Wnt/β-Catenin Signaling Inhibits Osteogenic Differentiation in Human Periodontal Ligament Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Western Blotting
2.4. Immunofluorescence Microscopy
2.5. Alkaline Phosphatase Staining
2.6. Alkaline Phosphatase Activity
2.7. Alizarin Red S Staining
2.8. Quantitative Real-Time PCR
2.9. Statistical Analysis
3. Results
3.1. Activation of Wnt/β-Catenin Signaling by Wnt-3a Stimulation in HPLFs
3.2. Effect of Wnt/β-Catenin Signaling on the Production of ALP, a Marker of Early Osteogenic Differentiation
3.3. Effect of Wnt/β-Catenin Signaling on the Formation of Calcified Nodules
3.4. Effect of Wnt/β-Catenin Signaling on Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Maeda, T.; Hayashi, S. Morphological basis of periodontal nerve endings. Kaibogaku Zasshi J. Anat. 2000, 75, 377–380. [Google Scholar]
- Li, M.; Zhang, C.; Yang, Y. Effects of mechanical forces on osteogenesis and osteoclastogenesis in human periodontal ligament fibroblasts: A systematic review of in vitro studies. Bone Jt. Res. 2019, 8, 19–31. [Google Scholar] [CrossRef]
- Raju, R.; Oshima, M.; Inoue, M.; Morita, T.; Huijiao, Y.; Waskitho, A.; Baba, O.; Inoue, M.; Matsuka, Y. Three-dimensional periodontal tissue regeneration using a bone-ligament complex cell sheet. Sci. Rep. 2020, 10, 1656. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.-M.G.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Seo, B.-M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lozano, F.J.; Bueno, C.R.; Insausti, C.L.; Meseguer-Olmo, L.; Ramírez, M.C.; Blanquer, M.B.; Marín, N.; Martinez, S.; Moraleda, J.M. Mesenchymal stem cells derived from dental tissues. Int. Endod. J. 2011, 44, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ruan, J.; Weir, M.D.; Ren, K.; Schneider, A.; Wang, P.; Oates, T.W.; Chang, X.; Xu, H.H.K. Periodontal Bone-Ligament-Cementum Regeneration via Scaffolds and Stem Cells. Cells 2019, 8, 537. [Google Scholar] [CrossRef]
- Yu, B.; Li, Q.; Zhou, M. LPS-induced upregulation of the TLR4 signaling pathway inhibits osteogenic differentiation of human periodontal ligament stem cells under inflammatory conditions. Int. J. Mol. Med. 2019, 43, 2341–2351. [Google Scholar] [CrossRef]
