Design, Manufacturing and Functions of Pore-Structured Materials: From Biomimetics to Artificial
Abstract
1. Introduction
| Matters | Porous Structure | Cell Morphology | SSA (m2/g) | Features | Biomimetic Applications | References |
|---|---|---|---|---|---|---|
| bone | hierarchical mineralization | bleed hole | 0.0394 | high compressive strength | scaffolds for bone regeneration and bone replacement, and other tissue engineering materials | [1,2] |
| sponge | 3D silicon network | bleed hole | 30–400 | elasticity, permeability, adsorption, | catalysts, energy separation | [3] |
| diatom | 3D periodicity | honeycomb pore | 9.7 | high mechanical properties, light orientation | catalysts, separation, optics | [4,5,6,7,8] |
| pummelo peel | fiber layered porous | honeycomb pore | - | moisture retention, pressure resistance, cushioning, space limited region | energy absorption, functional integration, solar evaporation thermal materials, bionic foam composites | [9] |
| block wood | fiber layered porous | hollow | 28.2 | high transmission, high specific strength, energy absorption | intelligent electronics, EMI shielding, heat insulation, TiC/C ceramics | [10,11] |
| bamboo chip | fiber layered porous | hollow | 0.0205 | high transmission, strong toughness and strength, energy absorption | energy dissipation, light weight and vibration reduction laminated structural materials, renewable catalytic system | [12,13,15,16,17] |
2. Design and Fabrication of the Bionic Porous Structure
2.1. Biomimetic Template Fabricated Pore Method
2.2. Biomimetic Porous Processing Technology
2.3. Biomimetic Mineralization Modified Pore Method
3. Structure-Function Relationship of Bionic Porous Structures
3.1. Effect of Pore Size on Performance
3.2. Effect of Pore Morphology on Performance
3.3. Effect of Pore Orientation on Performance
4. Design of Porous Meta-Structure
5. Summary and Outlook
- (1)
- The imitation accuracy gradually develops from the macroscopic micron level to the nanometer scale, and the bionic can manufacture complex porous structures more accurately.
- (2)
- The biomimetic structure level ranges from single-layer holes to multi-level sub-complex structures such as layered/fractal, enriching the diversity of structures and the integration of structural functions.
- (3)
- The pore scale imitation control range is extended, from micron to nano, to realize the refinement of pore structure and the expansion of performance matching.
- (4)
- The degree of order of the porous structure is from random to highly ordered, and the reproducibility and functional anisotropy of the structure are realized.
- The current bionic structure design and manufacturing still need to develop high-end manufacturing technology to enhance the accuracy and adaptability of bionic methods.
- The developed high-level substructure can overcome the contradiction between the inherent properties of the porous structure and the functional compatibility, and realizes the high-level integration of functions.
- The study of the deeper relationship between natural porous structure and func-tion will enhance the application value of biological porous structure.
- In the future, human beings will expand the types of porous meta-structure de-sign, establish a clear structure-activity theory system of meta-structure, and fur-ther develop porous meta-structure designs.
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Q.; He, J.; Sun, J.; Ho, J. Determining the specific surface area of coarse aggregate based on sieving curve via image-analysis approach. Constr. Build. Mater. 2021, 305, 124728. [Google Scholar] [CrossRef]
