Textile Design of an Intervertebral Disc Replacement Device from Silk Yarn
Abstract
1. Introduction
2. Materials and Methods
2.1. Silk Yarn
2.2. Textile Design of AF
2.3. Regenerated Silk Fibroin Adhesive for FAM
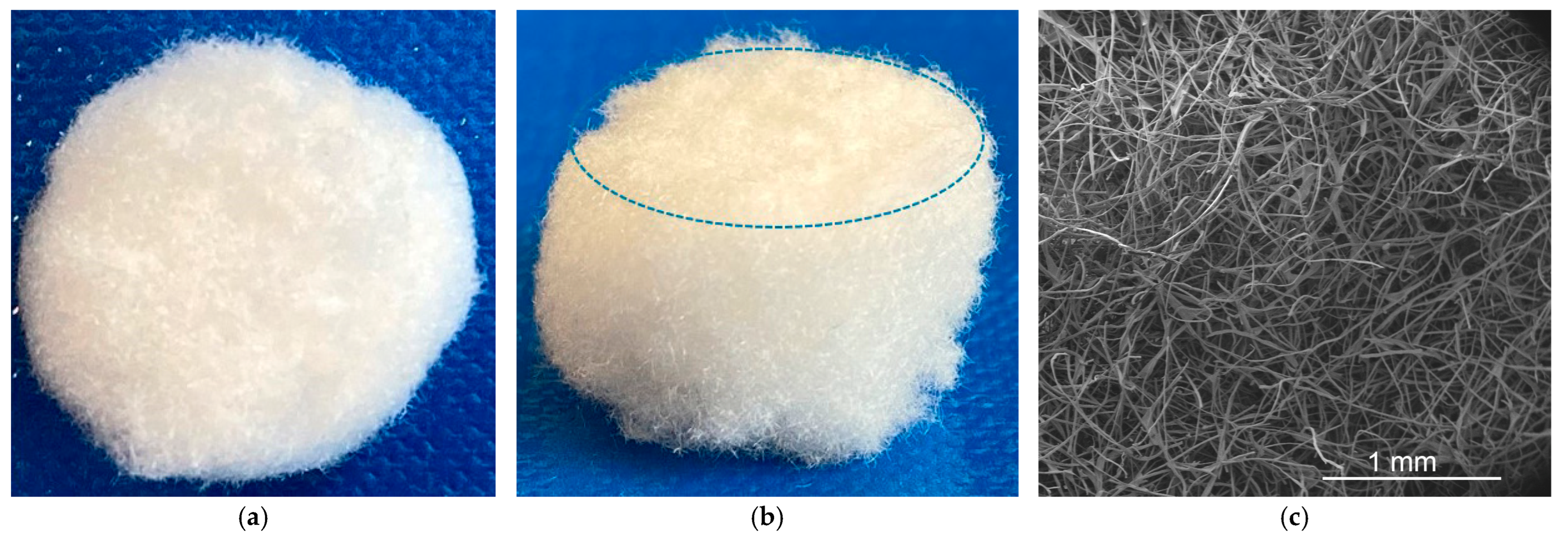
2.4. Textile Design of NP
2.5. Scanning Electron Microscopy (SEM)
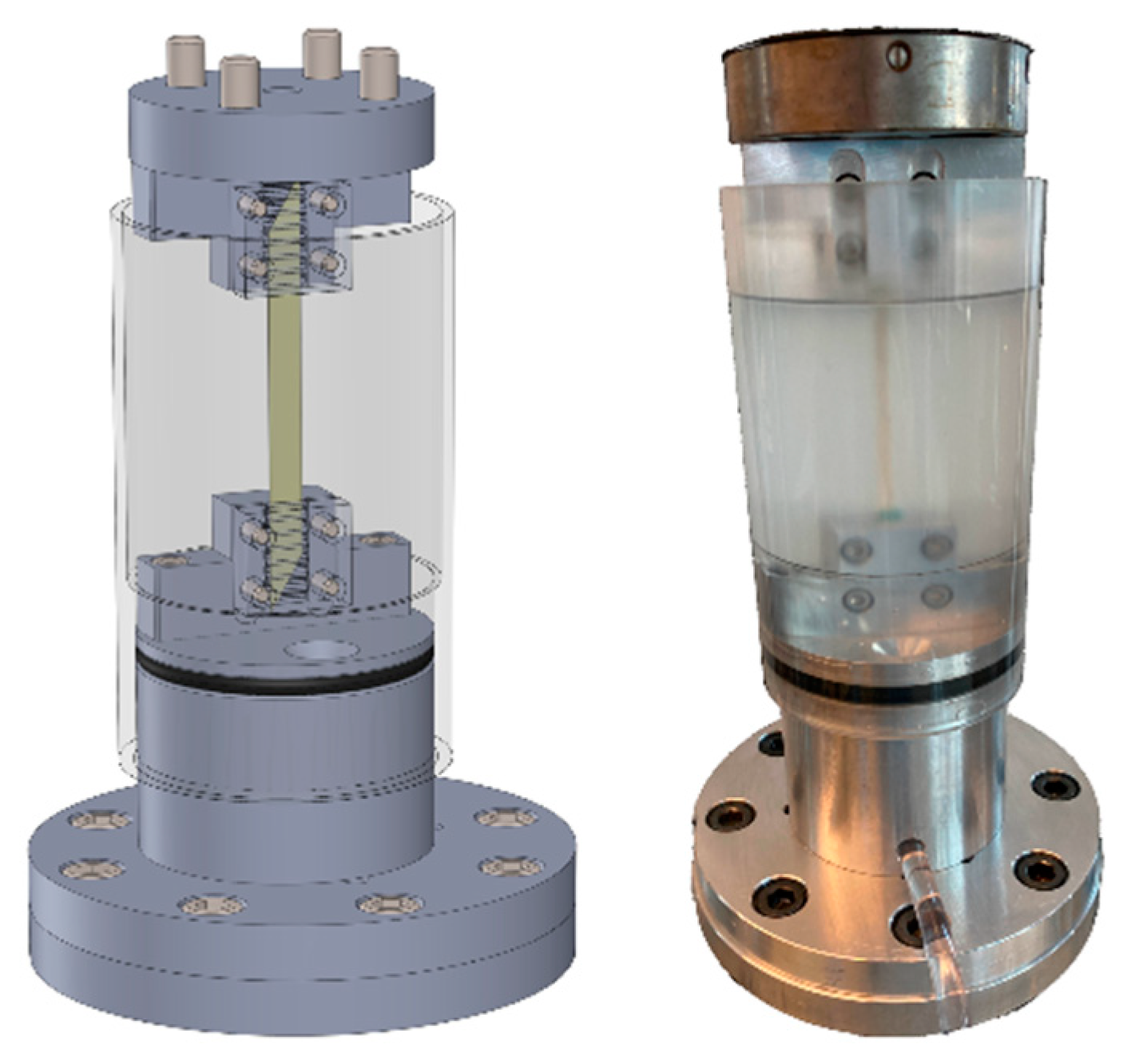
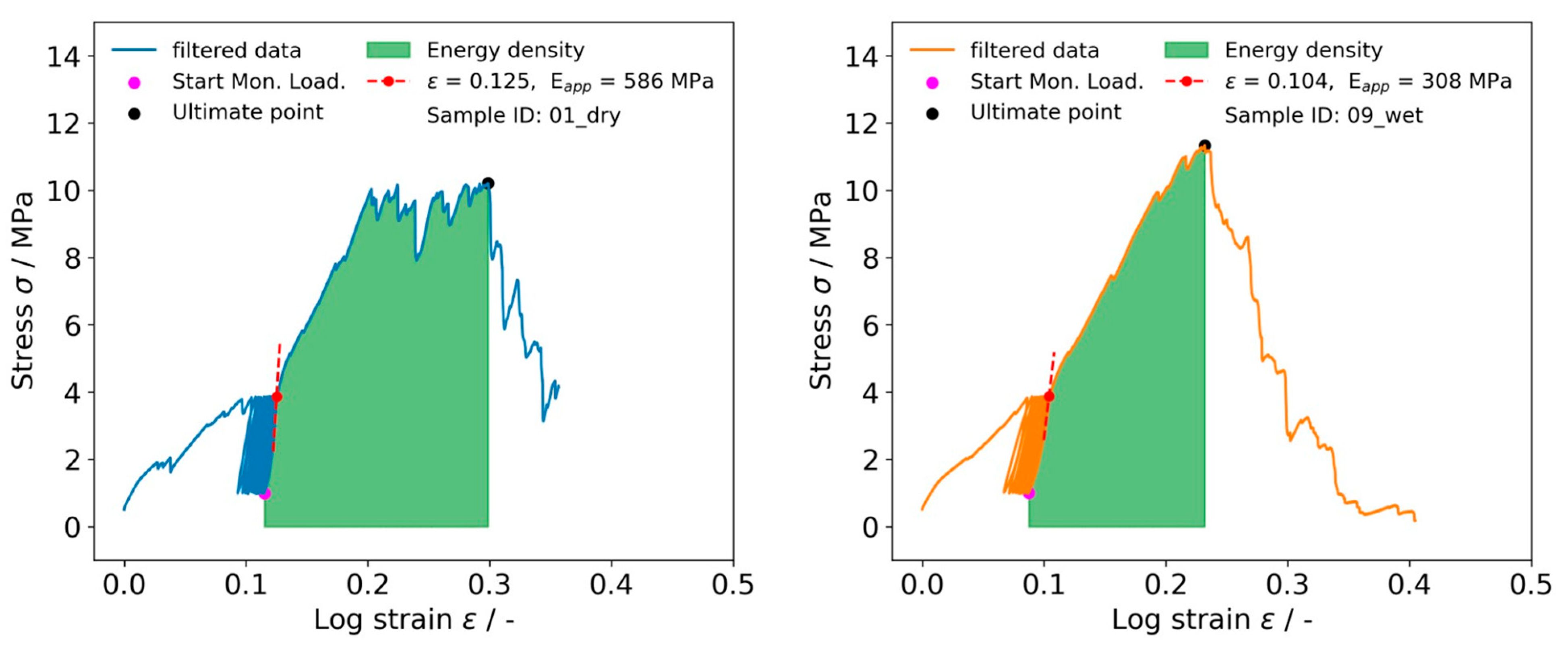
2.6. Tensile Testing of IVD Structures
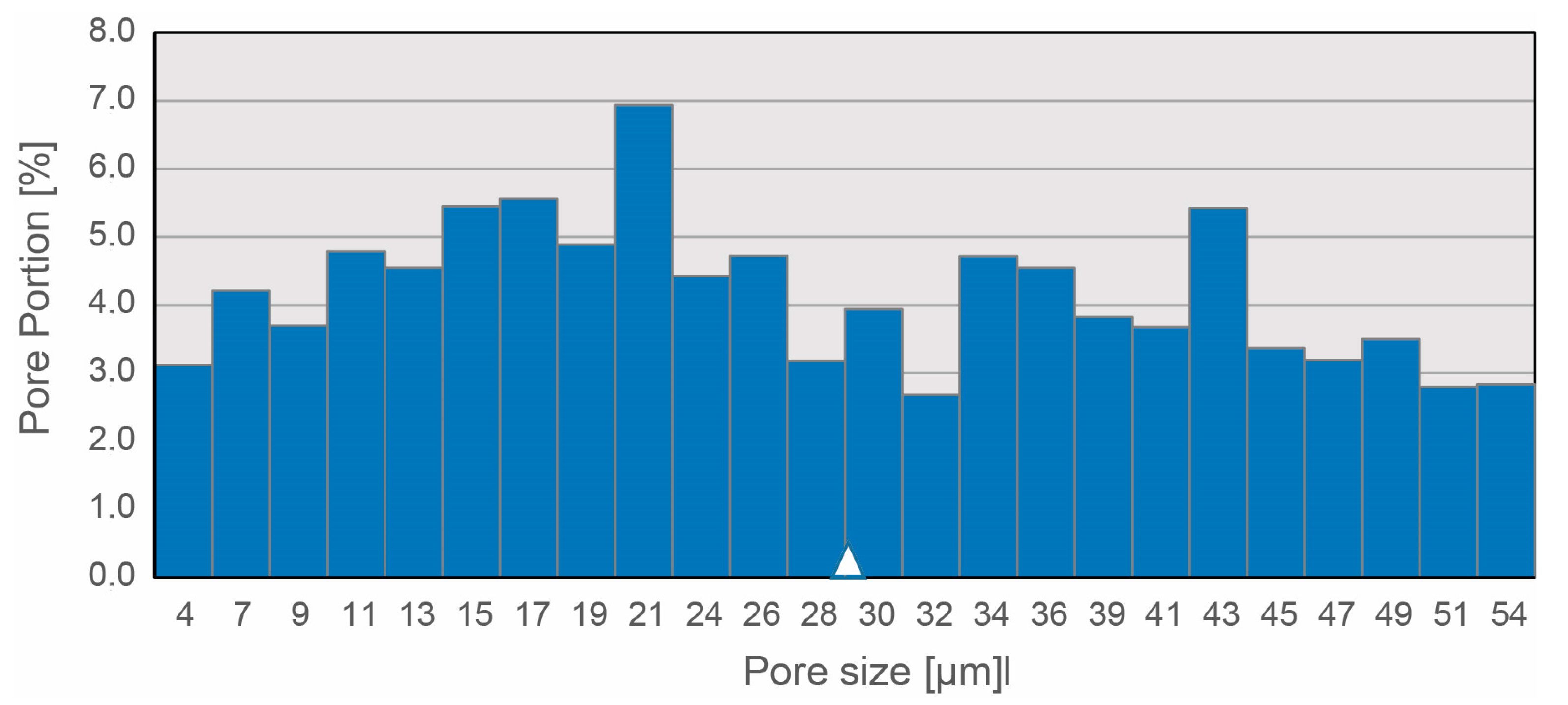
2.7. Determination of Porosity of NP-FAM Structures
2.8. Statistical Evaluation
3. Results
3.1. Textile Design of the Annulus Fibrosus
3.2. Textile Design of the Nucleus Pulposus
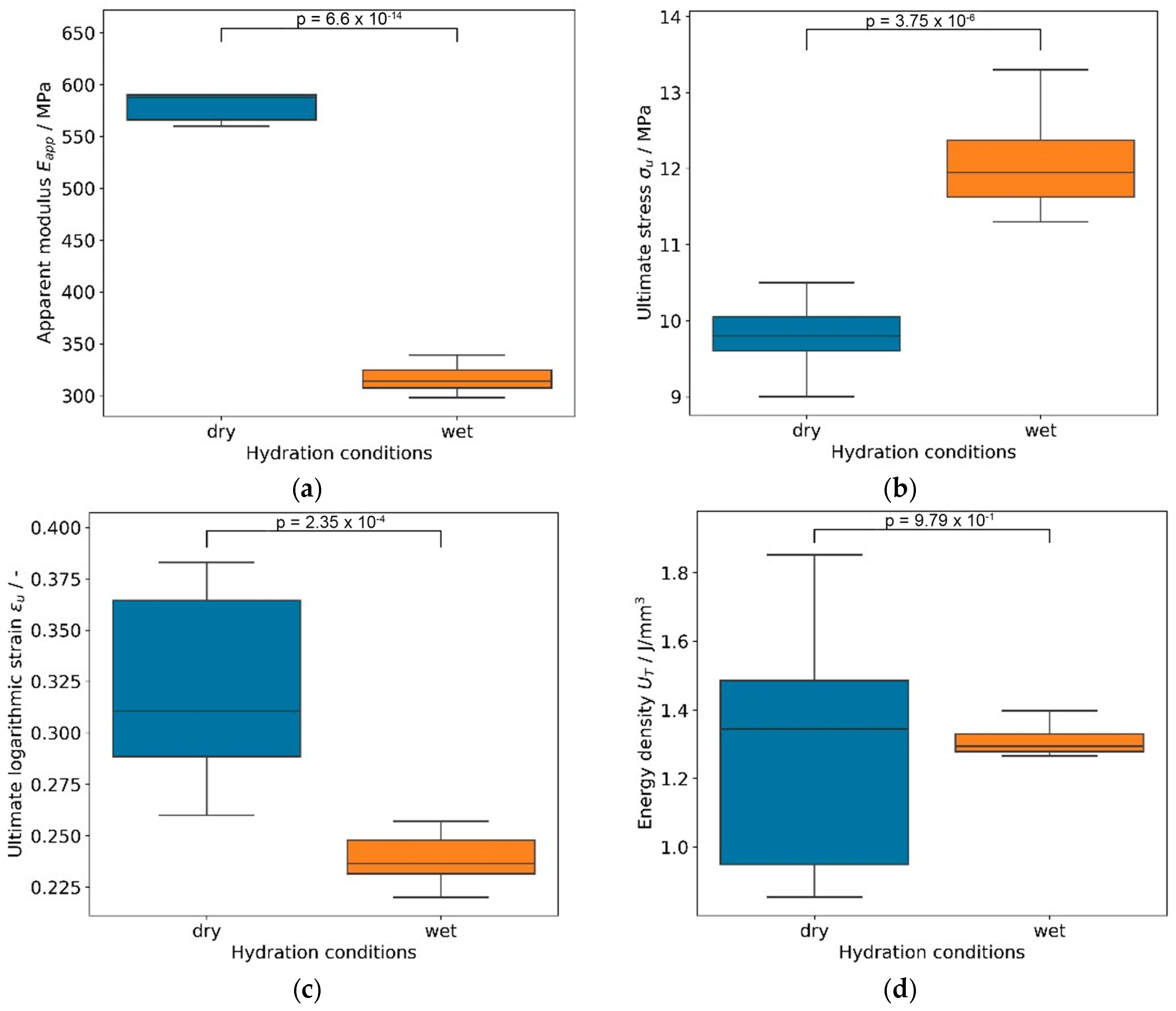
3.3. Characterization of Embroidered Annulus Fibrous Structures
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dagenais, S.; Caro, J.; Haldeman, S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008, 8, 8–20. [Google Scholar] [CrossRef]
- Hoy, D.; Brooks, P.; Blyth, F.; Buchbinder, R. The Epidemiology of low back pain. Best Pract. Res. Clin. Rheumatol. 2010, 24, 769–781. [Google Scholar] [CrossRef]
- Carregaro, R.L. Management of non-serious low back pain in the context of emergency care. Is it worth the cost? Lancet Reg. Health West. Pac. 2021, 7, 100105. [Google Scholar] [CrossRef] [PubMed]
- Long, R.G.; Bürki, A.; Zysset, P.; Eglin, D.; Grijpma, D.W.; Blanquer, S.B.G.; Hecht, A.C.; Iatridis, J.C. Mechanical restoration and failure analyses of a hydrogel and scaffold composite strategy for annulus fibrosus repair. Acta Biomater. 2016, 30, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Grad, S. Advancing the cellular and molecular therapy for intervertebral disc disease. Adv. Drug Deliv. Rev. 2015, 84, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, B.W.; Brielmaier, M.; Schwerdtfeger, K.; Oertel, J.M. Clinical outcome following anterior cervical discectomy and fusion with and without anterior cervical plating for the treatment of cervical disc herniation-a 25-year follow-up study. Neurosurg. Rev. 2018, 41, 473–482. [Google Scholar] [CrossRef]
- Donk, R.D.; Verhagen, W.I.M.; Hosman, A.J.F.; Verbeek, A.; Bartels, R.H.M.A. Symptomatic Adjacent Segment Disease After Anterior Cervical Discectomy for Single-level Degenerative Disk Disease. Clin. Spine Surg. 2018, 31, E50–E54. [Google Scholar] [CrossRef]
- Lee, C.S.; Hwang, C.J.; Lee, S.-W.; Ahn, Y.-J.; Kim, Y.-T.; Lee, D.-H.; Lee, M.Y. Risk factors for adjacent segment disease after lumbar fusion. Eur. Spine J. 2009, 18, 1637–1643. [Google Scholar] [CrossRef]
- Watkins, R.; Hanna, R. Non-union rate with stand-alone lateral lumbar interbody fusion. Medicine 2014, 93, e275. [Google Scholar] [CrossRef]
- Martin, B.I.; Mirza, S.K.; Comstock, B.A.; Gray, D.T.; Kreuter, W.; Deyo, R.A. Reoperation rates following lumbar spine surgery and the influence of spinal fusion procedures. Spine 2007, 32, 382–387. [Google Scholar] [CrossRef]
- Rajan, M.; Najman, S.; Rajendran, N.K. Editorial: Biomimetic Materials for Tissue Regenerations. Front. Cell Dev. Biol. 2022, 10, 825455. [Google Scholar] [CrossRef]
