Guided Bone Regeneration with Occlusive Titanium Barrier: A Case Report and Clinical Considerations
Abstract
1. Introduction
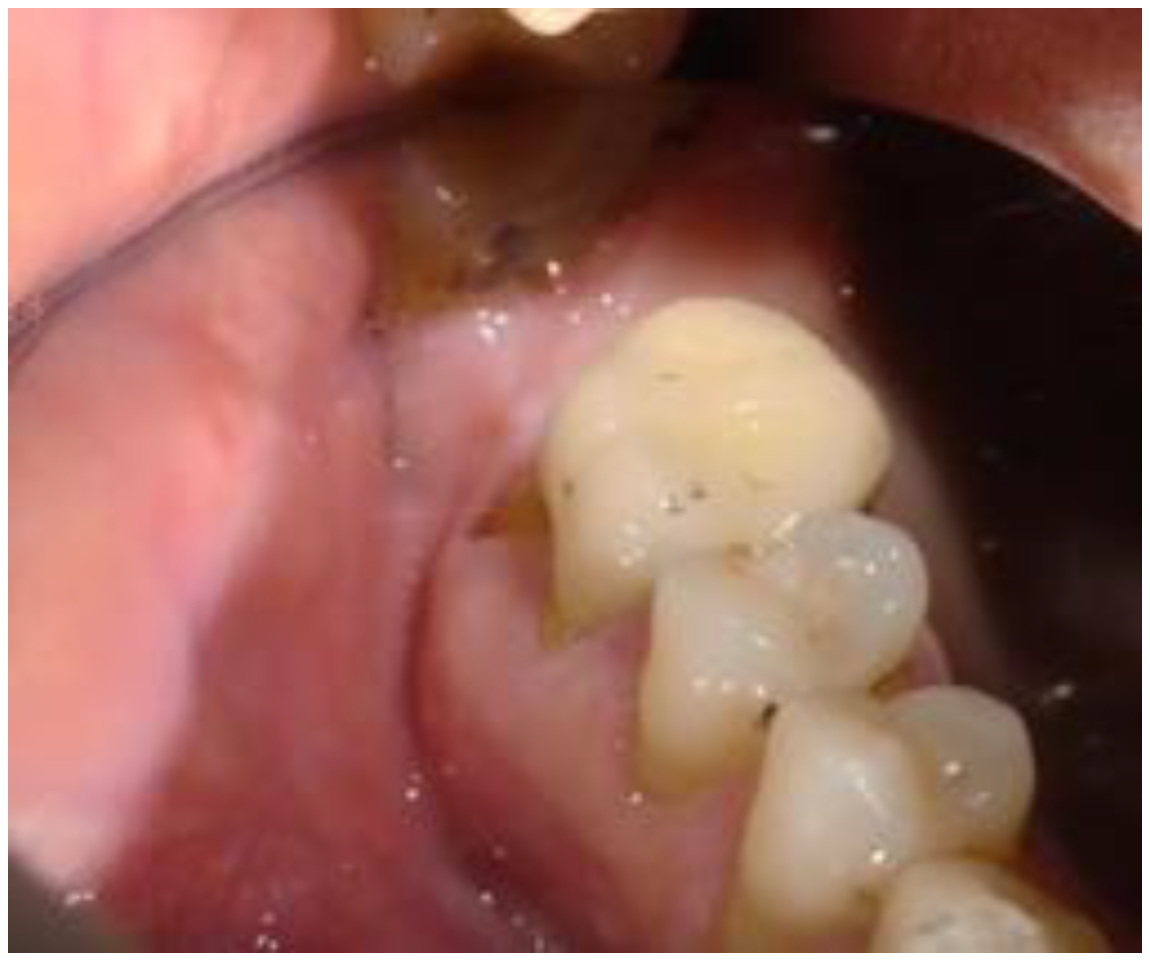
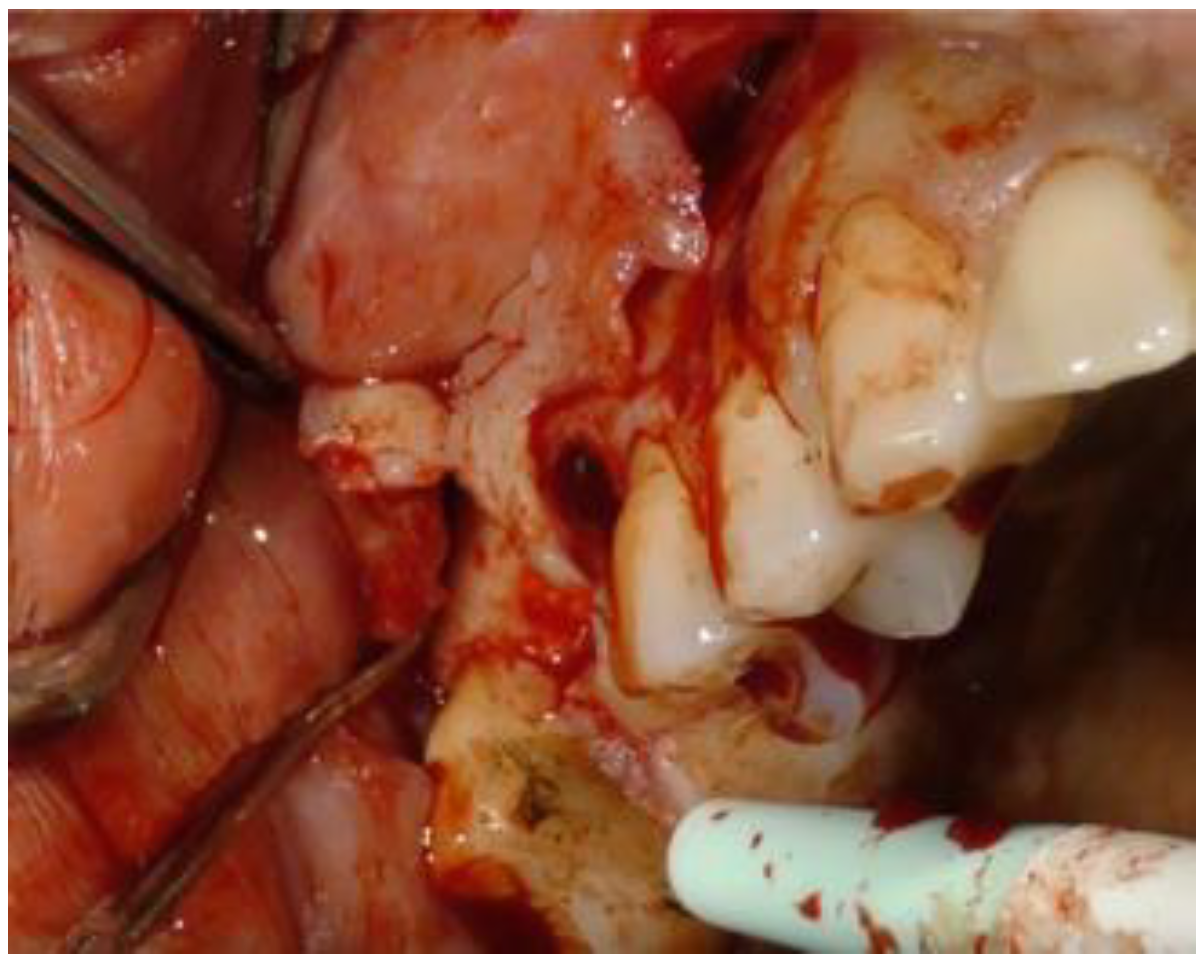
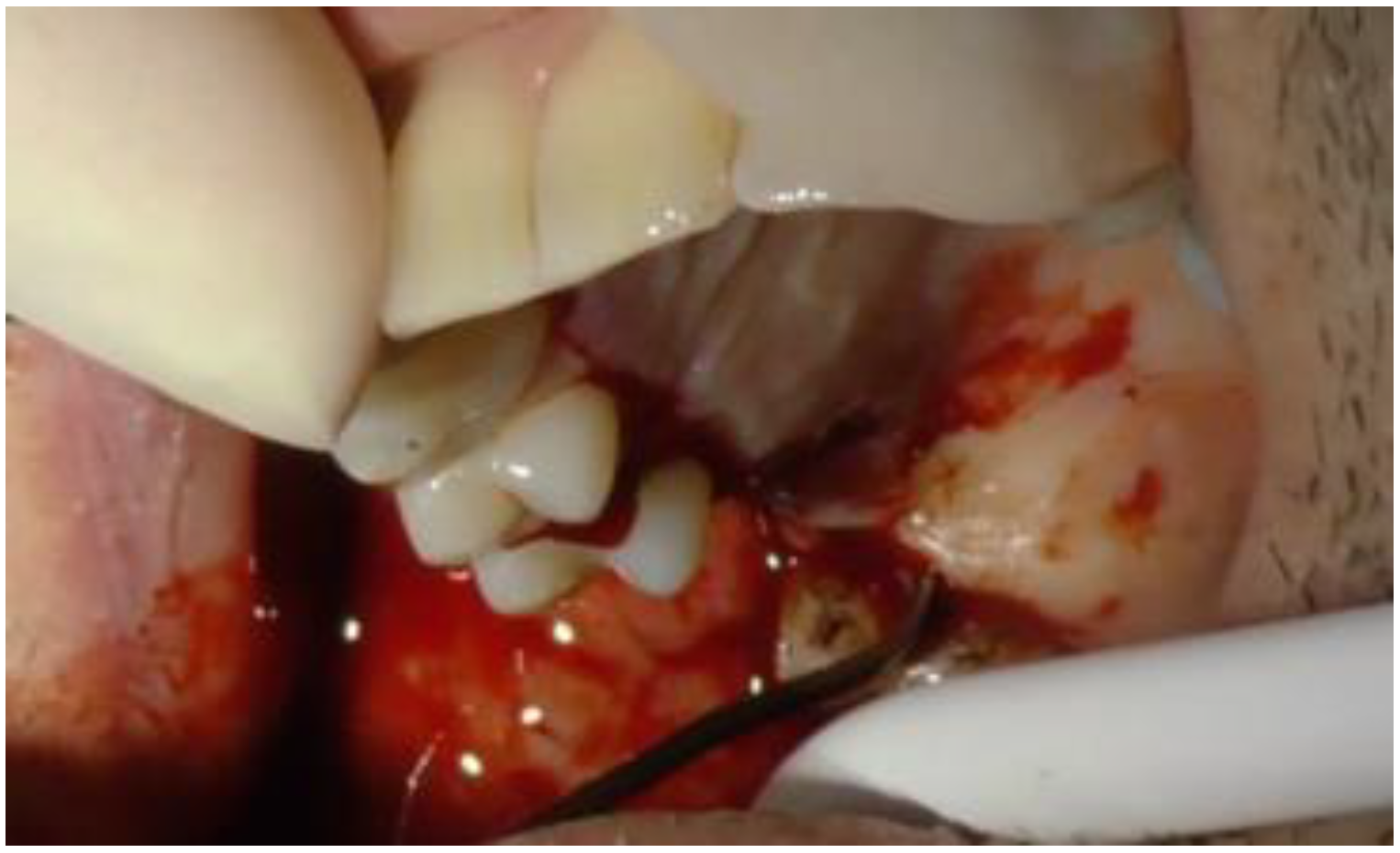
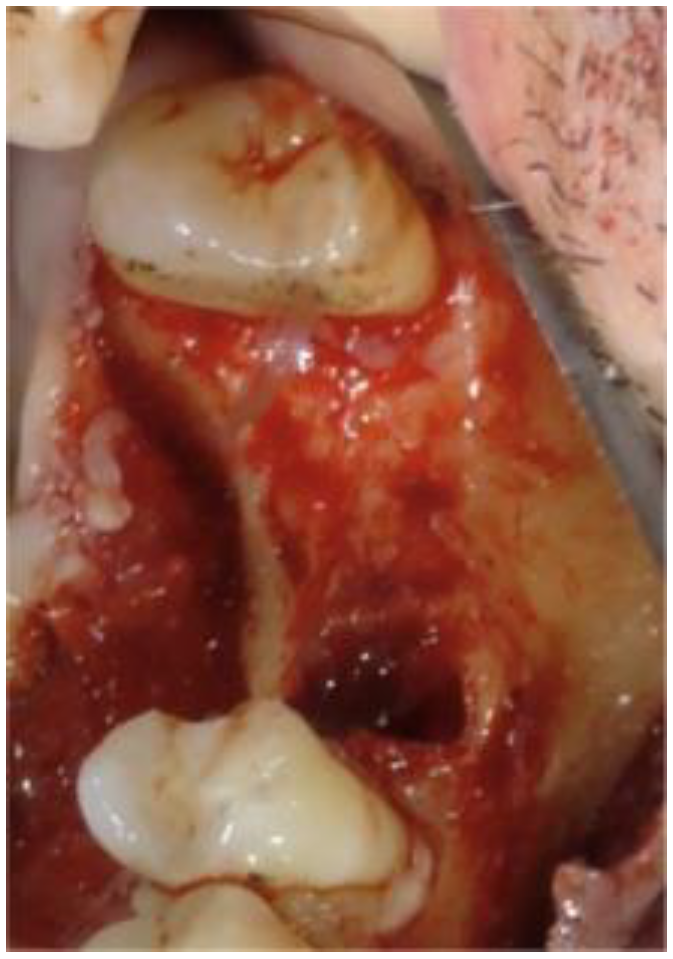
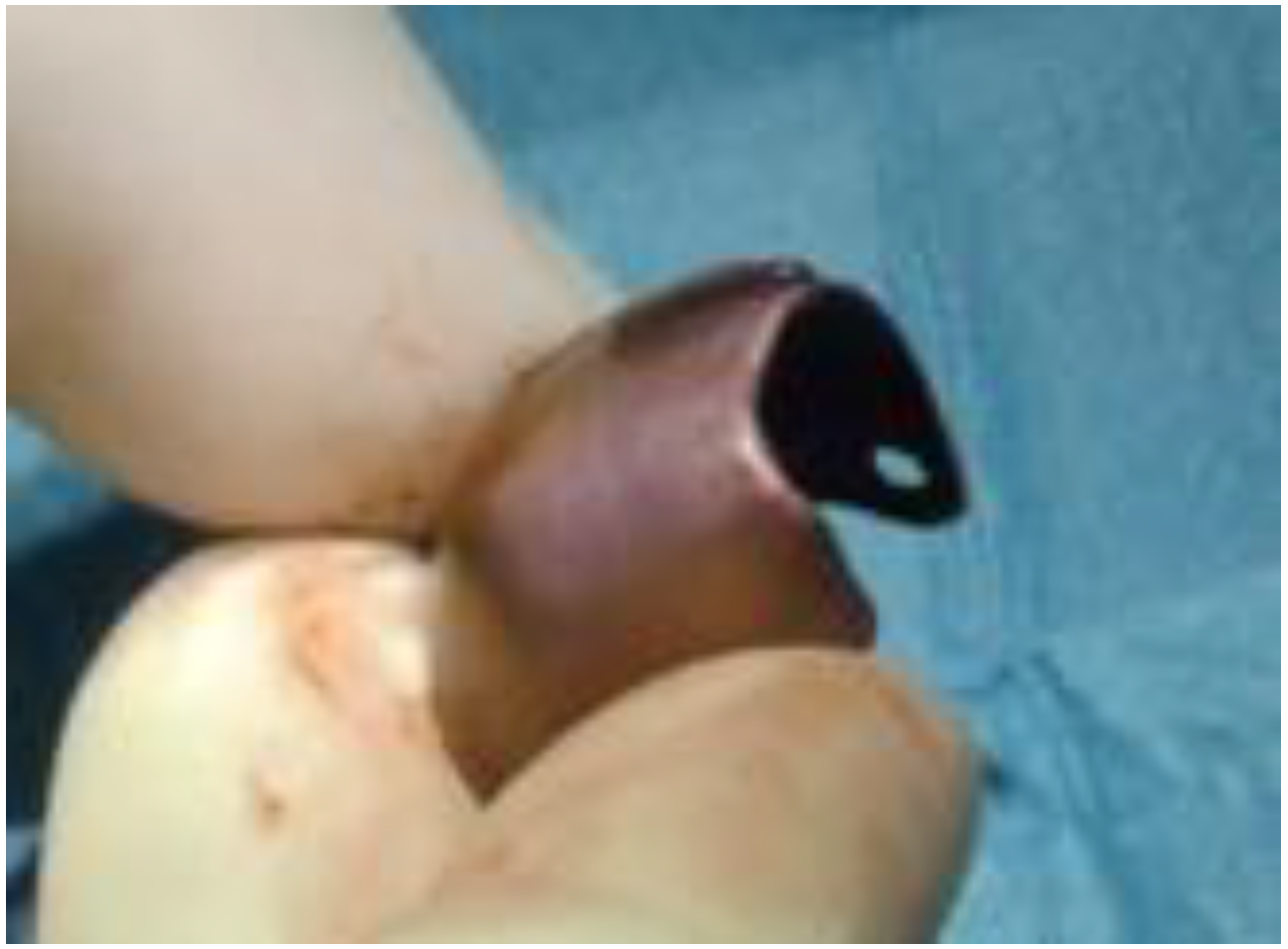
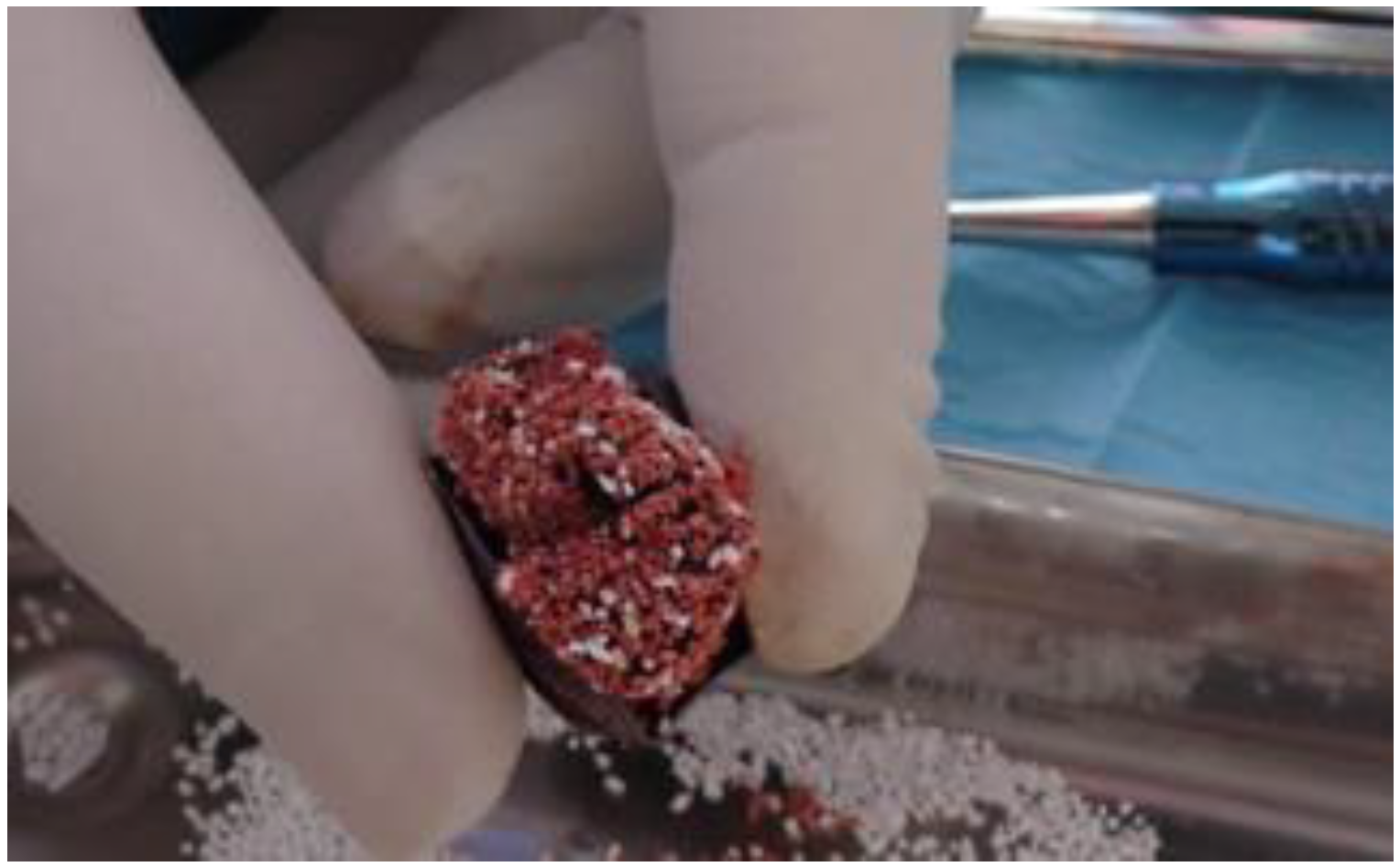
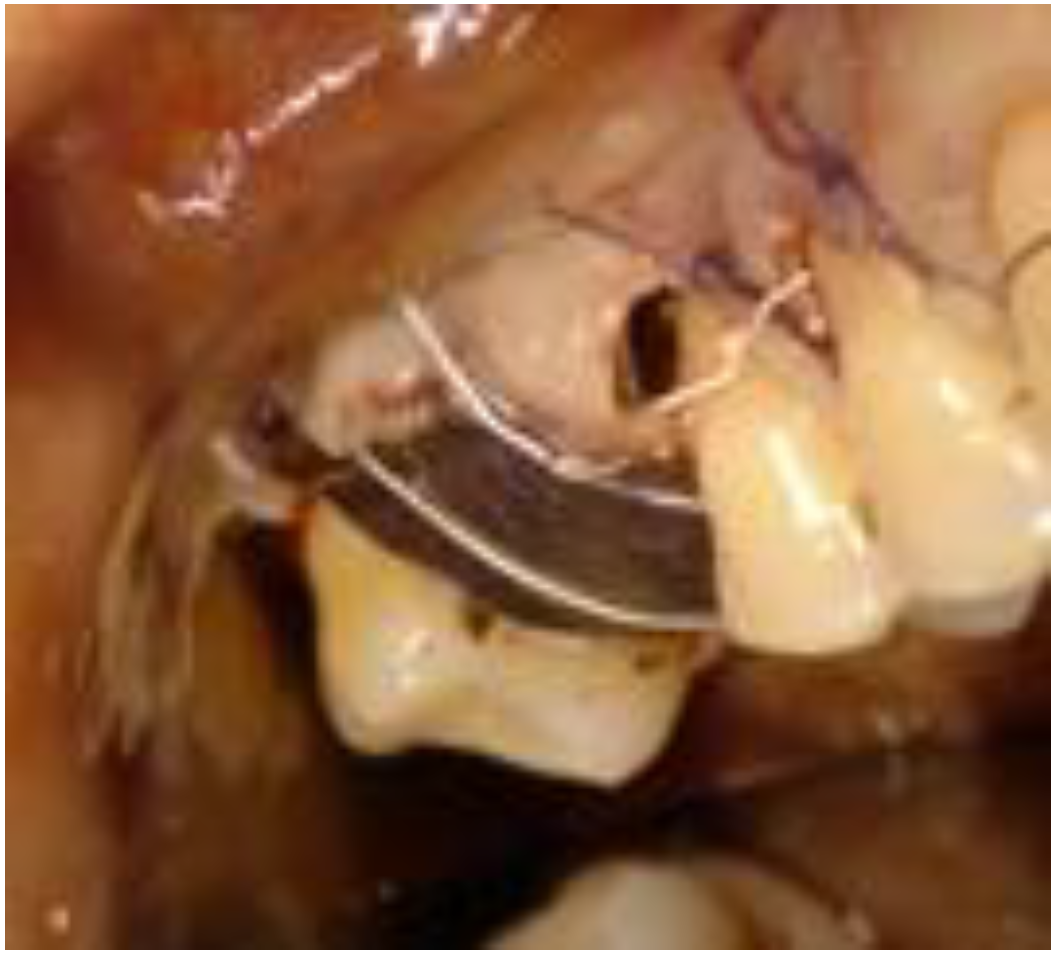
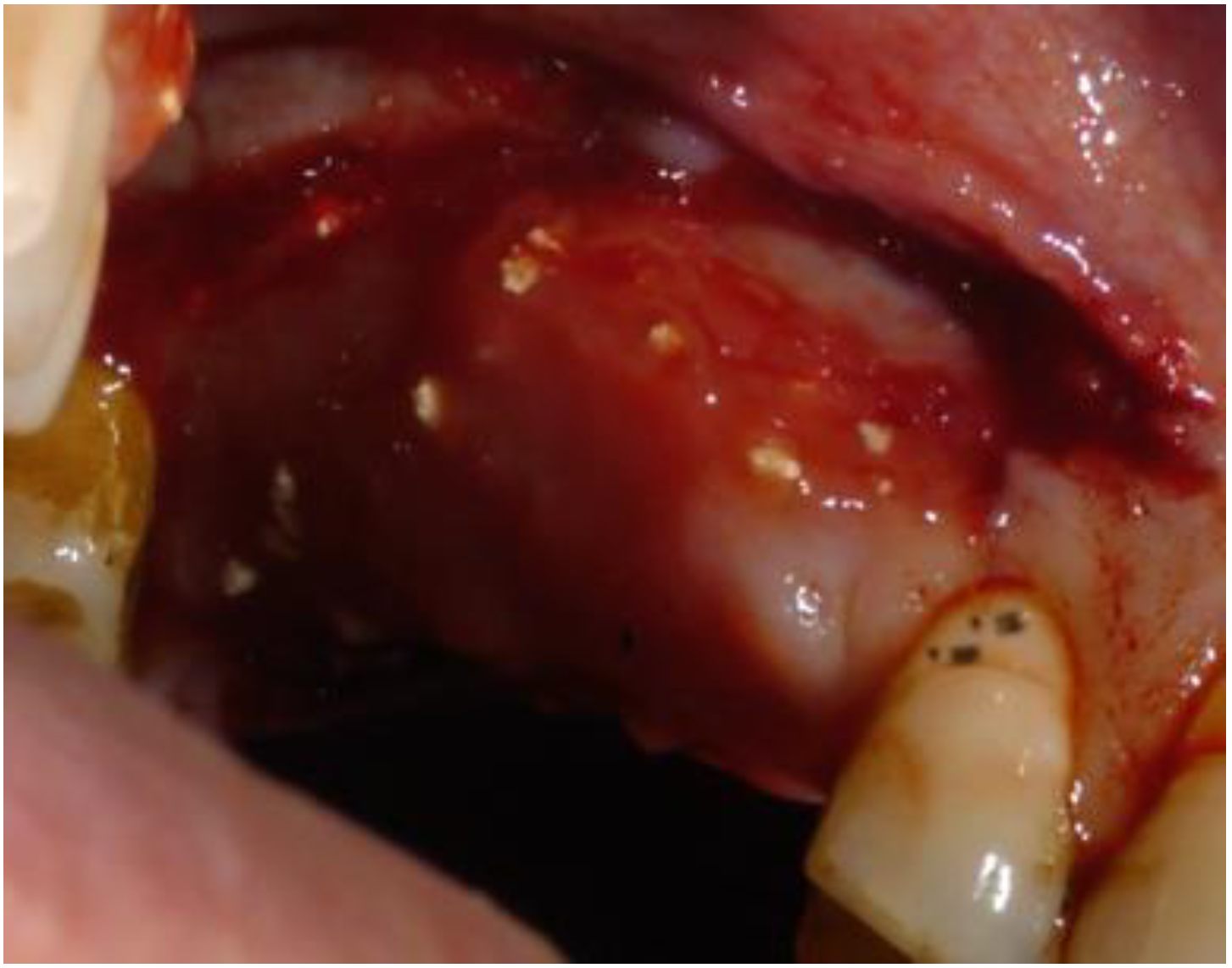
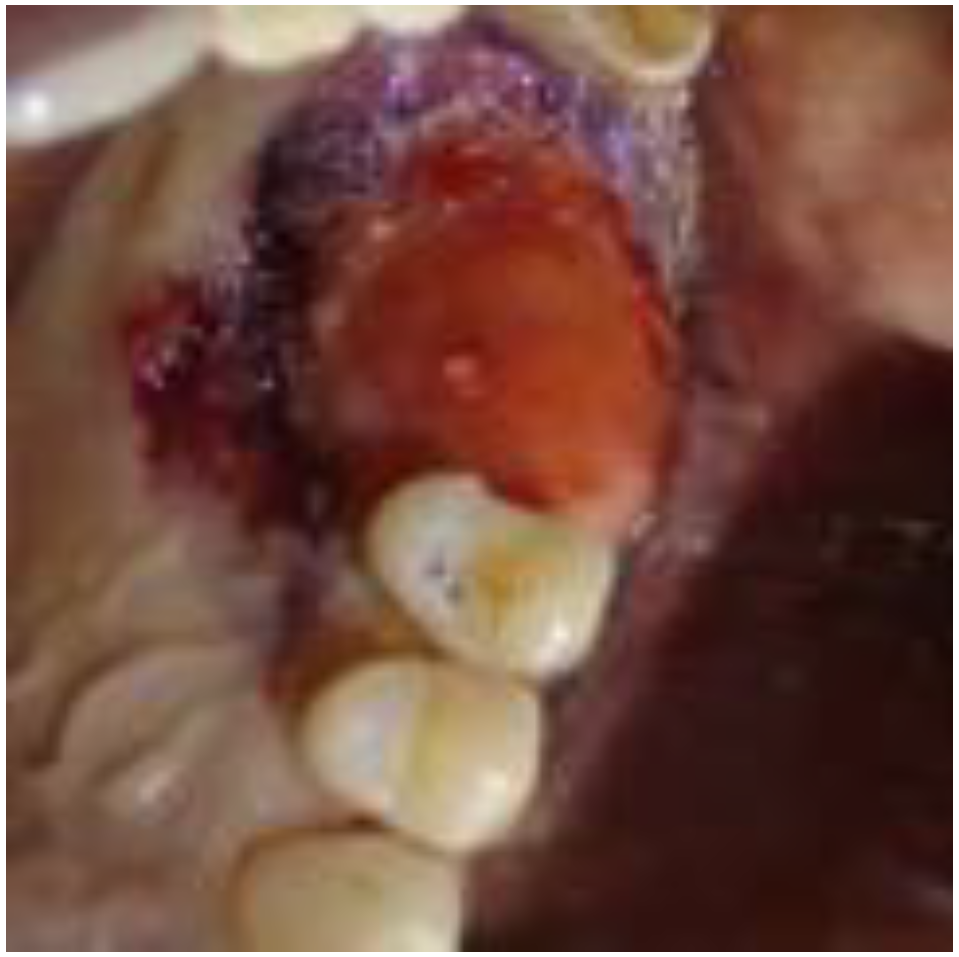
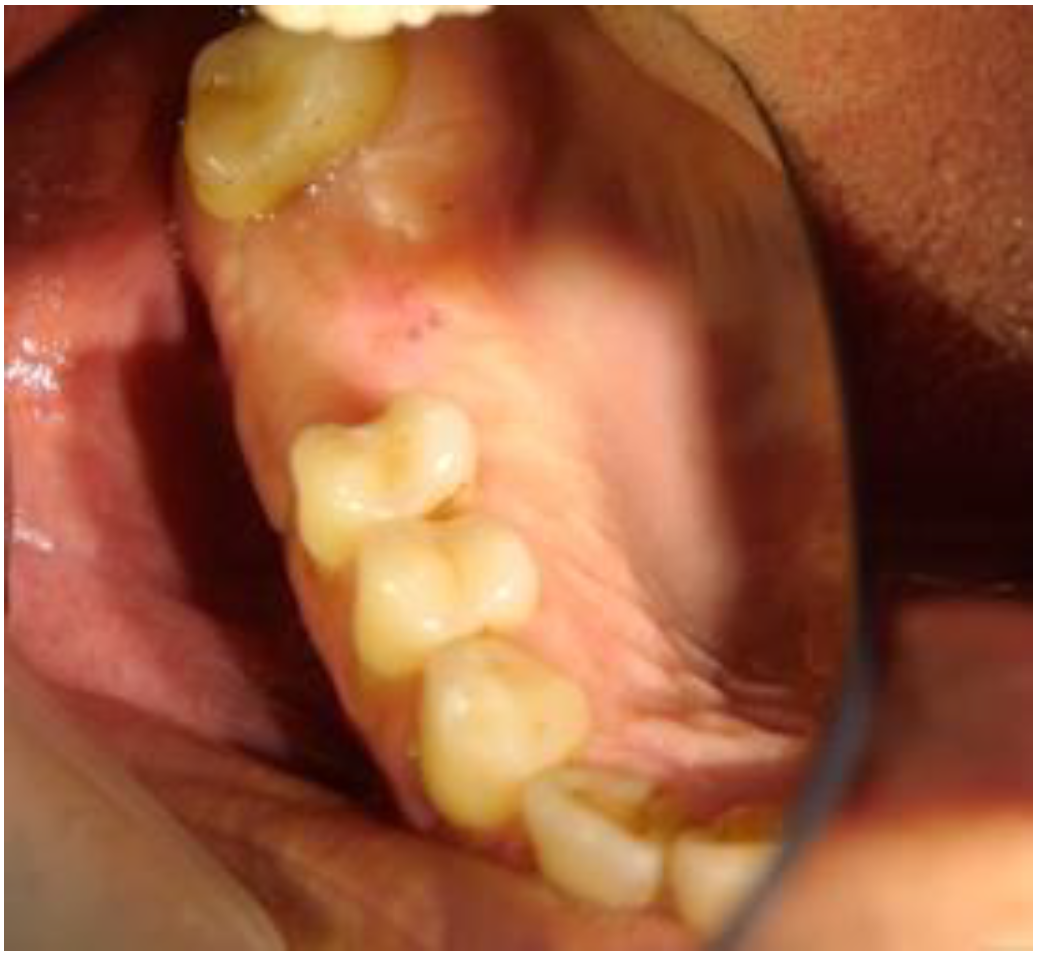
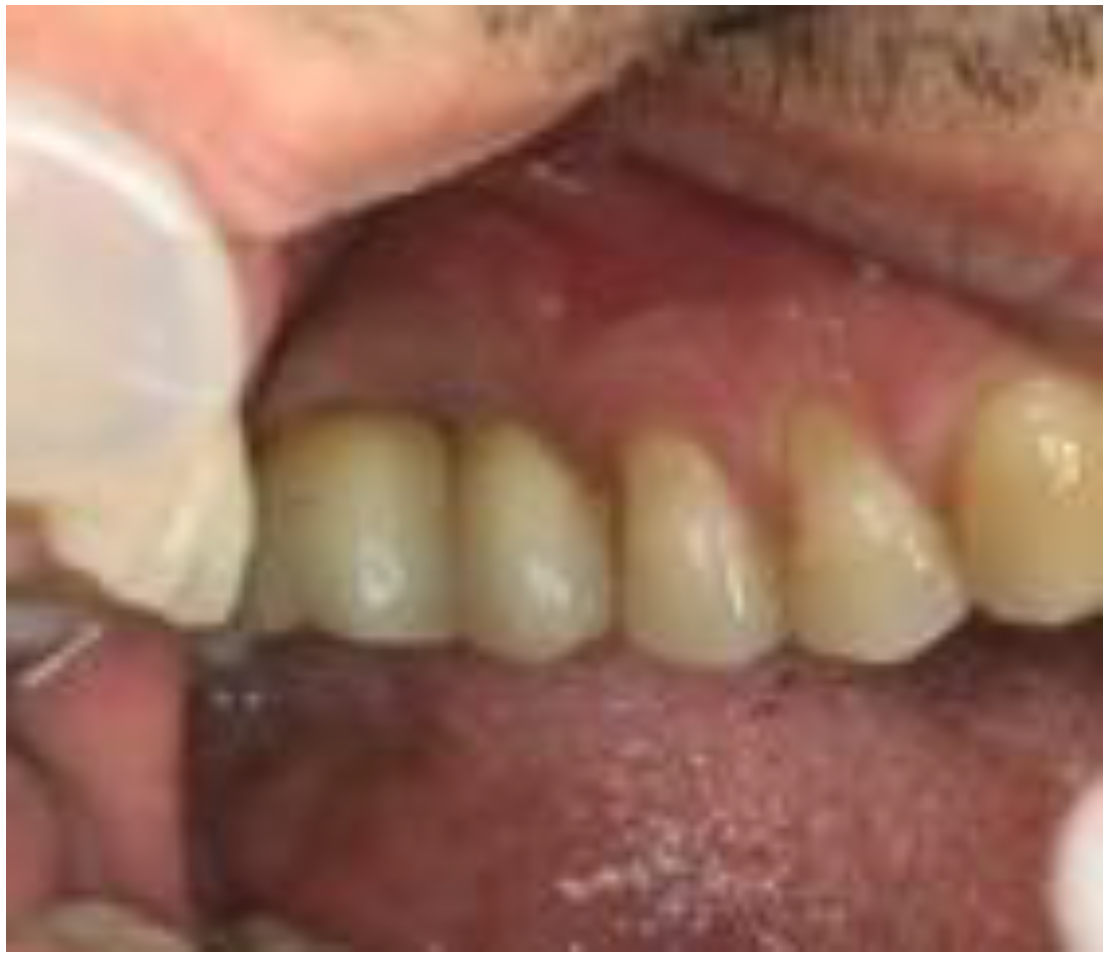
2. Case Report
2.1. Clinical and Surgical Procedures
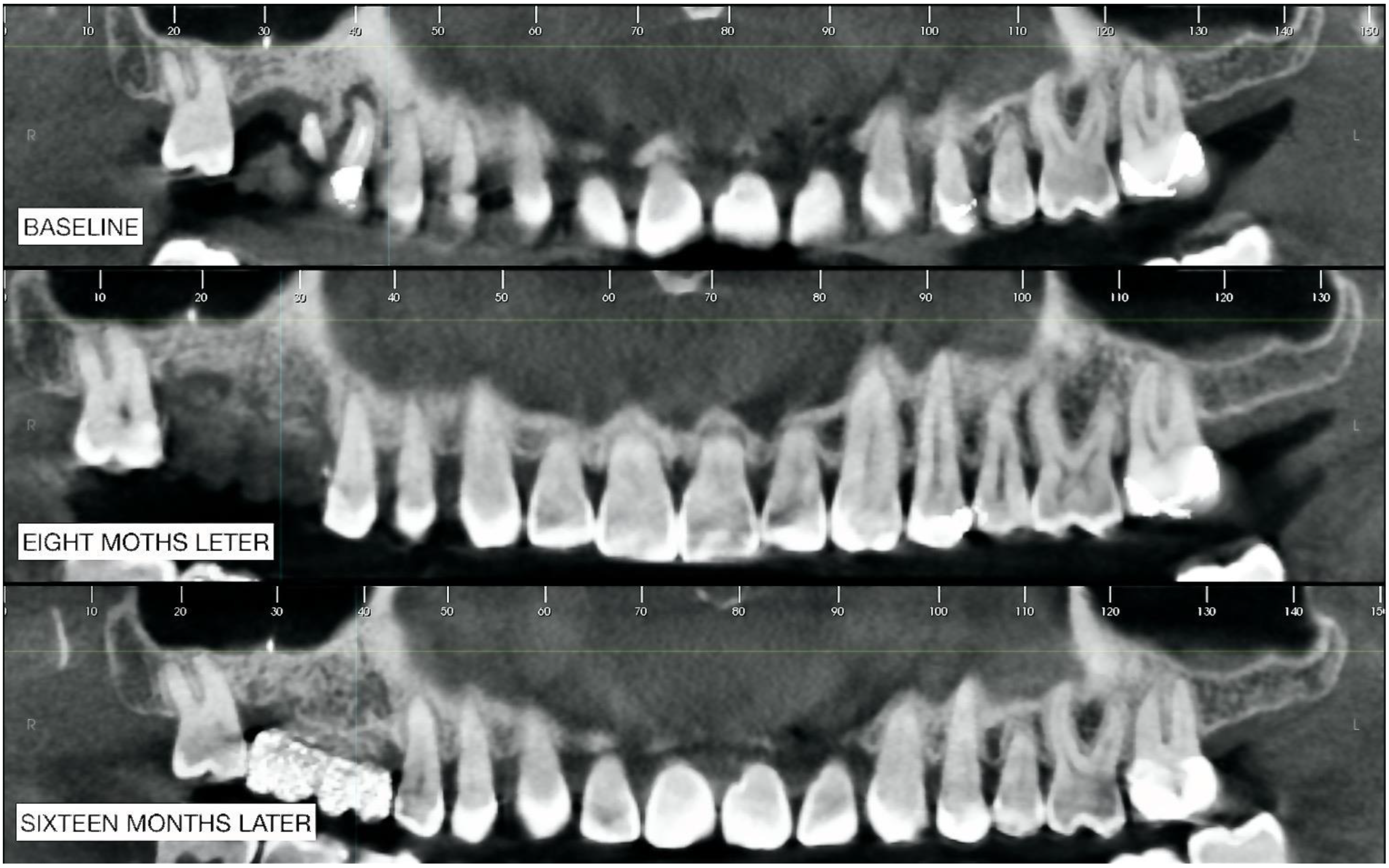
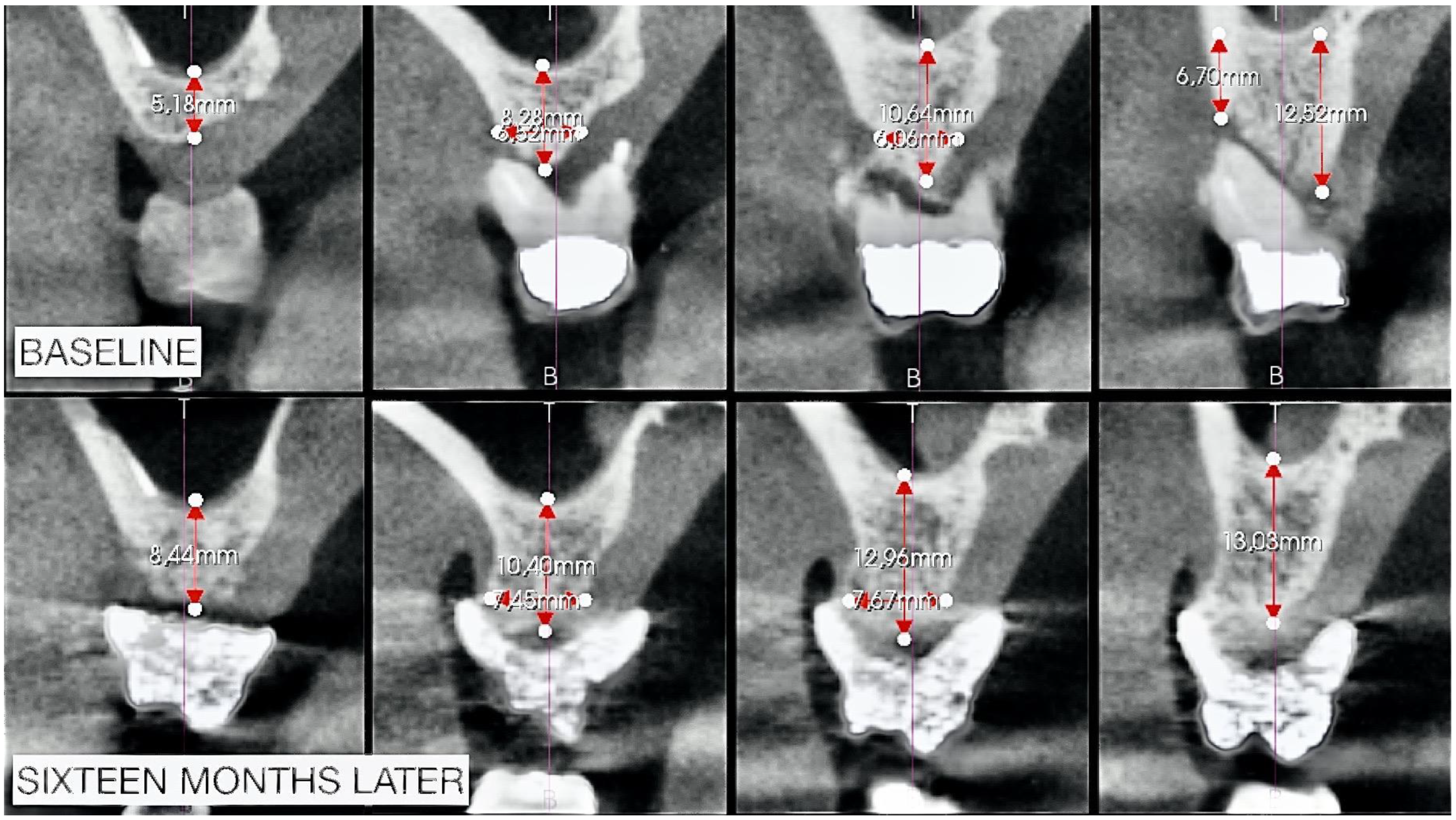
2.2. Radiographic Observations
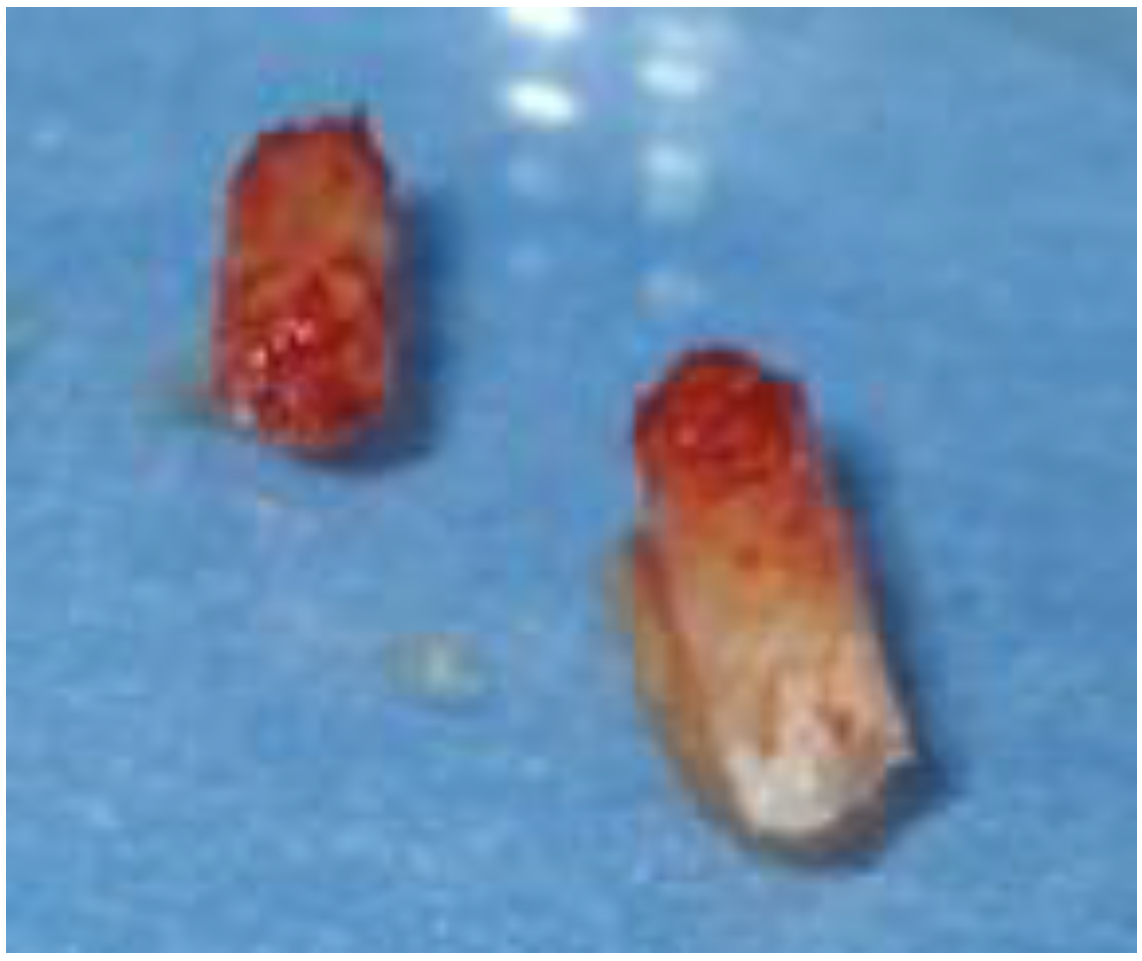
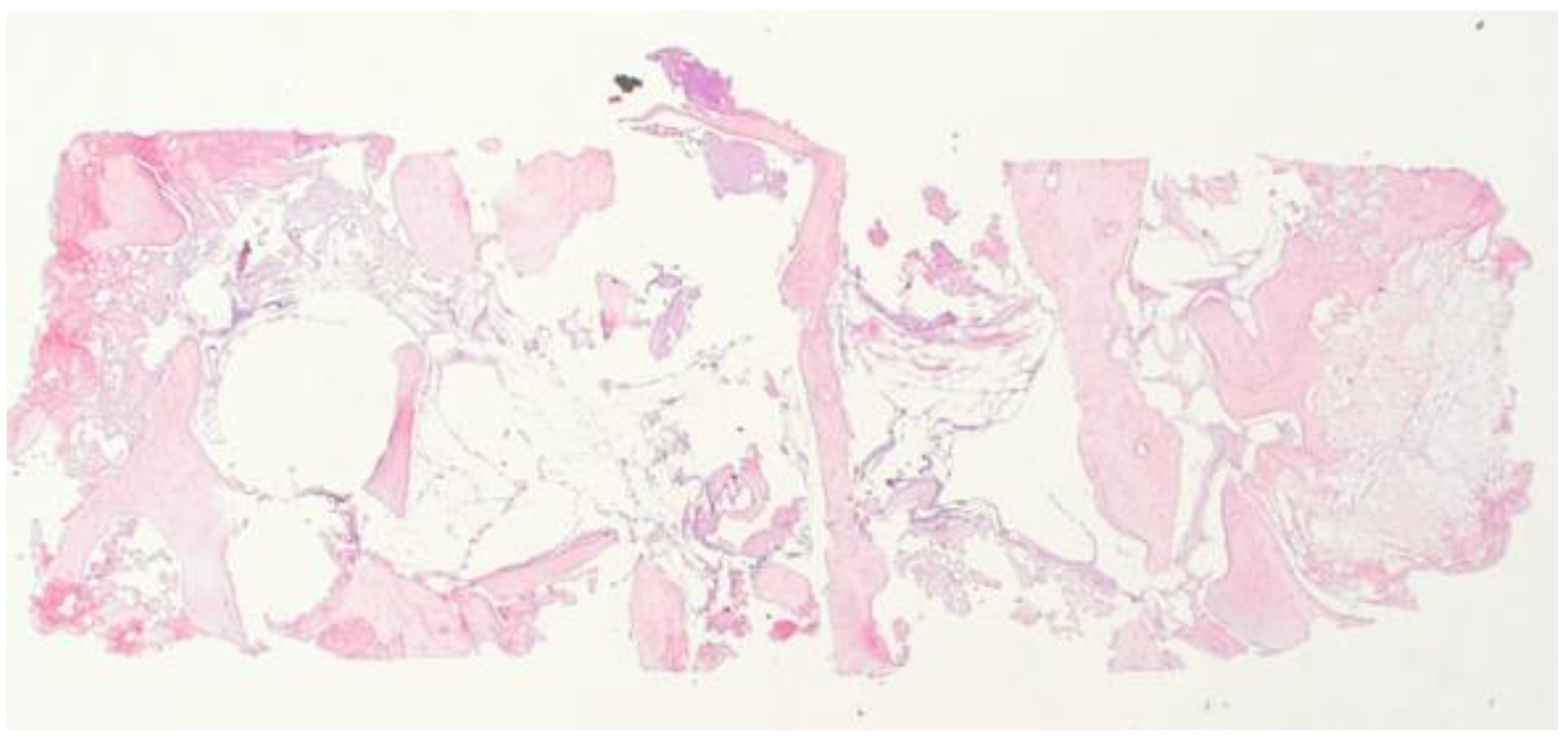
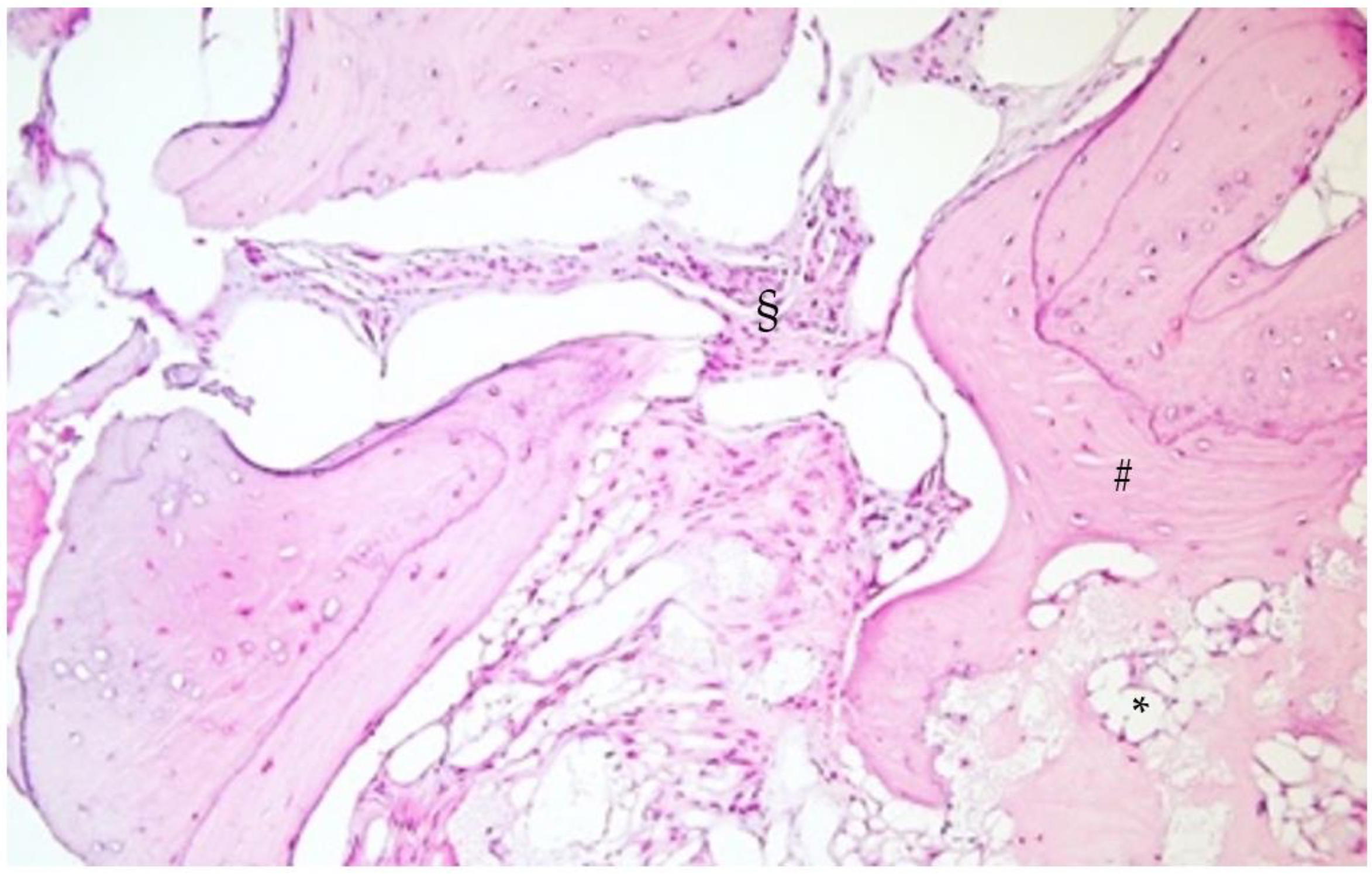
2.3. Histological Analysis
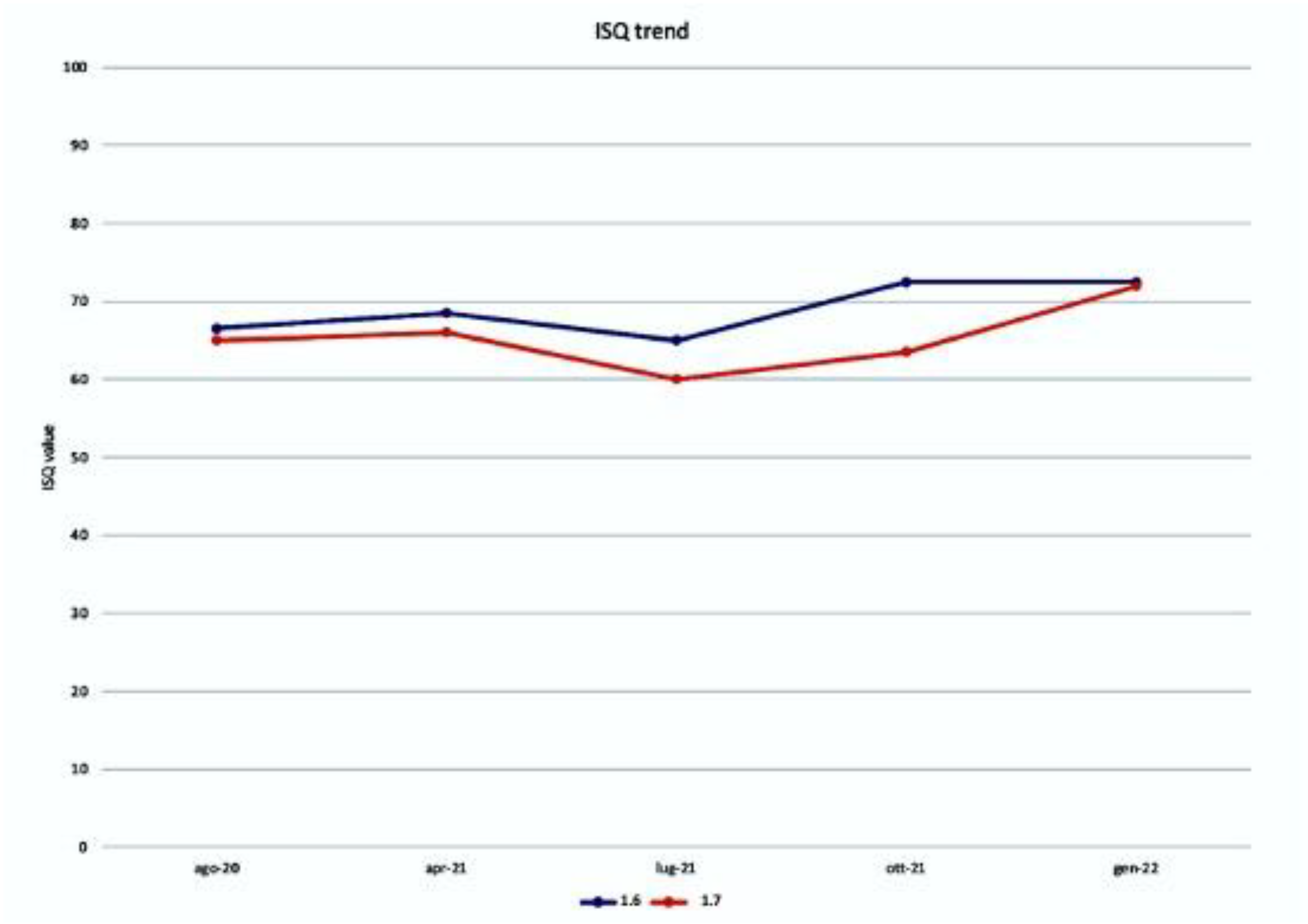
2.4. ISQ Measurements
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elian, N.; Cho, S.; Froum, S.; Smith, R.B.; Tarnow, D.P. A simplified socket classification and repair technique. Pract. Proced. Aesthet. Dent. 2007, 19, 99–104. [Google Scholar] [PubMed]
- Gultekin, B.A.; Bedeloglu, E.; Kose, T.E.; Mijiritsky, E. Comparison of Bone Resorption Rates after Intraoral Block Bone and Guided Bone Regeneration Augmentation for the Reconstruction of Horizontally Deficient Maxillary Alveolar Ridges. BioMed Res. Int. 2016, 2016, 4987437. [Google Scholar] [CrossRef]
