Abstract
Despite many efforts over the last few decades, cardiac-based drug delivery systems are experiencing major problems, such as the effective delivery of the precise amount of a drug. In the current study, an effort has been made to prepare a nano-herbformulation (NHF) to overcome the major problem of conventional intervention. Copper oxide-based NHF was prepared using plant extract of Alternanthera sessilis and characterized using physicochemical techniques such as Transmission electron microscopy (TEM), X-ray powder diffraction (XRD), Dynamic light scattering (DLS), UV-Vis spectroscopy, and Fourier-transform infrared spectroscopy (FTIR). TEM analysis revealed that spherical NHF obtained of size 20–50 nm. In addition, XRD and FTIR confirmed the presence of phytochemicals with biological properties over the surface of copper oxide-based NHF. It was demonstrated that dose-dependent antiapoptotic activity was shown against DOX-induced cardiomyocytes, where ROS levels were significantly reduced to 0.29% from 37.99%. The results of the flow cytometry analysis using PI and Annexin staining further confirmed the antiapoptotic activity of NHF against DOX-induced cardiomyocytes by ROS scavenging. Thus, NHF might be used for cardiovascular disease treatment.
1. Introduction
Nano-biotechnology combines nanotechnology with biotechnology to improve products and create new technologies. We manufactured several nanoparticle materials, including pigments, cosmetics, and biomedical devices. Nanomaterials such as copper oxide nanoparticles have made possible advances in pharmaceuticals, food packaging, and catalyst manufacturing [1,2]. There are several methods available for synthesizing nanoparticles [3]. To prevent agglomeration, we use toxic chemicals as reducing or stabilizing agents. CuO nanoparticles synthesized by chemical routes are also toxic to acute and chronic aquatic organisms [4]. Nano herb formulation contains a variety of active ingredients. Invade the targets of cells involved in developing various diseases [5]. The reason is that many of these nano-herbal compounds combine metal with many other bioactive compounds. The presence analysis of numerous chemical components to detect these presents a challenge for the chemist and pharmacist. These are due to the presence of the metal compound and the variety of herbal compounds. The present works were approached based on the Siddha principle.
Furthermore, researchers focus on developing a more reliable and green method of synthesizing nanoparticles with various herbal formulations acting against cardiovascular disease. Using optimized CuO nanoparticles with Nelumbo nucifera (leaf extract), Spharanthus indicus (Leaf extract), Azardica indica (flower extract), Magnolia champaca extract, this simple process provides nanoparticles of better physical and optical properties [6]. The search for traditional medicine novels and medicines continues [7]. Since paranoia has not been studied in any significant detail, it offers unique opportunities. Doxorubicin is a drug that can cause serious health problems and harm in many children and the elderly. The doxorubicin-induced cardiotoxicity is also. Patterns of proteomic signatures are used in serum analysis from rat models to detect anthracycline and anthracenedione-induced cardiotoxicity [8]. Although doxorubicin is present in the heart at relatively small concentrations, myocardial cells are susceptible to pharmacological effects [9]. Some enzymes are inhibited by doxorubicin, such as Na-K ATPase and myosin ATPase [10]. The biomarker, considered acute toxicity with nanoparticles, can be designed and harvested to absorb, enrich and multiply [11]. Doxorubicin (DOX) mediated cardiac toxicity involves alterations in the phosphate pool energy, disturbances in myocardial adrenergic signaling, and changes in endothelin-1 [12]. Doxycycline, a well-established tetracycline antibiotic and antimicrobial agent with minor side effects, even after long-term usage [13], can be employed as a probable novel therapeutic agent for cardiac failure.
All parts of N. nucifera have many medicinal uses. The leaf, rhizome, seed, and flower are traditionally used for the treatment of pectoralgia dysentery, cough, fever, pharyngoplasty, smallpox, spermatorrhoea, epistaxis, hyperdipsia, haematemesis, haemoptysis, haematuria, metrorrhagia, leucoderma, hyperlipidaemia, cholera, hepatopathy and has great medicinal properties as antidiarrheal, anti-obesity and hypocholesterolemic analgesic, hypolipidemic activity [14]. Sphaeranthus indicus Linn. is known as Koṭṭaikkarantai in Tamil. It is an important medicinal plant for treating nervous depression skin diseases, laxative, anthelmintic, antibiotics, styptic gastric disorders, glandular swelling, and analgesic, antifungal and diuretic properties [15]. A. indica flower is reviewed to be a strong antioxidant agent; it plays an essential role in preventing cancer progression and development, is antimicrobial, and has a major role in dentistry, inflammation, hepatoprotective effects, wound healing properties, antidiabetic, antimalarial, antiphrotoxicity, and neuroprotective [16]. M. champaca flower extract is used as a diaphoretic, purgative, diuretic, expectorant, cardiotonic, digestive, stomachic, carminative, stimulant, antipyretic and astringent [17].
In the field of Siddha medicine, natural remedies are used as medicine. They include herbs, metals, minerals, hydro chemicals, animal products, and arsenic. These are mainly used for pharmaceutical products [18]. It is used in biomedical medicine to treat many diseases, especially heart disease and cancer [19]. In the nanoparticles, copper chendhooram is a drug made of metals and minerals. These copper centaurs are said to retain their energy for 75 years. They are separated and combined with specific plant juices by nanotechnology, filtration or extraction, and sublimation or calculation or burning or frying or subjecting them to the process of exposure to insolation until the characteristic reddening of the product takes place.
Cardiomyoblast cells derived from mice are the H9C2 cell line. These similarities to different cardiomyocytes and have cardiac contractility and functional properties [20]. Heart disease is associated with a high level of ROS production [21]. This leads to oxidative stress, which causes significant damage to these ROS. Heart cells damage the oxidative system, leading to further apoptosis [22].
Nanotechnology has tremendous potential in treating and diagnosing genetic diseases/disorders with its medical implications as nanomedicine. It has revolutionary treatment with nanoparticles being directly incorporated into the genome to either treat/suppress/delete the disease-causing gene and thus cure the ailment with a high success rate commonly known as “target drug delivery” system [23], biosensors [24], cosmetic industry [25], and therapeutics [26].
