Synthesis of Inorganic Compounds in the Matrix of Polysaccharide Chitosan
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Material Characteristics Ni(OH)2/CS and Ni(OH)2/CS/ACF
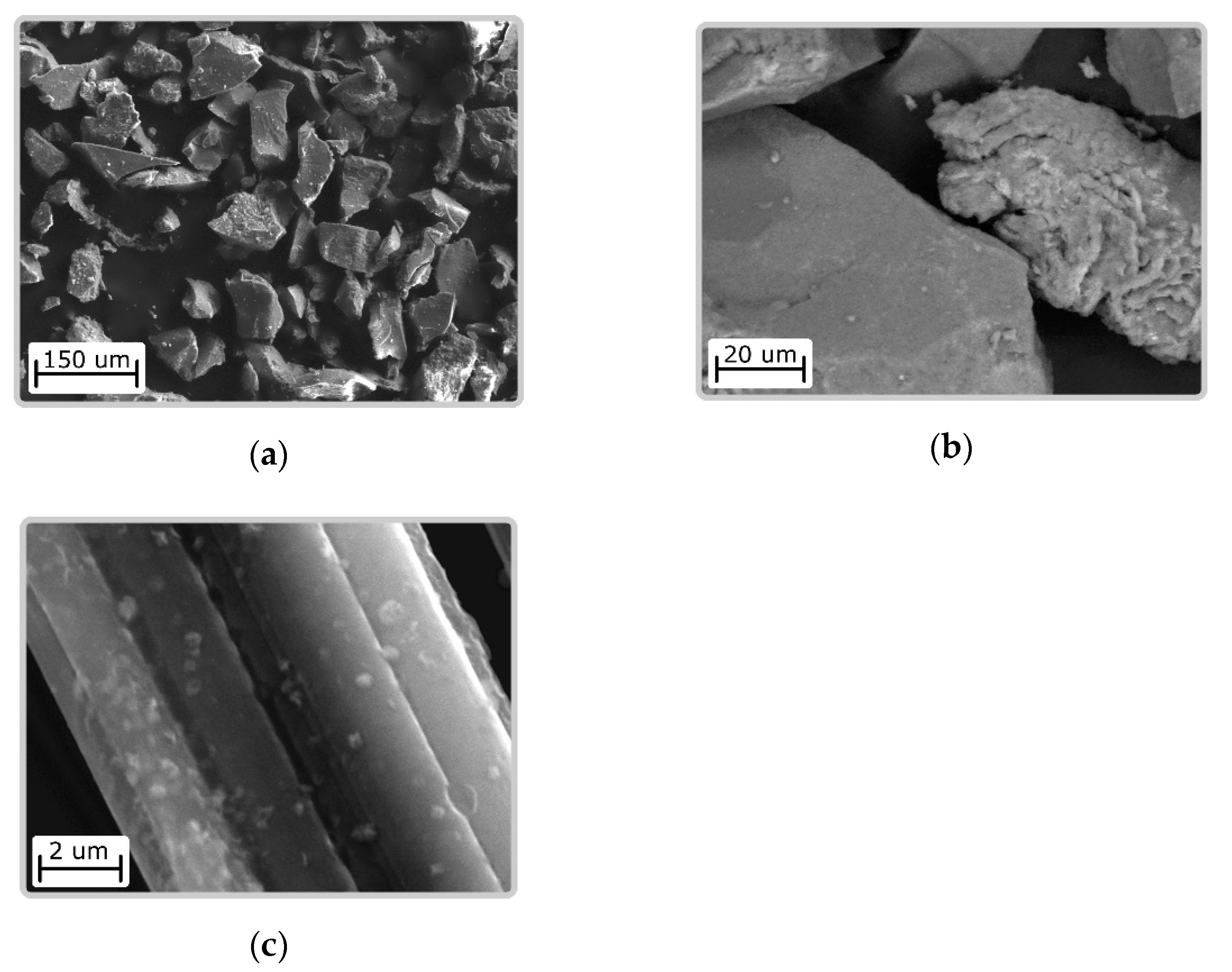
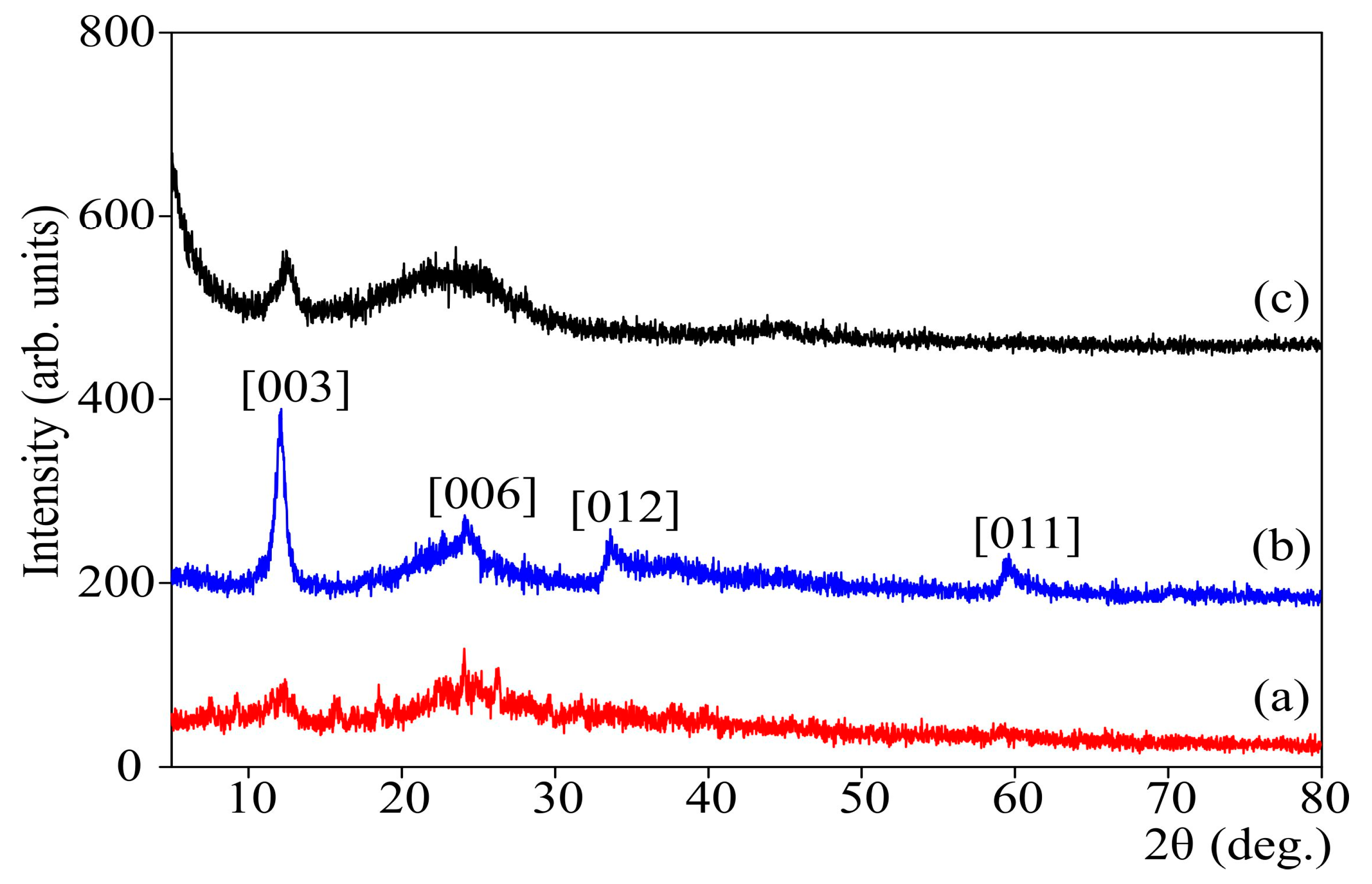
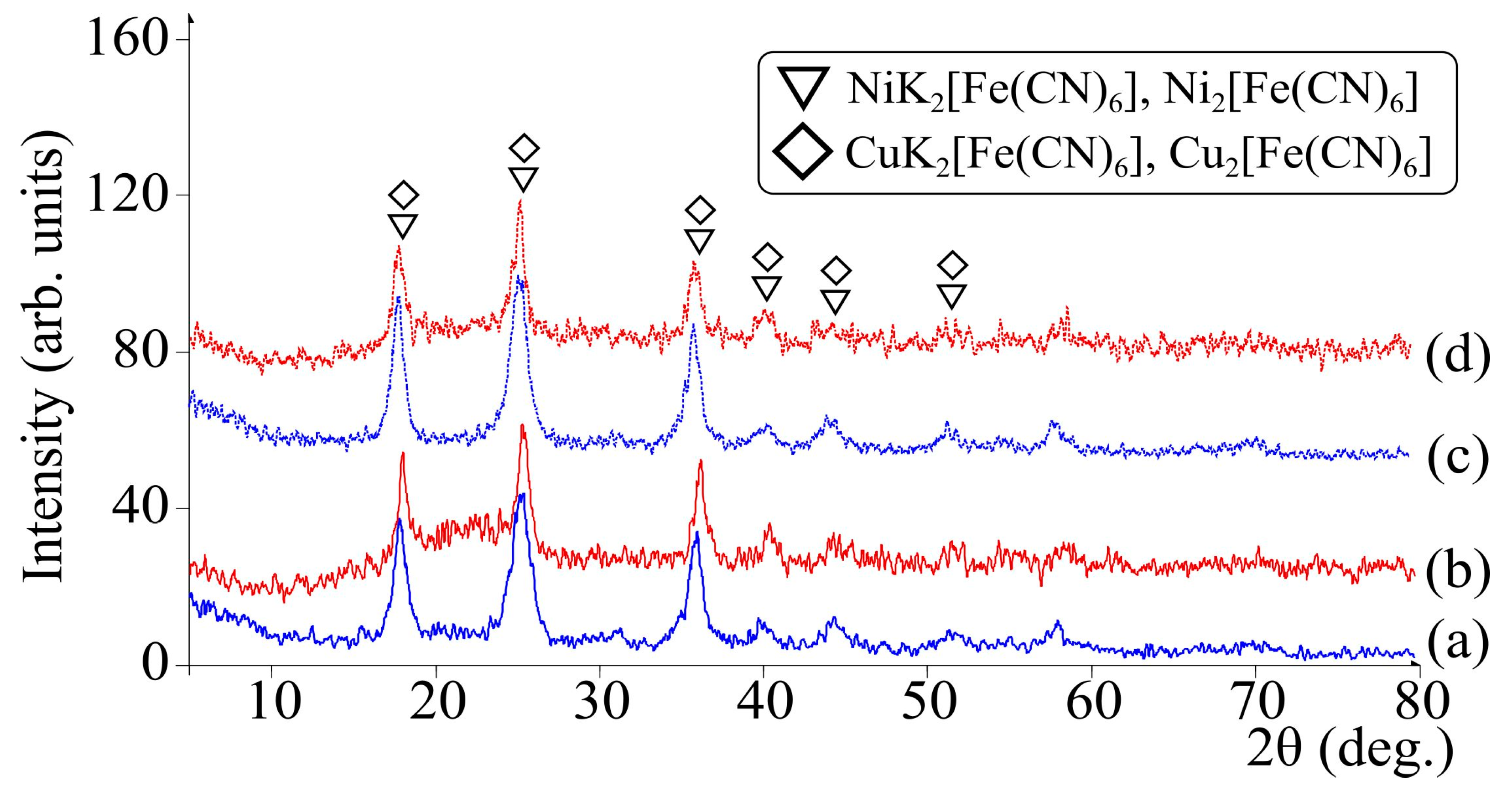
3.2. Characteristics of CFS—Chitosanferrocyanide Sorbent Ni-K, Cu-K, Zn-K
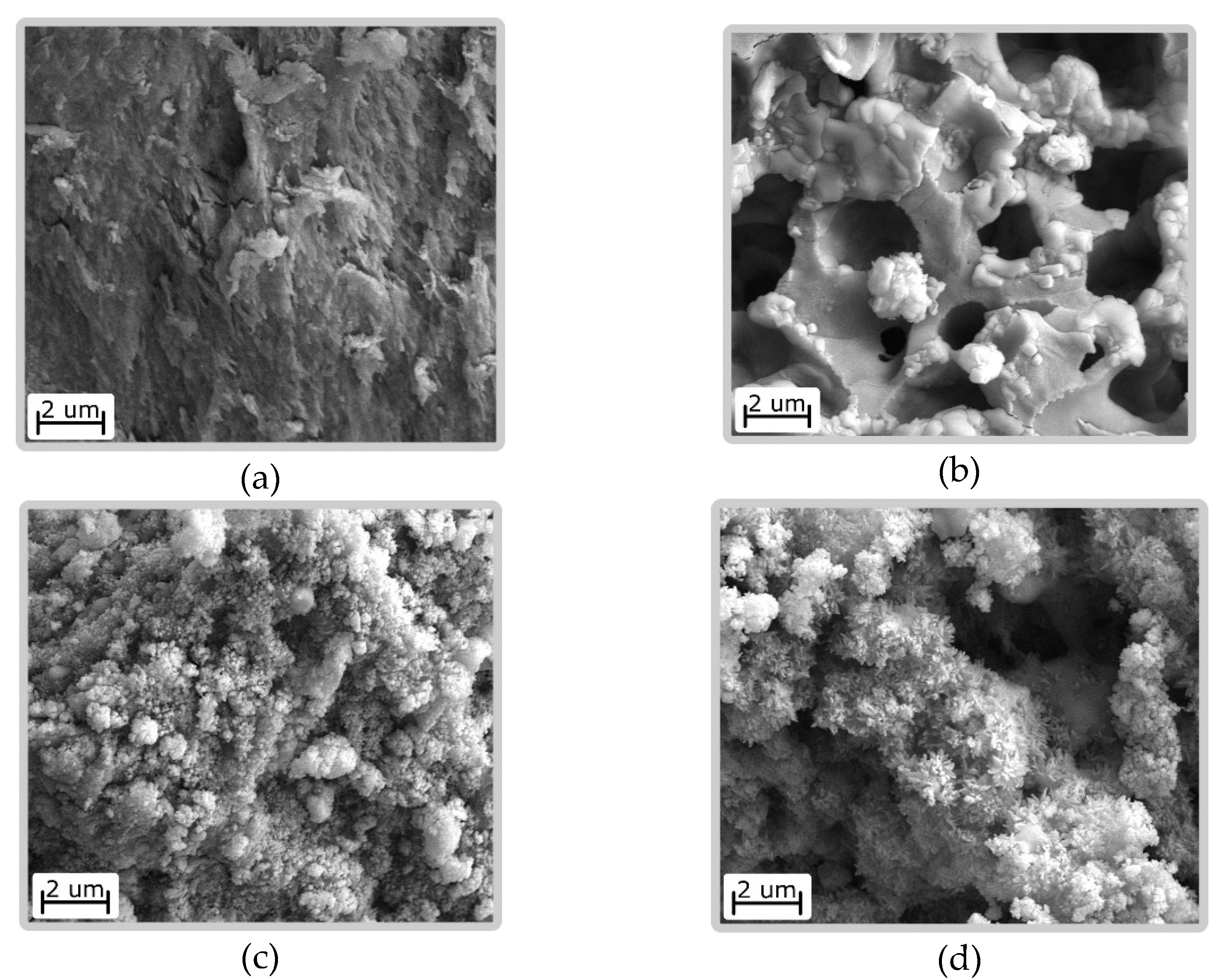
3.3. Material Characteristics HA/CS
4. Discussion
4.1. Ni(OH)2/Chitosan Nanosize Composites
4.2. Transition Metal Ferrocyanides/Chitosan Hybrid Sorbents
4.3. Hybrid Calcium Phosphates/Chitosan Composites
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarkar, S.; Guibal, E.; Quignard, F.; SenGupta, A.K. Polymer-Supported Metals and Metal Oxide Nanoparticles: Synthesis, Characterization, and Applications. J. Nanopart. Res. 2012, 14, 715. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, B.; Xu, H.; Liu, H.; Wang, M.; He, Y.; Pan, B. Nanomaterials-Enabled Water and Wastewater Treatment. Nanoimpact 2016, 3–4, 22–39. [Google Scholar] [CrossRef]