- Pistilli, R.; Checchi, V.; Sammartino, G.; Simion, M.; Felice, P. Safe New Approach to the Lingual Flap Management in Man-dibular Augmentation Procedures: The Digitoclastic Technique. Implant Dent. 2017, 26, 790–795. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Ronda, M.; Stacchi, C. A Novel Approach for the Coronal Advancement of the Buccal Flap. Int. J. Periodontics Restor. Dent. 2015, 35, 795–801. [Google Scholar] [CrossRef]
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier membranes: More than the barrier effect? J. Clin. Periodontol. 2019, 46 (Suppl. 21), 103–123. [Google Scholar] [CrossRef]
- Windisch, P.; Martin, A.; Shahbazi, A.; Molnar, B. Reconstruction of horizontovertical alveolar defects. Presentation of a nov-el split-thickness flap design for guided bone regeneration: A case report with 5-year follow-up. Quintessence Int. 2017, 48, 535–547. [Google Scholar] [CrossRef]
- Windisch, P.; Orban, K.; Salvi, G.E.; Sculean, A.; Molnar, B. Vertical-guided bone regeneration with a titanium-reinforced d-PTFE membrane utilizing a novel split-thickness flap design: A prospective case series. Clin. Oral Investig. 2021, 25, 2969–2980. [Google Scholar] [CrossRef]
- Cucchi, A.; Vignudelli, E.; Napolitano, A.; Marchetti, C.; Corinaldesi, G. Evaluation of complication rates and vertical bone gain after guided bone regeneration with non-resorbable membranes versus titanium meshes and resorbable membranes. A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2017, 19, 821–832. [Google Scholar] [CrossRef]