- Li, J.; Zhang, F.; Zhang, N.; Geng, X.; Meng, C.; Wang, X.; Yang, Y. Osteogenic capacity and cytotherapeutic potential of periodontal ligament cells for periodontal regeneration in vitro and in vivo. PeerJ 2019, 7, e6589. [Google Scholar] [CrossRef]
- Koda, N.; Sato, T.; Shinohara, M.; Ichinose, S.; Ito, Y.; Nakamichi, R.; Kayama, T.; Kataoka, K.; Suzuki, H.; Moriyama, K.; et al. The transcription factor mohawk homeobox regulates homeostasis of the periodontal ligament. Development 2017, 144, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, A.; Kawatsu, M.; Yoshimoto, Y.; Kawamoto, T.; Seiryu, M.; Takano-Yamamoto, T.; Hiraki, Y.; Shukunami, C. Scleraxis and osterix antagonistically regulate tensile force-responsive remodeling of the periodontal ligament and alveolar bone. Development 2015, 142, 787–796. [Google Scholar] [CrossRef]
- Hasegawa, D.; Wada, N.; Maeda, H.; Yoshida, S.; Mitarai, H.; Tomokiyo, A.; Monnouchi, S.; Hamano, S.; Yuda, A.; Akamine, A. Wnt5a Induces Collagen Production by Human Periodontal Ligament Cells Through TGFβ1-Mediated Upregulation of Periostin Expression. J. Cell. Physiol. 2015, 230, 2647–2660. [Google Scholar] [CrossRef]
- Norris, R.A.; Damon, B.; Mironov, V.; Kasyanov, V.; Ramamurthi, A.; Moreno-Rodriguez, R.; Trusk, T.; Potts, J.D.; Goodwin, R.L.; Davis, J.; et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J. Cell. Biochem. 2007, 101, 695–711. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Ohkawara, B.; Sakai, T.; Ito, M.; Masuda, A.; Ishiguro, N.; Shukunami, C.; Docheva, D.; Ohno, K. Wnt/β-catenin signaling suppresses expressions of Scx, Mkx, and Tnmd in tendon-derived cells. PLoS ONE 2017, 12, e0182051. [Google Scholar] [CrossRef]
- Qiu, W.; Chen, L.; Kassem, M. Activation of non-canonical Wnt/JNK pathway by Wnt3a is associated with differentiation fate determination of human bone marrow stromal (mesenchymal) stem cells. Biochem. Biophys. Res. Commun. 2011, 413, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Miyabara, S.; Yuda, Y.; Kasashima, Y.; Kuwano, A.; Arai, K. Regulation of Tenomodulin Expression Via Wnt/β-catenin Signaling in Equine Bone Marrow-derived Mesenchymal Stem Cells. J. Equine Sci. 2014, 25, 7–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, Y.; Ge, Y.; Chen, G.; Yan, Z.; Yu, M.; Feng, L.; Jiang, Z.; Guo, W.; Tian, W. Hertwig’s epithelial root sheath cells regulate osteogenic