- Šebesta, F. Composite Sorbents of Inorganic Ion-Exchangers and Polyacrylonitrile Binding Matrix. J. Radioanal. Nucl. Chem. 1997, 220, 77–88. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Molecular Nanotechnologies of Gelatin-Immobilization Using Macrocyclic Metal Chelates. Nano Rev. 2014, 5, 21485. [Google Scholar] [CrossRef]
- Barinov, S.M. Calcium Phosphate-Based Ceramic and Composite Materials for Medicine. Russ. Chem. Rev. 2010, 79, 13. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Nanodimensional and Nanocrystalline Apatites and Other Calcium Orthophosphates in Biomedical Engineering, Biology and Medicine. Materials 2009, 2, 1975–2045. [Google Scholar] [CrossRef] [Green Version]
- Pighinelli, L.; Kucharska, M. Chitosan–Hydroxyapatite Composites. Carbohydr. Polym. 2013, 93, 256–262. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of Heavy Metal Ions Using Chitosan and Modified Chitosan: A Review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Chitosan-Based Biosorbents: Modification and Application for Biosorption of Heavy Metals and Radionuclides. Bioresour. Technol. 2014, 160, 129–141. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zheng, J. Removal of As(III) and As(V) from Water by Chitosan and Chitosan Derivatives: A Review. Environ. Sci. Pollut. Res. 2016, 23, 13789–13801. [Google Scholar] [CrossRef]
