Functional Grading of Mycelium Materials with Inorganic Particles: The Effect of Nanoclay on the Biological, Chemical and Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Species
2.2. Materials
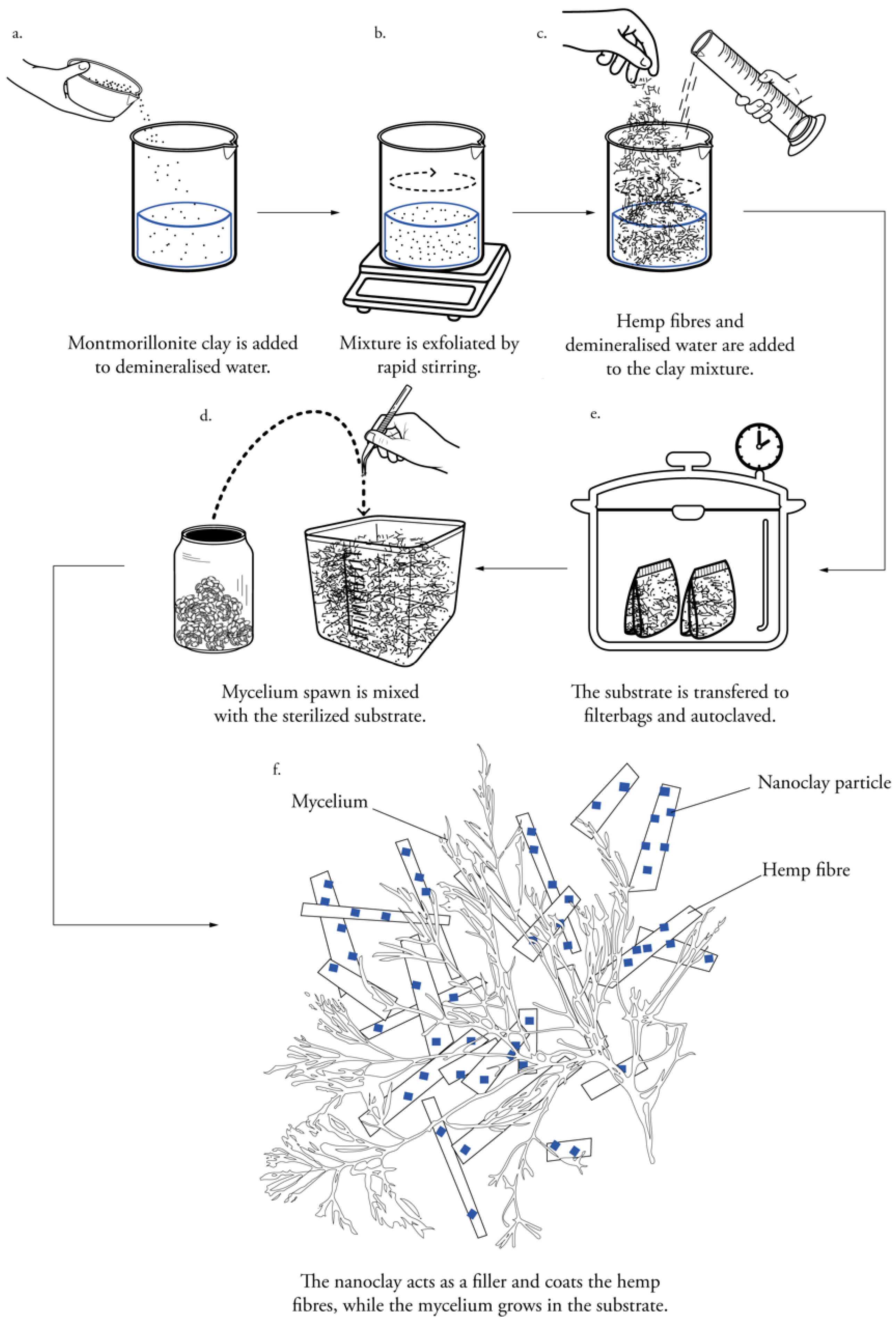
2.3. Preparation of Nanoclay-Coated Substrate
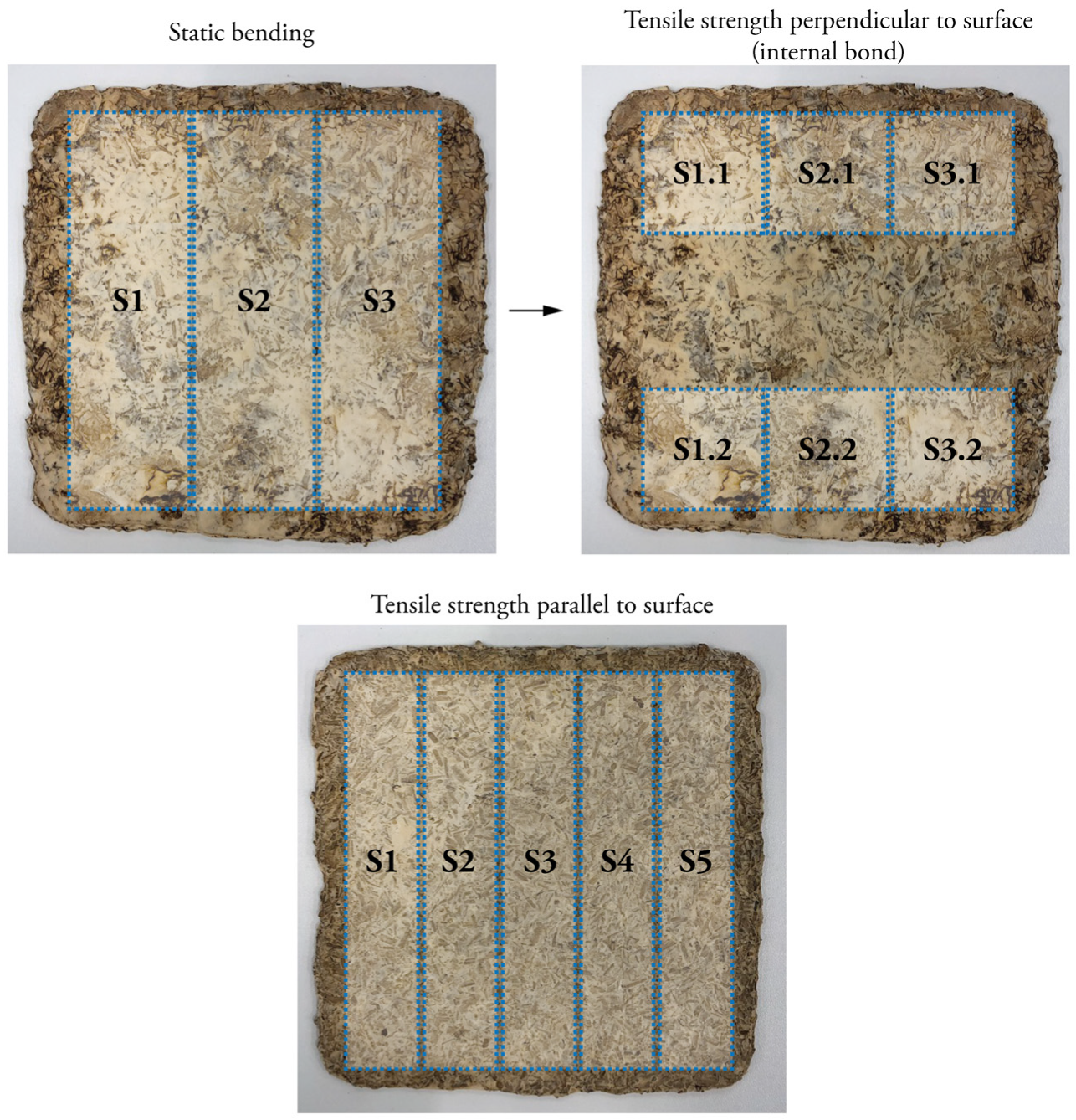
2.4. Particleboard Fabrication for Bending and Tensile Testing
2.5. Specimens for Compressive Testing
2.6. Substrate Inoculation for Growth Studies
2.7. Surface Colonisation Rate Measurements and Analysis
2.8. Scanning Electron Microscopy
2.9. Fourier Transform Infrared Spectroscopy
2.10. Determination of Bending Behaviour
2.11. Determination of Tensile Behaviour Parallel to the Surface
2.12. Determination of Tensile Behaviour Perpendicular to the Surface
2.13. Determination of Compressive Behaviour
2.14. Statistical Analysis
3. Results
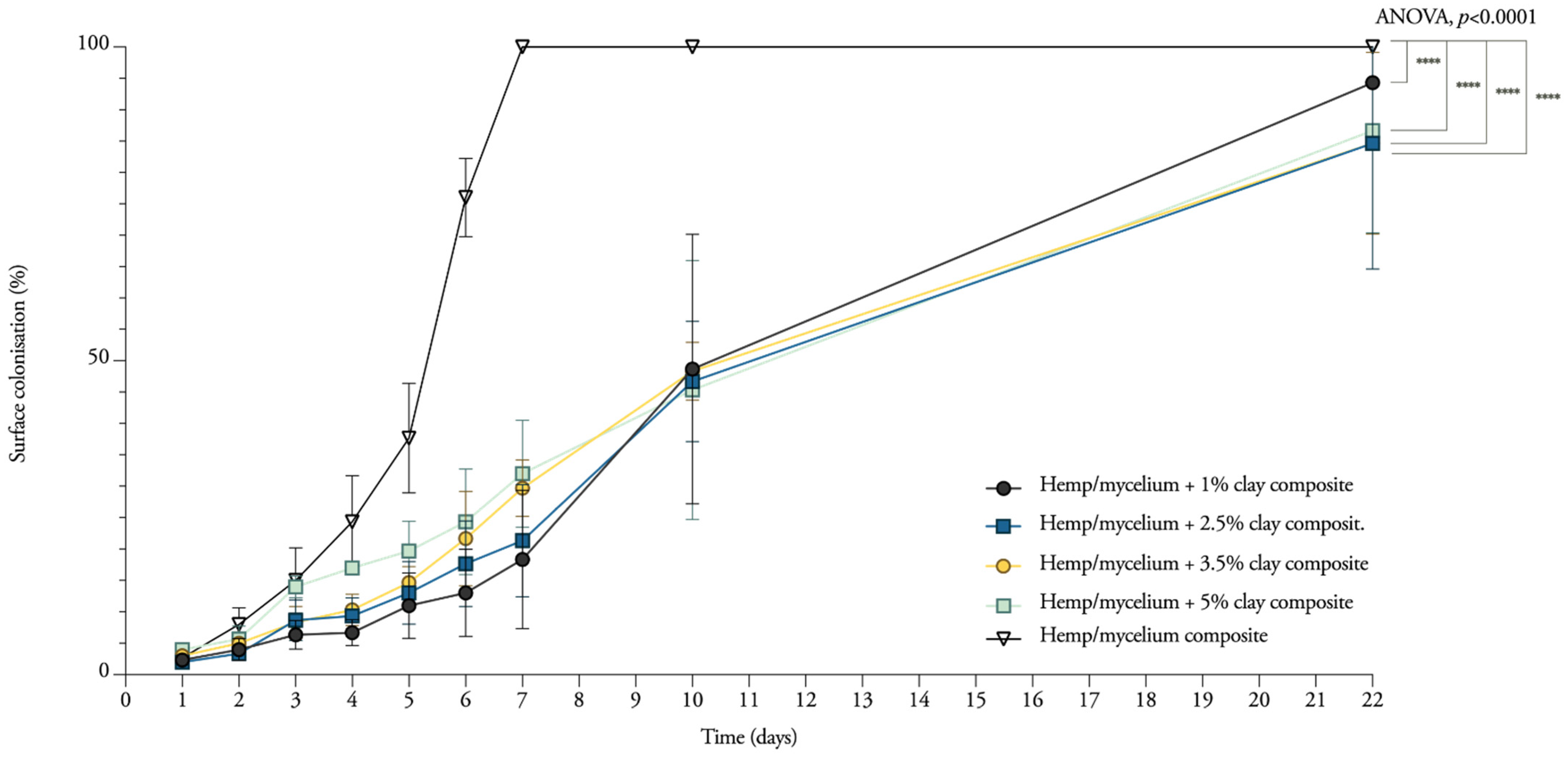
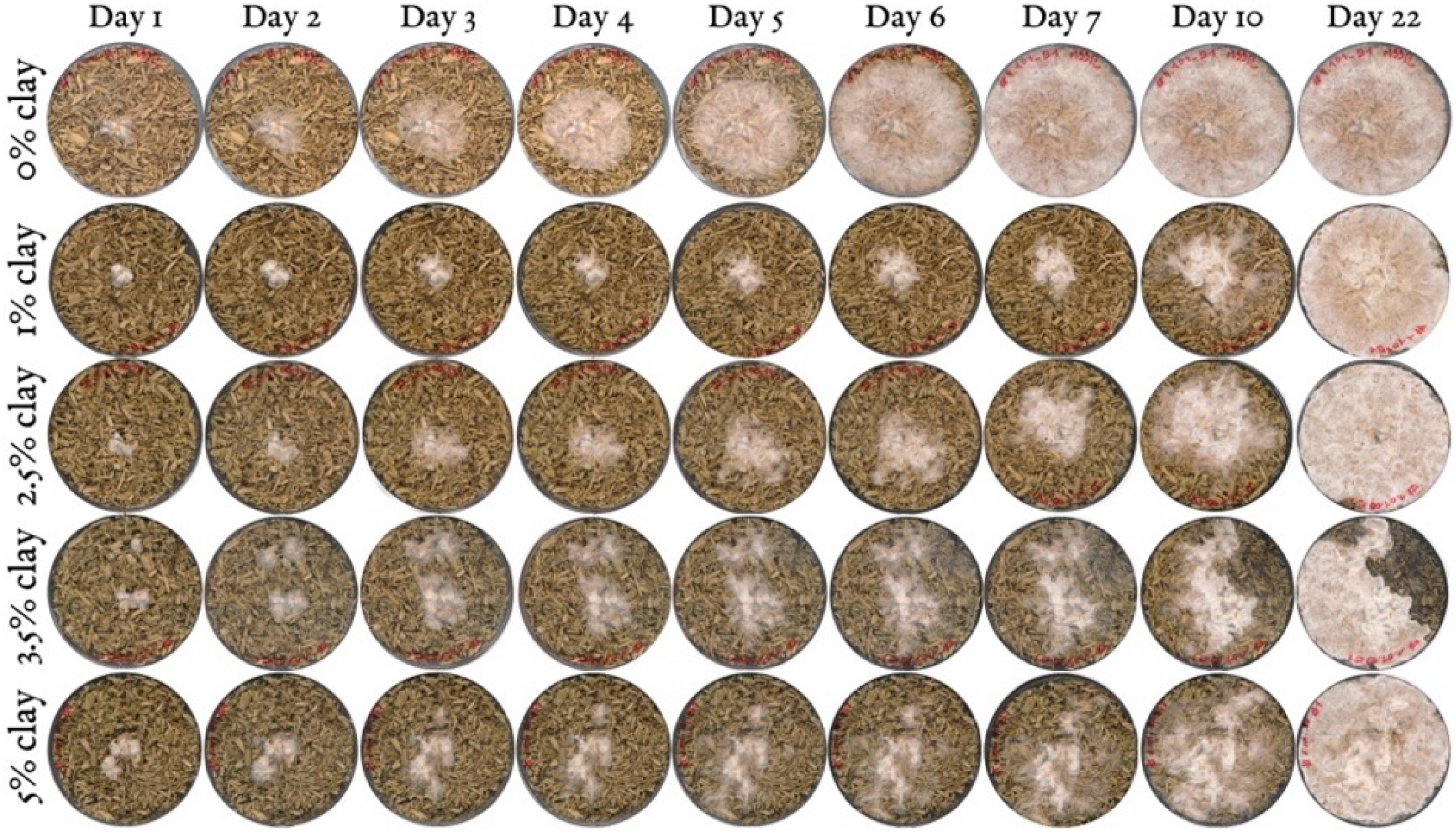
3.1. Mycelium Growth and Surface Colonisation
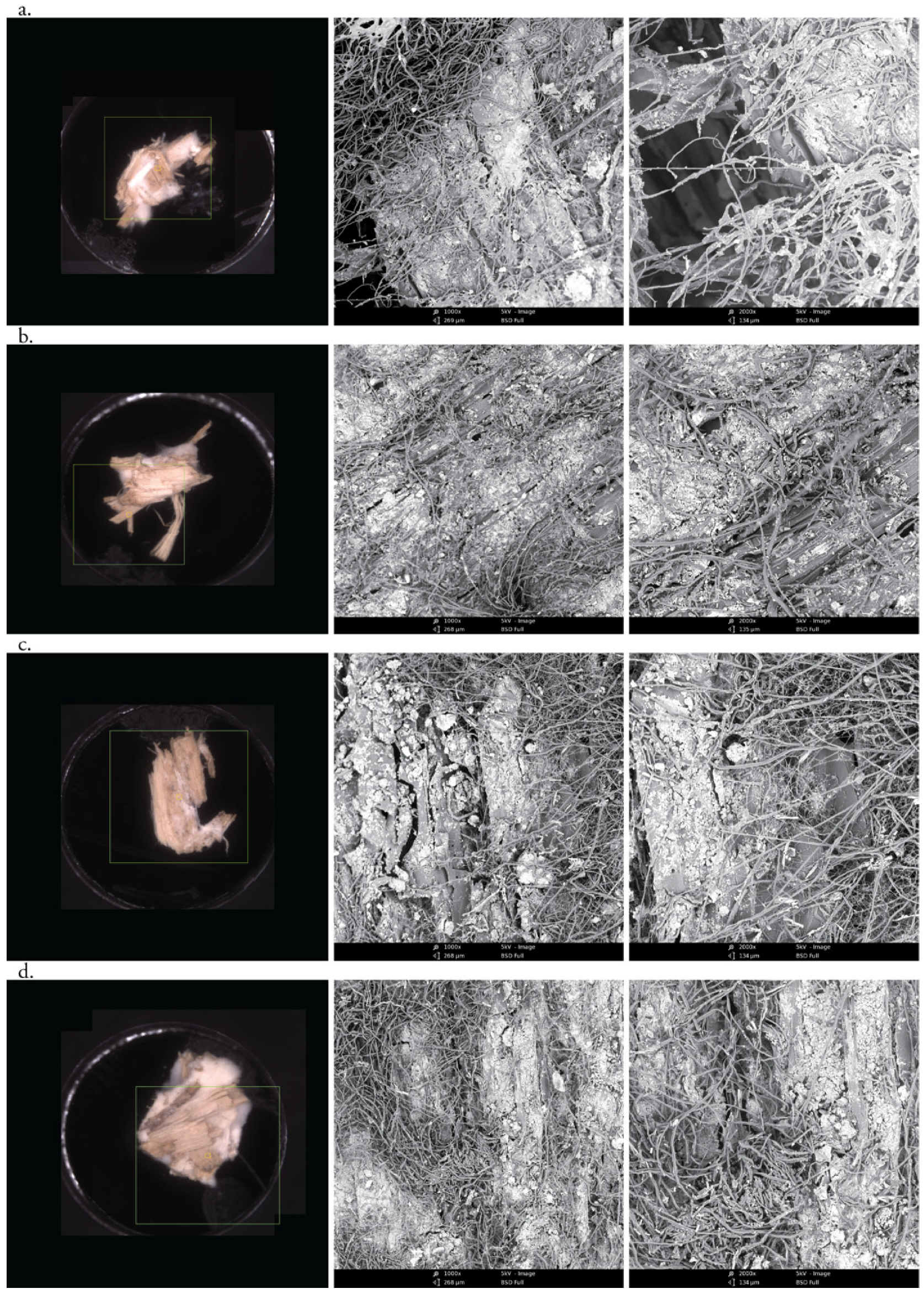
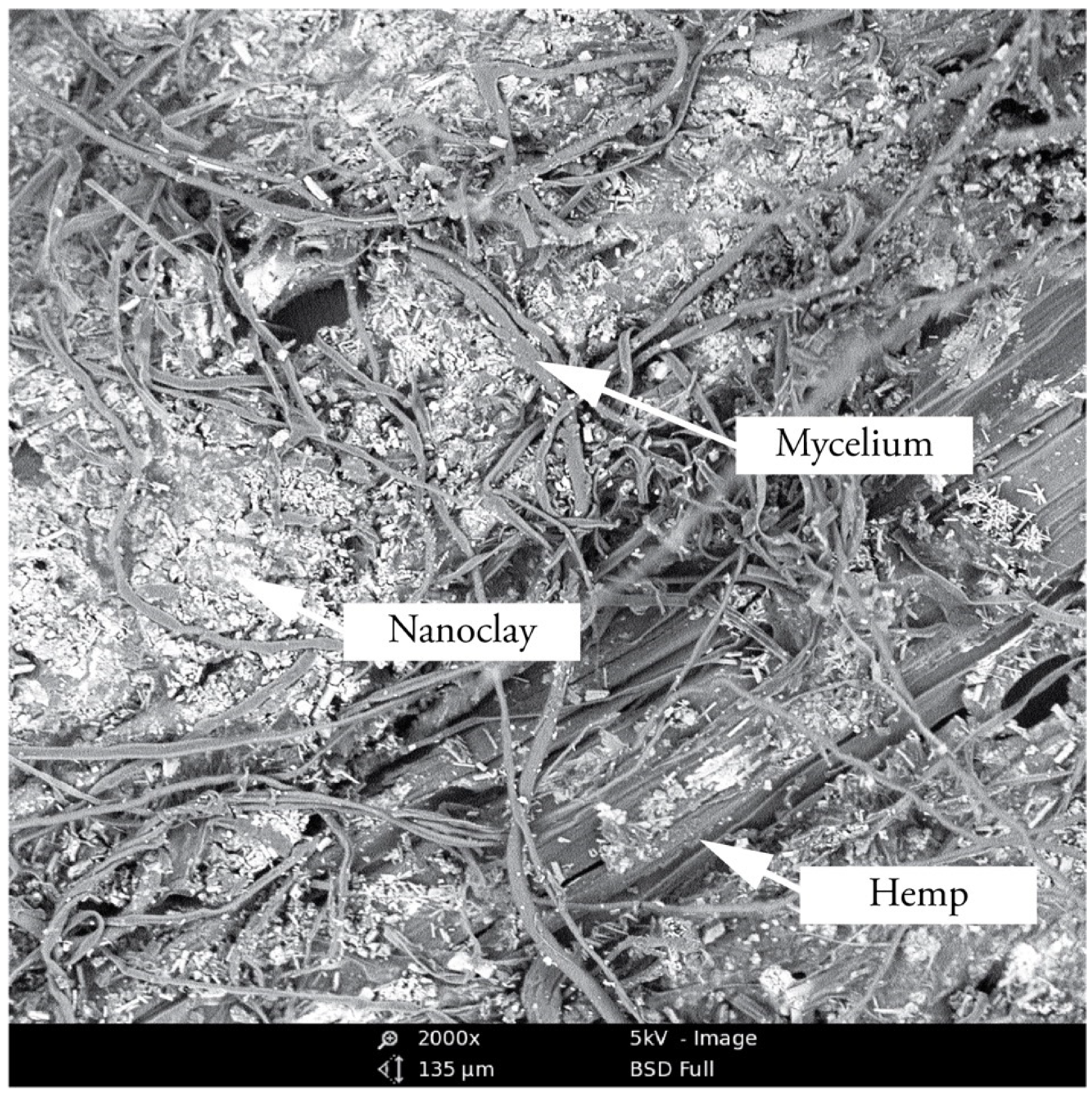
3.2. Morphological Analysis of Hyphae and Nanoclay
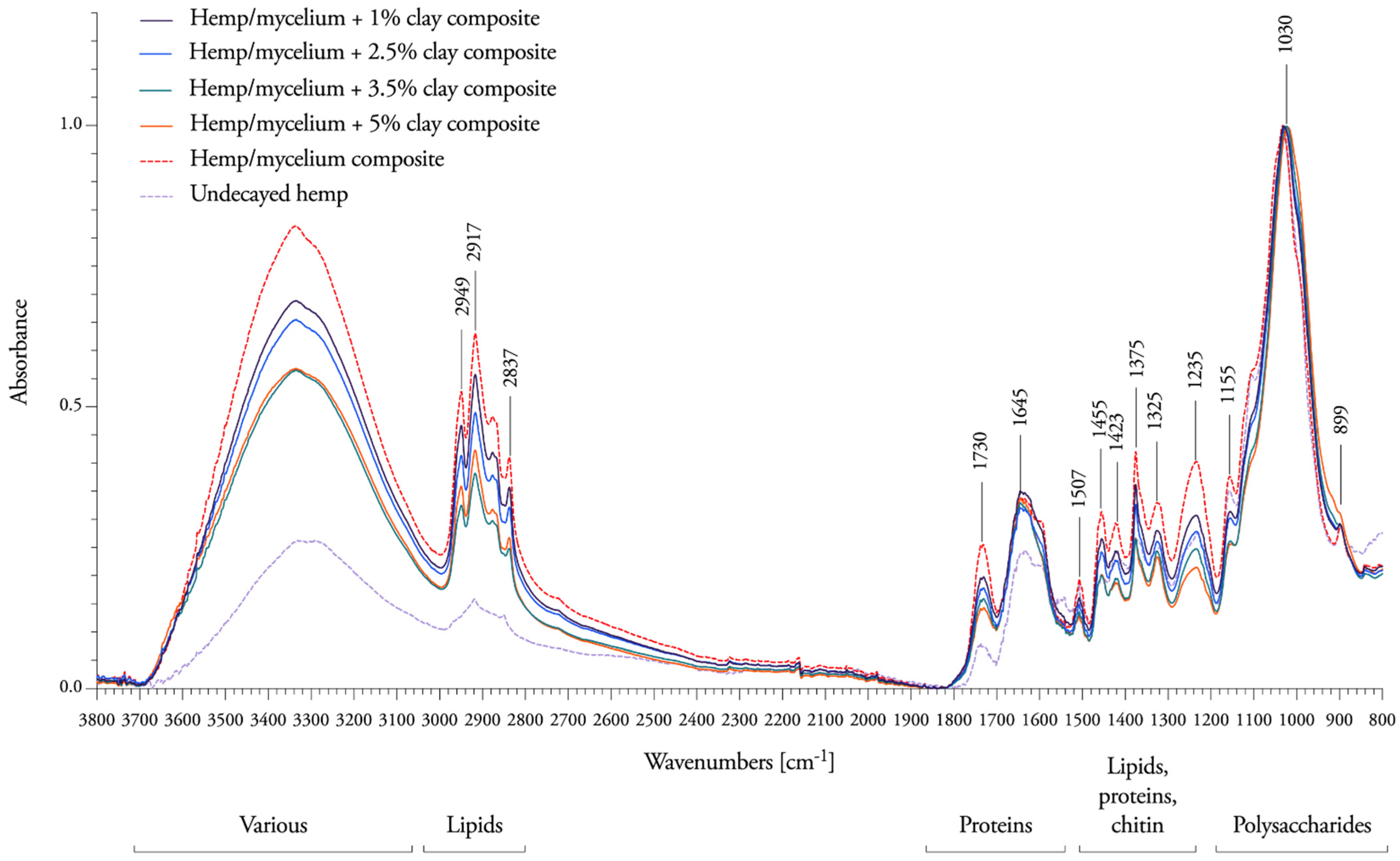
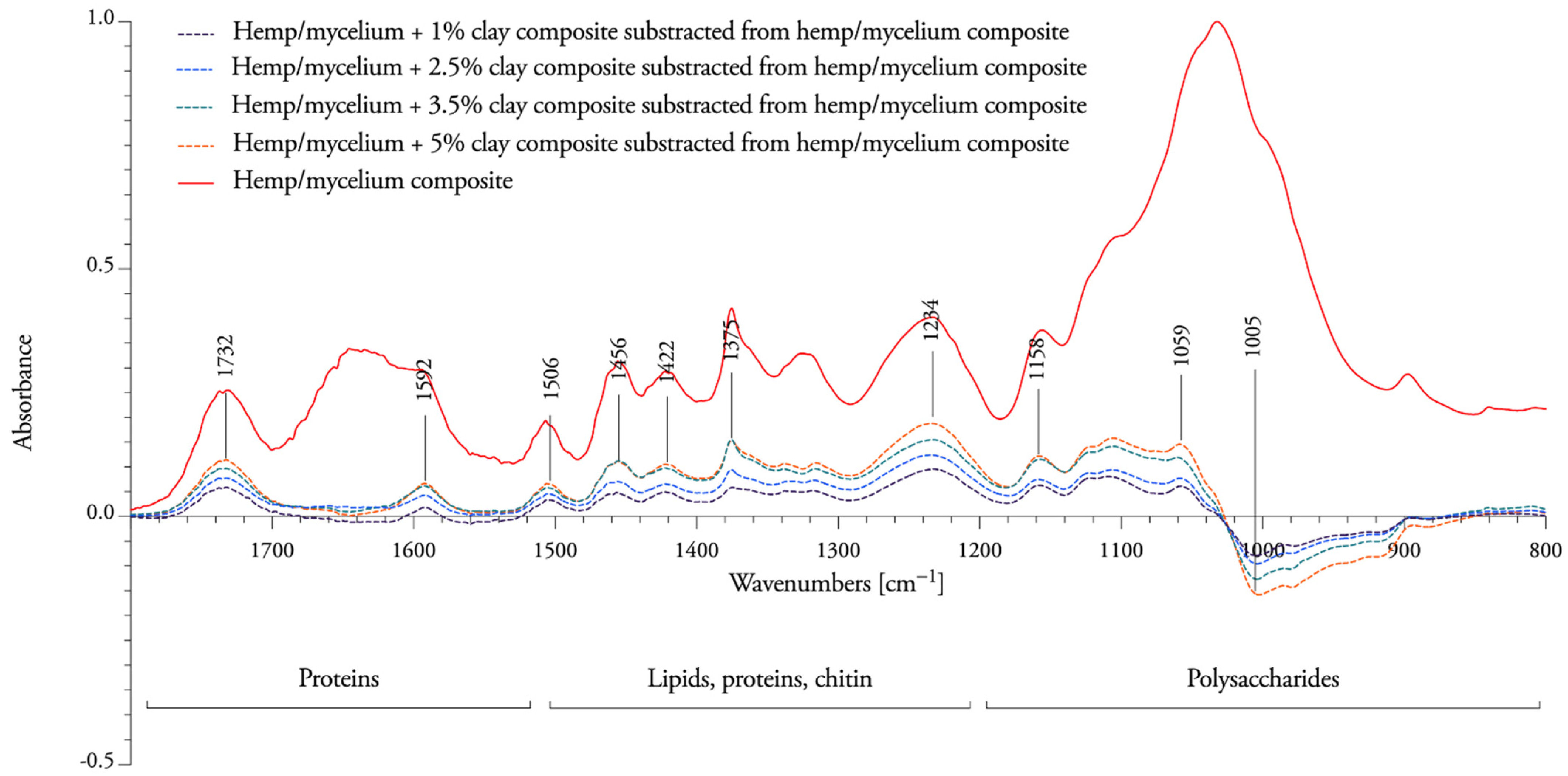
3.3. Spectral Response to Nanoclay Concentrations
3.4. Flexural Properties of Nanoclay-Mycelium Particleboards
3.5. Tensile Properties Parallel to the Surface