differentiation of dental follicle cells through the Wnt pathway. Bone 2014, 63, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, E.; Koshikawa, Y.; Kanaya, S.; Tsuchiya, M.; Tamura, M.; Somerman, M.J.; Shimauchi, H. Wnt signaling inhibits cementoblast differentiation and promotes proliferation. Bone 2009, 44, 805–812. [Google Scholar] [CrossRef]
- Krieghoff, E.; Behrens, J.; Mayr, B. Nucleo-cytoplasmic distribution of β-catenin is regulated by retention. J. Cell Sci. 2006, 119, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Cao, Y.; Yang, H.; Han, N.; Zhu, X.; Fan, Z.; Du, J.; Zhang, F. CB1 enhanced the osteo/dentinogenic differentiation ability of periodontal ligament stem cells via p38 MAPK and JNK in an inflammatory environment. Cell Prolif. 2019, 52, e12691. [Google Scholar] [CrossRef]
- Ibarra, B.; Machen, C.; Atit, R. Wnt-Dependent Activation of ERK Mediates Repression of Chondrocyte Fate during Calvarial Development. J. Dev. Biol. 2021, 9, 23. [Google Scholar] [CrossRef]
- Brunt, K.R.; Zhang, Y.; Mihic, A.; Li, M.; Li, S.-H.; Xue, P.; Zhang, W.; Basmaji, S.; Tsang, K.; Weisel, R.D.; et al. Role of WNT/β-Catenin Signaling in Rejuvenating Myogenic Differentiation of Aged Mesenchymal Stem Cells from Cardiac Patients. Am. J. Pathol. 2012, 181, 2067–2078. [Google Scholar] [CrossRef] [PubMed]
- Hessle, L.; Johnson, K.A.; Anderson, H.C.; Narisawa, S.; Sali, A.; Goding, J.W.; Terkeltaub, R.; Millán, J.L. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc. Natl. Acad. Sci. USA 2002, 99, 9445–9449. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Choukroun, J.; Ghanaati, S.; Miron, R.J. Effects of an injectable platelet-rich fibrin on osteoblast behavior and bone tissue formation in comparison to platelet-rich plasma. Platelets 2018, 29, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.W.; Kim, S.H.; Han, Y. Discovery and development of berberine derivatives as stimulants of osteoblast differentiation. Biochem. Biophys. Res. Commun. 2020, 527, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Sudo, H.; Kodama, H.A.; Amagai, Y.; Yamamoto, S.; Kasai, S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J. Cell Biol. 1983, 96, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Kuroiwa, M.; Chihara, K.; Higashi, S. Electron microscopic studies on Sharpey’s fibers in the alveolar bone of rat molars. Kaibogaku Zasshi J. Anat. 1994, 69, 776–782. [Google Scholar]
