Preparation, Physicochemical Assessment and the Antimicrobial Action of Hydroxyapatite–Gelatin/Curcumin Nanofibrous Composites as a Dental Biomaterial
Abstract
:1. Introduction
2. Materials
3. Methods
3.1. Nanocomposites Fabrication
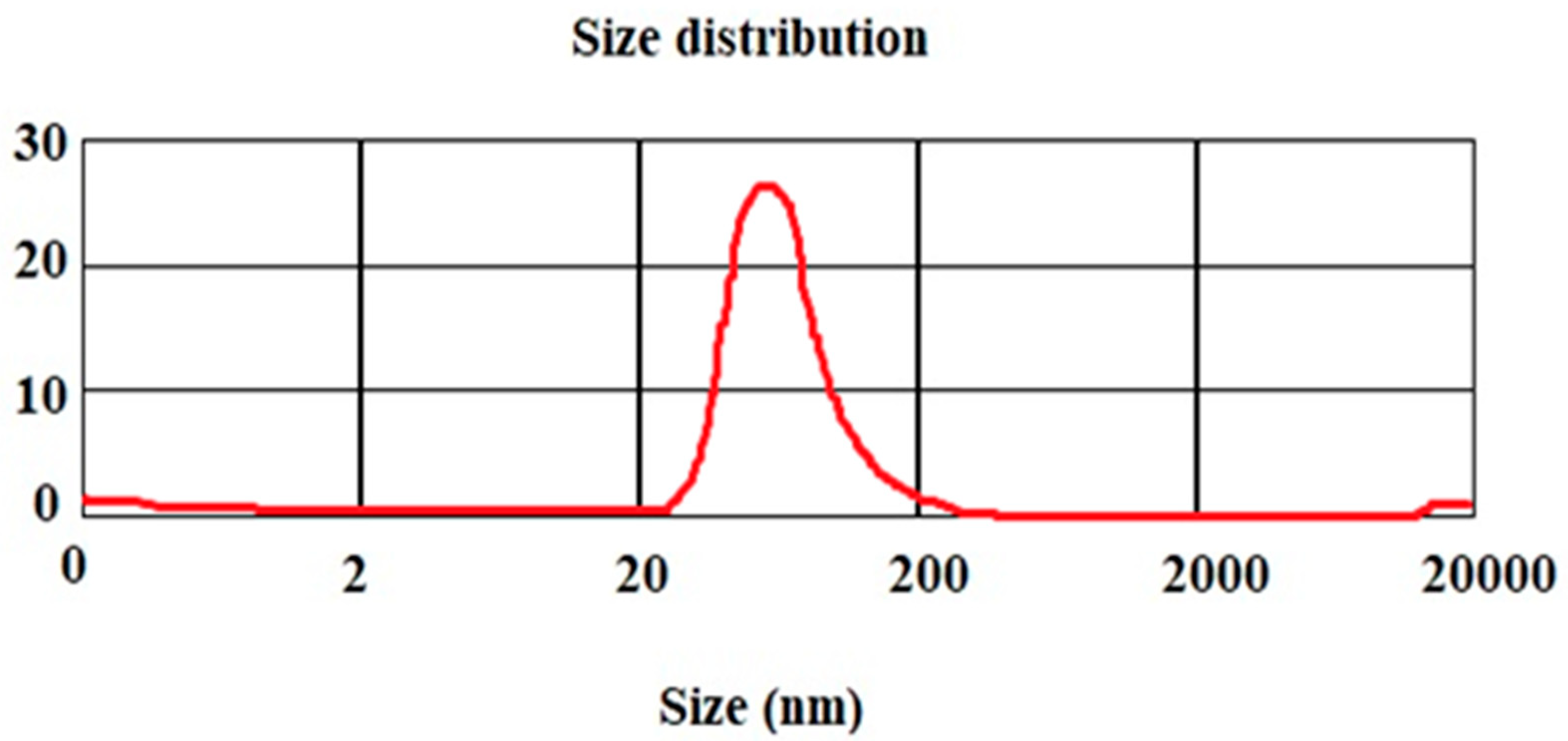
3.2. Nanoparticle’s Particle Size
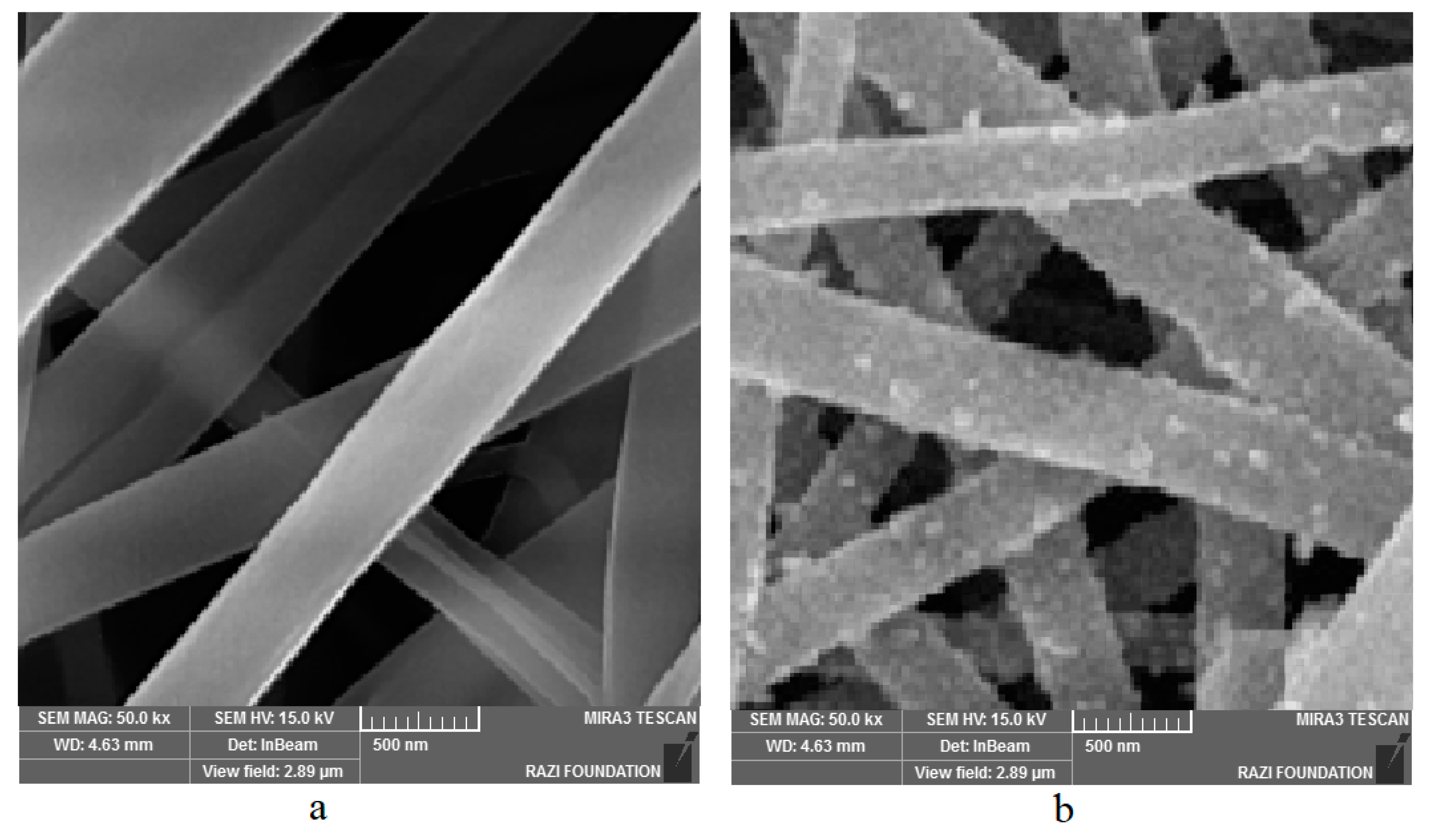
3.3. Morphology of Nanoparticles
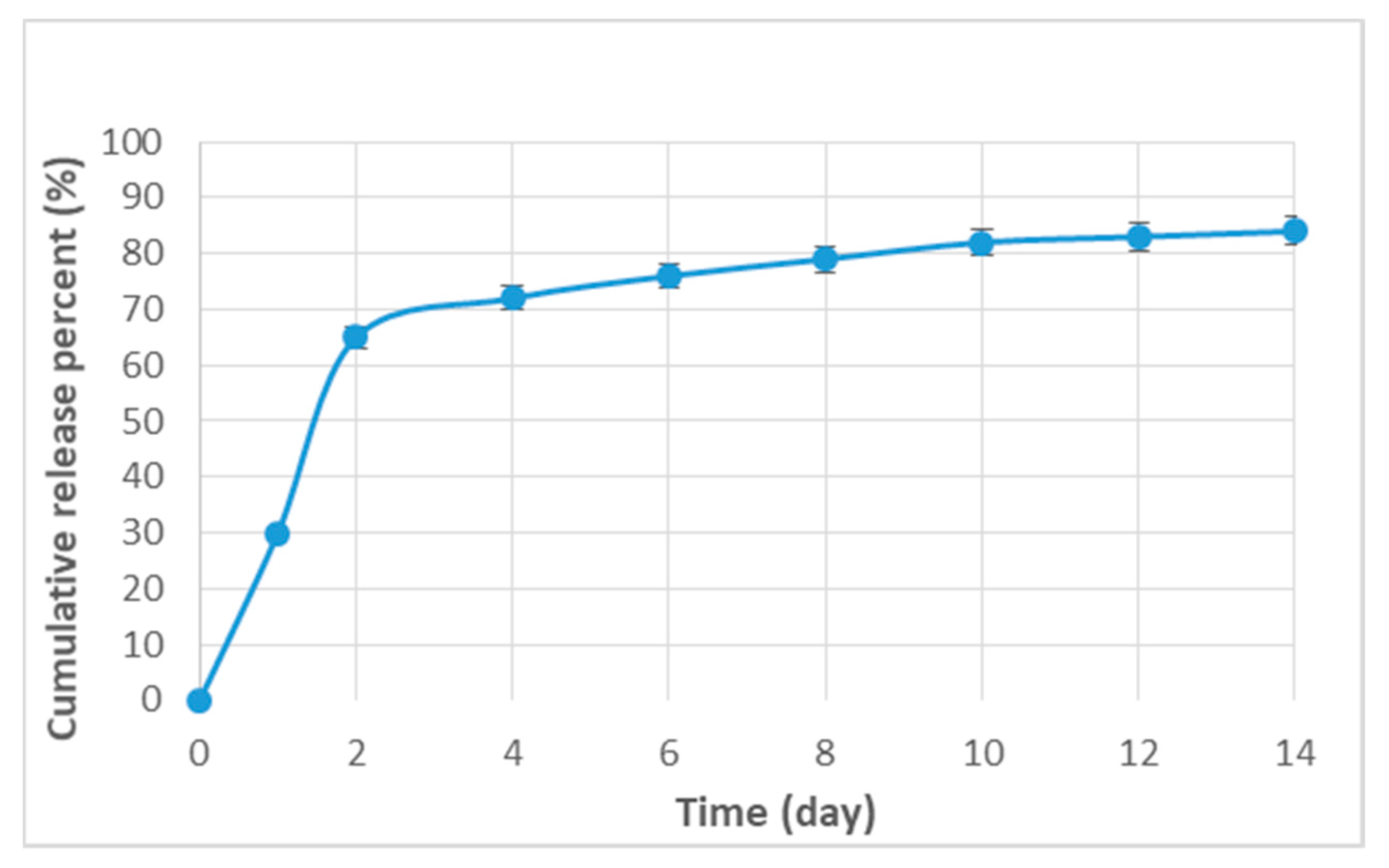
3.4. The Loading Efficiency and the Release Pattern of Curcumin
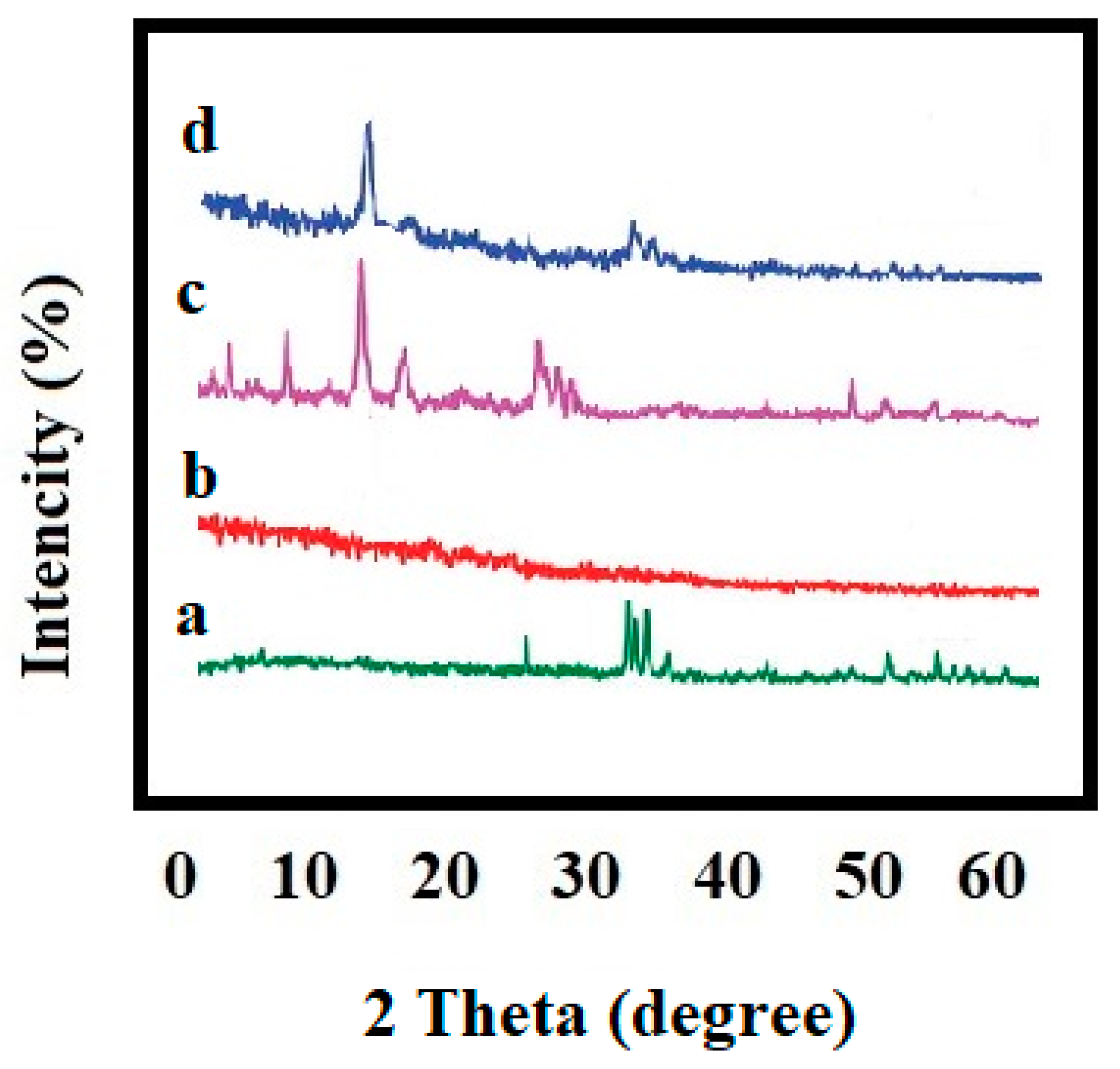
3.5. X-ray Diffraction (XRD) Analysis
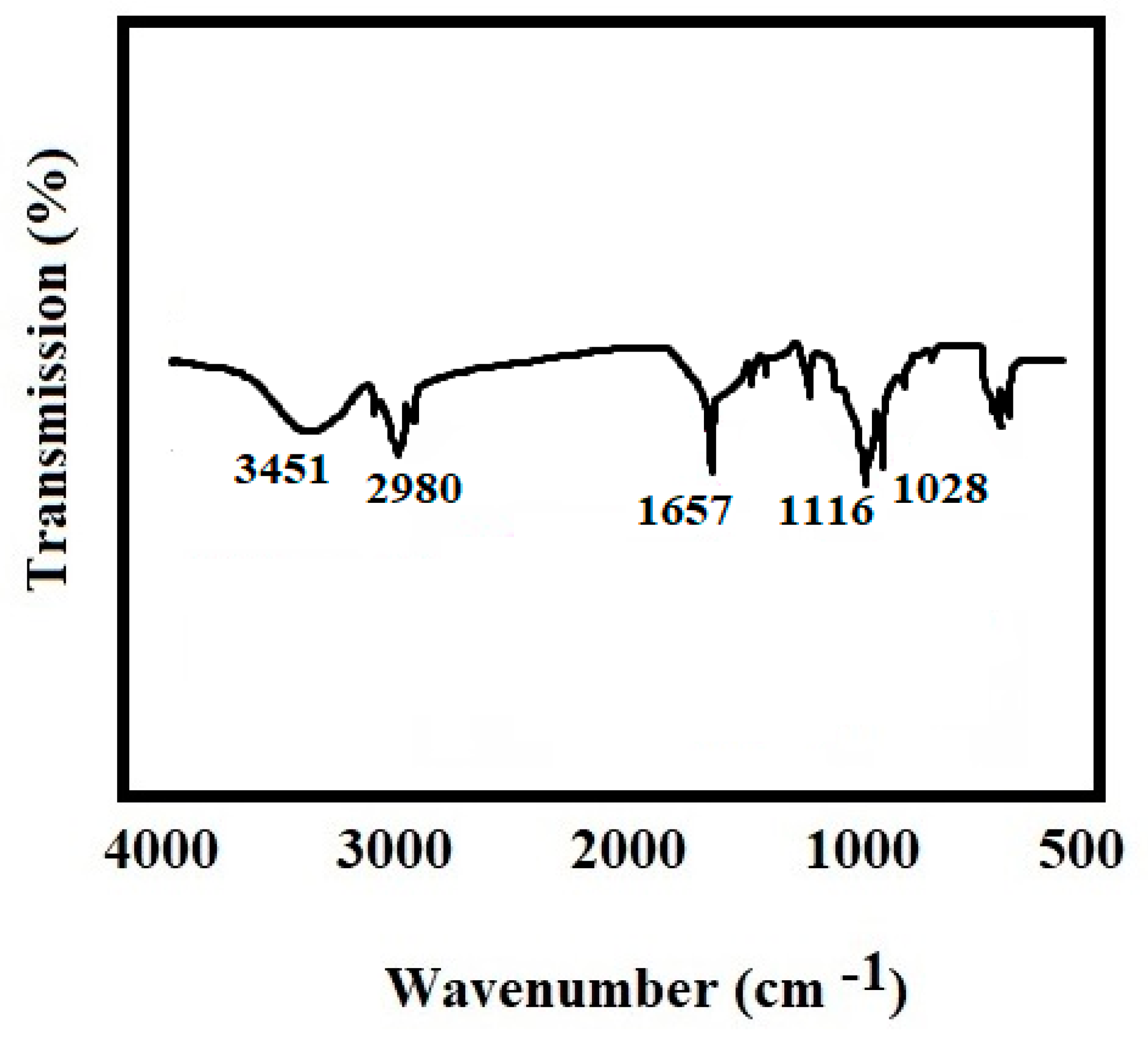
3.6. Fourier Transform Infrared Spectroscopy (FTIR)
3.7. Microbial Methods
3.8. Statistical Analysis
4. Results
4.1. Mean Particle Size
4.2. Morphological Assessment
4.3. Release Pattern of Curcumin
4.4. X-ray Diffraction (XRD) Analysis
4.5. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
4.6. Microbial Findings
4.7. Statistical Analysis for Microbial Findings
5. Discussion
Conclusions and the Future Perspectives
6. Ethical Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shirmohammadi, A.; Babaloo, A.; Maleki Dizaj, S.; Lotfipour, F.; Sharifi, S.; Ghavimi, M.A.; Khezri, K. A View on Polymerase Chain Reaction as an Outstanding Molecular Diagnostic Technique in Periodontology. BioMed Res. Int. 2021, 2021, 9979948. [Google Scholar] [CrossRef] [PubMed]
- Gholami, Z.; Hasanpour, S.; Sadigh, S.; Johari, S.; Shahveghar, Z.; Ataei, K.; Javari, E.; Amani, M.; Kia, L.J.; Akbari, Z.D. Antibacterial agent-releasing scaffolds in dental tissue engineering. J. Adv. Periodontol. Implant. Dent. 2021, 13, 43–47. [Google Scholar] [CrossRef]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine fight against antibacterial resistance: An overview of the recent pharmaceutical innovations. Pharmaceutics. 2020, 12, 142. [Google Scholar] [CrossRef] [Green Version]