3.6. Tensile Properties Perpendicular to the Surface
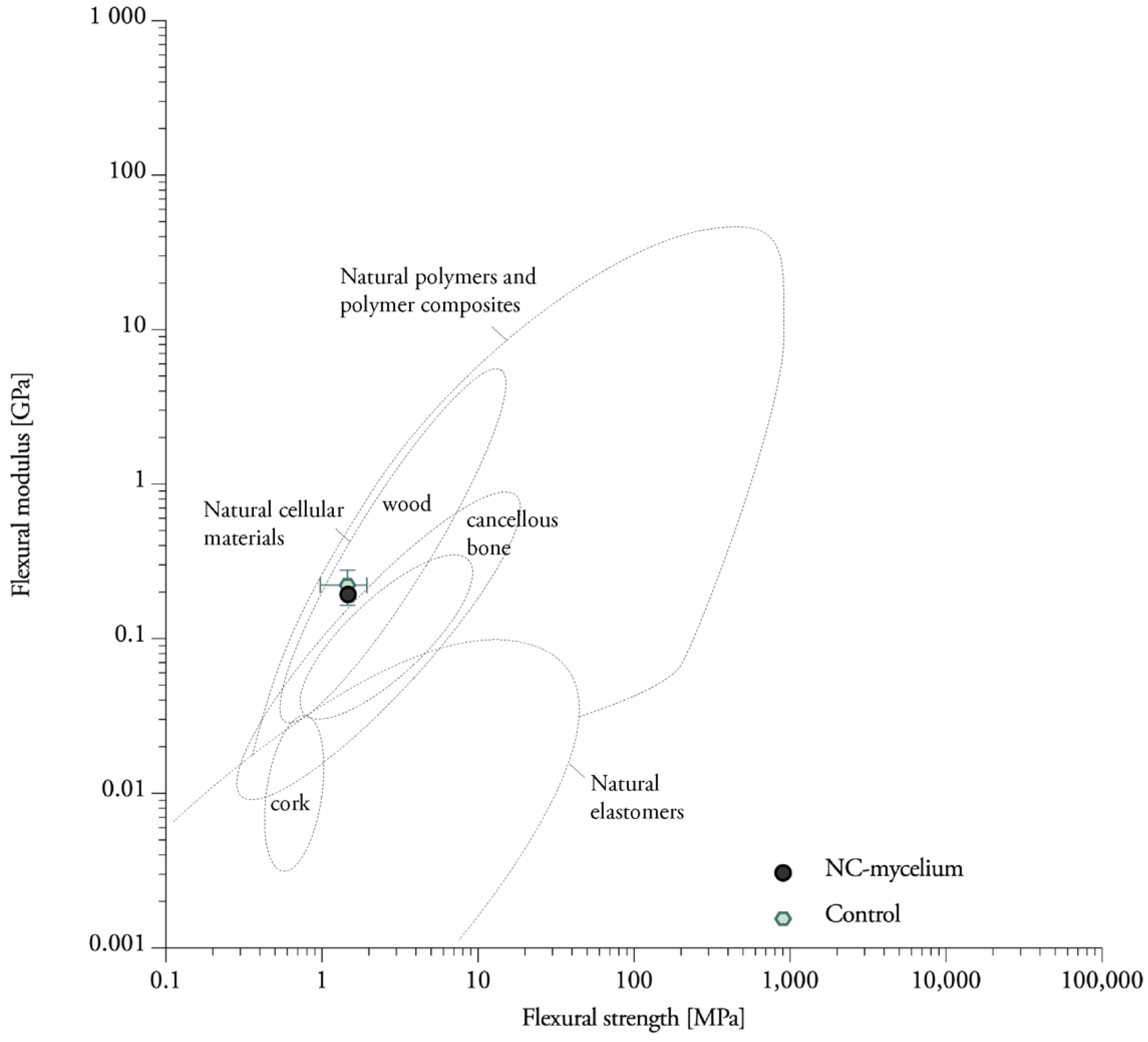
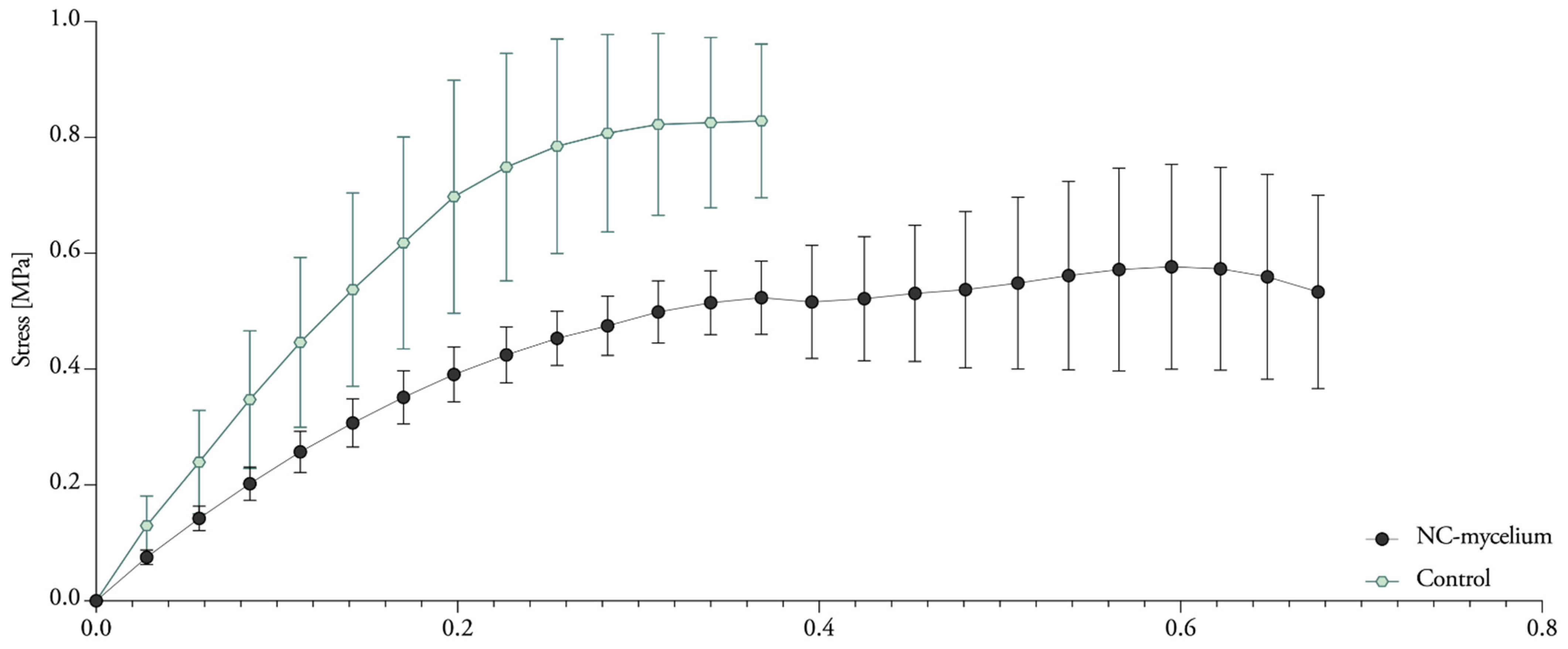
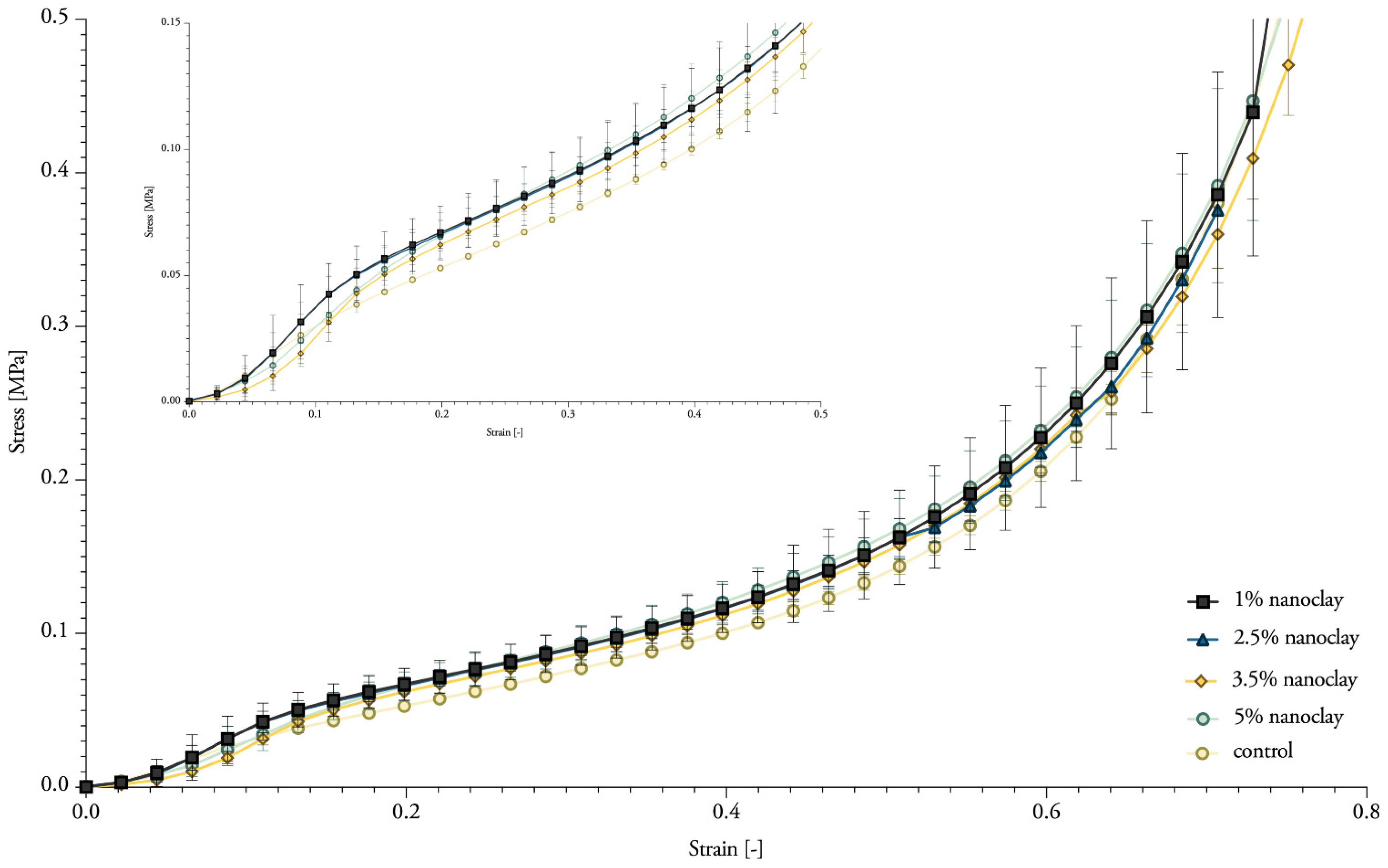
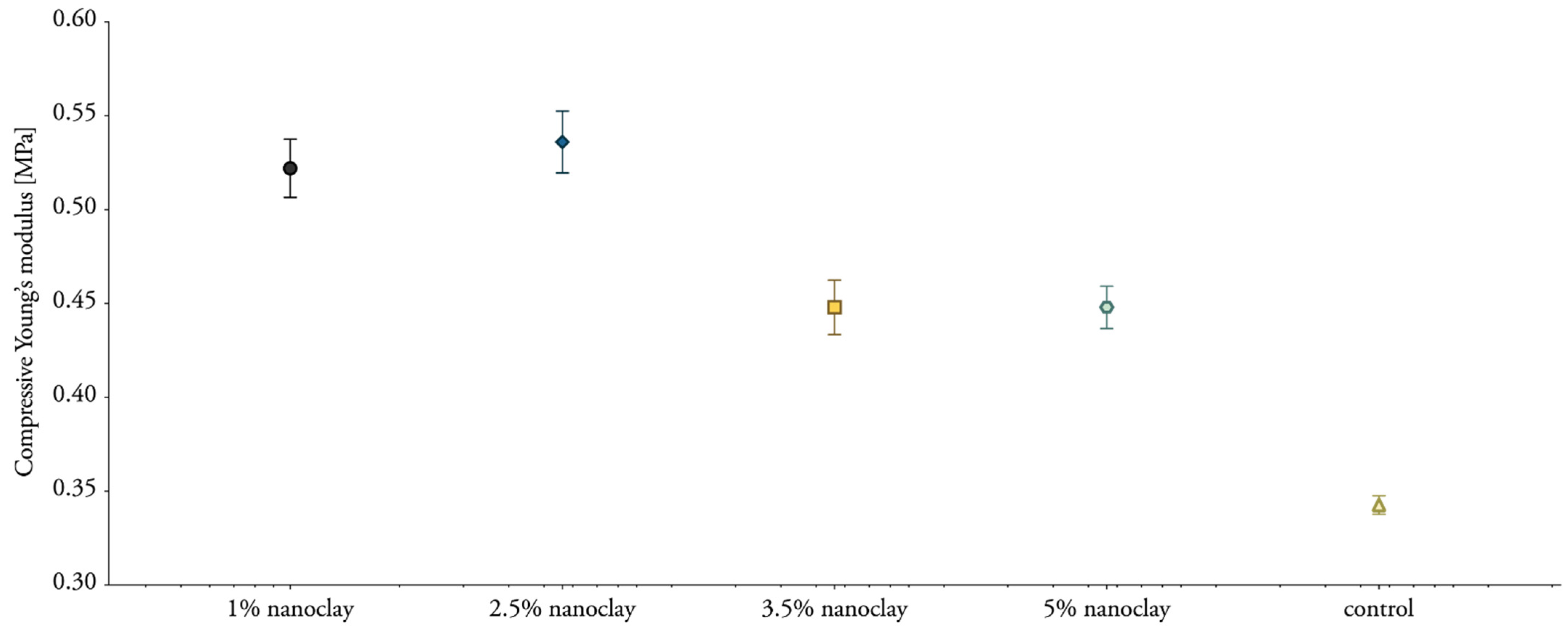
3.7. Compressive Properties
4. Discussion
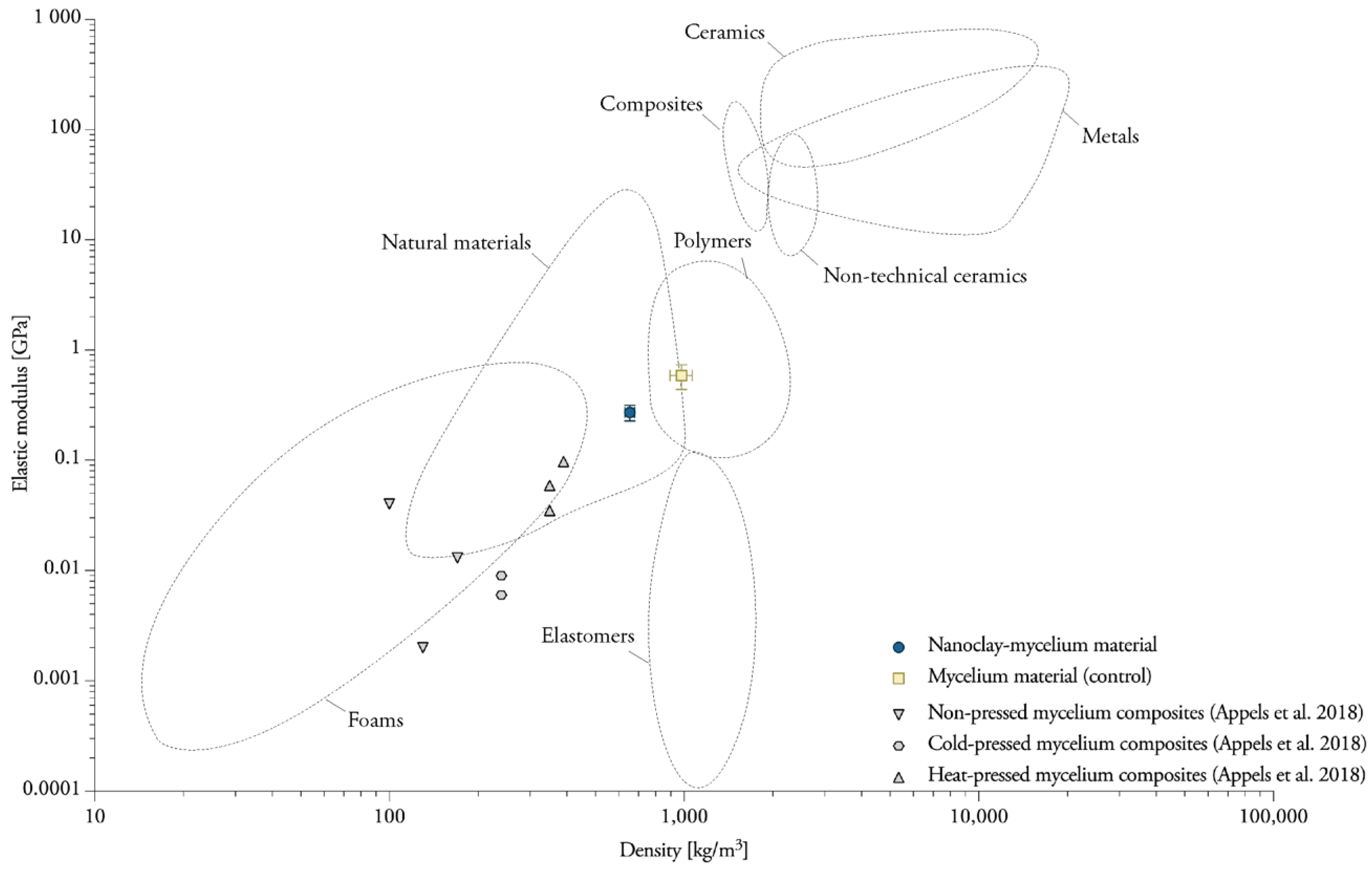
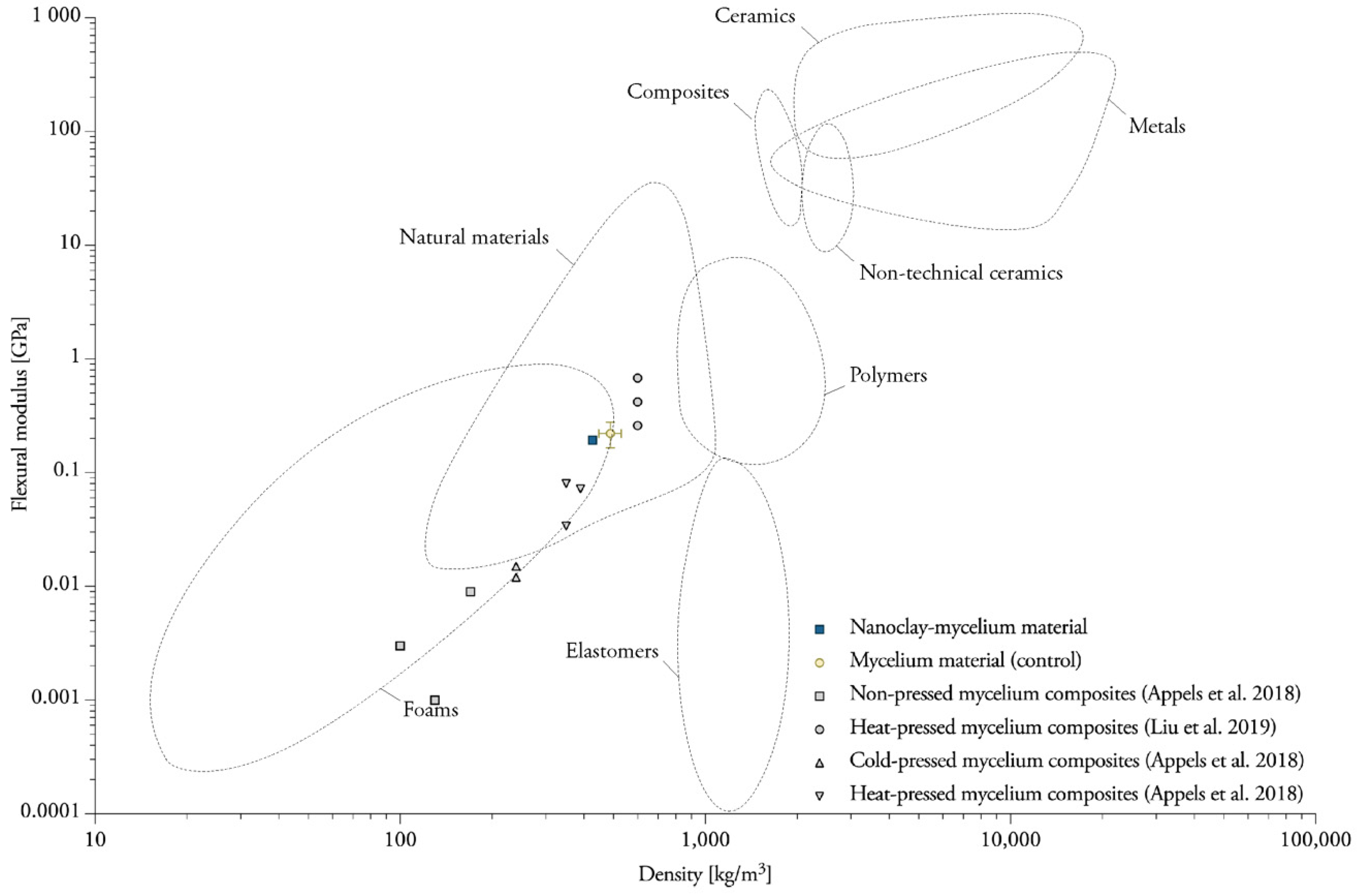
4.1. Nanoclay-Mycelium Materials Meet the Requirements of Softboards for the Flexure Index
4.2. Heat-Pressing Is a Major Factor in Increasing Tensile Properties
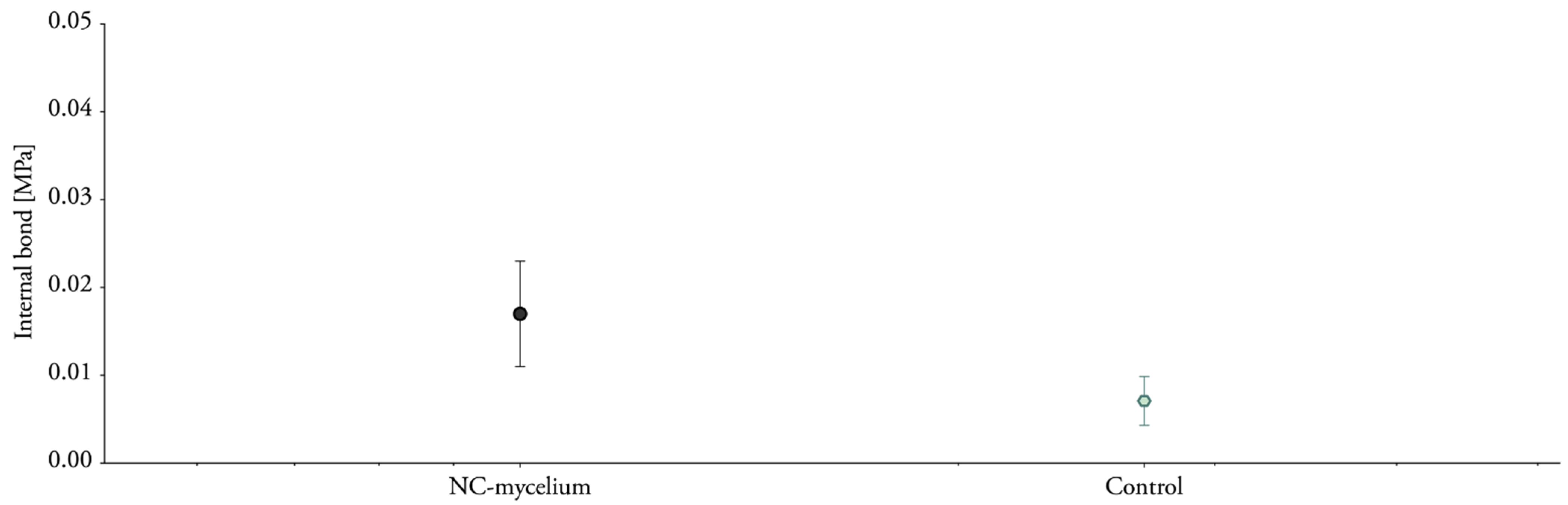
4.3. Mycelium Composites Have a Low Internal Bond
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elsacker, E.; Vandelook, S.; Brancart, J.; Peeters, E.; De Laet, L. Mechanical, Physical and Chemical Characterisation of Mycelium-Based Composites with Different Types of Lignocellulosic Substrates. PLoS ONE 2019, 14, e0213954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsacker, E. Mycelium Matters—An Interdisciplinary Exploration of the Fabrication and Properties of Mycelium-Based Materials. Ph.D. Thesis, VUBPRESS, Vrije Universiteit Brussel, Brussles, Belgium, 2021. [Google Scholar]
- Appels; Camere, S.; Montalti, M.; Karana, E.; Jansen, K.M.B.; Dijksterhuis, J.; Krijgsheld, P.; Wösten, H.A.B. Fabrication Factors Influencing Mechanical, Moisture- and Water-Related Properties of Mycelium-Based Composites. Mater. Des. 2018, 161, 64–71. [Google Scholar] [CrossRef]