- Sakai, S.; Takagi, Y.; Yamada, Y.; Yamaguchi, T.; Kawakami, K. Reinforcement of porous alginate scaffolds by incorporating electrospun fibres. Biomed. Mater. 2008, 3, 34102. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Johnson, J.K.; Bradley, P.A.; Parikh, K.S.; Lannutti, J.J.; Winter, J.O. Cell attachment to hydrogel-electrospun fiber mat composite materials. J. Funct. Biomater. 2012, 3, 497–513. [Google Scholar] [CrossRef] [PubMed]
- Panzavolta, S.; Torricelli, P.; Amadori, S.; Parrilli, A.; Rubini, K.; Della Bella, E.; Fini, M.; Bigi, A. 3D interconnected porous biomimetic scaffolds: In vitro cell response. J. Biomed. Mater. Res. A 2013, 101, 3560–3570. [Google Scholar] [CrossRef]
- McMurtrey, R.J. Patterned and functionalized nanofiber scaffolds in three-dimensional hydrogel constructs enhance neurite outgrowth and directional control. J. Neural Eng. 2014, 11, 66009. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Li, R.; Jiang, W.; Sun, Y.; Li, H. Comparison of three-dimensional printing and vacuum freeze-dried techniques for fabricating composite scaffolds. Biochem. Biophys. Res. Commun. 2016, 477, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Jeong, Y.; Park, J.M.; Lee, K.H.; Hong, J.W.; Choi, J. Biomimetics: Forecasting the future of science, engineering, and medicine. Int. J. Nanomed. 2015, 10, 5701–5713. [Google Scholar] [CrossRef]
- Owen, S.C.; Shoichet, M.S. Design of three-dimensional biomimetic scaffolds. J. Biomed. Mater. Res. A 2010, 94, 1321–1331. [Google Scholar] [CrossRef]
- Shoichet, M.S. Polymer Scaffolds for Biomaterials Applications. Macromolecules 2010, 43, 581–591. [Google Scholar] [CrossRef]
- Kim, T.G.; Shin, H.; Lim, D.W. Biomimetic Scaffolds for Tissue Engineering. Adv. Funct. Mater. 2012, 22, 2446–2468. [Google Scholar] [CrossRef]
- Lawson, L.Y.; Harfe, B.D. Developmental mechanisms of intervertebral disc and vertebral column formation. Wiley Interdiscip. Rev. Dev. Biol. 2017, 6, e283. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.R. Biochemistry of the intervertebral disc. Int. Rev. Connect. Tissue Res. 1979, 8, 227–291. [Google Scholar] [CrossRef] [PubMed]
- Oegema, T.R. Biochemistry of the Intervertebral Disc. Clin. Sport. Med. 1993, 12, 419–438. [Google Scholar] [CrossRef]
- Yu, J.; Tirlapur, U.; Fairbank, J.; Handford, P.; Roberts, S.; Winlove, C.P.; Cui, Z.; Urban, J. Microfibrils, elastin fibres and collagen fibres in the human intervertebral disc and bovine tail disc. J. Anat. 2007, 210, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.J.; Isaacs, M.D.; Hughes, C.; Caterson, B.; Ralphs, J.R. Collagen fibrillogenesis in the development of the annulus fibrosus of the intervertebral disc. Eur. Cell. Mater. 2011, 22, 226–241. [Google Scholar] [CrossRef]
- Cassidy, J.J.; Hiltner, A.; Baer, E. Hierarchical structure of the intervertebral disc. Connect. Tissue Res. 1989, 23, 75–88. [Google Scholar] [CrossRef]