- Memar, M.Y.; Yekani, M.; Ghanbari, H.; Nabizadeh, E.; Vahed, S.Z.; Dizaj, S.M.; Sharifi, S. Antimicrobial and antibiofilm activities of meropenem loaded-mesoporous silica nanoparticles against carbapenem-resistant Pseudomonas aeruginosa. J. Biomater. Appl. 2021, 36, 08853282211003848. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.-Y.; Wei, W.-C.; Jian, F.-Y.; Yang, N.-S. Therapeutic applications of herbal medicines for cancer patients. Evid. Based Complementary Altern. Med. 2013, 2013, 302426. [Google Scholar] [CrossRef]
- Sharifi, S.; Moghaddam, F.A.; Abedi, A.; Maleki Dizaj, S.; Ahmadian, S.; Abdolahinia, E.D.; Khatibi, S.M.H.; Samiei, M. Phytochemicals impact on osteogenic differentiation of mesenchymal stem cells. BioFactors 2020, 46, 874–893. [Google Scholar] [CrossRef]
- Mohseni, M.; Samadi, N.; Ghanbari, P.; Yousefi, B.; Tabasinezhad, M.; Sharifi, S.; Nazemiyeh, H. Co-treatment by docetaxel and vinblastine breaks down P-glycoprotein mediated chemo-resistance. Iran. J. Basic Med. Sci. 2016, 19, 300. [Google Scholar]
- Samadi, N.; Ghanbari, P.; Mohseni, M.; Tabasinezhad, M.; Sharifi, S.; Nazemieh, H.; Rashidi, M.R. Combination therapy increases the efficacy of docetaxel, vinblastine and tamoxifen in cancer cells. J. Cancer Res. Ther. 2014, 10, 715. [Google Scholar]
- Ghavimi, M.A.; Shahabadi, A.B.; Jarolmasjed, S.; Memar, M.Y.; Dizaj, S.M.; Sharifi, S. Nanofibrous asymmetric collagen/curcumin membrane containing aspirin-loaded PLGA nanoparticles for guided bone regeneration. Sci. Rep. 2020, 10, 18200. [Google Scholar] [CrossRef]
- Negahdari, R.; Ghavimi, M.A.; Barzegar, A.; Memar, M.Y.; Balazadeh, L.; Bohlouli, S.; Sharifi, S.; Maleki Dizaj, S. Antibacterial effect of nanocurcumin inside the implant fixture: An in vitro study. Clin. Exp. Dent. Res. 2020, 7, 163–169. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Sharifi, S.; Fathi, N.; Memar, M.Y.; Hosseiniyan Khatibi, S.M.; Khalilov, R.; Negahdari, R.; Zununi Vahed, S.; Maleki Dizaj, S. Anti-microbial activity of curcumin nanoformulations: New trends and future perspectives. Phytother. Res. 2020, 34, 1926–1946. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Curcumin nanoformulations: A future nanomedicine for cancer. Drug Discov. Today 2012, 17, 71–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negahdari, R.; Bohlouli, S.; Sharifi, S.; Maleki Dizaj, S.; Rahbar Saadat, Y.; Khezri, K.; Jafari, S.; Ahmadian, E.; Gorbani Jahandizi, N.; Raeesi, S. Therapeutic benefits of rutin and its nanoformulations. Phytother. Res. 2020, 35, 1719–1738. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, A.; Sharifi, S.; Davaran, S.; Ghasemi, S.; Omidi, Y.; Rashidi, M.-R. Novel aldehyde-terminated dendrimers; synthesis and cytotoxicity assay. BioImpacts BI 2012, 2, 97. [Google Scholar]
- Peddada, K.V.; Peddada, K.V.; Shukla, S.K.; Mishra, A.; Verma, V. Role of curcumin in common musculoskeletal disorders: A review of current laboratory, translational, and clinical data. Orthop. Surg. 2015, 7, 222–231. [Google Scholar] [CrossRef]
- Ghazi, I.F.; Salih, S.I.; Oleiwi, J.K.; Mutar, M.A. Evaluation of diametral tensile and compressive strengths of different types of nanocomposites for dental applications. Mater. Today Proc. 2021, in press. [Google Scholar] [CrossRef]
- El-Fattah, A.; Youssef, H.; Gepreel, M.A.H.; Abbas, R.; Kandil, S. Surface Morphology and Mechanical Properties of Polyether Ether Ketone (PEEK) Nanocomposites Reinforced by Nano-Sized Silica (SiO2) for Prosthodontics and Restorative Dentistry. Polymers 2021, 13, 3006. [Google Scholar] [CrossRef]
- Siang Soh, M.; Sellinger, A.; Uj Yap, A. Dental nanocomposites. Curr. Nanosci. 2006, 2, 373–381. [Google Scholar] [CrossRef]