- Jepsen, S.; Schwarz, F.; Cordaro, L.; Derks, J.; Hämmerle, C.H.F.; Heitz-Mayfield, L.J.; Hernández-Alfaro, F.; Meijer, H.J.A.; Naenni, N.; Ortiz-Vigón, A.; et al. Regeneration of alveolar ridge defects. Consensus report of group 4 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46 (Suppl. 21), 277–286. [Google Scholar] [CrossRef]
- Murray, G.; Holden, R.; Roschlau, W. Experimental and clinical study of new growth of bone in a cavity. Am. J. Surg. 1957, 93, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Van Steenberghe, D.; Johansson, C.; Quirynen, M.; Molly, L.; Albrektsson, T.; Naert, I. Bone augmentation by means of a stiff occlusive titanium barrier. Clin. Oral Implants Res. 2003, 14, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Roca-Millan, E.; Jané-Salas, E.; Estrugo-Devesa, A.; López-López, J. Evaluation of bone gain and complication rates after guided bone regeneration with titanium foils: A systematic review. Materials 2020, 13, 5346. [Google Scholar] [CrossRef]
- Bassi, M.A.; Andrisani, C.; Lico, S.; Ormanier, Z.; Ottria, L.; Gargari, M. Guided bone regeneration via a preformed titanium foil: Clinical, histological and histomorphometric outcome of a case series. ORAL Implant. 2016, 9, 164–174. [Google Scholar]
- Perret, F.; Romano, F.; Ferrarotti, F.; Aimetti, M. Occlusive Titanium Barrier for Immediate Bone Augmentation of Severely Resorbed Alveolar Sockets with Secondary Soft Tissue Healing: A 2-Year Case Series. Int. J. Periodontics Restor. Dent. 2019, 39, 97–105. [Google Scholar] [CrossRef]