2. Materials and Method
2.1. Collection and Identification of Plants
Our nano herbomineral formulation is synthesized using floral species such as Alternanthera sessilis, Azadirachta indica, Sphaeranthus indicus, Nelumbo nucifera, and Michelia champaca. Fresh leaves of Sphaeranthus indicus, Alternanthera sessilis, and Azadirachta indica, free from diseases, were collected from the Vellore institute of technology. Nelumbo nucifera and Michelia champaca were collected from Arulmigu Arunachaleswarar Temple, Thiruvannamalai, Tamilnadu, India.
2.2. Optimization and Formulation of Nano Herbomineral in Modified Siddha Approach
The CuONP required for our herboformulation was biosynthesized by a green method in Alternanthera sessilis and optimized using response surface methodology (RSM) [27]. RSM is an optimization approach using statistical analysis to optimize the parameters and determine the optimal conditions for obtaining nano-sized particles. Testing the existing drugs available in the market, followed by understanding the prime relationship based on polynomial mathematics and representing the results at optimum levels, are the main goals of this optimization method, as shown in Figure 1.
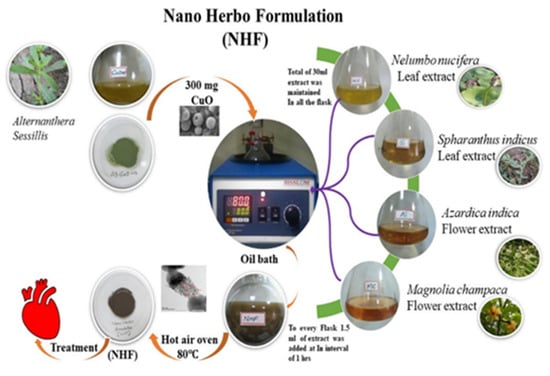
Figure 1.
Schematic representation of Nano Herboformulation (NHF).
Initially, plant extract of Alternanthera sessilis and cupric chloride was used to synthesize CuONP (to eliminate the toxicity), which was later optimized to a size of 10 nm. Our formulation is entirely inspired by the prevailing Siddha medicine formulation in which they take pure copper along with the extract of other plants and synthesize the particles for around 32 days to eliminate its toxicity and achieve efficiency. However, here in our formulation methodology, we tried to reduce the synthesis time by opting for suitable technology and following the same flow for formulation. After the optimization of CuONP in a powdered concentration of 300 mg, it is placed in an oil bath. Parallelly 30 mL of another essential extract (i.e.) Nelumbo nucifera (leaf extract), Spharanthus indicus (Leaf extract), Azardica indica (flower extract), and Mangolia champaca (flower extract) were prepared. A time interval of 1 h 1.5 mL of the essential extract was added consecutively to the CuO particles placed in the oil bath, ensuring room temperature and rotation. After adding all the extract, the setup is left to formulate for 24 h. After the incubation of 24 h, the vial contents had filtered using a 0.22 μm cellulosic membrane filter, and obtained NHF was converted to powder form with the help of a hot air oven. Moreover, the entrapment efficiency (EE) of phytochemicals in NHF was calculated based on the following Equation (1)
where UV-Vis measurements at 435 nm were used to assess content Vs (Standard Graph)
2.3. Physiochemical and Compound Identification
Gas Chromatography-Mass Spectrometry/Mass Spectrometry was used to analyze NHF samples following liquid-liquid extraction with hexane and ethanol followed by ethanol. The extract was analyzed with UV-Vis DRS, X-Ray diffraction, FTIR, SEM, HRTEM, and DLS to determine its size, shape, and morphology. Elemental analysis of NHF was performed through the EDAX method. A different part of the plant was used to synthesize the nano herboformulation.
2.4. In-Vitro Drug Release Study
The cumulative phytochemical release from NHF formulation was performed. The NHF formulation at the concentration of 100.0 µg /mL in a dialysis bag taken in a beaker having the required pH buffer solution. The dialysis bag containing the NHF formulation was kept inside the buffer solution at 37 °C, and samples of the buffer were collected at regular intervals. The amount of drug release was measured by measuring the absorbance of the collected external buffer at 435 nm. The cumulative phytochemical release from NHF was determined by the following Equation (2)
Furthermore, the kinetics of phytochemical release from NHF have been understood using the drug release dynamics using zero order, first order, and Korsmeyer-Peppas Model.
Zero-order is an independent kinetics model where the drug release rate is independent of its concentration and given by the Equation (3) as follows
where , is the initial amount of drug, is the cumulative amount of drug released at a time “t”, is the zero-order rate constant, and is the time.
Zero-order release kinetics is given by the graph plotted between the cumulative percentage of drugs released () versus time.
First order kinetics system describes that the drug release rate depends on its concentration and is given by the Equation (4) as follows
where initial amount of the drug is, is the cumulative amount of drug released at a time “t”, is the first order rate constant, and is the time? The rate constant is given by plotting as log cumulative percentage drug remaining versus time where the slop is equal to .
The Korsmeyer-Peppas kinetics Model is given by the Equation (5). As follows:
where the drug fraction released is denoted as F at a time ‘t’, Mt is the amount of drug released at a time ‘t’, M is the total amount of drug, Km is the kinetic constant, and n is the diffusion or release exponent. The kinetic constant ‘Km’ and n was given by plotting the graph of log (Mt/M) versus log t. The value of n indicates the type of diffusion to be either fickian diffusion (n = 0.45), fickian diffusion (0.45 < n < 0.89), or case-2 relaxation (n = 0.89).