- Brown, T.; Hansen, R.J.; Yorra, A.J. Some mechanical tests on the lumbosacral spine with particular reference to the intervertebral discs; a preliminary report. J. Bone Joint Surg. Am. 1957, 39-A, 1135–1164. [Google Scholar] [CrossRef]
- Newell, N.; Little, J.P.; Christou, A.; Adams, M.A.; Adam, C.J.; Masouros, S.D. Biomechanics of the human intervertebral disc: A review of testing techniques and results. J. Mech. Behav. Biomed. Mater. 2017, 69, 420–434. [Google Scholar] [CrossRef]
- Cyril, D.; Giugni, A.; Bangar, S.S.; Mirzaeipoueinak, M.; Shrivastav, D.; Sharabi, M.; Tipper, J.L.; Tavakoli, J. Elastic Fibers in the Intervertebral Disc: From Form to Function and toward Regeneration. Int. J. Mol. Sci. 2022, 23, 8931. [Google Scholar] [CrossRef]
- Frost, B.A.; Camarero-Espinosa, S.; Foster, E.J. Materials for the Spine: Anatomy, Problems, and Solutions. Materials 2019, 12, 253. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [PubMed]
- Ajisawa, A. Dissolution of silk fibroin with calciumchloride/ethanol aqueous solution Studies on the dissolution of silk fibroin. (IX). J. Serc. Sci. Jpn. 1998, 67, 91–94. [Google Scholar] [CrossRef]
- Hild, M.; Brünler, R.; Jäger, M.; Laourine, E.; Scheid, L.; Haupt, D.; Aibibu, D.; Cherif, C.; Hanke, T. Net Shape Nonwoven: A novel technique for porous three-dimensional nonwoven hybrid scaffolds. Text. Res. J. 2014, 84, 1084–1094. [Google Scholar] [CrossRef]
- Brünler, R.; Aibibu, D.; Wöltje, M.; Anthofer, A.-M.; Cherif, C. In silico modeling of structural and porosity properties of additive manufactured implants for regenerative medicine. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 810–817. [Google Scholar] [CrossRef]
- Holzapfel, G.A.; Schulze-Bauer, C.A.J.; Feigl, G.; Regitnig, P. Single lamellar mechanics of the human lumbar anulus fibrosus. Biomech. Model. Mechanobiol. 2005, 3, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Green, T.P.; Adams, M.A.; Dolan, P. Tensile properties of the annulus fibrosus II. Ultimate tensile strength and fatigue life. Eur. Spine J. 1993, 2, 209–214. [Google Scholar] [CrossRef]
- Skaggs, D.L.; Weidenbaum, M.; Iatridis, J.C.; Ratcliffe, A.; Mow, V.C. Regional variation in tensile properties and biochemical composition of the human lumbar anulus fibrosus. Spine 1994, 19, 1310–1319. [Google Scholar] [CrossRef]
- Acaroglu, E.R.; Iatridis, J.C.; Setton, L.A.; Foster, R.J.; Mow, V.C.; Weidenbaum, M. Degeneration and aging affect the tensile behavior of human lumbar anulus fibrosus. Spine 1995, 20, 2690–2701. [Google Scholar] [CrossRef]
- Elliott, D.M.; Setton, L.A. Anisotropic and inhomogeneous tensile behavior of the human anulus fibrosus: Experimental measurement and material model predictions. J. Biomech. Eng. 2001, 123, 256–263. [Google Scholar] [CrossRef]
- Wagner, D.R.; Lotz, J.C. Theoretical model and experimental results for the nonlinear elastic behavior of human annulus fibrosus. J. Orthop. Res. 2004, 22, 901–909. [Google Scholar] [CrossRef]