- Milillo, L.; Cinone, F.; Lo Presti, F.; Lauritano, D.; Petruzzi, M. The Role of Blood Clot in Guided Bone Regeneration: Biological Considerations and Clinical Applications with Titanium Foil. Materials 2021, 14, 6642. [Google Scholar] [CrossRef]
- Molly, L.; Quirynen, M.; Michiels, K.; van Steenberghe, D. Comparison between jaw bone augmentation by means of a stiff occlusive titanium membrane or an autologous hip graft: A retrospective clinical assessment. Clin Oral Implants Res. 2006, 17, 481–487. [Google Scholar] [CrossRef]
- Nyström, E.; Nilson, H.; Gunne, J.; Lundgren, S. A 9–14 year follow-up of onlay bone grafting in the atrophic maxilla. Int. J. Oral Maxillofac. Surg. 2009, 38, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Burchardt, H. The biology of bone graft repair. Clin. Orthop. Relat. Res. 1983, 174, 28–42. [Google Scholar] [CrossRef]
- Widmark, G.; Andersson, B.; Ivanoff, C.J. Mandibular bone graft in the anterior maxilla for single-tooth implants. Presentation of surgical method. Int. J. Oral Maxillofac. Surg. 1997, 26, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Stricker, A.; Jacobs, R.; Maes, F.; Fluegge, T.; Vach, K.; Fleiner, J. Resorption of retromolar bone grafts after alveolar ridge augmentation-volumetric changes after 12 months assessed by CBCT analysis. Int. J. Implant Dent. 2021, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Chiapasco, M.; Casentini, P.; Zaniboni, M. Bone augmentation procedures in implant dentistry. Int. J. Oral Maxillofac. Implants 2009, 24, 237–259. [Google Scholar] [PubMed]