- Choi, J.W.; Arai, C.; Ishikawa, M.; Shimoda, S.; Nakamura, Y. Fiber system degradation, and periostin and connective tissue growth factor level reduction, in the periodontal ligament of teeth in the absence of masticatory load. J. Periodontal Res. 2011, 46, 513–521. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lee, E.-H.; Park, S.-Y.; Kim, J.-E. Induction of fibrillin-2 and periostin expression in Osterix-knockdown MC3T3-E1 cells. Gene 2017, 596, 123–129. [Google Scholar] [CrossRef]
- Wilde, J.; Yokozeki, M.; Terai, K.; Kudo, A.; Moriyama, K. The divergent expression of periostin mRNA in the periodontal ligament during experimental tooth movement. Cell Tissue Res. 2003, 312, 345–351. [Google Scholar] [CrossRef]
- Yokoya, F.; Imamoto, N.; Tachibana, T.; Yoneda, Y. β-Catenin Can Be Transported into the Nucleus in a Ran-unassisted Manner. Mol. Biol. Cell 1999, 10, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Koike, M.; Kose, S.; Furuta, M.; Taniguchi, N.; Yokoya, F.; Yoneda, Y.; Imamoto, N. β-Catenin Shows an Overlapping Sequence Requirement but Distinct Molecular Interactions for Its Bidirectional Passage through Nuclear Pores. J. Biol. Chem. 2004, 279, 34038–34047. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.H.; Liu, B.; Cheng, D.; Williams, B.O.; Mah, S.J.; Helms, J.A. Wnt signaling regulates homeostasis of the periodontal ligament. J. Periodontal Res. 2014, 49, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhang, Y.; Li, D.; Zhang, W.; Zhang, D.; Xu, X. Analyses of key mRNAs and lncRNAs for different osteo-differentiation potentials of periodontal ligament stem cell and gingival mesenchymal stem cell. J. Cell. Mol. Med. 2021, 25, 6217–6231. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hutmacher, D.W.; Sae-Lim, V.; Zhou, Z.; Woodruff, M.; Lim, T.M. Osteogenic and Adipogenic Induction Potential of Human Periodontal Cells. J. Periodontol. 2008, 79, 525–534. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Dai, J.; Wang, X.; Shen, S.G. Overexpression of Dlx2 enhances osteogenic differentiation of BMSCs and MC3T3-E1 cells via direct upregulation of Osteocalcin and Alp. Int. J. Oral Sci. 2019, 11, 12. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Tan, L. Downregulation of lncRNA DANCR promotes osteogenic differentiation of periodontal ligament stem cells. BMC Dev. Biol. 2020, 20, 2. [Google Scholar] [CrossRef]