- O’Connell, G.D.; Guerin, H.L.; Elliott, D.M. Theoretical and uniaxial experimental evaluation of human annulus fibrosus degeneration. J. Biomech. Eng. 2009, 131, 111007. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, G.D.; Sen, S.; Elliott, D.M. Human annulus fibrosus material properties from biaxial testing and constitutive modeling are altered with degeneration. Biomech. Model. Mechanobiol. 2012, 11, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Prez-Rigueiro, J.; Viney, C.; Llorca, J.; Elices, M. Mechanical properties of single-brin silkworm silk. J. Appl. Polym. Sci. 2000, 75, 1270–1277. [Google Scholar] [CrossRef]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.-W.; et al. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Haut, R.C. The influence of specimen length on the tensile failure properties of tendon collagen. J. Biomech. 1986, 19, 951–955. [Google Scholar] [CrossRef]
- Shen, Z.L.; Dodge, M.R.; Kahn, H.; Ballarini, R.; Eppell, S.J. Stress-strain experiments on individual collagen fibrils. Biophys. J. 2008, 95, 3956–3963. [Google Scholar] [CrossRef]
- Kang, R.; Svend Le, D.Q.; Li, H.; Lysdahl, H.; Chen, M.; Besenbacher, F.; Bünger, C. Engineered three-dimensional nanofibrous multi-lamellar structure for annulus fibrosus repair. J. Mater. Chem. B 2013, 1, 5462–5468. [Google Scholar] [CrossRef]
- Shamsah, A.H.; Cartmell, S.H.; Richardson, S.M.; Bosworth, L.A. Tissue Engineering the Annulus Fibrosus Using 3D Rings of Electrospun PCL:PLLA Angle-Ply Nanofiber Sheets. Front. Bioeng. Biotechnol. 2019, 7, 437. [Google Scholar] [CrossRef]
- Nerurkar, N.L.; Baker, B.M.; Sen, S.; Wible, E.E.; Elliott, D.M.; Mauck, R.L. Nanofibrous biologic laminates replicate the form and function of the annulus fibrosus. Nat. Mater. 2009, 8, 986–992. [Google Scholar] [CrossRef]
- Nerurkar, N.L.; Sen, S.; Baker, B.M.; Elliott, D.M.; Mauck, R.L. Dynamic culture enhances stem cell infiltration and modulates extracellular matrix production on aligned electrospun nanofibrous scaffolds. Acta Biomater. 2011, 7, 485–491. [Google Scholar] [CrossRef]
- Wu, L.-C.; Chiang, C.-J.; Liu, Z.-H.; Tsuang, Y.-H.; Sun, J.-S.; Huang, Y.-Y. Fabrication and properties of acellular porcine anulus fibrosus for tissue engineering in spine surgery. J. Orthop. Surg. Res. 2014, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.L.; Jacobsen, T.D.; Emsbo, E.; Murali, A.; Anton, K.; Liu, J.Z.; Lu, H.H.; Chahine, N.O. Three-Dimensional-Printed Flexible Scaffolds Have Tunable Biomimetic Mechanical Properties for Intervertebral Disc Tissue Engineering. ACS Biomater. Sci. Eng. 2021, 7, 5836–5849. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Huang, X.; Yan, H.; Wang, Y.; Wang, Y.; Zhuo, S.; Wei, C.; Qiu, H.; Yang, X.; Zhang, Y.; et al. Biomimetic Design of 3D Fibrous Mesh Reinforced Hydrogel Replicating the Form and Function of the Intervertebral Disc. Small Struct. 2023, 4, 2200254. [Google Scholar] [CrossRef]
- Bhunia, B.K.; Dey, S.; Bandyopadhyay, A.; Mandal, B.B. 3D printing of annulus fibrosus anatomical equivalents recapitulating angle-ply architecture for intervertebral disc replacement. Appl. Mater. Today 2021, 23, 101031. [Google Scholar] [CrossRef]