- Chiapasco, M.; Zaniboni, M.; Rimondini, L. Autogenous onlay bone grafts vs. alveolar distraction osteogenesis for the correction of vertically deficient edentulous ridges: A 2-4-year prospective study on humans. Clin. Oral Implants Res. 2007, 18, 432–440. [Google Scholar] [CrossRef]
- Yildirim, M.; Spiekermann, H.; Biesterfeld, S.; Edelhoff, D. Maxillary sinus augmentation using xenogenic bone substitute material Bio-Oss in combination with venous blood. A histologic and histomorphometric study in humans. Clin. Oral Implants Res. 2000, 11, 217–229. [Google Scholar] [CrossRef]
- Traini, T.; Valentini, P.; Iezzi, G.; Piattelli, A. A histologic and histomorphometric evaluation of anorganic bovine bone retrieved 9 years after a sinus augmentation procedure. J. Periodontol. 2007, 78, 955–961. [Google Scholar] [CrossRef]
- Guarnieri, R.; Belleggia, F.; DeVillier, P.; Testarelli, L. Histologic and Histomorphometric Analysis of Bone Regeneration with Bovine Grafting Material after Months of Healing. A Case Report. J. Funct. Biomater. 2018, 9, 48. [Google Scholar] [CrossRef]
- Berglundh, T.; Abrahamsson, I.; Lang, N.P.; Lindhe, J. De novo alveolar bone formation adjacent to endosseous implants. Clin. Oral Implants Res. 2003, 14, 251–262. [Google Scholar] [CrossRef]