- Soda, M.; Saitoh, I.; Murakami, T.; Inada, E.; Iwase, Y.; Noguchi, H.; Shibasaki, S.; Kurosawa, M.; Sawami, T.; Terunuma, M.; et al. Repeated human deciduous tooth-derived dental pulp cell reprogramming factor transfection yields multipotent intermediate cells with enhanced iPS cell formation capability. Sci. Rep. 2019, 9, 1490. [Google Scholar] [CrossRef]
- Zhang, P.; Chang, W.-H.; Fong, B.; Gao, F.; Liu, C.; Al Alam, D.; Bellusci, S.; Lu, W. Regulation of Induced Pluripotent Stem (iPS) Cell Induction by Wnt/β-Catenin Signaling. J. Biol. Chem. 2014, 289, 9221–9232. [Google Scholar] [CrossRef]
- Hong, H.; Pischon, N.; Santana, R.; Palamakumbura, A.H.; Chase, H.B.; Gantz, D.; Guo, Y.; Uzel, M.I.; Ma, D.; Trackman, P.C. A role for lysyl oxidase regulation in the control of normal collagen deposition in differentiating osteoblast cultures. J. Cell. Physiol. 2004, 200, 53–62. [Google Scholar] [CrossRef]
- Aaron, J.E. Periosteal Sharpey’s fibers: A novel bone matrix regulatory system? Front. Endocrinol. 2012, 3, 98. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, T.; Tokutomi, K.; Sasaki, M.; Katafuchi, M.; Mizumachi, E.; Sato, H. Distinct Characteristics of Mandibular Bone Collagen Relative to Long Bone Collagen: Relevance to Clinical Dentistry. BioMed Res. Int. 2014, 2014, 769414. [Google Scholar] [CrossRef] [PubMed]
- Almalki, S.G.; Agrawal, D.K. Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation 2016, 92, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.S.; Cabral, J.M.; da Silva, C.L.; Vashishth, D. Synergistic effect of extracellularly supplemented osteopontin and osteocalcin on stem cell proliferation, osteogenic differentiation, and angiogenic properties. J. Cell. Biochem. 2019, 120, 6555–6569. [Google Scholar] [CrossRef]
- Komori, T.; Yagi, H.; Nomura, S.; Yamaguchi, A.; Sasaki, K.; Deguchi, K.; Shimizu, Y.; Bronson, R.T.; Gao, Y.H.; Inada, M.; et al. Targeted Disruption of Cbfa1 Results in a Complete Lack of Bone Formation owing to Maturational Arrest of Osteoblasts. Cell 1997, 89, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.N.; Hwang, H.-S.; Oh, S.