- Islam, M.R.; Tudryn, G.; Bucinell, R.; Schadler, L.; Picu, R.C. Morphology and Mechanics of Fungal Mycelium. Sci. Rep. 2017, 7, 13070. [Google Scholar] [CrossRef] [Green Version]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.A.; Athanassiou, A. Advanced Materials from Fungal Mycelium: Fabrication and Tuning of Physical Properties. Sci. Rep. 2017, 7, 41292. [Google Scholar] [CrossRef]
- Jones, M.; Mautner, A.; Luenco, S.; Bismarck, A.; John, S. Engineered Mycelium Composite Construction Materials from Fungal Biorefineries: A Critical Review. Mater. Des. 2020, 187, 108397. [Google Scholar] [CrossRef]
- Attias, N.; Danai, O.; Abitbol, T.; Tarazi, E.; Ezov, N.; Pereman, I.; Grobman, Y.J. Mycelium Bio-Composites in Industrial Design and Architecture: Comparative Review and Experimental Analysis. J. Clean. Prod. 2020, 246, 119037. [Google Scholar] [CrossRef]
- Elsacker, E.; Vandelook, S.; Van Wylick, A.; Ruytinx, J.; De Laet, L.; Peeters, E. A Comprehensive Framework for the Production of Mycelium-Based Lignocellulosic Composites. Sci. Total Environ. 2020, 725, 138431. [Google Scholar] [CrossRef]
- Elsacker, E.; Vandelook, S.; Damsin, B.; Van Wylick, A.; Peeters, E.; De Laet, L. Mechanical Characteristics of Bacterial Cellulose-Reinforced Mycelium Composite Materials. Fungal Biol. Biotechnol. 2021, 8, 18. [Google Scholar] [CrossRef]