- Du, L.; Yang, Q.; Zhang, J.; Zhu, M.; Ma, X.; Zhang, Y.; Wang, L.; Xu, B. Engineering a biomimetic integrated scaffold for intervertebral disc replacement. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 522–529. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, K.; Zhou, L.; He, M.; Zhu, C.; Wang, J.; Li, J.; Li, Y.; Liu, L.; Sun, D.; et al. 3D-printed auxetic-structured intervertebral disc implant for potential treatment of lumbar herniated disc. Bioact. Mater. 2023, 20, 528–538. [Google Scholar] [CrossRef]
- Wu, D.; Tan, J.; Yao, L.; Tian, J.; Luo, B.; Li, L.; Zhou, C.; Lu, L. Customized composite intervertebral disc scaffolds by integrated 3D bioprinting for therapeutic implantation. Compos. Part A Appl. Sci. Manuf. 2021, 147, 106468. [Google Scholar] [CrossRef]
- Chang, G.; Kim, H.J.; Vunjak-Novakovic, G.; Kaplan, D.L.; Kandel, R. Enhancing annulus fibrosus tissue formation in porous silk scaffolds. J. Biomed. Mater. Res. A 2010, 92, 43–51. [Google Scholar] [CrossRef]
- Park, S.-H.; Gil, E.S.; Cho, H.; Mandal, B.B.; Tien, L.W.; Min, B.-H.; Kaplan, D.L. Intervertebral disk tissue engineering using biphasic silk composite scaffolds. Tissue Eng. Part A 2012, 18, 447–458. [Google Scholar] [CrossRef]
- Park, S.-H.; Gil, E.S.; Mandal, B.B.; Cho, H.; Kluge, J.A.; Min, B.-H.; Kaplan, D.L. Annulus fibrosus tissue engineering using lamellar silk scaffolds. J. Tissue Eng. Regen. Med. 2012, 6 (Suppl. S3), s24–s33. [Google Scholar] [CrossRef]
- Bhunia, B.K.; Kaplan, D.L.; Mandal, B.B. Silk-based multilayered angle-ply annulus fibrosus construct to recapitulate form and function of the intervertebral disc. Proc. Natl. Acad. Sci. USA 2018, 115, 477–482. [Google Scholar] [CrossRef]
- Costa, J.B.; Silva-Correia, J.; Ribeiro, V.P.; Da Silva Morais, A.; Oliveira, J.M.; Reis, R.L. Engineering patient-specific bioprinted constructs for treatment of degenerated intervertebral disc. Mater. Today Commun. 2019, 19, 506–512. [Google Scholar] [CrossRef]
- Zhang, T.; Du, L.; Zhao, J.; Ding, J.; Zhang, P.; Wang, L.; Xu, B. Biomimetic angle-ply multi-lamellar scaffold for annulus fibrosus tissue engineering. J. Mater. Sci. Mater. Med. 2020, 31, 67. [Google Scholar] [CrossRef] [PubMed]
- See, E.Y.-S.; Toh, S.L.; Goh, J.C.-H. Effects of radial compression on a novel simulated intervertebral disc-like assembly using bone marrow-derived mesenchymal stem cell cell-sheets for annulus fibrosus regeneration. Spine 2011, 36, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, M.; Chameettachal, S.; Pahwa, S.; Ray, A.R.; Ghosh, S. Strategies for replicating anatomical cartilaginous tissue gradient in engineered intervertebral disc. ACS Appl. Mater. Interfaces 2014, 6, 183–193. [Google Scholar] [CrossRef]
- Heim, F.M.; Daspit, J.T.; Li, X. Quantifying the effect of tow architecture variability on the performance of biaxially braided composite tubes. Compos. Part B Eng. 2020, 201, 108383. [Google Scholar] [CrossRef]