-H.; Roshanzadeh, A.; Kim, J.-W.; Song, J.H.; Kim, E.-S.; Koh, J.-T. Elevated extracellular calcium ions promote proliferation and migration of mesenchymal stem cells via increasing osteopontin expression. Exp. Mol. Med. 2018, 50, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rittling, S.R.; Matsumoto, H.N.; McKee, M.D.; Nanci, A.; Novick, K.E.; Kowalski, A.J.; Noda, M.; Denhardt, D.T.; An, X.-R. Mice Lacking Osteopontin Show Normal Development and Bone Structure but Display Altered Osteoclast Formation In Vitro. J. Bone Miner. Res. 1998, 13, 1101–1111. [Google Scholar] [CrossRef]
- Bailey, S.; Karsenty, G.; Gundberg, C.; Vashishth, D. Osteocalcin and osteopontin influence bone morphology and mechanical properties. Ann. N. Y. Acad. Sci. 2017, 1409, 79–84. [Google Scholar] [CrossRef]
- Uribe-Etxebarria, V.; García-Gallastegui, P.; Pérez-Garrastachu, M.; Casado-Andrés, M.; Irastorza, I.; Unda, F.; Ibarretxe, G.; Subirán, N. Wnt-3a Induces Epigenetic Remodeling in Human Dental Pulp Stem Cells. Cells 2020, 9, 652. [Google Scholar] [CrossRef]
- Rolph, D.N.; Deb, M.; Kanji, S.; Greene, C.J.; Das, M.; Joseph, M.; Aggarwal, R.; Leblebicioglu, B.; Das, H. Ferutinin directs dental pulp-derived stem cells towards the osteogenic lineage by epigenetically regulating canonical Wnt signaling. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165314. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Deng, F.; Huang, E.; Yan, Z.; Wang, Z.; Deng, Y.; Zhang, Q.; Zhang, Z.; Ye, J.; et al. Canonical Wnt signaling acts synergistically on BMP9-induced osteo/odontoblastic differentiation of stem cells of dental apical papilla (SCAPs). Biomaterials 2015, 39, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Arzate, H.; Zeichner-David, M.; Mercado-Celis, G. Cementum proteins: Role in cementogenesis, biomineralization, periodontium formation and regeneration. Periodontology 2000 2015, 67, 211–233. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-H.; Zhang, X.-J.; Dang, N.-N.; Ma, Z.-F.; Xu, L.; Wu, J.-J.; Sun, Y.-J.; Duan, Y.-Z.; Lin, Z.; Jin, Y. Apical tooth germ cell-conditioned medium enhances the differentiation of periodontal ligament stem cells into cementum/periodontal ligament-like tissues. J. Periodontal Res. 2009, 44, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, P.; Yu, Z.; Tran, Q.T.; Bhatti, F.