- Pontoni, L.; Fabbricino, M. Use of Chitosan and Chitosan-Derivatives to Remove Arsenic from Aqueous Solutions—A Mini Review. Carbohydr. Res. 2012, 356, 86–92. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Lee, S.-M. Application of Magnetic Chitosan Composites for the Removal of Toxic Metal and Dyes from Aqueous Solutions. Adv. Colloid Interface Sci. 2013, 201–202, 68–93. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pastora, J.; Bringas, E.; Ortiz, I. Recent Progress and Future Challenges on the Use of High Performance Magnetic Nano-Adsorbents in Environmental Applications. Chem. Eng. J. 2014, 256, 187–204. [Google Scholar] [CrossRef]
- Liu, S.; Huang, B.; Chai, L.; Liu, Y.; Zeng, G.; Wang, X.; Zeng, W.; Shang, M.; Deng, J.; Zhou, Z. Enhancement of As( v ) Adsorption from Aqueous Solution by a Magnetic Chitosan/Biochar Composite. RSC Adv. 2017, 7, 10891–10900. [Google Scholar] [CrossRef] [Green Version]
- Boudemagh, D.; Venturini, P.; Fleutot, S.; Cleymand, F. Elaboration of Hydroxyapatite Nanoparticles and Chitosan/Hydroxyapatite Composites: A Present Status. Polym. Bull. 2019, 76, 2621–2653. [Google Scholar] [CrossRef]