- Attias, N.; Reid, M.; Mijowska, S.C.; Dobryden, I.; Isaksson, M.; Pokroy, B.; Grobman, Y.J.; Abitbol, T. Biofabrication of Nanocellulose–Mycelium Hybrid Materials. Adv. Sustain. Syst. 2020, 5, 2000196. [Google Scholar] [CrossRef]
- Jones, M.; Bhat, T.; Huynh, T.; Kandare, E.; Yuen, R.; Wang, C.H.; John, S. Waste-Derived Low-Cost Mycelium Composite Construction Materials with Improved Fire Safety. Fire Mater. 2018, 42, 816–825. [Google Scholar] [CrossRef]
- Moser, F.J.; LWormit, A.; Reimer, J.J.; Jacobs, G.; Trautz, M.; Hillringhaus, F. Fungal Mycelium as a Building Material; Fachgruppe Biologie, Lehrstuhl für Botanik und Institut für Biologie I (Botanik), Lehrstuhl und Institut für Allgemeine Konstruktionstechnik des Maschinenbaus, Lehrstuhl für Tragkonstruktionen. In Proceedings of the Annual Symposium of the International Association for Shell and Spatial Structures (IASS 2017), Hamburg, Germany, 25–28 September 2017. [Google Scholar]
- Ross, P. Method for Producing Fungus Structures. U.S. Patent 9,410,116, 9 August 2016. [Google Scholar]
- Abend, S.; Lagaly, G. Sol-Gel Transitions of Sodium Montmorillonite Dispersions. Appl. Clay Sci. 1999, 16, 201–227. [Google Scholar] [CrossRef]
