 sequestration. J. Mol. Catal. B Enzym. 2014, 109, 31–39. [Google Scholar] [CrossRef]
- Jo, B.H.; Kim, I.G.; Seo, J.H.; Kang, D.G.; Cha, H.J. Engineered Escherichia coli with periplasmic carbonic anhydrase as a biocatalyst for CO2 sequestration. Appl. Environ. Microbiol. 2013, 79, 6697–6705. [Google Scholar] [CrossRef] [PubMed]
- Laws and Regulations Retrieving System, Environmental Protection Administration, Taiwan. Available online: https://oaout.epa.gov.tw/Law/index.aspx (accessed on 28 March 2022).
- Compute pI/Mw Tool. Available online: https://web.expasy.org/compute_pi/ (accessed on 31 May 2021).
- Perfetto, R.; Del Prete, S.; Vullo, D.; Sansone, G.; Barone, C.M.A.; Rossi, M.; Supuran, C.T.; Capasso, C. Production and covalent immobilisation of the recombinant bacterial carbonic anhydrase (SspCA) onto magnetic nanoparticles. J. Enzym. Inhib. Med. Chem. 2017, 32, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.N.; Alissa Park, A.-H.; Banta, S. Surface display of small peptides on Escherichia coli for enhanced calcite precipitation rates. Biopolymers 2014, 102, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Barbero, R.; Carnelli, L.; Simon, A.; Kao, A.; Monforte, A.d.A.; Riccò, M.; Bianchi, D.; Belcher, A. Engineered yeast for enhanced CO2 mineralization. Energy Environ. Sci. 2013, 6, 660–674. [Google Scholar] [CrossRef] [PubMed]
- Elhadj, S.; De Yoreo, J.J.; Hoyer, J.R.; Dove, P.M. Role of molecular charge and hydrophilicity in regulating the kinetics of crystal growth. Proc. Natl. Acad. Sci. USA 2006, 103, 19237–19242. [Google Scholar] [CrossRef] [PubMed]

| Purification Method | Total Activity (WAU) 3 | Total Protein (mg) | Specific Activity (WAU/mg) | Purification (x) 4 | Yield (%) 5 |
|---|---|---|---|---|---|
| Crude lysate | 29,039 ± 4250 | 85 ± 2.6 | 342 ± 50 | 1 | 100 |
| One-step heating 1 | 23,460 ± 5974 | 10 ± 0.2 | 2441 ± 621 | 7.2 | 81 |
| Two-step heating 2 | 27,712 ± 3371 | 13 ± 0.3 | 2061 ± 251 | 6.0 | 95 |
| Ni-NTA column | 23,806 ± 4043 | 6 ± 0.2 | 3911 ± 664 | 12 | 82 |
| Purification Method | |||||||
|---|---|---|---|---|---|---|---|
| Ni-NTA Column | Heating | ||||||
| SspCA (µg/mL) | Control | 250 | 300 | 350 | 250 | 300 | 350 |
| Onset time (s) | 93 ± 15 | 67 ± 0 | 58 ± 0 | 40 ± 0 | 40 ± 0 | 37 ± 6 | 27 ± 6 |
| DCD (%) 1 | ||||
|---|---|---|---|---|
| SspCA (WAU) | 0 | 0.5 | 0.75 | 1 |
| Buffer | 49.8 ± 1.5 | 40.3 ± 2.3 | 33.1 ± 0.9 | 29 ± 2.3 |
| Industrial wastewater | 33.6 ± 0.7 | 15.5 ± 0.8 | 13.4 ± 0.1 | 14.1 ± 0.4 |
| Artificial wastewater | 9.6 ± 1.1 | 10.5 ± 1.7 | 8.6 ± 1.7 | 15.9 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, C.-J.; Hu, C.-J.; Yu, C.-Y. Biomimetic Carbon Sequestration and Cyanate Detoxification Using Heat-Purified Carbonic Anhydrase from Sulfurihydrogenibium yellowstonense. Biomimetics 2023, 8, 365. https://doi.org/10.3390/biomimetics8040365
Hsieh C-J, Hu C-J, Yu C-Y. Biomimetic Carbon Sequestration and Cyanate Detoxification Using Heat-Purified Carbonic Anhydrase from Sulfurihydrogenibium yellowstonense. Biomimetics. 2023; 8(4):365. https://doi.org/10.3390/biomimetics8040365
Chicago/Turabian StyleHsieh, Chia-Jung, Chia-Jung Hu, and Chi-Yang Yu. 2023. "Biomimetic Carbon Sequestration and Cyanate Detoxification Using Heat-Purified Carbonic Anhydrase from Sulfurihydrogenibium yellowstonense" Biomimetics 8, no. 4: 365. https://doi.org/10.3390/biomimetics8040365
APA StyleHsieh, C.-J., Hu, C.-J., & Yu, C.-Y. (2023). Biomimetic Carbon Sequestration and Cyanate Detoxification Using Heat-Purified Carbonic Anhydrase from Sulfurihydrogenibium yellowstonense. Biomimetics, 8(4), 365. https://doi.org/10.3390/biomimetics8040365