- de Calais, C.D.; Bordin, D.; Piattelli, A.; Iezzi, G.; Negretto, A.; Shibli, J.A. Lateral static overload on immediately restored implants decreases the osteocyte index in peri-implant bone: A secondary analysis of a pre-clinical study in dogs. Clin. Oral Investig. 2021, 25, 3297–3303. [Google Scholar] [CrossRef]
- Piattelli, A.; Artese, L.; Penitente, E.; Iaculli, F.; Degidi, M.; Mangano, C.; Shibli, J.A.; Coelho, P.G.; Perrotti, V.; Iezzi, G. Osteocyte density in the peri-implant bone of implants retrieved after different time periods (4 weeks to 27 years). J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 239–243. [Google Scholar] [CrossRef]
- Shah, F.A.; Thomsen, P.; Palmquist, A. A Review of the Impact of Implant Biomaterials on Osteocytes. J. Dent. Res. 2018, 97, 977–986. [Google Scholar] [CrossRef]
- Franchi, M.; Fini, M.; Martini, D.; Orsini, E.; Leonardi, L.; Ruggeri, A.; Giavaresi, G.; Ottani, V. Biological fixation of endosseous implants. Micron 2005, 36, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Meredith, N.; Shagaldi, F.; Alleyne, D.; Sennerby, L.; Cawley, P. The application of resonance frequency measurements to study the stability of titanium implants during healing in the rabbit tibia. Clin. Oral Implants Res. 1997, 8, 234–243. [Google Scholar] [CrossRef]
- Bischof, M.; Nedir, R.; Szmukler-Moncler, S.; Bernard, J.P.; Samson, J. Implant stability measurement of delayed and immediately loaded implants during healing. Clin. Oral Implants Res. 2004, 15, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Milillo, L.; Fiandaca, C.; Giannoulis, F.; Ottria, L.; Lucchese, A.; Silvestre, F.; Petruzzi, M. Immediate vs non-immediate loading post-extractive implants: A comparative study of implant stability quotient (ISQ). Oral Implantol. 