2.5. Cell Culture and Assessment of Cell Viability
In Dulbecco’s Modified Eagles Medium, H9C2 myocardial cells were purchased from National Centre for Cell Sciences, Pune, India. Under humidified 5% CO2 atmosphere at 37 °C, the cells at density 1 × 104 counts were grown in 10% FBS, 1% penicillin-streptomycin antibiotic solution. MTT assay was used to assess cell viability. To reduce toxicity, we stimulated H9C2 myocardial cells with doxorubicin. NHF at different concentrations was used to reduce the toxicity of myocardial cells. A 96-well cell culture plate was filled with NHF with doxorubicin (5, 10, 20, 30, 40, and 50 µg/mL) for 30 min. The cells were incubated with various NHF Cells concentrations and then set at 37 °C in the dark for 4 h with MTT. After discarding the supernatant, 100 mL DMSO was added to the plates, dissolving the formazan crystals caused by MTT’s interaction with the live cells. Microplate readers were used to measuring the absorbance at 570 nm.
2.6. Apoptosis and Cell Death Estimation
Following the manufacturer’s instructions, FITC Annexin V/Dead Cell Apoptosis Kit was used to analyze apoptosis. Following trypsinization, cells were harvested, maintained at 1 × 106 counts, and suspended in Annexin binding buffer containing FITC Annexin and PI for 20 min in the dark. After that, the cells were suspended in a buffer containing propidium iodide for 5 min. An FC500 flow cytometer (Beckman Coulter, Indianapolis, IN, USA.) was used to acquire data for 10,000 events using three cell suspensions (5, 10, and 20 µg/mL).
2.7. Intracellular ROS Generation Estimation Using Flow Cytometry
Monitoring intracellular ROS was accomplished using dichloro-dihydro-fluorescein diacetate fluorescent probes. After 24 h of incubation in 6-well plates maintained at 1 × 106 counts, cells were treated with NHF with doxorubicin (5, 10, or 20 µg/mL) and incubated for another 24 h. Incubation with DCFH-DA at 37 °C for about 30 min, followed by rinsing with fresh DMEM, was followed by analysis using a flow cytometer after cells were rinsed three times with fresh DMEM. Results were expressed as fold changes in fluorescence intensity based on a minimum of 10,000 events per sample. A comparison was made between the results and the control value.
2.8. Statistical Analysis
The experiments were run in triplicate, and the values were analyzed using the ANOVA statistical method. The results were statistically significant when the p-value was <0.05 and was expressed as the mean ± standard error.
3. Results
3.1. UV-Vis Spectra Studies
Nano Herboformulation (NHF) was prepared as per the protocol described above, where the capacity of phytochemical in NHF was investigated and found to be 305.51 µg/mg with a loading efficiency of around 61.58% in Figure 2. UV-Vis spectroscopy is a widely recognized methodology for studying powder-suspended nanoparticles [28]. At room temperature, the UV-Visible absorption spectra of the green synthesized nanoformulations are presented in Figure 3. As a result of surface plasmon resonance (SPR), the nanoformulations show high absorption below 350 nm and a well-defined absorbance peak at around 280 nm. Copper oxide nanoparticles synthesized from various plant precursors also showed a similar result [29].
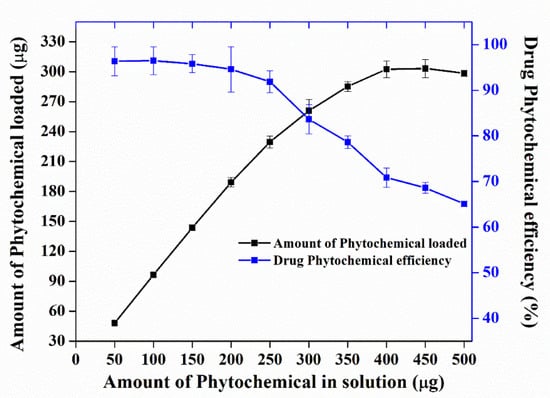
Figure 2.
Drug loading capacity and efficiency of nano herboformulations.
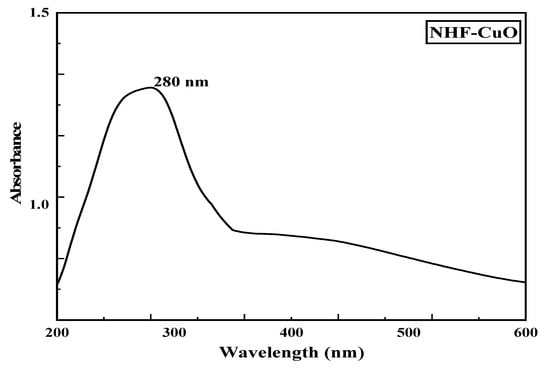
Figure 3.
UV-Vis spectra of Nano Herboformulation.
3.2. XRD and FTIR Analysis of Nano Herboformulation
XRD technique was used to analyze the microcrystalline structure of nano herboformulation. Characteristic XRD peaks of the nanoformulations were observed at 32.7, 40.2, 46.4, and 57.3, correspondings to 110, 200, 202, and 021 planes, respectively, as shown in Figure 4. This observation indicates the formation of monoclinic CuO. The NP system is seen as a typical result. Their sharp peaks confirm CuO NP. Reveals the crystalline nature of the further confirmation (JCPDS card number 89-2529). The XRD crystal structure provides the lattice parameters, nature of the phase, and crystal grain size [30]. Our Sharer equation is used to determine how broadening the most extreme peak of an XRD measurement is for a model. The average size of the nano herboformulation was 7.70 nm.
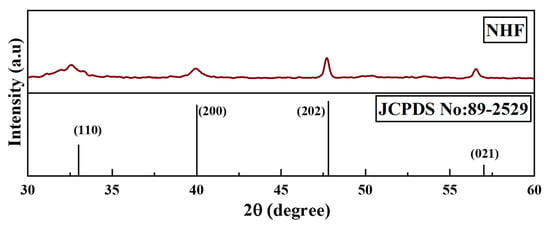
Figure 4.
X-ray diffraction pattern of Nano Herboformulation.