- Albuquerque, M.; Valera, M.; Nakashima, M.; Nör, J.; Bottino, M. Tissue-engineering-based strategies for regenerative endodontics. J. Dent. Res. 2014, 93, 1222–1231. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, R.; Fan, Y.; Liao, S.; Wang, Y.; Wen, Z.; Xu, X. Antibacterial dental composites with chlorhexidine and mesoporous silica. J. Dent. Res. 2014, 93, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Gupte, M.; Ma, P.X. Nanofibrous scaffolds for dental and craniofacial applications. J. Dent. Res. 2012, 91, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Hixon, K.R.; Eberlin, C.T.; Pendyala, M.; Alarcon de la Lastra, A.; Sell, S.A. Scaffolds for Use in Craniofacial Bone Regeneration. In Craniofacial Development; Springer: Berlin/Heidelberg, Germany, 2022; pp. 223–234. [Google Scholar]
- Blachowicz, T.; Ehrmann, A. Production and Application of Biodegradable Nanofibers Using Electrospinning Techniques. In Electrospun Nanofibers; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–24. [Google Scholar]
- Rosa, V.; Zhang, Z.; Grande, R.; Nör, J. Dental pulp tissue engineering in full-length human root canals. J. Dent. Res. 2013, 92, 970–975. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, M.T.; Valera, M.C.; Moreira, C.S.; Bresciani, E.; de Melo, R.M.; Bottino, M.C. Effects of ciprofloxacin-containing scaffolds on enterococcus faecalis biofilms. J. Endod. 2015, 41, 710–714. [Google Scholar] [CrossRef]
- Kamocki, K.; Nör, J.E.; Bottino, M.C. Effects of ciprofloxacin-containing antimicrobial scaffolds on dental pulp stem cell viability—In vitro studies. Arch. Oral Biol. 2015, 60, 1131–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pankajakshan, D.; Albuquerque, M.T.; Evans, J.D.; Kamocka, M.M.; Gregory, R.L.; Bottino, M.C. Triple antibiotic polymer nanofibers for intracanal drug delivery: Effects on dual species biofilm and cell function. J. Endod. 2016, 42, 1490–1495. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Gao, S.; Yu, H. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed. Mater. 2009, 4, 034104. [Google Scholar] [CrossRef] [Green Version]