- Bensadoun, F.; Kchit, N.; Billotte, C.; Bickerton, S.; Trochu, F.; Ruiz, E. A Study of Nanoclay Reinforcement of Biocomposites Made by Liquid Composite Molding. Int. J. Polym. Sci. 2011, 2011, 964193. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.-F.; Fu, Y.-T. Effect of Montmorillonite on the Swelling Behavior and Drug-Release Behavior of Nanocomposite Hydrogels. J. Appl. Polym. Sci. 2003, 89, 3652–3660. [Google Scholar] [CrossRef]
- Kristak, L.; Antov, P.; Bekhta, P.; Lubis, M.A.R.; Iswanto, A.H.; Reh, R.; Sedliacik, J.; Savov, V.; Taghiyari, H.R.; Papadopoulos, A.N.; et al. Recent Progress in Ultra-Low Formaldehyde Emitting Adhesive Systems and Formaldehyde Scavengers in Wood-Based Panels: A Review. Wood Mater. Sci. Eng. 2022, 1–20. [Google Scholar] [CrossRef]
- Yano, K.; Usuki, A.; Okada, A.; Kurauchi, T.; Kamigaito, O. Synthesis and Properties of Polyimide-Clay Hybrid. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 2493–2498. [Google Scholar] [CrossRef]
- Yano, K.; Usuki, A.; Okada, A. Synthesis and Properties of Polyimide-Clay Hybrid Films. J. Polym. Sci. Part A Polym. Chem. 1997, 35, 2289–2294. [Google Scholar] [CrossRef]
- Lee, D.C.; Jang, L.W. Preparation and Characterization of PMMA-Clay Hybrid Composite by Emulsion Polymerization. J. Appl. Polym. Sci. 1996, 61, 1117–1122. [Google Scholar] [CrossRef]