- Tang, S.; Dong, Z.; Ke, X.; Luo, J.; Li, J. Advances in biomineralization-inspired materials for hard tissue repair. Int. J. Oral Sci. 2021, 13, 42. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Qu, Y.; Wang, K.; Lv, K.; He, X.; Qin, S. Preparation of Super Absorbent and Highly Active Fish Collagen Sponge and its Hemostatic Effect In Vivo and In Vitro. Front. Bioeng. Biotechnol. 2022, 10, 862532. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, C.; Shi, L.; Gao, T.; Song, X.; Bachmatiuk, A.; Zou, Z.; Deng, B.; Ji, Q.; Ma, D.; et al. Growing three-dimensional biomorphic graphene powders using naturally abundant diatomite templates towards high solution processability. Nat. Commun. 2016, 7, 13440. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Song, M.-K.; Davis, S.C.; Cai, Y.; Liu, M.; Sandhage, K.H. High surface area, micro/mesoporous carbon particles with selectable 3-D biogenic morphologies for tailored catalysis, filtration, or adsorption. Energy Environ. Sci. 2011, 4, 3980–3984. [Google Scholar] [CrossRef]
- Li, K.; Li, Y.; Wang, X.; Cui, M.; An, B.; Pu, J.; Liu, J.; Zhang, B.; Ma, G.; Zhong, C. Diatom-inspired multiscale mineralization of patterned protein–polysaccharide complex structures. Natl. Sci. Rev. 2021, 8, nwaa191. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Guney, M.G.; Fedder, G.K.; Dávila, L.P. The role of hierarchical design and morphology in the mechanical response of diatom-inspired structures via simulation. Biomater. Sci. 2017, 6, 146–153. [Google Scholar] [CrossRef]
- Ragni, R.; Cicco, S.R.; Vona, D.; Farinola, G.M. Multiple Routes to Smart Nanostructured Materials from Diatom Microalgae: A Chemical Perspective. Adv. Mater. 2018, 30, 1704289. [Google Scholar] [CrossRef]
- Geng, Y.; Sun, W.; Ying, P.; Zheng, Y.; Ding, J.; Sun, K.; Li, L.; Li, M. Bioinspired Fractal Design of Waste Biomass-Derived Solar–Thermal Materials for Highly Efficient Solar Evaporation. Adv. Funct. Mater. 2020, 31, 2007648. [Google Scholar] [CrossRef]
- Sun, B.; Fan, T.; Zhang, D.; Okabe, T. The synthesis and microstructure of morph-genetic TiC/C ceramics. Carbon 2004, 42, 177–182. [Google Scholar] [CrossRef]
- Zhao, B.; Bai, P.; Wang, S.; Ji, H.; Fan, B.; Zhang, R.; Che, R. High-Performance Joule Heating and Electromagnetic Shielding Properties of Anisotropic Carbon Scaffolds. ACS Appl. Mater. Interfaces 2021, 13, 29101–29112. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Jiang, Y.; Rong, X.; Wei, W.; Wang, S.; Nie, S. Surface characterization and chemical analysis of bamboo substrates pretreated by alkali hydrogen peroxide. Bioresour. Technol. 2016, 216, 1098–1101. [Google Scholar] [CrossRef]
- Han, S.; Chen, F.; Yu, Y.; Zheng, Z.; Chen, L.; Wang, G. Bamboo-Inspired Renewable, Lightweight, and Vibration-Damping Laminated Structural Materials for the Floor of a Railroad Car. ACS Appl. Mater. Interfaces 2022, 14, 42645–42655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Z.; Liu, K.; Jiang, L. Bioinspired Multifunctional Foam with Self-Cleaning and Oil/Water Separation. Adv. Funct. Mater. 2013, 23, 2881–2886. [Google Scholar] [CrossRef]
- Ni, H.; Li, X.; Gao, H.; Nguyen, T.-P. Nanoscale structural and mechanical characterization of bamboo-like polymer/silicon nanocomposite films. Nanotechnology 2005, 16, 1746. [Google Scholar] [CrossRef]
- Zou, L.; Jin, H.; Lu, W.-Y.; Li, X. Nanoscale structural and mechanical characterization of the cell wall of bamboo fibers. Mater. Sci. Eng. C 2009, 29, 1375–1379. [Google Scholar] [CrossRef]
- Zou, L.; Li, X. In Situ Observation of Fracture Behavior of Bamboo Culm. JOM 2021, 73, 1705–1713. [Google Scholar] [CrossRef]