- Ciobanu, C.S.; Iconaru, S.L.; Le Coustumer, P.; Constantin, L.V.; Predoi, D. Antibacterial activity of silver-doped hydroxyapatite nanoparticles against gram-positive and gram-negative bacteria. Nanoscale Res. Lett. 2012, 7, 324. [Google Scholar] [CrossRef] [Green Version]
- Diba, M.; Kharaziha, M.; Fathi, M.; Gholipourmalekabadi, M.; Samadikuchaksaraei, A. Preparation and characterization of polycaprolactone/forsterite nanocomposite porous scaffolds designed for bone tissue regeneration. Compos. Sci. Technol. 2012, 72, 716–723. [Google Scholar] [CrossRef]
- Lien, S.-M.; Li, W.-T.; Huang, T.-J. Genipin-crosslinked gelatin scaffolds for articular cartilage tissue engineering with a novel crosslinking method. Mater. Sci. Eng. C 2008, 28, 36–43. [Google Scholar] [CrossRef]
- Thein-Han, W.; Saikhun, J.; Pholpramoo, C.; Misra, R.; Kitiyanant, Y. Chitosan–gelatin scaffolds for tissue engineering: Physico-chemical properties and biological response of buffalo embryonic stem cells and transfectant of GFP–buffalo embryonic stem cells. Acta Biomater. 2009, 5, 3453–3466. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Fu, X.; Liu, J.; Qi, Y.; Li, S.; Wang, H. The involvement of integrin β1 signaling in the migration and myofibroblastic differentiation of skin fibroblasts on anisotropic collagen-containing nanofibers. Biomaterials 2012, 33, 1791–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, L.S.; Xu, Z.K. Polymer surfaces structured with random or aligned electrospun nanofibers to promote the adhesion of blood platelets. J. Biomed. Mater. Res. Part A 2009, 89, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; MacEwan, M.R.; Ray, W.Z.; Liu, W.; Siewe, D.Y.; Xia, Y. Radially aligned, electrospun nanofibers as dural substitutes for wound closure and tissue regeneration applications. ACS Nano 2010, 4, 5027–5036. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Li, B. Biomimetic electrospun nanofibrous structures for tissue engineering. Mater. Today 2013, 16, 229–241. [Google Scholar] [CrossRef]