- Sunil, D. Recennt Advances on Chitosan-Metal Oxide Nanoparticles and Their Biological Application. Mater. Sci. Forum 2013, 754, 99–108. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-Based Nanomaterials: A State-of-the-Art Review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef]
- Zemskova, L.; Egorin, A.; Tokar, E.; Ivanov, V.; Bratskaya, S. New Chitosan/Iron Oxide Composites: Fabrication and Application for Removal of Sr2+ Radionuclide from Aqueous Solutions. Biomimetics 2018, 3, 39. [Google Scholar] [CrossRef] [Green Version]
- Egorin, A.; Tokar, E.; Matskevich, A.; Ivanov, N.; Tkachenko, I.; Sokolnitskaya, T.; Zemskova, L. Composite Magnetic Sorbents Based on Iron Oxides in Different Polymer Matrices: Comparison and Application for Removal of Strontium. Biomimetics 2020, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Choo, C.K.; Goh, T.L.; Shahcheraghi, L.; Ngoh, G.C.; Abdullah, A.Z.; Horri, B.A.; Salamatinia, B. Synthesis and Characterization of NiO Nano-Spheres by Templating on Chitosan as a Green Precursor. J. Am. Ceram. Soc. 2016, 99, 3874–3882. [Google Scholar] [CrossRef]
- Braga, T.P.; Gomes, E.C.C.; de Sousa, A.F.; Carreño, N.L.V.; Longhinotti, E.; Valentini, A. Synthesis of Hybrid Mesoporous Spheres Using the Chitosan as Template. J. Non-Cryst. Solids 2009, 355, 860–866. [Google Scholar] [CrossRef]
