Sponge-like Scaffolds for Colorectal Cancer 3D Models: Substrate-Driven Difference in Micro-Tumors Morphology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Cryogels
2.3. Characterization of Hydrogels
2.3.1. Elemental Analysis
2.3.2. Rheological Properties and Swelling
2.3.3. Cell Cultivation
2.3.4. Permeability of Cryogels
2.3.5. Cell Staining and Flow Cytometry
2.3.6. Confocal Laser Scanning Microscopy (CLSM)
3. Results and Discussion
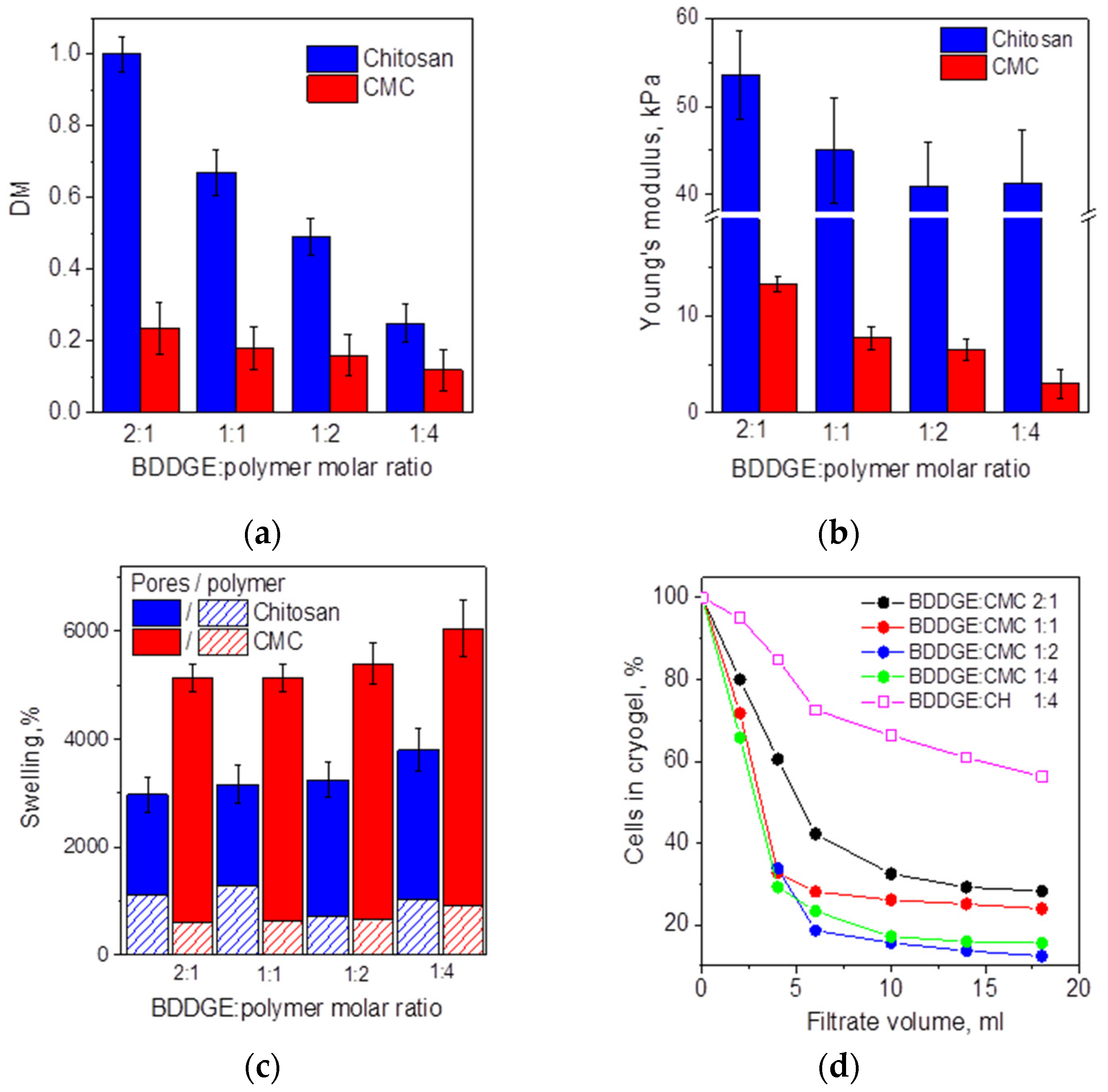
3.1. Fabrication of Cryogels via Chitosan and CMC Cross-Linking with BDDGE
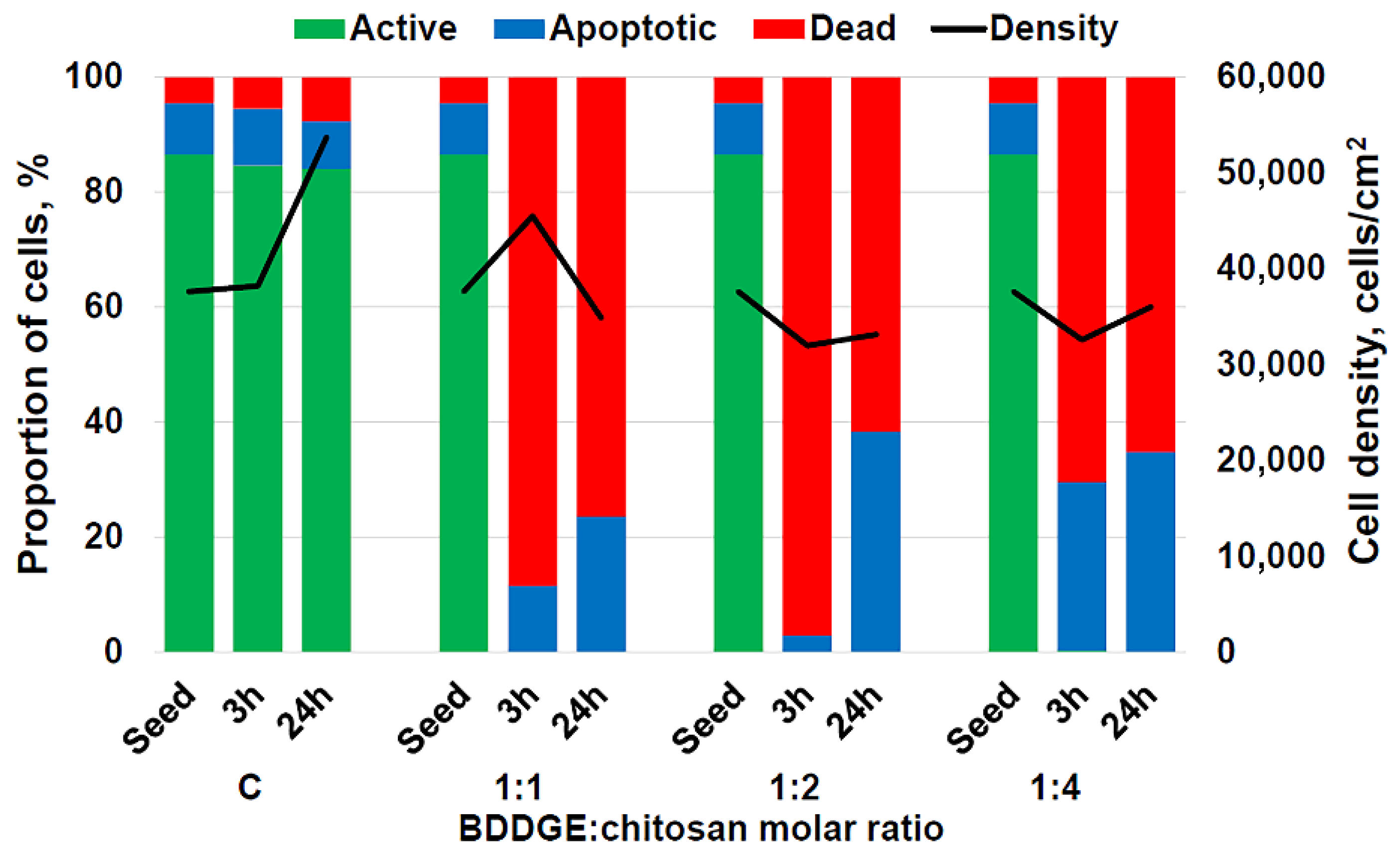
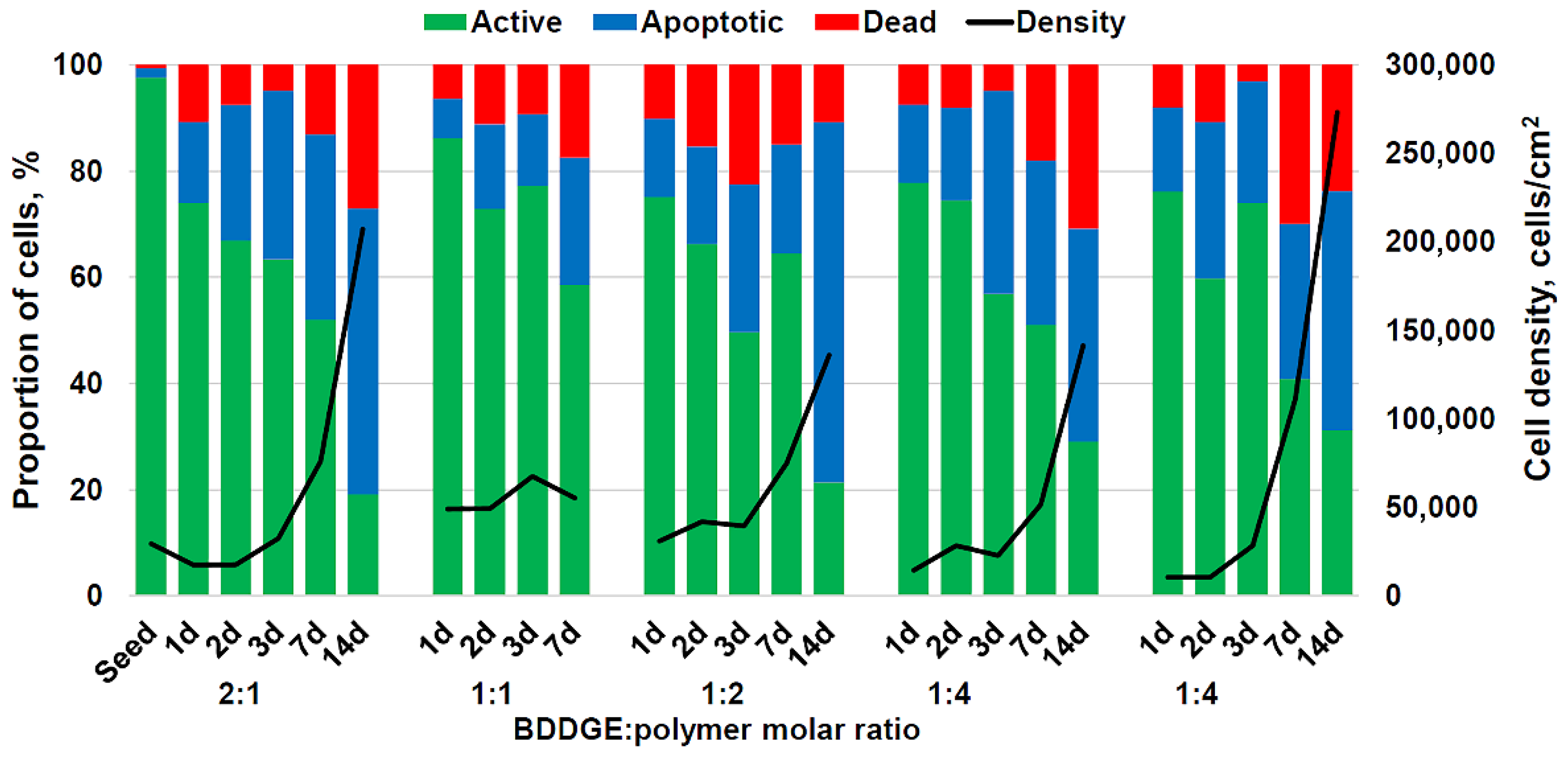
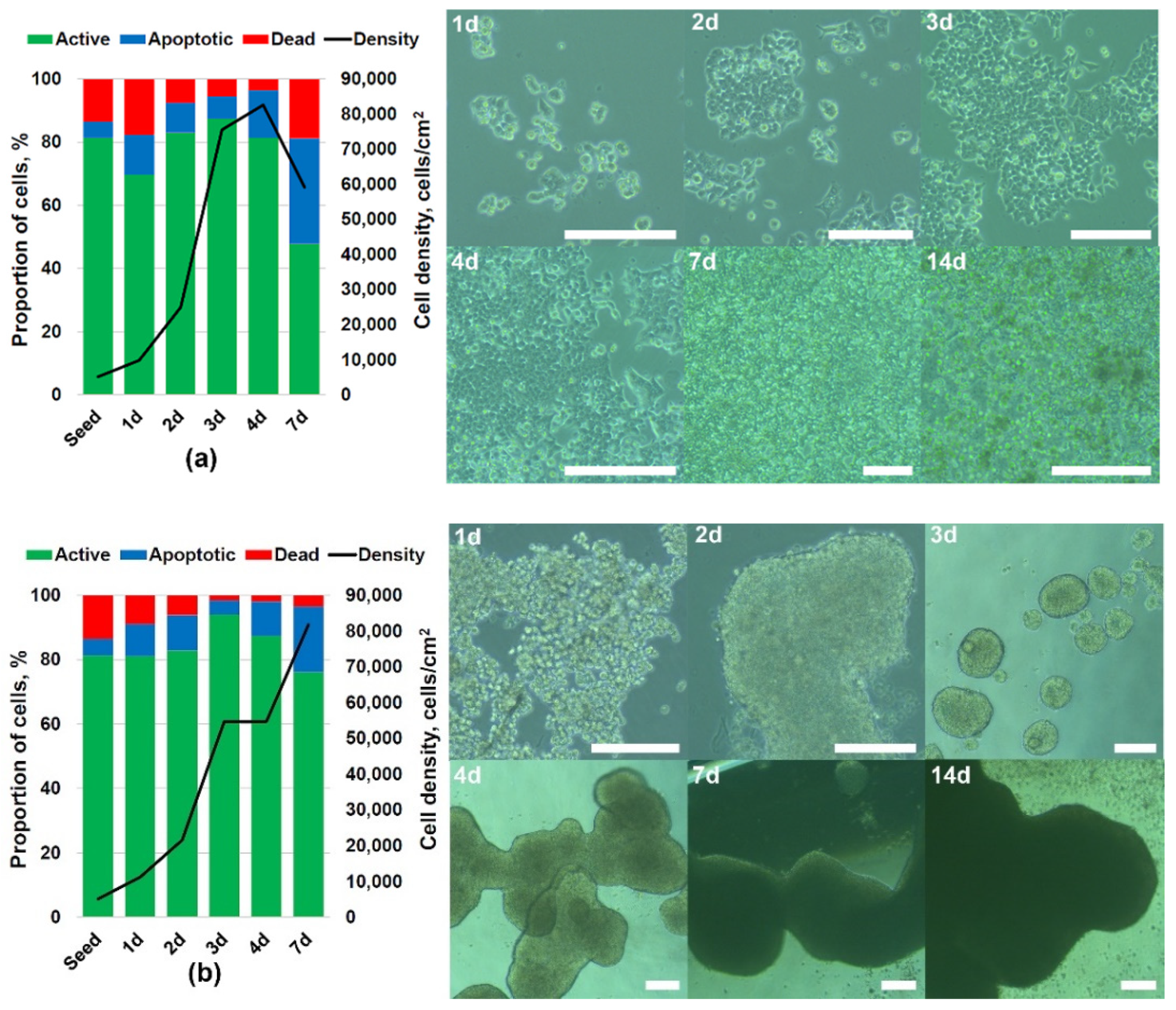
3.2. 3D Culturing of HCT 116 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, J.Y.; Tan, S.J.; Yang, Z.; Tayag, C.; Han, B. Tumor bioengineering using a transglutaminase crosslinked hydrogel. PLoS ONE 2014, 9, e105616. [Google Scholar] [CrossRef]
- Ivanovska, J.; Zehnder, T.; Lennert, P.; Sarker, B.; Boccaccini, A.R.; Hartmann, A.; Schneider-Stock, R.; Detsch, R. Biofabrication of 3D Alginate-Based Hydrogel for Cancer Research: Comparison of Cell Spreading, Viability, and Adhesion Characteristics of Colorectal HCT116 Tumor Cells. Tissue Eng.