2016, 9, 123–131. [Google Scholar] [CrossRef]
- Janyaphadungpong, R.; Serichetaphongse, P.; Pimkhaokham, A. A Clinical Resonance Frequency Analysis of Implants Placed at Dehiscence-type Defects with Simultaneous Guided Bone Regeneration During Early Healing. Int. J. Oral Maxillofac. Implants 2019, 34, 772–777. [Google Scholar] [CrossRef]
- Farias, D.; Caceres, F.; Sanz, A.; Olate, S. Horizontal Bone Augmentation in the Posterior Atrophic Mandible and Dental Implant Stability Using the Tenting Screw Technique. Int. J. Periodontics Restor. Dent. 2021, 41, e147–e155. [Google Scholar] [CrossRef]
- Vallecillo-Rivas, M.; Reyes-Botella, C.; Vallecillo, C.; Lisbona-González, M.J.; Vallecillo-Capilla, M.; Olmedo-Gaya, M.V. Comparison of Implant Stability between Regenerated and Non-Regenerated Bone. A Prospective Cohort Study. J. Clin. Med. 2021, 10, 3220. [Google Scholar] [CrossRef] [PubMed]
- Deli, G.; Petrone, V.; De Risi, V.; Tadic, D.; Zafiropoulos, G.G. Longitudinal implant stability measurements based on resonance frequency analysis after placement in healed or regenerated bone. J. Oral Implantol. 2014, 40, 438–447. [Google Scholar] [CrossRef]
- Chipaila, N.; Marini, R.; Sfasciotti, G.L.; Cielo, A.; Bonanome, L.; Monaco, A. Graftless sinus augmentation technique with contextual placement of implants: A case report. J. Med. Case Rep. 2014, 8, 437. [Google Scholar] [CrossRef] [PubMed]