- Heim, F.M.; Daspit, J.T.; Holzmond, O.B.; Croom, B.P.; Li, X. Analysis of tow architecture variability in biaxially braided composite tubes. Compos. Part B Eng. 2020, 190, 107938. [Google Scholar] [CrossRef]
- Halloran, D.O.; Grad, S.; Stoddart, M.; Dockery, P.; Alini, M.; Pandit, A.S. An injectable cross-linked scaffold for nucleus pulposus regeneration. Biomaterials 2008, 29, 438–447. [Google Scholar] [CrossRef]
- Zhuang, Y.; Huang, B.; Li, C.Q.; Liu, L.T.; Pan, Y.; Zheng, W.J.; Luo, G.; Zhou, Y. Construction of tissue-engineered composite intervertebral disc and preliminary morphological and biochemical evaluation. Biochem. Biophys. Res. Commun. 2011, 407, 327–332. [Google Scholar] [CrossRef]
- Chik, T.K.; Chooi, W.H.; Li, Y.Y.; Ho, F.C.; Cheng, H.W.; Choy, T.H.; Sze, K.Y.; Luk, K.K.D.; Cheung, K.M.C.; Chan, B.P. Bioengineering a multicomponent spinal motion segment construct—A 3D model for complex tissue engineering. Adv. Healthc. Mater. 2015, 4, 99–112. [Google Scholar] [CrossRef]
- Friedmann, A.; Baertel, A.; Schmitt, C.; Ludtka, C.; Milosevic, J.; Meisel, H.-J.; Goehre, F.; Schwan, S. Intervertebral Disc Regeneration Injection of a Cell-Loaded Collagen Hydrogel in a Sheep Model. Int. J. Mol. Sci. 2021, 22, 4248. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Roy, A.K.; Vacanti, C.A.; Kojima, K.; Ueda, M.; Bonassar, L.J. Tissue-engineered composites of anulus fibrosus and nucleus pulposus for intervertebral disc replacement. Spine 2004, 29, 1290–1297; discussion 1297-8. [Google Scholar] [CrossRef] [PubMed]
- Bowles, R.D.; Gebhard, H.H.; Härtl, R.; Bonassar, L.J. Tissue-engineered intervertebral discs produce new matrix, maintain disc height, and restore biomechanical function to the rodent spine. Proc. Natl. Acad. Sci. USA 2011, 108, 13106–13111. [Google Scholar] [CrossRef] [PubMed]
- Bowles, R.D.; Gebhard, H.H.; Dyke, J.P.; Ballon, D.J.; Tomasino, A.; Cunningham, M.E.; Härtl, R.; Bonassar, L.J. Image-based tissue engineering of a total intervertebral disc implant for restoration of function to the rat lumbar spine. NMR Biomed. 2012, 25, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Roughley, P.; Hoemann, C.; DesRosiers, E.; Mwale, F.; Antoniou, J.; Alini, M. The potential of chitosan-based gels containing intervertebral disc cells for nucleus pulposus supplementation. Biomaterials 2006, 27, 388–396. [Google Scholar] [CrossRef]
- Alinejad, Y.; Adoungotchodo, A.; Grant, M.P.; Epure, L.M.; Antoniou, J.; Mwale, F.; Lerouge, S. Injectable Chitosan Hydrogels with Enhanced Mechanical Properties for Nucleus Pulposus Regeneration. Tissue Eng. Part A 2019, 25, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, J.; Tang, X.; Liu, Y.; Bian, G.; Shi, J.; Zhang, Y.; Zhao, B.; Zhao, H.; Sui, K.; et al. The Thermosensitive Injectable Celecoxib-Loaded Chitosan Hydrogel for Repairing Postoperative Intervertebral Disc Defect. Front. Bioeng. Biotechnol. 2022, 10, 876157. [Google Scholar] [CrossRef]
- Panebianco, C.J.; Rao, S.; Hom, W.W.; Meyers, J.H.; Lim, T.Y.; Laudier, D.M.; Hecht, A.C.; Weir, M.D.; Weiser, J.R.; Iatridis, J.C. Genipin-crosslinked fibrin seeded with oxidized alginate microbeads as a novel composite biomaterial strategy for intervertebral disc cell therapy. Biomaterials 2022, 287, 121641. [Google Scholar] [CrossRef]