- Zhitomirsky, I.; Hashambhoy, A. Chitosan-Mediated Electrosynthesis of Organic–Inorganic Nanocomposites. J. Mater. Process. Technol. 2007, 191, 68–72. [Google Scholar] [CrossRef]
- Zemskova, L.A.; Nikolenko, Y.M.; Sheveleva, I.V.; Voit, A.V.; Kuryavyi, V.G.; Sergienko, V.I. Hybrid Nickel Oxide-Carbon Fiber Composites Obtained in the Presence of Surfactants. Glass Phys. Chem. 2011, 37, 555. [Google Scholar] [CrossRef]
- Zemskova, L.A.; Voit, A.V.; Kaidalova, T.A.; Barinov, N.N.; Nikolenko, Y.M.; Ziatdinov, A.M. Organic-Mineral Composites Copper Oxide/Chitosan/Carbon Fiber Obtained by the Electrodeposition Method. Russ. J. Appl. Chem. 2012, 85, 1212–1219. [Google Scholar] [CrossRef]
- Zemskova, L.A.; Voyt, A.V.; Barinov, N.N.; Kaydalova, T.A. Functional Materials Based on Manganese Dioxide Deposited on Carbon Fiber. Glass Phys. Chem. 2014, 40, 1–7. [Google Scholar] [CrossRef]
- Rumyantseva, E.V.; Veleshko, A.N.; Kulyukhin, S.A.; Veleshko, I.E.; Shaitura, D.S.; Rozanov, K.V.; Dmitrieva, N.A. Preparation and Properties of Modified Spherically Granulated Chitosan for Sorption of 137Cs from Solutions. Radiochemistry 2009, 51, 496. [Google Scholar] [CrossRef]