- Yang, L.; Xiao, H.; Qian, Y.; Zhao, X.; Kong, X.-Y.; Liu, P.; Xin, W.; Fu, L.; Jiang, L.; Wen, L. Bioinspired hierarchical porous membrane for efficient uranium extraction from seawater. Nat. Sustain. 2021, 5, 71–80. [Google Scholar] [CrossRef]
- Bennett, N.; Seddon, A.M.; Hallett, J.E.; Kockelmann, W.; Ting, V.P.; Sadasivan, S.; Tooze, R.P.; Hall, S.R. Mesoporous tertiary oxides via a novel amphiphilic approach. APL Mater. 2016, 4, 015701. [Google Scholar] [CrossRef]
- Feng, G.; Cheng, X.; Xie, D.; Wang, K.; Zhang, B. Fabrication and characterization of nano prism-like hydroxyapatite coating on porous titanium substrate by combined biomimetic-hydrothermal method. Mater. Lett. 2016, 163, 134–137. [Google Scholar] [CrossRef]
- Qiu, P.; Ma, B.; Hung, C.-T.; Li, W.; Zhao, D. Spherical Mesoporous Materials from Single to Multilevel Architectures. Accounts Chem. Res. 2019, 52, 2928–2938. [Google Scholar] [CrossRef]
- Deville, S. Wood-like polymeric materials by ice templating. Natl. Sci. Rev. 2018, 6, 184–185. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, G.; Jiao, D.; Zhang, J.; Wang, S.; Liu, F.; Liu, Z.; Zhuo, L.; Zhang, Z.; Deville, S.; et al. Ice-templated porous tungsten and tungsten carbide inspired by natural wood. J. Mater. Sci. Technol. 2020, 45, 187–197. [Google Scholar] [CrossRef]
- Shao, G.; Hanaor, D.A.H.; Shen, X.; Gurlo, A. Freeze Casting: From Low-Dimensional Building Blocks to Aligned Porous Structures-A Review of Novel Materials, Methods, and Applications. Adv. Mater. 2020, 32, 1907176. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Pereira, R.F.P.; Coradin, T.; Bermudez, V.D.Z.; Fernandes, F.M. Biomimetic Silk Macroporous Materials for Drug Delivery Obtained via Ice-Templating. ACS Appl. Bio Mater. 2022, 5, 2556–2566. [Google Scholar] [CrossRef]
- Naglieri, V.; Bale, H.A.; Gludovatz, B.; Tomsia, A.P.; Ritchie, R.O. On the development of ice-templated silicon carbide scaffolds for nature-inspired structural materials. Acta Mater. 2013, 61, 6948–6957. [Google Scholar] [CrossRef]
- Sun, M.; Elkhodiry, M.; Shi, L.; Xue, Y.; Abyaneh, M.H.; Kossar, A.P.; Giuglaris, C.; Carter, S.L.; Li, R.L.; Bacha, E.; et al. A biomimetic multilayered polymeric material designed for heart valve repair and replacement. Biomaterials 2022, 288, 121756. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, N.; Wang, M.; Dai, X.; Bai, H. Conch-Shell-Inspired Tough Ceramic. Adv. Funct. Mater. 2022, 32, 2205309. [Google Scholar] [CrossRef]
- Qiu, W.; Zhang, J.; Tan, G.; Gao, K.; Zhang, M.; Liu, Z.; Zhang, Z. Continuous ice-templating of macro-porous materials with uniformly ordered architecture. Sci. China Mater. 2022, 65, 3134–3143. [Google Scholar] [CrossRef]
- Wu, B.; Shan, C.; Zhang, X.; Zhao, H.; Ma, S.; Shi, Y.; Yang, J.; Bai, H.; Liu, Q. CeO2/Co3O4 porous nanosheet prepared using rose petal as biotemplate for photo-catalytic degradation of organic contaminants. Appl. Surf. Sci. 2021, 543, 148677. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, F.; Lv, X.; Xu, L.; Huang, R.; Chen, R.; Li, L. Achieving Sustainable and Stable Potassium-Ion Batteries by Leaf-Bioinspired Nanofluidic Flow. Adv. Mater. 2022, 34, 2204370. [Google Scholar] [CrossRef]
- Sun, J.; Yu, S.; Cui, Z.; Hu, L.; Sun, B.; Chen, B. Synthesis, characterization, and photocatalytic antibacterial activities of porous Ce-doped TiO2 microspheres using pine pollen as novel biotemplates. J. Mater. Sci. 2022, 57, 15276–15297. [Google Scholar] [CrossRef]
- Jia, X.; Wang, N.; Tian, J.; Zhang, Y.; Lu, D.; Tan, J.; Qiao, R.; Chen, L.; Zhang, W.; Zhong, J. A highly sensitive gas sensor employing biomorphic SnO2 with multi-level tubes/pores structure: Bio-templated from waste of flax. RSC Adv. 2019, 9, 19993–20001. [Google Scholar] [CrossRef]