- Okamoto, M.; Morita, S.; Taguchi, H.; Kim, Y.H.; Kotaka, T.; Tateyama, H. Synthesis and Structure of Smectic Clay/Poly(Methyl Methacrylate) and Clay/Polystyrene Nanocomposites via in Situ Intercalative Polymerization. Polymer 2000, 41, 3887–3890. [Google Scholar] [CrossRef]
- Fu, X.; Qutubuddin, S. Synthesis of Polystyrene-Clay Nanocomposites. Mater. Lett. 2000, 42, 12–15. [Google Scholar] [CrossRef]
- DePolo, W.S.; Baird, D.G. Particulate Reinforced PC/PBT Composites. II. Effect of Nano-Clay Particles on Dimensional Stability and Structure-Property Relationships. Polym. Compos. 2009, 30, 200–213. [Google Scholar] [CrossRef]
- Rangavar, H.; Taghiyari, H.R.; Oromiehie, A.; Gholipour, T.; Safarpour, A. Effects of Nanoclay on Physical and Mechanical Properties of Wood-Plastic Composites. Wood Mater. Sci. Eng. 2017, 12, 211–219. [Google Scholar] [CrossRef]
- Delhom, C.D.; White-Ghoorahoo, L.A.; Pang, S.S. Development and Characterization of Cellulose/Clay Nanocomposites. Compos. Part B Eng. 2010, 41, 475–481. [Google Scholar] [CrossRef]
- Gao, F. Clay/Polymer Composites: The Story. Mater. Today 2004, 7, 50–55. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, M. Polymer/Layered Silicate Nanocomposites: A Review from Preparation to Processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Utracki, L.A.; Sepehr, M.; Boccaleri, E. Synthetic, Layered Nanoparticles for Polymeric Nanocomposites (PNCs). Polym. Adv. Technol. 2007, 18, 1–37. [Google Scholar] [CrossRef] [Green Version]