- Jeong, C.G.; Francisco, A.T.; Niu, Z.; Mancino, R.L.; Craig, S.L.; Setton, L.A. Screening of hyaluronic acid-poly(ethylene glycol) composite hydrogels to support intervertebral disc cell biosynthesis using artificial neural network analysis. Acta Biomater. 2014, 10, 3421–3430. [Google Scholar] [CrossRef]
- Inoue, M.; Isa, I.L.M.; Orita, S.; Suzuki-Narita, M.; Inage, K.; Shiga, Y.; Norimoto, M.; Umimura, T.; Sakai, T.; Eguchi, Y.; et al. An Injectable Hyaluronic Acid Hydrogel Promotes Intervertebral Disc Repair in a Rabbit Model. Spine 2021, 46, E810–E816. [Google Scholar] [CrossRef]
- Zeng, C.; Yang, Q.; Zhu, M.; Du, L.; Zhang, J.; Ma, X.; Xu, B.; Wang, L. Silk fibroin porous scaffolds for nucleus pulposus tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 37, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Cho, H.; Gil, E.S.; Mandal, B.B.; Min, B.-H.; Kaplan, D.L. Silk-fibrin/hyaluronic acid composite gels for nucleus pulposus tissue regeneration. Tissue Eng. Part A 2011, 17, 2999–3009. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, B.; Guo, F.; Du, J.; Gu, P.; Lin, X.; Yang, W.; Zhang, H.; Lu, M.; Huang, Y.; et al. Injectable silk fibroin/polyurethane composite hydrogel for nucleus pulposus replacement. J. Mater. Sci. Mater. Med. 2012, 23, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.-W.; Chen, W.-P.; Tai, P.-W.; Lo, H.-Y.; Wu, T.-Y. Roles of Silk Fibroin on Characteristics of Hyaluronic Acid/Silk Fibroin Hydrogels for Tissue Engineering of Nucleus Pulposus. Materials 2020, 13, 2750. [Google Scholar] [CrossRef] [PubMed]
- Murab, S.; Samal, J.; Shrivastava, A.; Ray, A.R.; Pandit, A.; Ghosh, S. Glucosamine loaded injectable silk-in-silk integrated system modulate mechanical properties in bovine ex-vivo degenerated intervertebral disc model. Biomaterials 2015, 55, 64–83. [Google Scholar] [CrossRef]
- Ghorbani, M.; Ai, J.; Nourani, M.R.; Azami, M.; Hashemi Beni, B.; Asadpour, S.; Bordbar, S. Injectable natural polymer compound for tissue engineering of intervertebral disc: In vitro study. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Diwan, A.D.; Tipper, J.L. Elastic fibers: The missing key to improve engineering concepts for reconstruction of the Nucleus Pulposus in the intervertebral disc. Acta Biomater. 2020, 113, 407–416. [Google Scholar] [CrossRef]
- Shau, Y.W.; Wang, C.L.; Shieh, J.Y.; Hsu, T.C. Noninvasive assessment of the viscoelasticity of peripheral arteries. Ultrasound Med. Biol. 1999, 25, 1377–1388. [Google Scholar] [CrossRef]
- Vesely, I. The role of elastin in aortic valve mechanics. J. Biomech. 1998, 31, 115–123. [Google Scholar] [CrossRef]