-Part C Methods 2016, 22, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Dainiak, M.B.; Savina, I.N.; Musolino, I.; Kumar, A.; Mattiasson, B.; Galaev, I.Y. Biomimetic macroporous hydrogel scaffolds in a high-throughput screening format for cell-based assays. Biotechnol. Prog. 2008, 24, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Sedlačík, T.; Proks, V.; Šlouf, M.; Dušková-Smrčková, M.; Studenovská, H.; Rypáček, F. Macroporous Biodegradable Cryogels of Synthetic Poly(α-amino acids). Biomacromolecules 2015, 16, 3455–3465. [Google Scholar] [CrossRef] [PubMed]
- Reidy, E.; Leonard, N.A.; Treacy, O.; Ryan, A.E. A 3D view of colorectal cancer models in predicting therapeutic responses and resistance. Cancers 2021, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Ling, Y.; Deng, W.; Xue, J.; Sun, P.; Wang, D.-A. A novel cell encapsulatable cryogel (CECG) with macro-porous structures and high permeability: A three-dimensional cell culture scaffold for enhanced cell adhesion and proliferation. Biomed. Mater. 2019, 14, 055006. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Abdeen, A.A.; Wycislo, K.L.; Fan, T.M.; Kilian, K.A. Interfacial geometry dictates cancer cell tumorigenicity. Nat. Mater. 2016, 15, 856–862. [Google Scholar] [CrossRef]
- Hamada, K.; Monnai, M.; Kawai, K.; Nishime, C.; Kito, C.; Miyazaki, N.; Ohnishi, Y.; Nakamura, M.; Suemizu, H. Liver metastasis models of colon cancer for evaluation of drug efficacy using NOD/Shi-scid IL2Rgammanull (NOG) mice. Int. J. Oncol. 2008, 32, 153–159. [Google Scholar]
- Baker, A.M.; Bird, D.; Lang, G.; Cox, T.R.; Erler, J.T. Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene 2013, 32, 1863–1868. [Google Scholar] [CrossRef] [Green Version]