In FTIR analysis, we were able to classify nano-sized substances by the composition of nano-herboformulation. Figure 5 shows FTIR spectra of nano herboformulation. Nano herboformulation FTIR spectra show Cu-O vibrations at frequencies less than 700 cm−1 [31]. Small peaks (592.17 cm−1), O-H stretching vibration (3321.42 cm−1), frequency-independent (2927.94 cm−1), vibration resulting from C=C stretching (1600.92 cm−1), O-H deformation vibration (1359.82 cm−1), and bands corresponding to the characteristic frequency of inorganic ions are observed in CuO NPs [32]. Alkane, aromatic, ketone compounds, carboxylic acids, and aromatic nitro compounds are attributed to C-H deformation vibration (1317.38 cm−1) and CH3 rocking vibration (1031.92 cm−1). Deflation of the capping caused a comparatively high peak in CuO NPs due to C-O stretching [33].
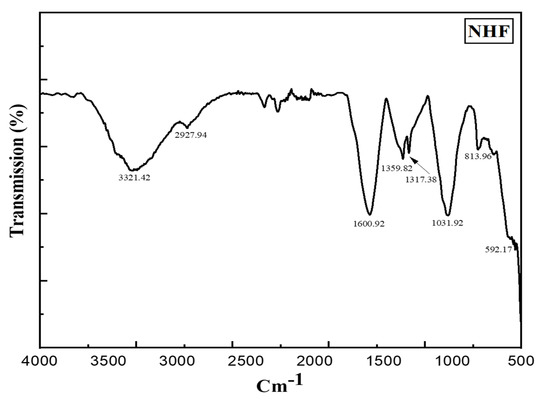
Figure 5.
FT-IR spectrum of Nano Herboformulation (1317.38 cm−1peak corresponding C-H deformation vibration).
3.3. Size and Shape Determination of Nano Herboformulation
The standard physicochemical characterization used for integrated nano-herb formation. As shown in Figure 6A of TEM and Figure 6B of SEM, spherical particles of size ranging between 5–10 nm were identified. The amount of nano-herb formulation differs depending on the solvent medium as plant juices are attached to them. Thus, the cytotoxicity effect and the extent of its medium [34]. SEM showed that the nano herboformulation’s nano shape is approximately 300 nm Figure 5B. The shape of nanoparticles in the nano herboformulation plays a significant role in influencing the distinctive process of nanoparticle interaction with living patterns [35]. In in vivo tests, nanoparticles less than 10–20 nm are rapidly distributed over neuronal administration between all organs and tissues, while larger nanoparticles ranging from 50–250 nm are found in the blood, liver, spleen, and heart [36].
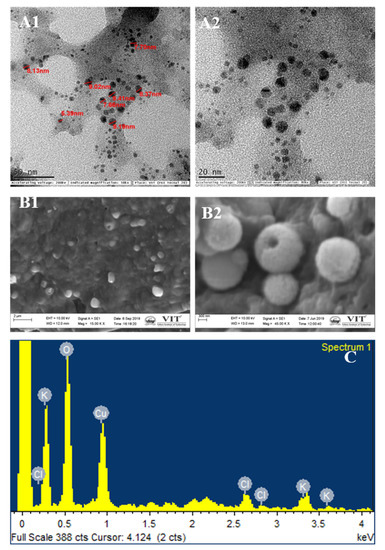
Figure 6.
Electron micrographs of Nano Herboformulation. (A1,A2) TEM analysis. (B1,B2) SEM analysis, (C) EDX analysis of Nano Herboformulation, where O—48.41%, Cl—5.77%, K—8.29%, Cu—37.23%.
EDX microanalysis is used to create maps of various chemical components. This map is generated using software that evaluates the required element concentration at each imaging stage (X-ray spectra) in scanning mode. EDX analysis confirmed copper oxide nanoparticles and trivial quantities of Cl and K Figure 6C. The occurrence of copper and oxygen confirmed the formation of copper oxide nanoparticles in the reaction system. However, minor amounts of other reserves were found, which may be the molecule used in the analysis.
3.4. Zeta Potential and Particle Size of Nano Herboformulation
The size and stability of nano herboformulation are essential for medicinal application. To assess the stability of nanofluids, Zeta observes the electrophoretic behavior of the potential analysis fluid [37]. As shown in Figure 7B, a high Zeta potential value represents the stability of nano herboformulations in an aqueous medium. A zeta potential value of −20.58 mV shows colloidal stability. The nanoparticle size and colloidal stability were determined using DLS [38]. The hydrodynamic diameter of the nano herboformulation was determined to be 12.9 nm Figure 7A.
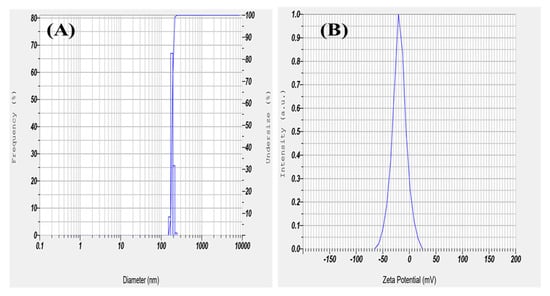
Figure 7.
(A) Dynamic light scattering and (B) Zeta Potential of Herboformulation.
3.5. GC-MS Analysis of Nano Herboformulation
The presence of a compound responsible for a different type of plant reacts with copper oxide. The Gas chromatography-mass spectrometry of nano herboformulation showed 13 peaks that were identified after comparing the mass spectra with Library used NIST Version-2011, indicating the presence of 13 phytocompounds. Figure 8 represents the formulation’s possible components (based on the NIST Library). The compounds are listed in Table 1. along with their peak area and RT molecular weight. Represents compound from 1H-Inden-1-one,2,3-dihydro 5,6-dimethody-3-methyl (RT 10.07), performed experiments revealed that 10e has an anti-β amyloid effect can reduce ROS, LDH, and MDA also a positive posing impact on TAC [39]. Similarly, 9-octadecenoic acid resists doxorubicin and cytarabine-induced oxidative stress. Oleic acid (RT), as reported in our present findings (RT 20.46) compound had more favorable effects similar effects on non-HDL-cholesterol concentrations and fasting LDL-cholesterol in comparison to hexadecenoic acid [40].