-U.; Zhu, X.; Huang, G.T.-J. Cementogenic genes in human periodontal ligament stem cells are downregulated in response to osteogenic stimulation while upregulated by vitamin C treatment. Cell Tissue Res. 2017, 368, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Komaki, M.; Iwasaki, K.; Arzate, H.; Narayanan, A.S.; Izumi, Y.; Morita, I. Cementum protein 1 (CEMP1) induces a cementoblastic phenotype and reduces osteoblastic differentiation in periodontal ligament cells. J. Cell. Physiol. 2012, 227, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Kadokura, H.; Yamazaki, T.; Masuda, Y.; Kato, Y.; Hasegawa, A.; Sakagami, H.; Yokose, S. Establishment of a Primary Culture System of Human Periodontal Ligament Cells that Differentiate into Cementum Protein 1-expressing Cementoblast-like Cells. In Vivo 2019, 33, 349–352. [Google Scholar] [CrossRef]
- Du, J.; Li, M. Functions of Periostin in dental tissues and its role in periodontal tissues’ regeneration. Cell. Mol. Life Sci. 2017, 74, 4279–4286. [Google Scholar] [CrossRef]
- Afanador, E.; Yokozeki, M.; Oba, Y.; Kitase, Y.; Takahashi, T.; Kudo, A.; Moriyama, K. Messenger RNA expression of periostin and Twist transiently decrease by occlusal hypofunction in mouse periodontal ligament. Arch. Oral Biol. 2005, 50, 1023–1031. [Google Scholar] [CrossRef]
- Xu, H.-Y.; Nie, E.-M.; Deng, G.; Lai, L.-Z.; Sun, F.-Y.; Tian, H.; Fang, F.-C.; Zou, Y.-G.; Wu, B.-L.; Ou-Yang, J. Periostin is essential for periodontal ligament remodeling during orthodontic treatment. Mol. Med. Rep. 2017, 15, 1800–1806. [Google Scholar] [CrossRef][Green Version]
- Yoshimoto, Y.; Takimoto, A.; Watanabe, H.; Hiraki, Y.; Kondoh, G.; Shukunami, C. Scleraxis is required for maturation of tissue domains for proper integration of the musculoskeletal system. Sci. Rep. 2017, 7, 45010. [Google Scholar] [CrossRef]
- Huang, A.H.; Watson, S.S.; Wang, L.; Baker, B.M.; Akiyama, H.; Brigande, J.V.; Schweitzer, R. Requirement for Scleraxis in the recruitment of mesenchymal progenitors during embryonic tendon elongation. Development 2019, 146, dev182782. [Google Scholar] [CrossRef] [PubMed]