- Mei, J.; Peng, X.; Zhang, Q.; Zhang, X.; Liao, T.; Mitic, V.; Sun, Z. Bamboo-Membrane Inspired Multilevel Ultrafast Interlayer Ion Transport for Superior Volumetric Energy Storage. Adv. Funct. Mater. 2021, 31, 2100299. [Google Scholar] [CrossRef]
- Tao, X.; Li, Y.; Du, J.; Xia, Y.; Yang, Y.; Huang, H.; Gan, Y.; Zhang, W.; Li, X. A generic bamboo-based carbothermal method for preparing carbide (SiC, B4C, TiC, TaC, NbC, TixNb1−xC, and TaxNb1−xC) nanowires. J. Mater. Chem. 2011, 21, 9095–9102. [Google Scholar] [CrossRef]
- Tao, X.; Du, J.; Li, Y.; Yang, Y.; Fan, Z.; Gan, Y.; Huang, H.; Zhang, W.; Dong, L.; Li, X. TaC Nanowire/Activated Carbon Microfiber Hybrid Structures from Bamboo Fibers. Adv. Energy Mater. 2011, 1, 534–539. [Google Scholar] [CrossRef]
- Du, J.; Yang, Y.; Fan, Z.; Xia, Y.; Cheng, X.; Gan, Y.; Hang, H.; Dong, L.; Li, X.; Zhang, W.; et al. Biotemplating fabrication, mechanical and electrical characterizations of NbC nanowire arrays from the bamboo substrate. J. Alloys Compd. 2013, 560, 142–146. [Google Scholar] [CrossRef]
- Du, L.; Li, Y.; Tong, Y.; Zhang, M. Biotemplates based preparation of hierarchical ZnSnO3 porous nanostructures for fast detection of formaldehyde. Ceram. Int. 2021, 47, 13139–13146. [Google Scholar] [CrossRef]
- Qiang, T.; Zhu, R. Bio-templated synthesis of porous silica nano adsorbents to wastewater treatment inspired by a circular economy. Sci. Total. Environ. 2022, 819, 152929. [Google Scholar] [CrossRef]
- El Hajam, M.; Kandri, N.I.; Zerouale, A.; Wang, X.; Gustafsson, J.; Wang, L.; Mäkilä, E.; Hupa, L.; Xu, C. Lignocellulosic Nanocrystals from Sawmill Waste as Biotemplates for Free-Surfactant Synthesis of Photocatalytically Active Porous Silica. ACS Appl. Mater. Interfaces 2022, 14, 19547–19560. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, J.; Dong, H.; Dong, J.H.; Qiu, K.; Jansen-Varnum, S.A. Preparation and Physisorption Characterization of d-Glucose-Templated Mesoporous Silica Sol−Gel Materials. Chem. Mater. 1999, 11, 2023–2029. [Google Scholar] [CrossRef]
- Lalchhingpuii; Tiwari, D.; Lalhmunsiama; Lee, S.M. Chitosan templated synthesis of mesoporous silica and its application in the treatment of aqueous solutions contaminated with cadmium(II) and lead(II). Chem. Eng. J. 2017, 328, 434–444. [Google Scholar] [CrossRef]
- Lyu, Y.; Asoh, T.-A.; Uyama, H. Hierarchically porous TiO2 monolith prepared using a cellulose monolith as a template. Mater. Chem. Front. 2021, 5, 3877–3885. [Google Scholar] [CrossRef]
- Gao, Y.; Yuan, Z.; Li, G.; Xu, Q.; Li, Z.; Fu, Y.; Qin, M. Cellulose liquid crystal templated TiO2 chiral nematic foams. Colloids Surf. A 2022, 646, 128988. [Google Scholar] [CrossRef]
- Karthikeyan, K.T.; Nithya, A.; Jothivenkatachalam, K. Photocatalytic and antimicrobial activities of chitosan-TiO2 nanocomposite. Int. J. Biol. Macromol. 2017, 104, 1762–1773. [Google Scholar] [CrossRef] [PubMed]
- Nursam, N.M.; Wang, X.; Tan, J.Z.Y.; Caruso, R.A. Probing the Effects of Templating on the UV and Visible Light Photocatalytic Activity of Porous Nitrogen-Modified Titania Monoliths for Dye Removal. ACS Appl. Mater. Interfaces 2016, 8, 17194–17204. [Google Scholar] [CrossRef]
- Shopsowitz, K.E.; Qi, H.; Hamad, W.Y.; MacLachlan, M.J. Free-standing mesoporous silica films with tunable chiral nematic structures. Nature 2010, 468, 422–425. [Google Scholar] [CrossRef]
- Duan, Y.; Che, S. Chiral Mesostructured Inorganic Materials with Optical Chiral Response. Adv. Mater. 2022, 2205088. [Google Scholar] [CrossRef]
- Li, X. Nanoscale structural and mechanical characterization of natural nanocomposites: Seashells. JOM 2007, 59, 71–74. [Google Scholar] [CrossRef]