- Micek, H.M.; Visetsouk, M.R.; Masters, K.S.; Kreeger, P.K. Engineering the Extracellular Matrix to Model the Evolving Tumor Microenvironment. iScience 2020, 23, 101742. [Google Scholar] [CrossRef]
- Berger, A.J.; Linsmeier, K.M.; Kreeger, P.K.; Masters, K.S. Decoupling the effects of stiffness and fiber density on cellular behaviors via an interpenetrating network of gelatin-methacrylate and collagen. Biomaterials 2017, 141, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Kojima, M.; Higuchi, Y.; Sugimoto, M.; Ikeda, K.; Sakuyama, N.; Takahashi, S.; Hayashi, R.; Ochiai, A.; Saito, N. Assessment of elasticity of colorectal cancer tissue, clinical utility, pathological and phenotypical relevance. Cancer Sci. 2015, 106, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Morello, G.; Quarta, A.; Gaballo, A.; Moroni, L.; Gigli, G.; Polini, A.; Gervaso, F. A thermo-sensitive chitosan/pectin hydrogel for long-term tumor spheroid culture. Carbohydr. Polym. 2021, 274, 118633. [Google Scholar] [CrossRef] [PubMed]
- Bednarzig, V.; Karakaya, E.; Egaña, A.L.; Teßmar, J.; Boccaccini, A.R.; Detsch, R. Advanced ADA-GEL bioink for bioprinted artificial cancer models. Bioprinting 2021, 23, e00145. [Google Scholar] [CrossRef]
- Mahboubian, A.R.; Vllasaliu, D.; Dorkoosh, F.A.; Stolnik, S. Temperature-Responsive Methylcellulose-Hyaluronic Hydrogel as a 3D Cell Culture Matrix. Biomacromolecules 2020, 21, 4737–4746. [Google Scholar] [CrossRef]