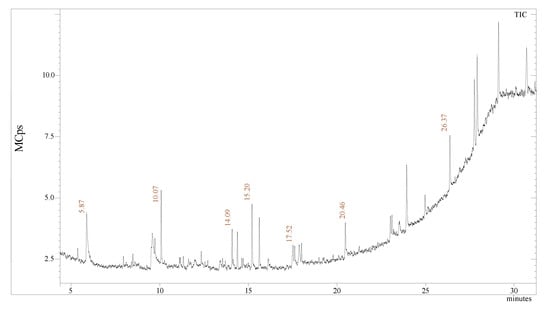
Figure 8.
GC-MS analysis: chromatogram of the NHF.

Table 1.
Compound identification from Nano herboformulation.
3.6. In-Vitro Phytochemical Release Study of NHF Formulation
We have also studied the in-vitro phytochemical release study of NHF formulation, nearly 94.2 % of the phytochemical release (Figure 9A). The phytochemical release kinetics data from NHF formulation were also fitted to the different kinetics models (Table 2). It was found best to follow the Korsmeyer-Peppas model, and hence, the release of the drug must be mediated by diffusion mechanism, especially non-fickian diffusion (0.45 < n < 0.89) type of transport mechanism (Figure 9B–D).
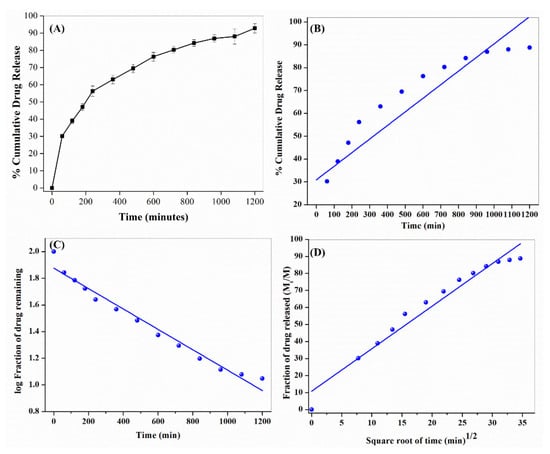
Figure 9.
(A) Phytochemical release study from NHF. Kinetics of study phytochemical release study from NHF (B) Zero-Order, (C) First-Order & (D) Korsmeyer–Peppas models.

Table 2.
Drug release data were obtained from various kinetic models.
3.7. Cell Viability, Oxidative Stress, and Apoptosis in Doxorubicin-Induced h9c2 Cell Line
The study of different cell biology techniques, the analysis of reactive oxygen species, the analysis of apoptosis, and the identification of morphological changes. Using H9C2 cells to induce doxorubicin treatment, we explored the role of nano herbal formulations in reducing cardiotoxicity. We then examined the protective capacity of the nano herboformulations in myocardial cells from H9C2.
3.7.1. Dose-Response Curve Using MTT Assay—Cell Viability Assay
H9C2 cardiomyoblast cells viability to various concentrations of doxorubicin-induced nano herboformulation was studied for 24 h. Dose-dependent cytotoxicity was observed with doxorubicin treatment (Figure 10). DOX-induced cells showed stable cytotoxicity after NHF treatment and higher mitochondrial oxidative pressure after NHF treatment. An MTT assay can distinguish membrane lysis from non-membrane lysis by changing MTT reductase activity.
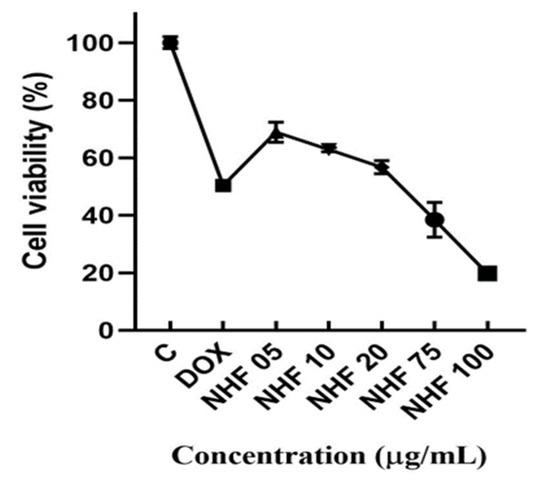
Figure 10.
MTT analysis for NHF against DOX-induced H9c2 cell lines. All the data are expressed as mean ± SD (n = 3). (p < 0.05).
Cell viability was found to be increased in NHF-treated cells in a concentration-dependent manner Figure 10. Cells were treated with (1 µM) of doxorubicin to induce cytotoxicity in H9C2 cells and with NHF at different concentrations (5, 10, 20, 30, 40, and 50 µg/mL) to revert the induced toxicity. As shown in the graph, we found 5, 10, and 20 µg/mL concentrations to have high % cell viability; thus, we chose this concentration for further in-vitro studies. NHF of higher concentrations (5, 10, 20 µg/mL). Based on earlier studies, doxorubicin and levosimendan increased cell endurance and prevented oxidative stress-induced cell death in rodent hepatocytes [41]. Evaluation of the cytotoxic effects due to various nanoparticles for cells H9C2, A549, HEK293, and MCF-7 shows that fundamentally distinct nanoparticles impose unique biological and toxic effects [42]. Cells treated with NHF for 24 h showed no significant toxic effects. Co-treatment using a similar cocktail formula did not show a significant improvement in cell viability, which is also noticeable compared to free dox (p > 0.05) [43]. The experiments were carried out in triplicates, and the calculated values are mean ± SD. On the contrary, NHF at (5, 10, 20 µg/mL) dosage increased the viability of H9C2 cells to near normal.
3.7.2. NHF in Oxidative Stress Induced by DOX-Treatment in H9c2 Cells
Purine nucleotide acts as an intermediate to DOX metabolize quinone by flavoproteins. Doxorubicin damaged approximately 37.99% of the cells. After DOX treatment of NHF cell damage, DOX-induced H9C2 cells showed a low level of (0.83%, 0.56%, and 0.29%) (Figure 11 C-E) compared to control cells. Figure 11 A-B shows how NHF affects Dox-induced ROS production within cells. Here we have treated doxorubicin-induced toxicity with NHF at 5, 10, and 20 µg/mL Concentrations.