- Boroumand, S.; Hosseini, S.; Pashandi, Z.; Faridi-Majidi, R.; Salehi, M. Curcumin-loaded nanofibers for targeting endometriosis in the peritoneum of a mouse model. J. Mater. Sci. Mater. Med. 2020, 31, 8. [Google Scholar] [CrossRef]
- Shababdoust, A.; Ehsani, M.; Shokrollahi, P.; Zandi, M. Fabrication of curcumin-loaded electrospun nanofiberous polyurethanes with anti-bacterial activity. Prog. Biomater. 2018, 7, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Hezma, A.; Abdelrazzak, A.B.; El-Bahy, G.S. Preparation and spectroscopic investigations of hydroxyapatite-curcumin nanoparticles-loaded polylactic acid for biomedical application. Egypt. J. Basic Appl. Sci. 2019, 6, 1–9. [Google Scholar] [CrossRef]
- El-Rahman, S.; Al-Jameel, S. Protection of curcumin and curcumin nanoparticles against cisplatin induced nephrotoxicity in male rats. Sch. Acad. J. Biosci 2014, 2, 214–223. [Google Scholar]
- Muyonga, J.; Cole, C.; Duodu, K. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Gopal, J.; Muthu, M.; Chun, S. Bactericidal property of macro-, micro-and nanocurcumin: An assessment. Arab. J. Sci. Eng. 2016, 41, 2087–2093. [Google Scholar] [CrossRef]
- Ngwabebhoh, F.A.; Erdagi, S.I.; Yildiz, U. Pickering emulsions stabilized nanocellulosic-based nanoparticles for coumarin and curcumin nanoencapsulations: In vitro release, anticancer and antimicrobial activities. Carbohydr. Polym. 2018, 201, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Adahoun, M.a.A.; Al-Akhras, M.-A.H.; Jaafar, M.S.; Bououdina, M. Enhanced anti-cancer and antimicrobial activities of curcumin nanoparticles. Artif. Cells Nanomed. Biotechnol. 