- Li, H.; Xu, Z.-H.; Li, X. Multiscale hierarchical assembly strategy and mechanical prowess in conch shells (Busycon carica). J. Struct. Biol. 2013, 184, 409–416. [Google Scholar] [CrossRef]
- Li, H.; Jin, D.; Li, R.; Li, X. Structural and Mechanical Characterization of Thermally Treated Conch Shells. JOM 2015, 67, 720–725. [Google Scholar] [CrossRef]
- Li, H.; Shen, J.; Wei, Q.; Li, X. Dynamic self-strengthening of a bio-nanostructured armor—conch shell. Mater. Sci. Eng. C 2019, 103, 109820. [Google Scholar] [CrossRef]
- Chen, H.; Di, J.; Wang, N.; Dong, H.; Wu, J.; Zhao, Y.; Yu, J.; Jiang, L. Fabrication of Hierarchically Porous Inorganic Nanofibers by a General Microemulsion Electrospinning Approach. Small 2011, 7, 1779–1783. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qi, M.; Xu, X.; Liu, T.; Hu, Y. Fine pore tailoring of PSf-b-PEG membrane in sub-5 nm via phase-inversion. J. Membr. Sci. 2023, 672, 121427. [Google Scholar] [CrossRef]
- Huang, P.; Wu, F.; Shen, B.; Ma, X.; Zhao, Y.; Wu, M.; Wang, J.; Liu, Z.; Luo, H.; Zheng, W. Bio-inspired lightweight polypropylene foams with tunable hierarchical tubular porous structure and its application for oil-water separation. Chem. Eng. J. 2019, 370, 1322–1330. [Google Scholar] [CrossRef]
- Mao, A.; Zhao, N.; Liang, Y.; Bai, H. Mechanically Efficient Cellular Materials Inspired by Cuttlebone. Adv. Mater. 2021, 33, e2007348. [Google Scholar] [CrossRef]
- Lei, D.; Yang, Y.; Liu, Z.; Yang, B.; Gong, W.; Chen, S.; Wang, S.; Sun, L.; Song, B.; Xuan, H.; et al. 3D printing of biomimetic vasculature for tissue regeneration. Mater. Horiz. 2019, 6, 1197–1206. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Y.; Ding, K.; Lai, Y.; Mao, R.; Luo, F.; Zhang, B.; Zhu, J.; Fan, Y.; Zhou, C.; et al. Polyphosphate enhanced biomimetic mineralization of 3D printing scaffolds for bone regeneration. Compos. Part B 2022, 239, 109989. [Google Scholar] [CrossRef]
- Peng, M.; Wen, Z.; Xie, L.; Cheng, J.; Jia, Z.; Shi, D.; Zeng, H.; Zhao, B.; Liang, Z.; Li, T.; et al. 3D Printing of Ultralight Biomimetic Hierarchical Graphene Materials with Exceptional Stiffness and Resilience. Adv. Mater. 2019, 31, 1902930. [Google Scholar] [CrossRef]
- Siddique, S.H.; Hazell, P.J.; Wang, H.; Escobedo, J.P.; Ameri, A.A. Lessons from nature: 3D printed bio-inspired porous structures for impact energy absorption—A review. Addit. Manuf. 2022, 58, 103051. [Google Scholar] [CrossRef]
- Teng, L.; Shao, Z.; Bai, Q.; Zhang, X.; He, Y.; Lu, J.; Zou, D.; Feng, C.; Dong, C. Biomimetic Glycopolypeptide Hydrogels with Tunable Adhesion and Microporous Structure for Fast Hemostasis and Highly Efficient Wound Healing. Adv. Funct. Mater. 2021, 31, 2105628. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.-N.; Zhu, C.-Y.; Huang, X.-J.; Greiner, A.; Xu, Z.-K. Biomimetic gill-inspired membranes with direct-through micropores for water remediation by efficiently removing microplastic particles. Chem. Eng. J. 2022, 434, 134758. [Google Scholar] [CrossRef]
- Lacey, S.; Kirsch, D.J.; Li, Y.; Morgenstern, J.T.; Zarket, B.; Yao, Y.; Dai, J.; Garcia, L.Q.; Liu, B.; Gao, T.; et al. Extrusion-Based 3D Printing of Hierarchically Porous Advanced Battery Electrodes. Adv. Mater. 2018, 30, e1705651. [Google Scholar] [CrossRef]
- Yuan, J.; Lei, X.; Yi, C.; Jiang, H.; Liu, F.; Cheng, G.J. 3D-printed hierarchical porous cellulose/alginate/carbon black hydrogel for high-efficiency solar steam generation. Chem. Eng. J. 2021, 430, 132765. [Google Scholar] [CrossRef]
- Dey, A.; de With, G.; Sommerdijk, N.A.J.M. In situ techniques in biomimetic mineralization studies of calcium carbonate. Chem. Soc. Rev. 2010, 39, 397–409. [Google Scholar] [CrossRef]