- Subramanian, A.; Lin, H.Y. Crosslinked chitosan: Its physical properties and the effects of matrix stiffness on chondrocyte cell morphology and proliferation. J. Biomed. Mater. Res.-Part A 2005, 75, 742–753. [Google Scholar] [CrossRef] [Green Version]
- Fan, C.; Wang, D.-A. Effects of Permeability and Living Space on Cell Fate and Neo-Tissue Development in Hydrogel-Based Scaffolds: A Study With Cartilaginous Model. Macromol. Biosci. 2015, 15, 535–545. [Google Scholar] [CrossRef]
- Toh, T.B.; Liu, Z.; Yu, H.; Fong, E.L.S. Three-Dimensional Macroporous Sponge for the Culture of Hepatocellular Carcinoma Patient-Derived Xenograft Organoids. SLAS Technol. 2021, 26, 249–254. [Google Scholar] [CrossRef]
- Kumar, A.; Bansal, V.; Nandakumar, K.S.; Galaev, I.Y.; Roychoudhury, P.K.; Holmdahl, R.; Mattiasson, B. Integrated bioprocess for the production and isolation of urokinase from animal cell culture using supermacroporous cryogel matrices. Biotechnol. Bioeng. 2006, 93, 636–646. [Google Scholar] [CrossRef]
- Wartenberg, A.; Weisser, J.; Schnabelrauch, M. Glycosaminoglycan-Based Cryogels as Scaffolds for Cell Cultivation and Tissue Regeneration. Molecules 2021, 26, 5597. [Google Scholar] [CrossRef]