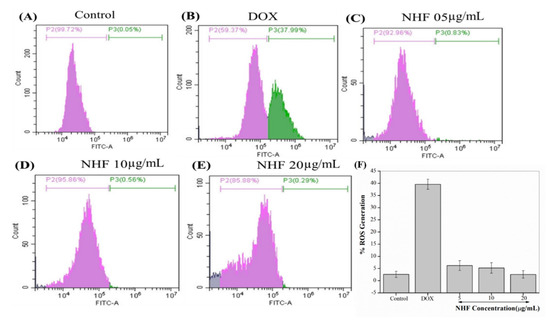
Figure 11.
NHF-induced oxidative stress in H9C2 cardiomyocytes. (A) Control (B) Dox-induced ROS (C) NHF 5 µg/mL (D) NHF 10 µg/mL (E) NHF 20 µg/mL (F) complementary representation of ROS.
The formation of the DOX-iron complex stimulates ROS generation. The results in hydrogen peroxide (H2O2) and superoxide ions [44]. Moreover, H2O2 is degraded by low molecular weight clay irons into common reaction hydroxyl radical [45]. Hyper generation of ROS leads to antioxidant pressure, which is activated by DOX and stimulates the apoptotic signaling layer in cardiomyocytes [46]. Figure 11 F complementary graph of ROS. As shown in the image, all treated groups showed reduced ROS levels compared to control cells. The ROS levels were drastically reduced in NHF treatment.
3.7.3. NHF in Apoptosis Induced by DOX-Treatment in H9c2 Cells
Here we have seen apoptosis assay for NHF. As shown in Figure 12A, the Control and doxorubicin-treated cell are the same as before. Figure 12C–E showed cells treated with 5, 10, and 20 µg/mL Figure 12C concentration showed late apoptosis cells. Figure 12D showed fewer late apoptotic cells treated with 10 µg/mL. Figure 12E shows 20 µg/mL concentration when we found reduced apoptosis cells compared to all other treatments, and Figure 12F complementary graph of apoptosis.
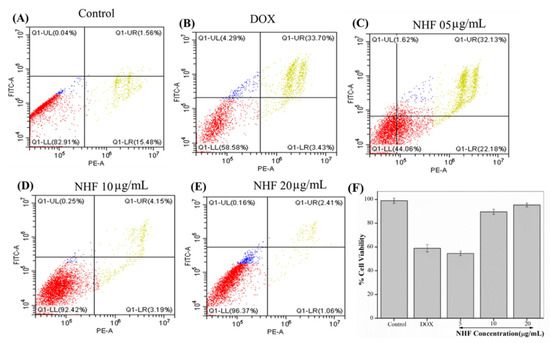
Figure 12.
Analyses of flow cytometry using PI and Annexin staining of H9C2 cells. (A) Control (B) Dox-induced apoptosis (C) NHF 5 µg/mL (D) NHF 10 µg/mL (E) NHF 20 µg/mL (F) Complementary representation of apoptosis.
The results of NHF with doxorubicin-induced antioxidant pressure are a significant factor in toxicity. The potential role of oxidative stress in mediation with apoptosis induction and DNA damage has been documented [47]. At the end of these studies, our results demonstrate that NHF oxidative stress, DNA damage, and apoptosis triggers are lower than in previous reports [48].
4. Conclusions
Cardiovascular disease poses a significant threat to society as it holds the highest mortality rate. Here, we have encountered the formation of nano herbal has shown good progress in cardiac function. From this study, it seems possible to shunt CVD harmlessly and healthily using Nano herbo-formulation with reduced toxicity and increased therapeutic support. The overall analysis of the relationship between nano herboformulation revealed by the transformation of value in H9C2 and zebrafish is given with doxorubicin, confirming the reduced toxicity while using nano herboformulation. It provides the exact measures and the role of ROS and the apoptotic analysis in H9C2. Overall, this gives a clear output for further expanding the nano herboformulation in the treatment of CVD. In future studies, the most common preclinical model, the nano herboformulations showing the highest potential in this in vitro model, can be further assessed in mice. It is possible to reduce research time and costs associated with high numbers of mice using this approach. The addition of this animal model would strengthen and improve the approval process for nano herboformulation-based cardiac and cancer treatments.