- Chen, C.-L.; Qi, J.; Zuckermann, R.N.; DeYoreo, J.J. Engineered Biomimetic Polymers as Tunable Agents for Controlling CaCO3 Mineralization. J. Am. Chem. Soc. 2011, 133, 5214–5217. [Google Scholar] [CrossRef]
- Xiao, C.; Li, M.; Wang, B.; Liu, M.-F.; Shao, C.; Pan, H.; Lu, Y.; Xu, B.-B.; Li, S.; Zhan, D.; et al. Total morphosynthesis of biomimetic prismatic-type CaCO3 thin films. Nat. Commun. 2017, 8, 1398. [Google Scholar] [CrossRef]
- Yang, T.; Fu, J.; Ma, L.; Du, H.; Yue, X.; Zhao, B.; Wang, C. Biomimetic synthesis of calcium carbonate under phenylalanine: Control of polymorph and morphology. Mater. Sci. Eng. C 2020, 114, 111019. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Koons, G.L.; Mikos, A.G.; Qiu, Z.; Song, T.; Cui, F.; Wang, X. Tuning pore features of mineralized collagen/PCL scaffolds for cranial bone regeneration in a rat model. Mater. Sci. Eng. C 2020, 106, 110186. [Google Scholar] [CrossRef]
- Xie, P.; Du, J.; Li, Y.; Wu, J.; He, H.; Jiang, X.; Liu, C. Robust hierarchical porous MBG scaffolds with promoted biomineralization ability. Colloids Surf. B 2019, 178, 22–31. [Google Scholar] [CrossRef]
- Zhu, W.; Lin, J.; Cai, C.; Lu, Y. Biomimetic mineralization of calcium carbonate mediated by a polypeptide-based copolymer. J. Mater. Chem. B 2013, 1, 841–849. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Y.; Zhou, Y.; Pei, C.; Yin, G. A promising hybrid scaffold material: Bacterial cellulose in-situ assembling biomimetic lamellar CaCO3. Mater. Lett. 2013, 102, 91–93. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, T.; Hua, W.; Li, P.; Wang, X. 3D Porous poly(lactic acid)/regenerated cellulose composite scaffolds based on electrospun nanofibers for biomineralization. Colloids Surf. A. 2020, 585, 124048. [Google Scholar] [CrossRef]
- Kong, J.; Wei, B.; Groth, T.; Chen, Z.; Li, L.; He, D.; Huang, R.; Chu, J.; Zhao, M. Biomineralization improves mechanical and osteogenic properties of multilayer-modified PLGA porous scaffolds. J. Biomed. Mater. Res. Part A 2018, 106, 2714–2725. [Google Scholar] [CrossRef]
- Wei, W.; Ma, G.-H.; Hu, G.; Yu, D.; Mcleish, T.; Su, Z.-G.; Shen, Z.-Y. Preparation of Hierarchical Hollow CaCO3 Particles and the Application as Anticancer Drug Carrier. J. Am. Chem. Soc. 2008, 130, 15808–15810. [Google Scholar] [CrossRef]
- Gelinsky, M.; Welzel, P.; Simon, P.; Bernhardt, A.; König, U. Porous three-dimensional scaffolds made of mineralised collagen: Preparation and properties of a biomimetic nanocomposite material for tissue engineering of bone. Chem. Eng. J. 2008, 137, 84–96. [Google Scholar] [CrossRef]
- Penk, A.; Förster, Y.; Scheidt, H.A.; Nimptsch, A.; Hacker, M.C.; Schulz-Siegmund, M.; Ahnert, P.; Schiller, J.; Rammelt, S.; Huster, D. The pore size of PLGA bone implants determines the de novo formation of bone tissue in tibial head defects in rats. Magn. Reson. Med. 2013, 70, 925–935. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Wang, J.; Yan, H.; Chen, T.; Wang, Y.; Li, H.; Zhi, W.; Feng, B.; Weng, J.; Zhu, M. Porous nanoapatite scaffolds synthesized using an approach of interfacial mineralization reaction and their bioactivity. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1749–1761. [Google Scholar] [CrossRef]
- Mohammadi, H.; Sepantafar, M.; Muhamad, N.; Bakar Sulong, A. How Does Scaffold Porosity Conduct Bone Tissue Regeneration? Adv. Eng. Mater. 2021, 23, 2100463. [Google Scholar] [CrossRef]