- Privar, Y.; Kodess, M.I.; Modin, E.; Nesterov, D.; Pestov, A.V.; Slobodyuk, A.; Marinin, D.V.; Bratskaya, S. Chitosan gels and cryogels cross-linked with diglycidyl ethers of ethylene glycol and polyethylene glycol in acidic media. Biomacromolecules 2019, 20, 1635–1643. [Google Scholar]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef] [PubMed]
- Bratskaya, S.; Skatova, A.; Privar, Y.; Boroda, A.; Kantemirova, E.; Maiorova, M.; Pestov, A. Stimuli-Responsive Dual Cross-Linked N-Carboxyethylchitosan Hydrogels with Tunable Dissolution Rate. Gels 2021, 7, 188. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Jeong, C.H.; Kim, D.H.; Yune, J.H.; Kwon, H.C.; Shin, D.M.; Sohn, H.; Lee, K.H.; Choi, B.; Kim, E.S.; Kang, J.H.; et al. In vitro toxicity assessment of crosslinking agents used in hyaluronic acid dermal filler. Toxicol. Vitr. 2021, 70, 105034. [Google Scholar] [CrossRef]
- Oelschlaeger, C.; Bossler, F.; Willenbacher, N. Synthesis, Structural and Micromechanical Properties of 3D Hyaluronic Acid-Based Cryogel Scaffolds. Biomacromolecules 2016, 17, 580–589. [Google Scholar] [CrossRef]
- Wende, F.J.; Gohil, S.; Nord, L.I.; Karlsson, A.; Kenne, A.H.; Sandström, C. Insights on the reactivity of chondroitin and hyaluronan toward 1,4-butanediol diglycidyl ether. Int. J. Biol. Macromol. 2019, 131, 812–820. [Google Scholar] [CrossRef]
- Shechter, L.; Wynstra, J. Glycidyl Ether Reactions with Alcohols, Phenols, Carboxylic Acids, and Acid Anhydrides. Ind. Eng. Chem. 1956, 48, 86–93. [Google Scholar] [CrossRef]