- Gelețu, G.L.; Burlacu, A.; Murariu, A.; Andrian, S.; Golovcencu, L.; Baciu, E.-R.; Maftei, G.; Onica, N. Customized 3D-Printed Titanium Mesh Developed for an Aesthetic Zone to Regenerate a Complex Bone Defect Resulting after a Deficient Odontectomy: A Case Report. Medicina 2022, 58, 1192. [Google Scholar] [CrossRef] [PubMed]
- Aceves-Argemí, R.; Roca-Millan, E.; González-Navarro, B.; Marí-Roig, A.; Velasco-Ortega, E.; López-López, J. Titanium Meshes in Guided Bone Regeneration: A Systematic Review. Coatings 2021, 11, 316. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milillo, L.; Petruzzi, M. Guided Bone Regeneration with Occlusive Titanium Barrier: A Case Report and Clinical Considerations. Biomimetics 2023, 8, 106. https://doi.org/10.3390/biomimetics8010106
Milillo L, Petruzzi M. Guided Bone Regeneration with Occlusive Titanium Barrier: A Case Report and Clinical Considerations. Biomimetics. 2023; 8(1):106. https://doi.org/10.3390/biomimetics8010106
Chicago/Turabian StyleMilillo, Lucio, and Massimo Petruzzi. 2023. "Guided Bone Regeneration with Occlusive Titanium Barrier: A Case Report and Clinical Considerations" Biomimetics 8, no. 1: 106. https://doi.org/10.3390/biomimetics8010106
APA StyleMilillo, L., & Petruzzi, M. (2023). Guided Bone Regeneration with Occlusive Titanium Barrier: A Case Report and Clinical Considerations. Biomimetics, 8(1), 106. https://doi.org/10.3390/biomimetics8010106