2017, 45, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Negahdari, R.; Sharifi, S.; Ghavimi, M.A.; Memar, M.Y.; Khaneshi, B.; Maleki Dizaj, S.; Eftekhari, A.; Cucchiarini, M. Curcumin nanocrystals: Production, physicochemical assessment, and in vitro evaluation of the antimicrobial effects against bacterial loading of the implant fixture. Appl. Sci. 2020, 10, 8356. [Google Scholar] [CrossRef]
- Khezri, K.; Maleki Dizaj, S.; Rahbar Saadat, Y.; Sharifi, S.; Shahi, S.; Ahmadian, E.; Eftekhari, A.; Dalir Abdolahinia, E.; Lotfipour, F.J.S.C.I. Osteogenic differentiation of mesenchymal stem cells via curcumin-containing nanoscaffolds. Stem Cells Int. 2021, 2021, 1520052. [Google Scholar] [CrossRef] [PubMed]
- Dillen, K.; Vandervoort, J.; Van den Mooter, G.; Verheyden, L.; Ludwig, A. Factorial design, physicochemical characterisation and activity of ciprofloxacin-PLGA nanoparticles. Int. J. Pharma. 2004, 275, 171–187. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Bouafia, A.; Laouini, S.E. Plant-mediated synthesis of iron oxide nanoparticles and evaluation of the antimicrobial activity: A review. Mini-Rev. Org. Chem. 2021, 18, 725–734. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Kim, D.; Ren, Z.; Oh, M.J.; Cormode, D.P.; Hara, A.T.; Zero, D.T.; Koo, H. Ferumoxytol Nanoparticles Target Biofilms Causing Tooth Decay in the Human Mouth. Nano Lett. 2021, 21, 9442–9449. [Google Scholar] [CrossRef]
- Çelik, P.A.; Derkuş, B.; Erdoğan, K.; Barut, D.; Manga, E.B.; Yıldırım, Y.; Pecha, S.; Çabuk, A. Bacterial membrane vesicle functions, laboratory methods, and applications. Biotechnol. Adv. 2022, 54, 107869. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.-H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Canalli Bortolassi, A.C.; Guerra, V.G.; Aguiar, M.L.; Soussan, L.; Cornu, D.; Miele, P.; Bechelany, M. Composites based on nanoparticle and pan electrospun nanofiber membranes for air filtration and bacterial removal. Nanomaterials 2019, 9, 1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamdan, N.; Yamin, A.; Hamid, S.A.; Khodir, W.K.W.A.; Guarino, V. Functionalized Antimicrobial Nanofibers: Design Criteria and Recent Advances. J. Funct. Biomater. 2021, 12, 59. [Google Scholar] [CrossRef] [PubMed]