- Gámiz González, M.A.; Edlund, U.; Vidaurre, A.; Gómez Ribelles, J.L. Synthesis of highly swellable hydrogels of water-soluble carboxymethyl chitosan and poly(ethylene glycol). Polym. Int. 2017, 66, 1624–1632. [Google Scholar] [CrossRef] [Green Version]
- Kono, H. Characterization and properties of carboxymethyl cellulose hydrogels crosslinked by polyethylene glycol. Carbohydr. Polym. 2014, 106, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Chen, H.; Xu, C.; Yu, D.; Xu, H.; Hu, Y. Synthesis of hyaluronic acid hydrogels by crosslinking the mixture of high-molecular-weight hyaluronic acid and low-molecular-weight hyaluronic acid with 1,4-butanediol diglycidyl ether. RSC Adv. 2020, 10, 7206–7213. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I.; Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. The potential of polymeric cryogels in bioseparation. Bioseparation 2001, 10, 163–188. [Google Scholar] [CrossRef] [PubMed]
- Rimann, M.; Angres, B.; Patocchi-Tenzer, I.; Braum, S.; Graf-Hausner, U. Automation of 3D Cell Culture Using Chemically Defined Hydrogels. J. Lab. Autom. 2014, 19, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.B.; Le, V.M.; Gu, C.H.; Zheng, Y.H.; Lang, M.D.; Lu, Y.H.; Liu, J.W. Overcoming multidrug resistance in 2D and 3D culture models by controlled drug chitosan-graft poly(caprolactone)-based nanoparticles. J. Pharm. Sci. 2014, 103, 1064–1074. [Google Scholar] [CrossRef]