Author Contributions
S.J.: Contributed to the conceptualization, methodology, validation, and investigation and to the writing—original draft preparation. H.K., R.B., S.M., M.R.S.: Methodology, formal analysis, validation. V.K.S., S.S.H., I.C. Supervision, editing resource, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) (#2020R1A2C1012586, #2020R1A6A1A03044512, & # 2020R1A6A3A01100150) funded by the Korean Ministry of Education, Science and Technology. The authors also thank the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, and Forestry (IPET) through the High Value-added Food Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (321027-5).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the Vellore Institute of Technology, School of Biosciences and Technology, India for supporting this research. This work was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1A6A1A03044512) and by the Ministry of Education, Science and Technology (2020R1A2C1012586).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Y.; Zhou, J.; Liu, L.; Huang, C.; Zhou, D.; Fu, L. Characterization and toxicology evaluation of chitosan nanoparticles on the embryonic development of zebrafish, Danio rerio. Carbohydr. Polym. 2016, 141, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.S.; Panwar, J.; Yun, Y.-S. Biogenic Synthesis of Metallic Nanoparticles by Plant Extracts. ACS Sustain. Chem. Eng. 2013, 1, 591–602. [Google Scholar] [CrossRef]
- Zahoor, M.; Nazir, N.; Iftikhar, M.; Naz, S.; Zekker, I.; Burlakovs, J.; Uddin, F.; Kamran, A.W.; Kallistova, A.; Pimenov, N.; et al. A Review on Silver Nanoparticles: Classification, Various Methods of Synthesis, and Their Potential Roles in Biomedical Applications and Water Treatment. Water 2021, 13, 2216. [Google Scholar] [CrossRef]
- Naz, S.; Gul, A.; Zia, M. Toxicity of copper oxide nanoparticles: A review study. IET Nanobiotechnol. 2019, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sundarrajan, S.; Arumugam, M. A systems pharmacology perspective to decipher the mechanism of action of Parangichakkai chooranam, a Siddha formulation for the treatment of psoriasis. Biomed. Pharmacother. 2017, 88, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.-L.; Li, X.-G.; Zhu, F.; Lei, C.-L. Structural Characterization of Nanoparticles Loaded with Garlic Essential Oil and Their Insecticidal Activity against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Agric. Food Chem. 2009, 57, 10156–10162. [Google Scholar] [CrossRef] [PubMed]
- Marles, R.; Farnsworth, N. Antidiabetic plants and their active constituents. Phytomedicine 1995, 2, 137–189. [Google Scholar] [CrossRef]
- Jain, K.K. Biomarkers for Drug Discovery and Development. In The Handbook of Biomarkers; Springer: Berlin/Heidelberg, Germany, 2017; pp. 113–145. [Google Scholar]
- Green, P.S.; Leeuwenburgh, C. Mitochondrial dysfunction is an early indicator of doxorubicin-induced apoptosis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2002, 1588, 94–101. [Google Scholar] [CrossRef]
- Someya, A.; Akiyama, T.; Misumi, M.; Tanaka, N. Interaction of anthracycline antibiotics with actin and heavy meromyosin. Biochem. Biophys. Res. Commun. 1978, 85, 1542–1550. [Google Scholar] [CrossRef]
- Morey, T.E.; Varshney, M.; Flint, J.A.; Rajasekaran, S.; Shah, D.O.; Dennis, D.M. Treatment of Local Anesthetic-Induced Cardiotoxicity Using Drug Scavenging Nanoparticles. Nano Lett. 2004, 4, 757–759. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Liang, E.; Zhou, L.; Dong, Z.; Liang, P.; Weng, Q.; Yang, M. Thrombopoietin protects H9C2 cells from excessive autophagy and apoptosis in doxorubicin-induced cardiotoxicity. Oncol. Lett. 2017, 15, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Sayles, H.; Mikuls, T.R.; Michaud, K. Minocycline and doxycycline therapy in community patients with rheumatoid arthritis: Prescribing patterns, patient-level determinants of use, and patient-reported side effects. Arthritis Res. Ther. 2011, 13, R168. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.-K.; Yang, H.J.; Ma, J.Y. Lotus (Nelumbo nucifera Gaertn.) leaf water extracts suppress influenza a viral infection via inhibition of neuraminidase and hemagglutinin. J. Funct. Foods 2022, 91, 105019. [Google Scholar] [CrossRef]
- Rajendra, V.K.P.; Kurapati, S.; Balineni, S.K.; Gogineni, N.T.T. A blend of Sphaeranthus indicus flower head and Terminalia chebula fruit extracts reduces fatty liver and improves liver function in non-alcoholic, overweight adults. Funct. Foods Health Dis. 2022, 12, 361. [Google Scholar] [CrossRef]
- Jayakodi, S.; Shanmugam, R.; Almutairi, B.O.; Almutairi, M.H.; Mahboob, S.; Kavipriya, M.; Gandusekar, R.; Nicoletti, M.; Govindarajan, M. Azadirachta indica-wrapped copper oxide nanoparticles as a novel functional material in cardiomyocyte cells: An ecotoxicity assessment on the embryonic development of Danio rerio. Environ. Res. 2022, 212, 113153. [Google Scholar] [CrossRef]
- Santhosh Kumar, J.; Shanmugam, V. Green synthesis of copper oxide nanoparticles from magnolia champaca floral extract and its antioxidant & toxicity assay using Danio Rerio. Int. J. Recent Technol. Eng. 2020, 8, 5444–5449. [Google Scholar]
- Uthamarayan, C. Siddha Maruthuvanga Churukkam. 1953. Available online: https://www.tknsiddha.com/medicine/siddha-books-free/ (accessed on 30 October 2022).
- Shailaja, R.; Sugunthan, S. Concept of nanotechnology in siddha medical literature. World J. Pharm. Res. 2016, 5, 276–284. [Google Scholar]
- Onódi, Z.; Visnovitz, T.; Kiss, B.; Hambalkó, S.; Koncz, A.; Ágg, B.; Váradi, B.; Tóth, V.É.; Nagy, R.N.; Gergely, T.G. Systematic transcriptomic and phenotypic characterization of human and murine cardiac myocyte cell lines and primary cardiomyocytes reveals serious limitations and low resemblances to adult cardiac phenotype. J. Mol. Cell. Cardiol. 2022, 165, 19–30. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Khotina, V.A.; Sukhorukov, V.N.; Kalmykov, V.A.; Mikhaleva, L.M.; Orekhov, A.N. The Role of Mitochondrial DNA Mutations in Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 952. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef]