- Minardi, S.; Taraballi, F.; Cabrera, F.J.; Van Eps, J.; Wang, X.; Gazze, S.A.; Fernandez-Mourev, J.S.; Tampieri, A.; Francis, L.; Weiner, B.K.; et al. Biomimetic hydroxyapatite/collagen composite drives bone niche recapitulation in a rabbit orthotopic model. Mater. Today Bio 2019, 2, 100005. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Zhang, W.; Ma, D.; Li, F.; Jia, R.; Shi, M.; Wang, Y.; Ma, G.; Wei, W. Shielding Ferritin with a Biomineralized Shell Enables Efficient Modulation of Tumor Microenvironment and Targeted Delivery of Diverse Therapeutic Agents. Adv. Mater. 2021, 34, 2107150. [Google Scholar] [CrossRef]
- He, X.; Tang, K.; Li, X.; Wang, F.; Liu, J.; Zou, F.; Yang, M.; Li, M. A porous collagen-carboxymethyl cellulose/hydroxyapatite composite for bone tissue engineering by bi-molecular template method. Int. J. Biol. Macromol. 2019, 137, 45–53. [Google Scholar] [CrossRef]
- Meng, F.; Ye, X.; Chen, W.; Liu, L.; Hu, F.; Qi, Y.; Yu, W.; Lin, Z.; Yan, J.; Guo, Z.; et al. A Mild Bioinspiration Route to Bacillus-Shaped Silica with Enhanced Immune Responses. ACS Sustain. Chem. Eng. 2023, 11, 1324–1332. [Google Scholar] [CrossRef]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic Systems for Hydroxyapatite Mineralization Inspired By Bone and Enamel. J. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef]
- Quan, Y.; Song, Y.; Shi, W.; Xu, Z.; Chen, J.S.; Jiang, X.; Wang, C.; Lin, W. Metal–Organic Framework with Dual Active Sites in Engineered Mesopores for Bioinspired Synergistic Catalysis. J. Am. Chem. Soc. 2020, 142, 8602–8607. [Google Scholar] [CrossRef]
- Huang, C.; Guan, X.; Lin, H.; Liang, L.; Miao, Y.; Wu, Y.; Bao, H.; Wu, X.; Shen, A.; Wei, M.; et al. Efficient Photoacoustic Imaging with Biomimetic Mesoporous Silica-Based Nanoparticles. Front. Bioeng. Biotechnol. 2021, 9, 762956. [Google Scholar] [CrossRef]
- Zhao, H.-Y.; Huang, J.; Zhou, J.; Chen, L.-F.; Wang, C.; Bai, Y.; Zhou, J.; Deng, Y.; Dong, W.-X.; Li, Y.-S.; et al. Biomimetic Design of Macroporous 3D Truss Materials for Efficient Interfacial Solar Steam Generation. ACS Nano 2022, 16, 3554–3562. [Google Scholar] [CrossRef]
- Xu, C.; Wei, Z.; Gao, H.; Bai, Y.; Liu, H.; Yang, H.; Lai, Y.; Yang, L. Bioinspired Mechano-Sensitive Macroporous Ceramic Sponge for Logical Drug and Cell Delivery. Adv. Sci. 2017, 4, 1600410. [Google Scholar] [CrossRef]
- Chen, L.-H. Hierarchically porous materials and green chemistry—An interview with Ming-Yuan He. Natl. Sci. Rev. 2020, 7, 1759–1761. [Google Scholar] [CrossRef]
- Chen, L.; Bin Tu, B.; Lu, X.; Li, F.; Jiang, L.; Antonietti, M.; Xiao, K. Unidirectional ion transport in nanoporous carbon membranes with a hierarchical pore architecture. Nat. Commun. 2021, 12, 4650. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, L.; Wei, F.; Ding, T.; Zhang, M.; Zhu, R. Insights into the adsorption mechanism of tetracycline on hierarchically porous carbon and the effect of nanoporous geometry. Chem. Eng. J. 2022, 437, 135454. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, M.-M.; Liu, Z.; Shi, J.; Huang, F.; Luo, X. Constructing a Continuous Flow Bioreactor Based on a Hierarchically Porous Cellulose Monolith for Ultrafast and Nonstop Enzymatic Esterification/Transesterification. ACS Sustain. Chem. Eng. 2018, 7, 2056–2063. [Google Scholar] [CrossRef]
- Dong, X.; Wei, Y.; Chen, S.; Lin, Y.; Liu, L.; Li, J. A linear and large-range pressure sensor based on a graphene/silver nanowires nanobiocomposites network and a hierarchical structural sponge. Compos. Sci. Technol. 2018, 155, 108–116. [Google Scholar] [CrossRef]
- Lee, J.; Jung, Y.; Lee, M.; Hwang, J.S.; Guo, J.; Shin, W.; Min, J.; Pyun, K.R.; Lee, H.S.; Lee, Y.; et al. Biomimetic reconstruction of butterfly wing scale nanostructures for radiative cooling and structural coloration. Nanoscale Horiz. 2022, 7, 1054–1064. [Google Scholar] [CrossRef]
- Xiao, K.; Wu, K.; Chen, L.; Kong, X.-Y.; Zhang, Y.; Wen, L.; Jiang, L. Biomimetic Peptide-Gated Nanoporous Membrane for On-Demand Molecule Transport. Angew. Chem. Int. Ed. 2018, 57, 151–155. [Google Scholar] [CrossRef]