- Dean, D.; Obore, A.M.; Richmond, S.; Nyairo, E. Multiscale Fiber-Reinforced Nanocomposites: Synthesis, Processing and Properties. Compos. Sci. Technol. 2006, 66, 2135–2142. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.-M.; Dubois, P. New Prospects in Flame Retardant Polymer Materials: From Fundamentals to Nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Bee, S.-L.; Abdullah, M.A.A.; Bee, S.-T.; Sin, L.T.; Rahmat, A.R. Polymer Nanocomposites Based on Silylated-Montmorillonite: A Review. Prog. Polym. Sci. 2018, 85, 57–82. [Google Scholar] [CrossRef]
- White, L.A. Preparation and Thermal Analysis of Cotton-Clay Nanocomposites. J. Appl. Polym. Sci. 2003, 92, 2125–2131. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- ISO 16978; Wood-Based Panels—Determination of Modulus of Elasticity in Bending and of Bending Strength. BSI: London, UK, 2003.
- ISO 12344; Thermal Insulating Products for Building Applications—Determination of Bending Behaviour. BSI: London, UK, 2010.
- ASTM D1037; Test Methods for Evaluating Properties of Wood-Base Fiber and Particle Panel Materials. ASTM International: West Conshohocken, PA, USA, 2020.
- EN 319; Particleboards and Fibreboards. Determination of Tensile Strength Perpendicular to the Plane of the Board. BSI: London, UK, 1993.