- Kawatsu, M.; Takeshita, N.; Takimoto, A.; Yoshimoto, Y.; Seiryu, M.; Ito, A.; Kimura, S.; Kawamoto, T.; Hiraki, Y.; Shukunami, C.; et al. Scleraxis upregulated by transforming growth factor-β1 signaling inhibits tension-induced osteoblast differentiation of priodontal ligament cells via ephrin A2. Bone 2021, 149, 115969. [Google Scholar] [CrossRef] [PubMed]
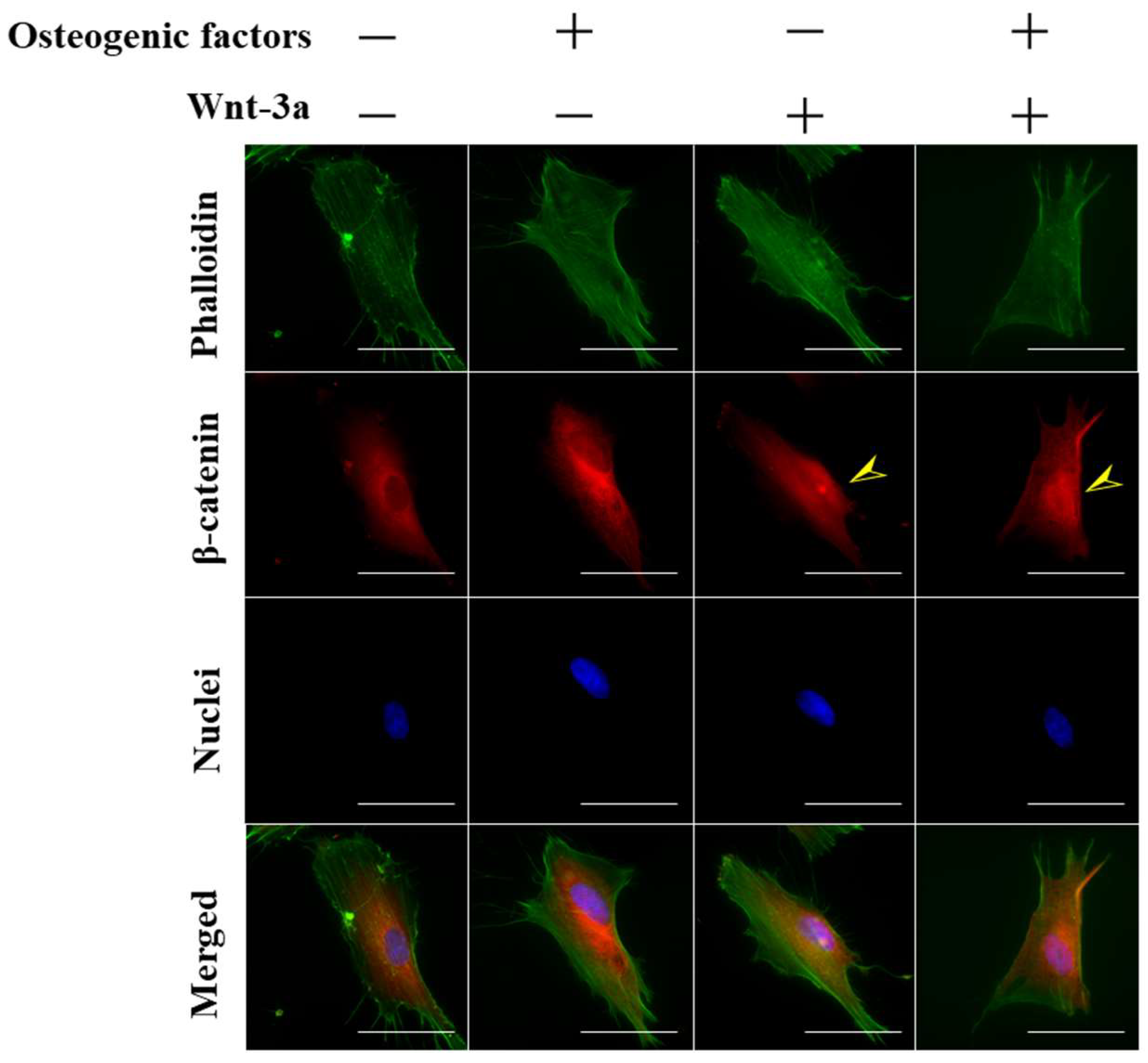
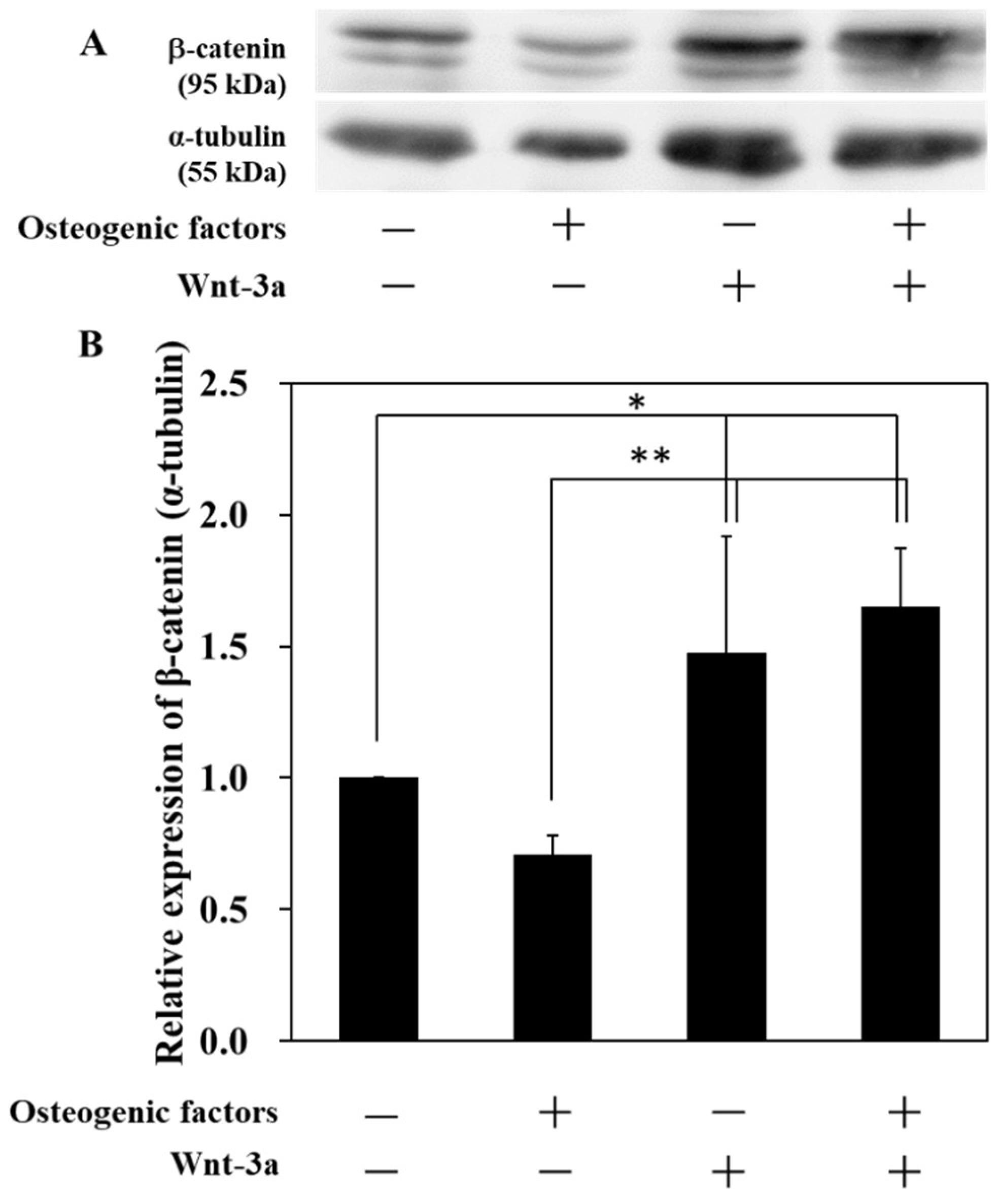
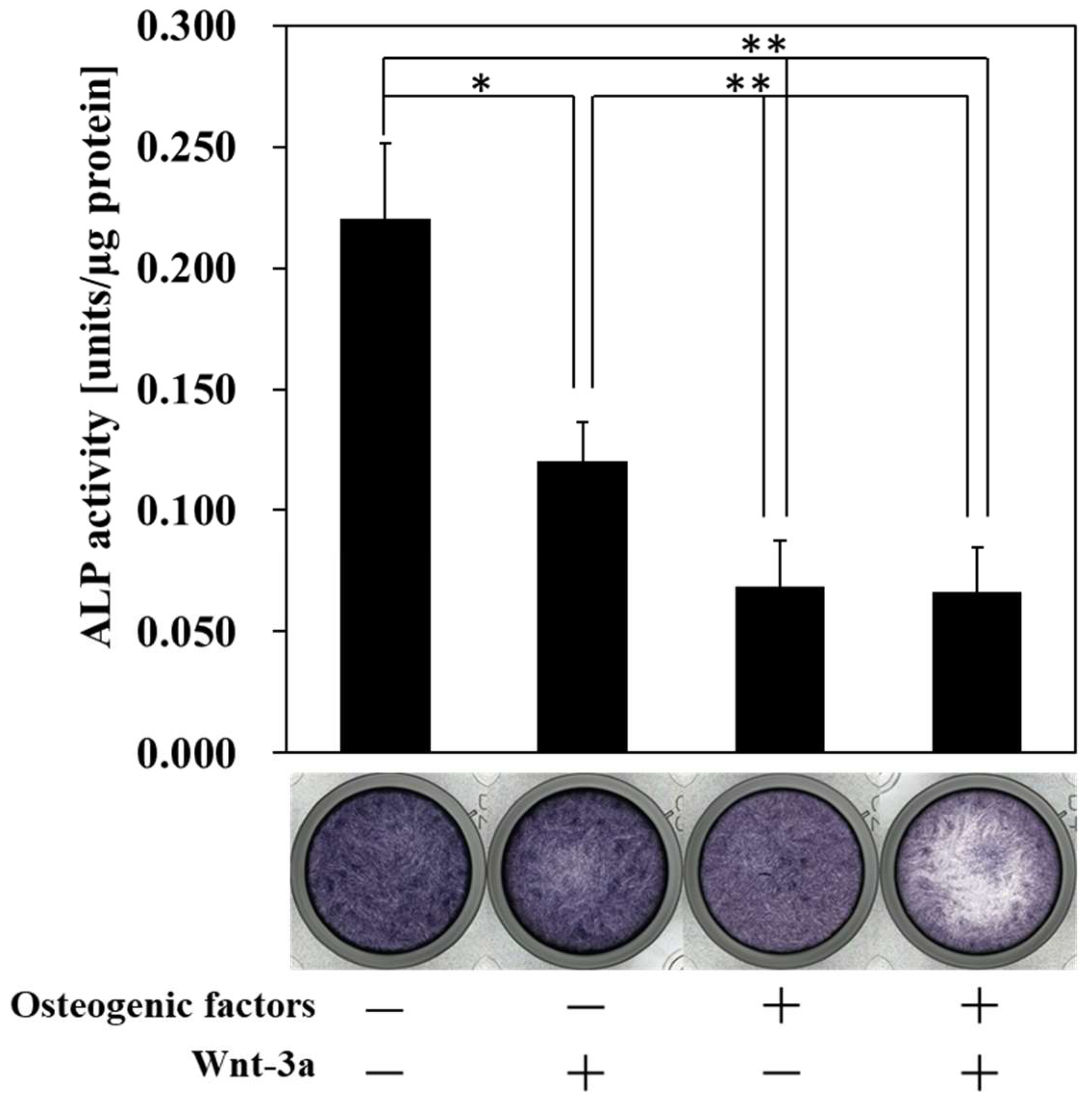
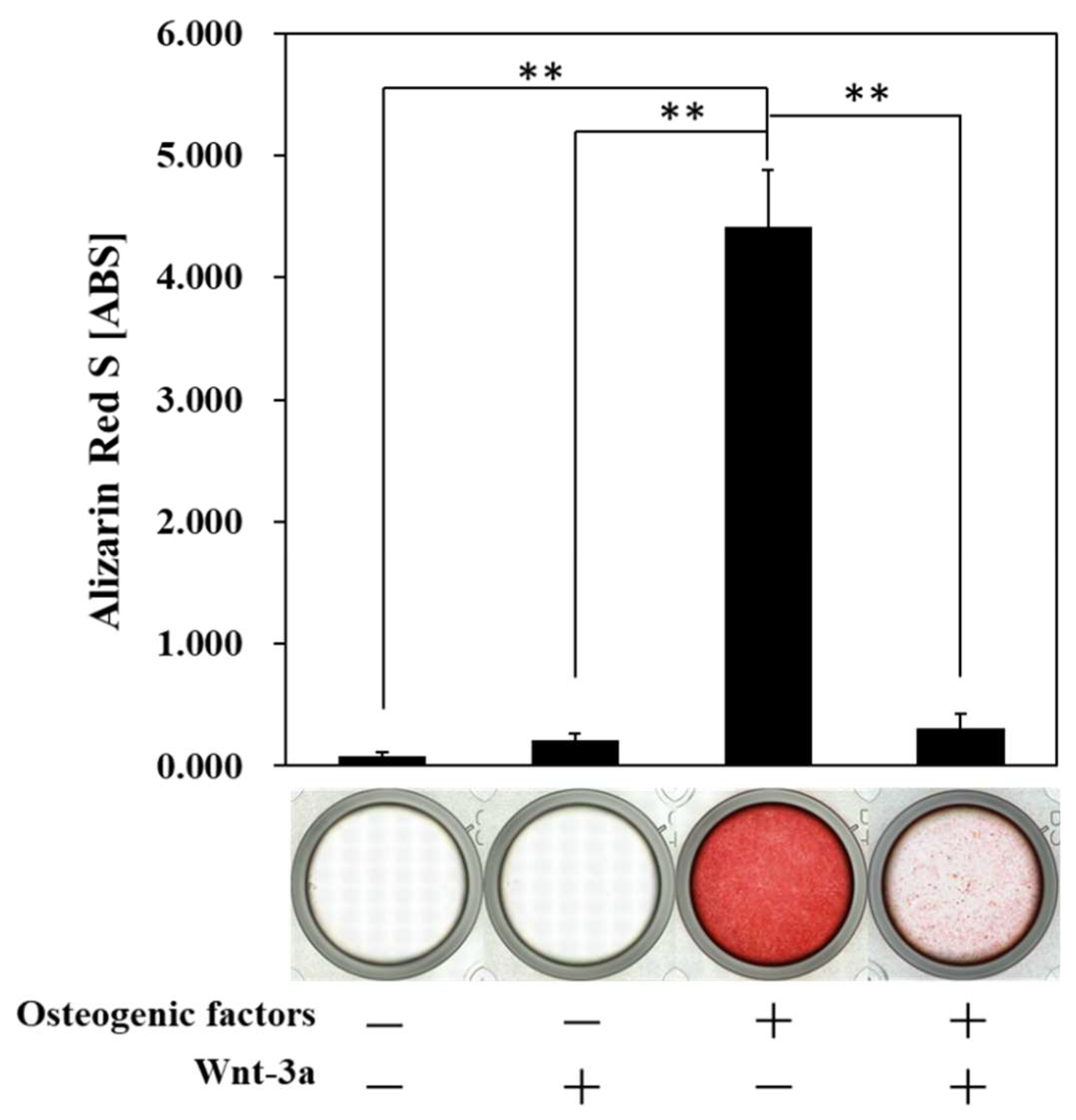
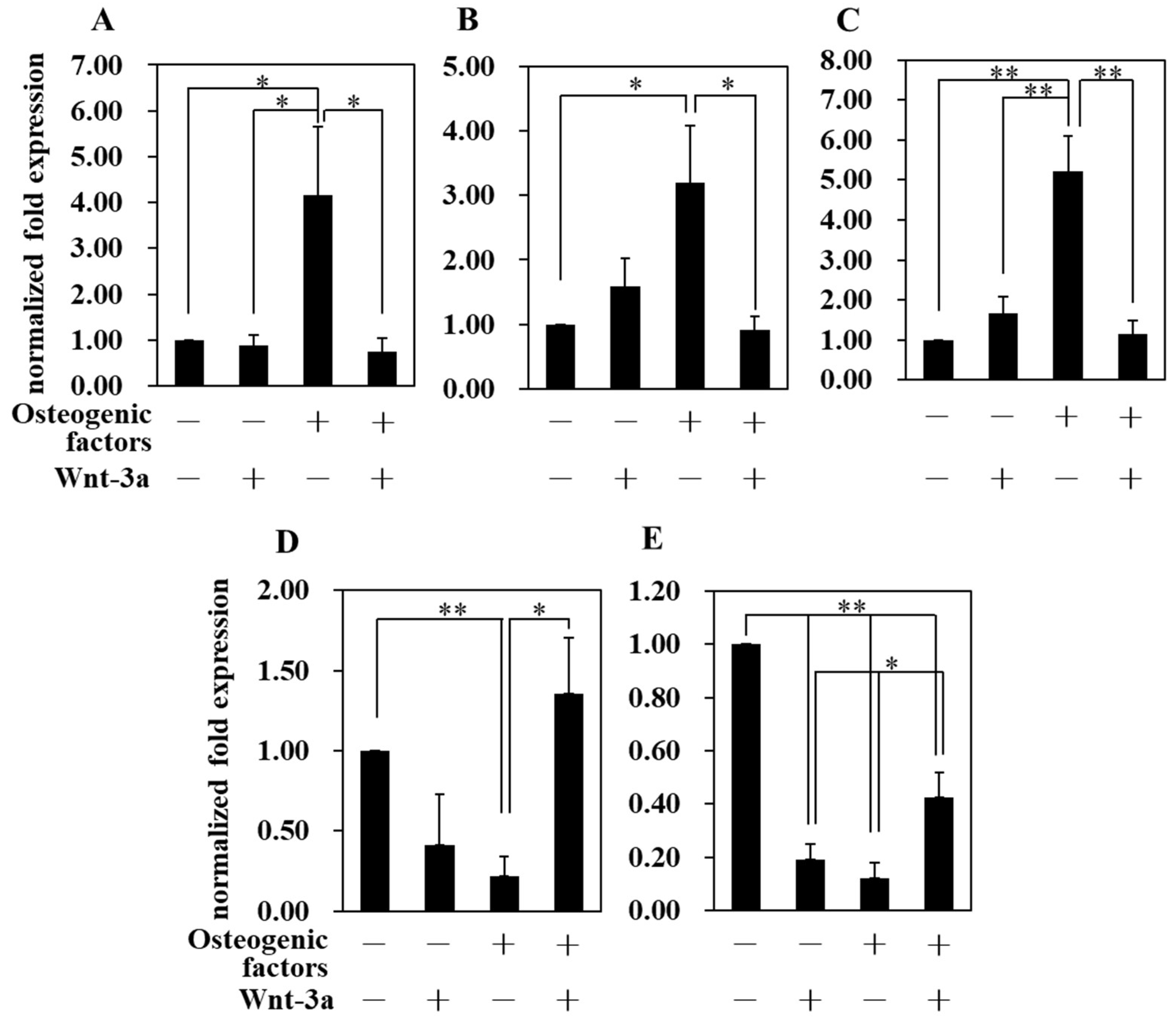
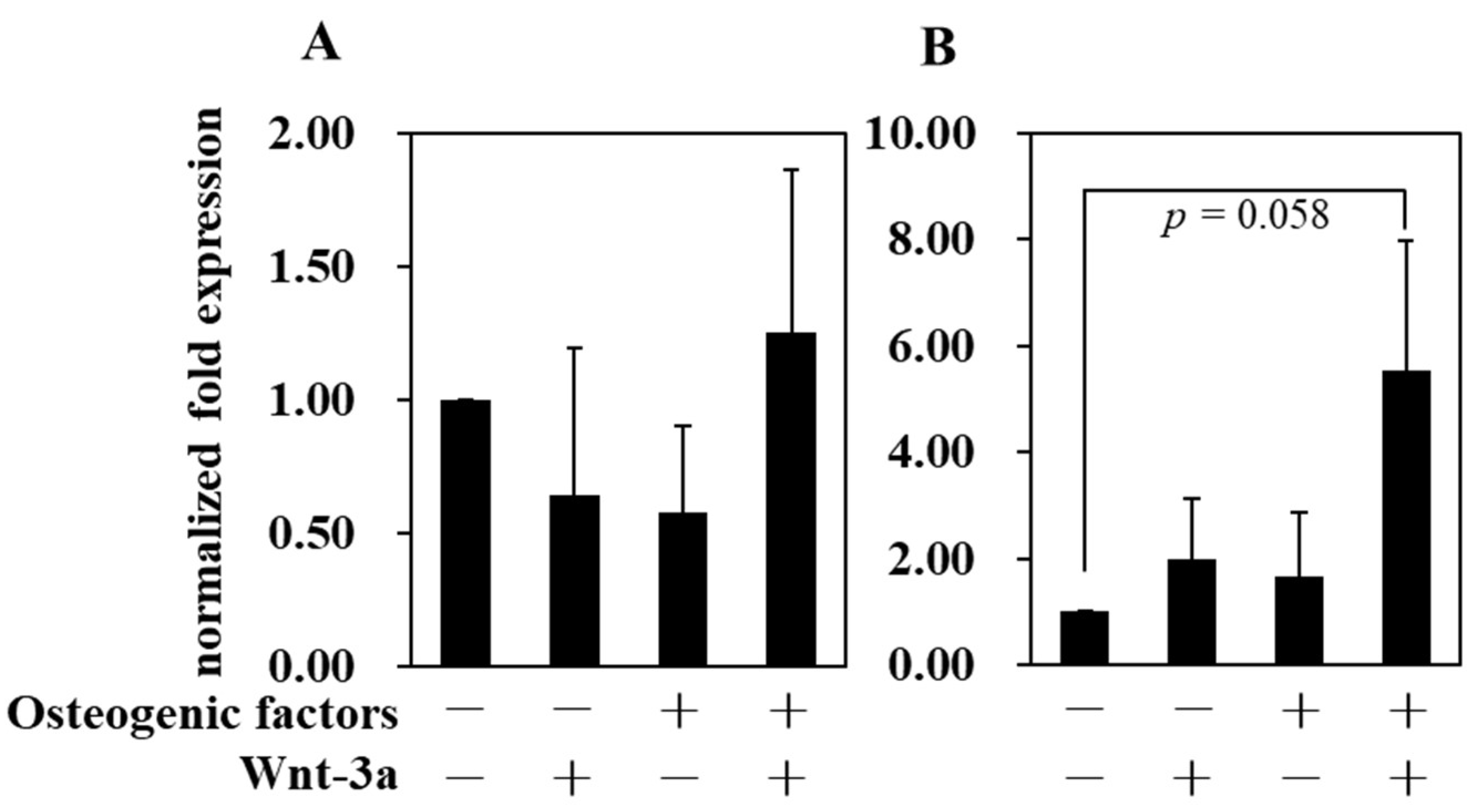
| Abbreviation | −/− (DMEM only) | −/+ | +/− | +/+ |
| Osteogenic factors | − | − | + | + |
| Wnt−3a (50 ng/mL) | − | + | − | + |
| GENE | ID | Sequence | |
|---|---|---|---|
| 18S rRNA | NR_145820.1 | Forward | GTAACCCGTTGAACCCCATTC |
| Reverse | CCATCCAATCGGTAGTAGCG | ||
| Runx2 | NM_001015051.3 | Forward | TTCGTCAGGATCCTATCAGTTTC |
| Reverse | TTTAATAGCGTGCTGCCATTC | ||
| Osteopontin | NM_000582.2 | Forward | CTGGATGACCAGAGTGCTGA |
| Reverse | TTGCTCTCATCATTGGCTTTC | ||
| Osteocalcin | NM_199173.6 | Forward | CCTCACACTCCTCGCCCTATT |
| Reverse | CCCTCCTGCTTGGACACAAA | ||
| Type-I collagen | NM_000088.4 | Forward | GGGATTCCCTGGACCTAAAG |
| Reverse | TCCCTGAGCTCCAGCCTCTCC | ||
| Periostin | NM_001135934.2 | Forward | AAGCTCAGGATCCTATCAGTTTC |
| Reverse | TGGTTGGCACAAATAATGTCC | ||
| Mkx | NM_001242702.2 | Forward | TTACAAGCACCGTGACAACC |
| Reverse | AAGCCGACGTCTTGCATTAG | ||
| Scx | NM_001080514.3 | Forward | GAGAACACCCAGCCCAAAC |
| Reverse | CTGCGAATCGCTGTCTTTCT | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iizumi, R.; Honda, M. Wnt/β-Catenin Signaling Inhibits Osteogenic Differentiation in Human Periodontal Ligament Fibroblasts. Biomimetics 2022, 7, 224. https://doi.org/10.3390/biomimetics7040224
Iizumi R, Honda M. Wnt/β-Catenin Signaling Inhibits Osteogenic Differentiation in Human Periodontal Ligament Fibroblasts. Biomimetics. 2022; 7(4):224. https://doi.org/10.3390/biomimetics7040224
Chicago/Turabian StyleIizumi, Ryoya, and Michiyo Honda. 2022. "Wnt/β-Catenin Signaling Inhibits Osteogenic Differentiation in Human Periodontal Ligament Fibroblasts" Biomimetics 7, no. 4: 224. https://doi.org/10.3390/biomimetics7040224
APA StyleIizumi, R., & Honda, M. (2022). Wnt/β-Catenin Signaling Inhibits Osteogenic Differentiation in Human Periodontal Ligament Fibroblasts. Biomimetics, 7(4), 224. https://doi.org/10.3390/biomimetics7040224