- Jiang, Z.; Dong, R.; Evans, A.M.; Biere, N.; Ebrahim, M.A.; Li, S.; Anselmetti, D.; Dichtel, W.R.; Livingston, A.G. Aligned macrocycle pores in ultrathin films for accurate molecular sieving. Nature 2022, 609, 58–64. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Mei, C.; Li, Y.; Duan, G.; Agarwal, S.; Greiner, A.; Ma, C.; Jiang, S. Wood-Inspired Anisotropic Cellulose Nanofibril Composite Sponges for Multifunctional Applications. ACS Appl. Mater. Interfaces 2020, 12, 35513–35522. [Google Scholar] [CrossRef]
- Li, S.; He, Y.; Ye, X.; Fu, X.; Hou, Y.; Tian, H.; Huang, J.; Gan, L. Improved piezoelectricity of porous cellulose material via flexible polarization-initiate bridge for self-powered sensor. Carbohydr. Polym. 2022, 298, 120099. [Google Scholar] [CrossRef]
- Li, D.; Bu, X.; Xu, Z.; Luo, Y.; Bai, H. Bioinspired Multifunctional Cellular Plastics with a Negative Poisson’s Ratio for High Energy Dissipation. Adv. Mater. 2020, 32, 2001222. [Google Scholar] [CrossRef]
- He, Y.; Li, D.; Zhou, N.; Gan, L.; Huang, J. Reversing Poisson’s Ratio of Biomass Foam to Be Negative to Achieve Super Mechanical Properties via Viscoelastic Compression. ACS Appl. Polym. Mater. 2021, 3, 599–603. [Google Scholar] [CrossRef]
- Mizzi, L.; Mahdi, E.M.; Titov, K.; Gatt, R.; Attard, D.; Evans, K.E.; Grima, J.N.; Tan, J.-C. Mechanical metamaterials with star-shaped pores exhibiting negative and zero Poisson’s ratio. Mater. Des. 2018, 146, 28–37. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, F.; Jing, X.; Pan, M.; Liu, P.; Li, W.; Zhu, B.; Li, J.; Chen, H.; Wang, L.; et al. Complex silica composite nanomaterials templated with DNA origami. Nature 2018, 559, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Weiden, J.; Bastings, M.M. DNA origami nanostructures for controlled therapeutic drug delivery. Curr. Opin. Colloid Interface Sci. 2021, 52, 101411. [Google Scholar] [CrossRef]
- Farimani, A.B.; Dibaeinia, P.; Aluru, N.R. DNA Origami–Graphene Hybrid Nanopore for DNA Detection. ACS Appl. Mater. Interfaces 2017, 9, 92–100. [Google Scholar] [CrossRef] [PubMed]

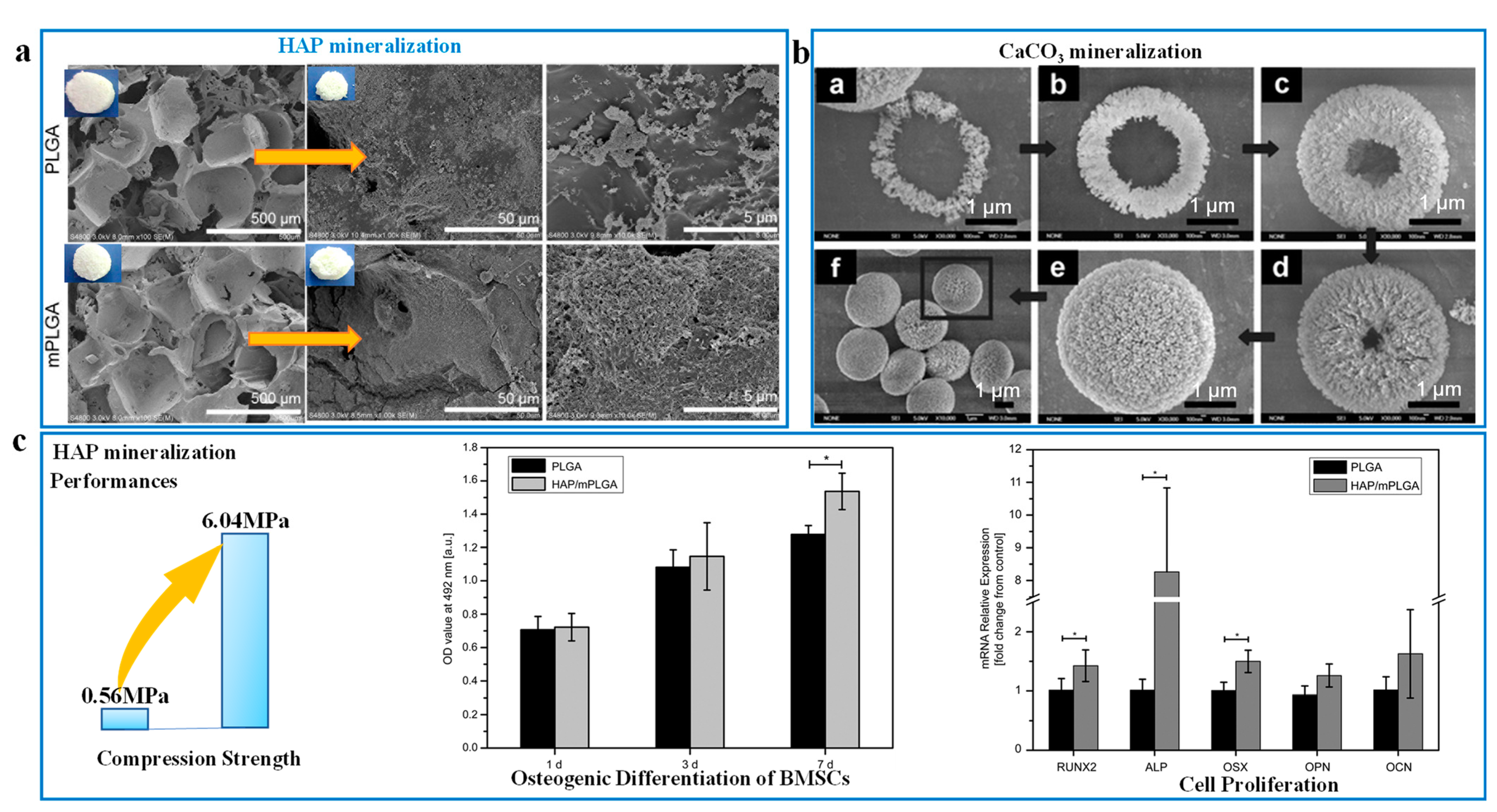


| Nanomaterials | Biological Template | Pore Size (nm) | SSA (m2/g) | Porous Structure | References |
|---|---|---|---|---|---|
| SiO2 | collagen | 2.1–4.5 | 907–1096 | hierarchical pores | [39] |
| cellulose nanocrystal | - | 348–1107 | hollow | [40] | |
| glucose | 3.2–3.5 | 800 | mesopores | [41] | |
| chitosan | 3.38 | 511.77 | mesopores | [42] | |
| TiO2 | cellulose nanocrystal | 5.6 | 13 | macropores, mesopores | [43] |
| cellulose nanofiber | 6.8–7.5 | 53–67 | hierarchical pores | [44] | |
| chitosan | 9 | 4 | mesopores | [45] | |
| agarose | 4.7 | 121 | porous network | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Gan, L.; Huang, J. Design, Manufacturing and Functions of Pore-Structured Materials: From Biomimetics to Artificial. Biomimetics 2023, 8, 140. https://doi.org/10.3390/biomimetics8020140
Chen W, Gan L, Huang J. Design, Manufacturing and Functions of Pore-Structured Materials: From Biomimetics to Artificial. Biomimetics. 2023; 8(2):140. https://doi.org/10.3390/biomimetics8020140
Chicago/Turabian StyleChen, Weiwei, Lin Gan, and Jin Huang. 2023. "Design, Manufacturing and Functions of Pore-Structured Materials: From Biomimetics to Artificial" Biomimetics 8, no. 2: 140. https://doi.org/10.3390/biomimetics8020140
APA StyleChen, W., Gan, L., & Huang, J. (2023). Design, Manufacturing and Functions of Pore-Structured Materials: From Biomimetics to Artificial. Biomimetics, 8(2), 140. https://doi.org/10.3390/biomimetics8020140