- Silver, F.H.; Freeman, J.W.; DeVore, D. Viscoelastic properties of human skin and processed dermis. Skin Res. Technol. 2001, 7, 18–23. [Google Scholar] [CrossRef]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; van Vlierberghe, S.; Dubruel, P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.A.; Caves, J.M.; Haller, C.A.; Dai, E.; Li, L.; Grainger, S.; Chaikof, E.L. Collagen-Based Substrates with Tunable Strength for Soft Tissue Engineering. Biomater. Sci. 2013, 1, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Dong, W.; Feng, Q.; Cui, F.; Uo, M.; Akasaka, T.; Watari, F. In vitro evaluation of porous poly(L-lactic acid) scaffold reinforced by chitin fibers. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Moffat, K.L.; Kwei, A.S.-P.; Spalazzi, J.P.; Doty, S.B.; Levine, W.N.; Lu, H.H. Novel nanofiber-based scaffold for rotator cuff repair and augmentation. Tissue Eng. Part A 2009, 15, 115–126. [Google Scholar] [CrossRef]
- Li, X.; Feng, Q.; Jiao, Y.; Cui, F. Collagen-based scaffolds reinforced by chitosan fibres for bone tissue engineering. Polym. Int. 2005, 54, 1034–1040. [Google Scholar] [CrossRef]
- Séguin, C.A.; Grynpas, M.D.; Pilliar, R.M.; Waldman, S.D.; Kandel, R.A. Tissue engineered nucleus pulposus tissue formed on a porous calcium polyphosphate substrate. Spine 2004, 29, 1299–1306; discussion 1306-7. [Google Scholar] [CrossRef]
- Zhu, Y.; Tan, J.; Zhu, H.; Lin, G.; Yin, F.; Wang, L.; Song, K.; Wang, Y.; Zhou, G.; Yi, W. Development of kartogenin-conjugated chitosan-hyaluronic acid hydrogel for nucleus pulposus regeneration. Biomater. Sci. 2017, 5, 784–791. [Google Scholar] [CrossRef]
- Vanawati, N.; Barlian, A.; Judawisastra, H.; Wibowo, I. The combinatory effect of scaffold topography and culture condition: An approach to nucleus pulposus tissue engineering. Future Sci. OA 2022, 8, FSO810. [Google Scholar] [CrossRef]
- Wöltje, M.; Brünler, R.; Böbel, M.; Ernst, S.; Neuss, S.; Aibibu, D.; Cherif, C. Functionalization of Silk Fibers by PDGF and Bioceramics for Bone Tissue Regeneration. Coatings 2020, 10, 8. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wöltje, M.; Künzelmann, L.; Belgücan, B.; Croft, A.S.; Voumard, B.; Bracher, S.; Zysset, P.; Gantenbein, B.; Cherif, C.; Aibibu, D. Textile Design of an Intervertebral Disc Replacement Device from Silk Yarn. Biomimetics 2023, 8, 152. https://doi.org/10.3390/biomimetics8020152
Wöltje M, Künzelmann L, Belgücan B, Croft AS, Voumard B, Bracher S, Zysset P, Gantenbein B, Cherif C, Aibibu D. Textile Design of an Intervertebral Disc Replacement Device from Silk Yarn. Biomimetics. 2023; 8(2):152. https://doi.org/10.3390/biomimetics8020152
Chicago/Turabian StyleWöltje, Michael, Liesa Künzelmann, Basak Belgücan, Andreas S. Croft, Benjamin Voumard, Stefan Bracher, Philippe Zysset, Benjamin Gantenbein, Chokri Cherif, and Dilbar Aibibu. 2023. "Textile Design of an Intervertebral Disc Replacement Device from Silk Yarn" Biomimetics 8, no. 2: 152. https://doi.org/10.3390/biomimetics8020152
APA StyleWöltje, M., Künzelmann, L., Belgücan, B., Croft, A. S., Voumard, B., Bracher, S., Zysset, P., Gantenbein, B., Cherif, C., & Aibibu, D. (2023). Textile Design of an Intervertebral Disc Replacement Device from Silk Yarn. Biomimetics, 8(2), 152. https://doi.org/10.3390/biomimetics8020152