- ISO 29469; Thermal Insulating Products for Building Applications—Determination of Compression Behaviour. BSI: London, UK, 2008.
- Bari, E.; Taghiyari, H.R.; Schmidt, O.; Ghorbani, A.; Aghababaei, H. Effects of Nano-Clay on Biological Resistance of Wood-Plastic Composite against Five Wood-Deteriorating Fungi. Maderas Cienc. Tecnol. 2015, 17, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Kord, B.; Jari, E.; Najafi, A.; Tazakorrezaie, V. Effect of Nanoclay on the Decay Resistance and Physicomechanical Properties of Natural Fiber-Reinforced Plastic Composites against White-Rot Fungi (Trametes Versicolor). J. Thermoplast. Compos. Mater. 2014, 27, 1085–1096. [Google Scholar] [CrossRef]
- Luo, F.; Zhong, Z.; Liu, L.; Igarashi, Y.; Xie, D.; Li, N. Metabolomic Differential Analysis of Interspecific Interactions among White Rot Fungi Trametes Versicolor, Dichomitus Squalens and Pleurotus Ostreatus. Sci. Rep. 2017, 7, 5265. [Google Scholar] [CrossRef] [Green Version]
- Wegst, U.G.K.; Ashby, M.F. The Mechanical Efficiency of Natural Materials. Philos. Mag. 2004, 84, 2167–2186. [Google Scholar] [CrossRef]
- Ng, B.H.; Chou, S.M.; Krishna, V. The Influence of Gripping Techniques on the Tensile Properties of Tendons. Proc. Inst. Mech. Eng. H 2005, 219, 349–354. [Google Scholar] [CrossRef]
- Liu, R.; Long, L.; Sheng, Y.; Xu, J.; Qiu, H.; Li, X.; Wang, Y.; Wu, H. Preparation of a Kind of Novel Sustainable Mycelium/Cotton Stalk Composites and Effects of Pressing Temperature on the Properties. Ind. Crops Prod. 2019, 141, 111732. [Google Scholar] [CrossRef]
- Holt, G.A.; Mcintyre, G.; Flagg, D.; Bayer, E.; Wanjura, J.D.; Pelletier, M.G. Fungal Mycelium and Cotton Plant Materials in the Manufacture of Biodegradable Molded Packaging Material: Evaluation Study of Select Blends of Cotton Byproducts. J. Biobased Mater. Bioenergy 2012, 6, 431–439. [Google Scholar] [CrossRef]
- López Nava, J.A.; Méndez González, J.; Ruelas Chacón, X.; Nájera Luna, J.A. Assessment of Edible Fungi and Films Bio-Based Material Simulating Expanded Polystyrene. Mater. Manuf. Processes 2016, 31, 1085–1090. [Google Scholar] [CrossRef]
- Ziegler, A.R.; Bajwa, S.G.; Holt, G.A.; McIntyre, G.; Bajwa, D.S. Evaluation of Physico-Mechanical Properties of Mycelium Reinforced Green Biocomposites Made from Cellulosic Fibers. Appl. Eng. Agric. 2016, 32, 931–938. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Cao, J. Effects of Modifier Type on Properties of in Situ Organo-Montmorillonite Modified Wood Flour/Poly(Lactic Acid) Composites. ACS Appl. Mater. Interfaces 2016, 8, 161–168. [Google Scholar] [CrossRef]
- Appels; Dijksterhuis, J.; Lukasiewicz, C.E.; Jansen, K.M.B.; Wösten, H.A.B.; Krijgsheld, P. Hydrophobin Gene Deletion and Environmental Growth Conditions Impact Mechanical Properties of Mycelium by Affecting the Density of the Material. Sci. Rep. 2018, 8, 4704. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liu, M.; Hu, S.; Huang, A.; Ma, E. Comparison of Six WPCs Made of Organo-Montmorillonite-Modified Fibers of Four Trees, Moso Bamboo and Wheat Straw and Poly(Lactic Acid) (PLA). Holzforschung 2018, 72, 735–744. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. Effect of Reactive Organoclay on Physicochemical Properties of Vegetable Oil-Based Waterborne Polyurethane Nanocomposites. RSC Adv. 2015, 5, 11524–11533. [Google Scholar] [CrossRef]
- Sun, W.; Tajvidi, M.; Hunt, C.G.; McIntyre, G.; Gardner, D.J. Fully Bio-Based Hybrid Composites Made of Wood, Fungal Mycelium and Cellulose Nanofibrils. Sci. Rep. 2019, 9, 3766. [Google Scholar] [CrossRef] [PubMed]
- Ashori, A.; Nourbakhsh, A. Effects of Nanoclay as a Reinforcement Filler on the Physical and Mechanical Properties of Wood-Based Composite. J. Compos. Mater. 2009, 43, 1869–1875. [Google Scholar] [CrossRef]
- Salari, A.; Tabarsa, T.; Khazaeian, A.; Saraeian, A. Effect of Nanoclay on Some Applied Properties of Oriented Strand Board (OSB) Made from Underutilized Low Quality Paulownia (Paulownia Fortunei) Wood. J. Wood Sci. 2012, 58, 513–524. [Google Scholar] [CrossRef]
- Ismita, N.; Lokesh, C. Effects of Different Nanoclay Loadings on the Physical and Mechanical Properties of Melia Composita Particle Board. Bois. Trop. 2018, 334, 7. [Google Scholar] [CrossRef] [Green Version]
- Lei, H.; Du, G.; Pizzi, A.; Celzard, A. Influence of Nanoclay on Urea-Formaldehyde Resins for Wood Adhesives and Its Model. J. Appl. Polym. Sci. 2008, 109, 2442–2451. [Google Scholar] [CrossRef]
- Ashby, M. Designing Architectured Materials. Scr. Mater. 2013, 68, 4–7. [Google Scholar] [CrossRef]

| Label | Dry Density [kg/m3] | Flexural Strength [MPa] | Flexural Modulus [GPa] |
|---|---|---|---|
| NC-mycelium hemp composite | 426.98 ± 9.19 | 1.47 ± 0.02 | 0.19 ± 0.002 |
| Mycelium hemp composite (control) | 488.89 ± 41.09 | 1.46 ± 0.48 | 0.22 ± 0.06 |
| Label | Dry Density [kg/m3] | Ultimate Tensile Strength [MPa] | Specific Tensile Strength [kN·m/kg] | Elastic Modulus [GPa] | Specific Modulus [106 m2 s−2] |
|---|---|---|---|---|---|
| NC-mycelium hemp composite | 654.55 ± 24.39 | 0.62 ± 0.10 | 0.95 ± 0.17 | 0.27 ± 0.04 | 0.42 ± 0.08 |
| Mycelium hemp composite (control) | 980.67 ± 84.77 | 1.14 ± 0.13 | 1.15 ± 0.07 | 0.59 ± 0.15 | 0.61 ± 0.17 |
| Label | Dry Density [kg/m3] | Ultimate Strength [MPa] | Specific Strength [kN·m/kg] | Elastic Modulus [GPa] | Specific Modulus [106 m2 s−2] |
|---|---|---|---|---|---|
| NC-mycelium | 340.85 ± 2.09 | 0.017 ± 0.006 | 0.049 ± 0.017 | 0.003 ± 0.000 | 0.009 ± 0.001 |
| Control | 492.32 ± 45.40 | 0.007 ± 0.003 | 0.01 ± 0.006 | 0.005 ± 0.002 | 0.01 ± 0.0007 |
| Label | Dry Density [kg/m3] | Compressive Strength [Mpa] | Corresponding Strain [%] | Young’s Modulus [Mpa] |
|---|---|---|---|---|
| 1% NC-mycelium | 180.14 ± 0.01 | 0.12354 | 42.85 | 0.52 ± 0.02 |
| 2.5% NC-mycelium | 183.16 ± 0.08 | 0.12356 | 42.52 | 0.54 ± 0.02 |
| 3.5% NC-mycelium | 176.82 ± 0.02 | 0.11932 | 42.41 | 0.45 ± 0.01 |
| 5% NC-mycelium | 175.31 ± 0.02 | 0.12804 | 43.00 | 0.45 ± 0.01 |
| Control | 172.34 ± 0.04 | 0.10715 | 43.50 | 0.34 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsacker, E.; De Laet, L.; Peeters, E. Functional Grading of Mycelium Materials with Inorganic Particles: The Effect of Nanoclay on the Biological, Chemical and Mechanical Properties. Biomimetics 2022, 7, 57. https://doi.org/10.3390/biomimetics7020057
Elsacker E, De Laet L, Peeters E. Functional Grading of Mycelium Materials with Inorganic Particles: The Effect of Nanoclay on the Biological, Chemical and Mechanical Properties. Biomimetics. 2022; 7(2):57. https://doi.org/10.3390/biomimetics7020057
Chicago/Turabian StyleElsacker, Elise, Lars De Laet, and Eveline Peeters. 2022. "Functional Grading of Mycelium Materials with Inorganic Particles: The Effect of Nanoclay on the Biological, Chemical and Mechanical Properties" Biomimetics 7, no. 2: 57. https://doi.org/10.3390/biomimetics7020057
APA StyleElsacker, E., De Laet, L., & Peeters, E. (2022). Functional Grading of Mycelium Materials with Inorganic Particles: The Effect of Nanoclay on the Biological, Chemical and Mechanical Properties. Biomimetics, 7(2), 57. https://doi.org/10.3390/biomimetics7020057