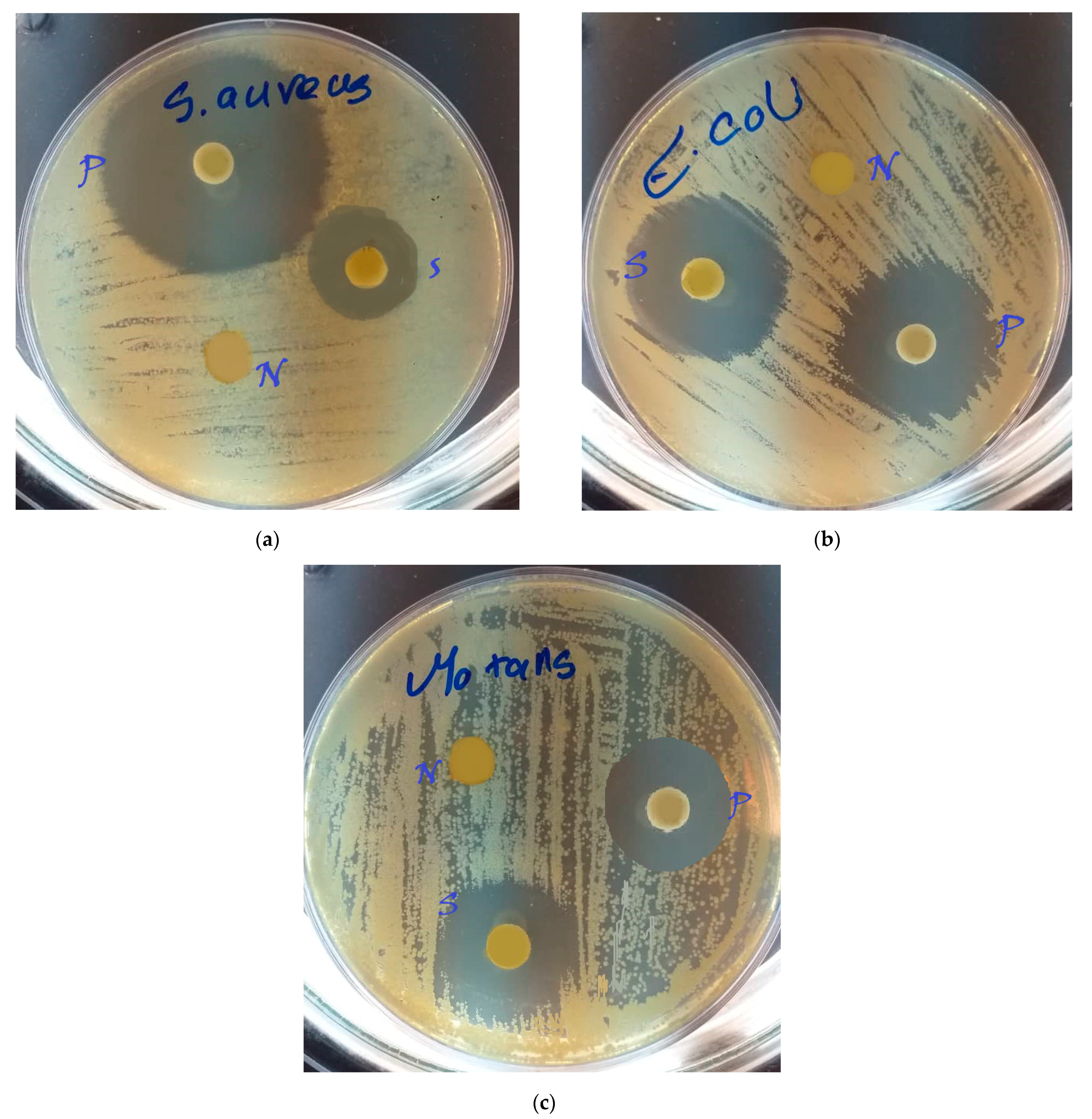
| Zone Size of Sample | Zone Size of Positive Control | Zone Size of Negative Control | |
|---|---|---|---|
| S. mutans | 13.17 ± 0.05 | 12.6 ± 0.03 | 0 |
| E. coli | 14.43 ± 0.01 | 14.1 ± 0.08 | 0 |
| S. aureus | 10.7 ± 0.05 | 16.13 ± 0.02 | 0 |
| ANOVA Summary | |
|---|---|
| F | 115.1 |
| p value | 0.0086 |
| p value summary | ** |
| Significant diff. among means (p < 0.05)? | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharifi, S.; Zaheri Khosroshahi, A.; Maleki Dizaj, S.; Rezaei, Y. Preparation, Physicochemical Assessment and the Antimicrobial Action of Hydroxyapatite–Gelatin/Curcumin Nanofibrous Composites as a Dental Biomaterial. Biomimetics 2022, 7, 4. https://doi.org/10.3390/biomimetics7010004
Sharifi S, Zaheri Khosroshahi A, Maleki Dizaj S, Rezaei Y. Preparation, Physicochemical Assessment and the Antimicrobial Action of Hydroxyapatite–Gelatin/Curcumin Nanofibrous Composites as a Dental Biomaterial. Biomimetics. 2022; 7(1):4. https://doi.org/10.3390/biomimetics7010004
Chicago/Turabian StyleSharifi, Simin, Asma Zaheri Khosroshahi, Solmaz Maleki Dizaj, and Yashar Rezaei. 2022. "Preparation, Physicochemical Assessment and the Antimicrobial Action of Hydroxyapatite–Gelatin/Curcumin Nanofibrous Composites as a Dental Biomaterial" Biomimetics 7, no. 1: 4. https://doi.org/10.3390/biomimetics7010004
APA StyleSharifi, S., Zaheri Khosroshahi, A., Maleki Dizaj, S., & Rezaei, Y. (2022). Preparation, Physicochemical Assessment and the Antimicrobial Action of Hydroxyapatite–Gelatin/Curcumin Nanofibrous Composites as a Dental Biomaterial. Biomimetics, 7(1), 4. https://doi.org/10.3390/biomimetics7010004