- Jin, S.; Ye, K. Nanoparticle-Mediated Drug Delivery and Gene Therapy. Biotechnol. Prog. 2007, 23, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Prow, T.; Grebe, R.; Merges, C.; Smith, J.; McLeod, S.; Leary, J.; Lutty, G. Nanoparticle tethered biosensors for autoregulated gene therapy in hyperoxic endothelium. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 276. [Google Scholar] [CrossRef]
- Perugini, P.; Simeoni, S.; Scalia, S.; Genta, I.; Modena, T.; Conti, B.; Pavanetto, F. Effect of nanoparticle encapsulation on the photostability of the sunscreen agent, 2-ethylhexyl-p-methoxycinnamate. Int. J. Pharm. 2002, 246, 37–45. [Google Scholar] [CrossRef]
- Czupryna, J.; Tsourkas, A. Suicide gene delivery by calcium phosphate nanoparticles: A novel method of targeted therapy for gastric cancer. Cancer Biol. Ther. 2006, 5, 1691–1692. [Google Scholar] [CrossRef]
- Jayakodi, S.; Shanmugam, V.K. Statistical Optimization of Copper Oxide Nanoparticles Using Response Surface Methodology and Box–Behnken Design Towards In Vitro and In Vivo Toxicity Assessment. Biointerface Res. Appl. Chem. 2020, 11, 10027–10039. [Google Scholar]
- Swamy, M.K.; Sudipta, K.M.; Jayanta, K.; Balasubramanya, S. The green synthesis, characterization, and evaluation of the biological activities of silver nanoparticles synthesized from Leptadenia reticulata leaf extract. Appl. Nanosci. 2014, 5, 73–81. [Google Scholar] [CrossRef]
- Sarkar, J.; Chakraborty, N.; Chatterjee, A.; Bhattacharjee, A.; Dasgupta, D.; Acharya, K. Green Synthesized Copper Oxide Nanoparticles Ameliorate Defence and Antioxidant Enzymes in Lens culinaris. Nanomaterials 2020, 10, 312. [Google Scholar] [CrossRef]
- Yan, W.; Petkov, V.; Mahurin, S.M.; Overbury, S.H.; Dai, S. Powder XRD analysis and catalysis characterization of ultra-small gold nanoparticles deposited on titania-modified SBA-15. Catal. Commun. 2005, 6, 404–408. [Google Scholar] [CrossRef]
- Shanmugam, V.; Jas, J.S.; Jayakodi, S. Biomimetic Copper Oxide Nanoparticles and its Validation Through In-silico Approach on Cardiac Enzymes. Curr. Nanosci. 2022, 18, 86–93. [Google Scholar] [CrossRef]
- Sabeena, G.; Rajaduraipandian, S.; Pushpalakshmi, E.; Alhadlaq, H.A.; Mohan, R.; Annadurai, G.; Ahamed, M. Green and chemical synthesis of CuO nanoparticles: A comparative study for several in vitro bioactivities and in vivo toxicity in zebrafish embryos. J. King Saud Univ.-Sci. 2022, 5, 102092. [Google Scholar]
- Nasrullah, M.; Gul, F.Z.; Hanif, S.; Mannan, A.; Naz, S.; Ali, J.S.; Zia, M. Green and Chemical Syntheses of CdO NPs: A Comparative Study for Yield Attributes, Biological Characteristics, and Toxicity Concerns. ACS Omega 2020, 5, 5739–5747. [Google Scholar] [CrossRef] [PubMed]
- Sayes, C.M.; Warheit, D.B. Characterization of nanomaterials for toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2009, 1, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Jin, S.; Ma, X.; Xue, X.; Yang, K.; Kumar, A.; Wang, P.C.; Zhang, J.; Hu, Z.; Liang, X.-J. Ultrasmall Gold Nanoparticles as Carriers for Nucleus-Based Gene Therapy Due to Size-Dependent Nuclear Entry. ACS Nano 2014, 8, 5852–5862. [Google Scholar] [CrossRef]
- De Jong, W.H.; Hagens, W.I.; Krystek, P.; Burger, M.C.; Sips, A.J.; Geertsma, R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 2008, 29, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Ghadimi, A.; Saidur, R.; Metselaar, H. A review of nanofluid stability properties and characterization in stationary conditions. Int. J. Heat Mass Transf. 2011, 54, 4051–4068. [Google Scholar] [CrossRef]
- Balamurugan, B.; Mehta, B.R.; Shivaprasad, S.M. Surface-modified CuO layer in size-stabilized single-phase Cu2O nanoparticles. Appl. Phys. Lett. 2001, 79, 3176–3178. [Google Scholar] [CrossRef]
- Mozaffarnia, S.; Teimuri-Mofrad, R.; Rashidi, M.-R. Design, synthesis and biological evaluation of 2,3-dihydro-5,6-dimethoxy-1H-inden-1-one and piperazinium salt hybrid derivatives as hAChE and hBuChE enzyme inhibitors. Eur. J. Med. Chem. 2020, 191, 112140. [Google Scholar] [CrossRef]
- Calderón Guzmán, D.; Osnaya Brizuela, N.; Ortiz Herrera, M.; Juarez Olguin, H.; Hernández García, E.; Valenzuela Peraza, A.; Barragan Mejia, G. Oleic acid protects against oxidative stress exacerbated by cytarabine and doxorubicin in rat brain. Anti-Cancer Agents Med. Chem. (Former Curr. Med. Chem.-Anti-Cancer Agents) 2016, 16, 1491–1495. [Google Scholar]
- Grossini, E.; Bellofatto, K.; Farruggio, S.; Sigaudo, L.; Marotta, P.; Raina, G.; De Giuli, V.; Mary, D.; Pollesello, P.; Minisini, R.; et al. Levosimendan Inhibits Peroxidation in Hepatocytes by Modulating Apoptosis/Autophagy Interplay. PLoS ONE 2015, 10, e0124742. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, Q.; Wang, N.; Yang, Y.; Liu, J.; Yu, G.; Yang, X.; Xu, H.; Wang, H. A complex micellar system co-delivering curcumin with doxorubicin against cardiotoxicity and tumor growth. Int. J. Nanomed. 2018, 13, 4549–4561. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.L.; Rosenzweig, B.A.; Zhang, J.; Knapton, A.D.; Honchel, R.; Lipshultz, S.E.; Retief, J.; Sistare, F.D.; Herman, E.H. Early alterations in heart gene expression profiles associated with doxorubicin cardiotoxicity in rats. Cancer Chemother. Pharmacol. 2009, 66, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Kruger, A.; Kleschyov, A.L.; Kalinowski, L.; Daiber, A.; Wojnowski, L. Gp91phox-containing N.A.D. (P) H oxidase increases superoxide formation by doxorubicin and NADPH. Free Radic. Biol. Med. 2007, 42, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Nitobe, J.; Yamaguchi, S.; Okuyama, M.; Nozaki, N.; Sata, M.; Miyamoto, T.; Takeishi, Y.; Kubota, I.; Tomoike, H. Reactive oxygen species regulate FLICE inhibitory protein (FLIP) and susceptibility to Fas-mediated apoptosis in cardiac myocytes. Cardiovasc. Res. 2003, 57, 119–128. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Beal, M.F. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann. Neurol. 1995, 38, 357–366. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).