- Pape, J.; Magdeldin, T.; Ali, M.; Walsh, C.; Lythgoe, M.; Emberton, M.; Cheema, U. Cancer invasion regulates vascular complexity in a three-dimensional biomimetic model. Eur. J. Cancer 2019, 119, 179–193. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Deng, H.; Fang, Y. PTEN deletion potentiates invasion of colorectal cancer spheroidal cells through 3D Matrigel. Integr. Biol. 2015, 7, 324–334. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boroda, A.; Privar, Y.; Maiorova, M.; Skatova, A.; Bratskaya, S. Sponge-like Scaffolds for Colorectal Cancer 3D Models: Substrate-Driven Difference in Micro-Tumors Morphology. Biomimetics 2022, 7, 56. https://doi.org/10.3390/biomimetics7020056
Boroda A, Privar Y, Maiorova M, Skatova A, Bratskaya S. Sponge-like Scaffolds for Colorectal Cancer 3D Models: Substrate-Driven Difference in Micro-Tumors Morphology. Biomimetics. 2022; 7(2):56. https://doi.org/10.3390/biomimetics7020056
Chicago/Turabian StyleBoroda, Andrey, Yuliya Privar, Mariya Maiorova, Anna Skatova, and Svetlana Bratskaya. 2022. "Sponge-like Scaffolds for Colorectal Cancer 3D Models: Substrate-Driven Difference in Micro-Tumors Morphology" Biomimetics 7, no. 2: 56. https://doi.org/10.3390/biomimetics7020056
APA StyleBoroda, A., Privar, Y., Maiorova, M., Skatova, A., & Bratskaya, S. (2022). Sponge-like Scaffolds for Colorectal Cancer 3D Models: Substrate-Driven Difference in Micro-Tumors Morphology. Biomimetics, 7(2), 56. https://doi.org/10.3390/biomimetics7020056