- Egorin, A.; Tokar, E.; Zemskova, L. Chitosan-Ferrocyanide Sorbent for Cs-137 Removal from Mineralized Alkaline Media. Radiochim. Acta 2016, 104, 657–661. [Google Scholar] [CrossRef]
- Zemskova, L.; Egorin, A.; Tokar, E.; Ivanov, V. Chitosan-Based Biosorbents: Immobilization of Metal Hexacyanoferrates and Application for Removal of Cesium Radionuclide from Aqueous Solutions. J. Sol-Gel Sci. Technol. 2019, 92, 459–466. [Google Scholar] [CrossRef]
- Yamaguchi, I.; Tokuchi, K.; Fukuzaki, H.; Koyama, Y.; Takakuda, K.; Monma, H.; Tanaka, J. Preparation and Microstructure Analysis of Chitosan/Hydroxyapatite Nanocomposites. J. Biomed. Mater. Res. 2001, 55, 20–27. [Google Scholar] [CrossRef]
- Chen, J.; Nan, K.; Yin, S.; Wang, Y.; Wu, T.; Zhang, Q. Characterization and Biocompatibility of Nanohybrid Scaffold Prepared via in Situ Crystallization of Hydroxyapatite in Chitosan Matrix. Colloids Surf. B Biointerfaces 2010, 81, 640–647. [Google Scholar] [CrossRef]
- Nikpour, M.R.; Rabiee, S.M.; Jahanshahi, M. Synthesis and Characterization of Hydroxyapatite/Chitosan Nanocomposite Materials for Medical Engineering Applications. Compos. Part B Eng. 2012, 43, 1881–1886. [Google Scholar] [CrossRef]
- Kong, L.; Gao, Y.; Cao, W.; Gong, Y.; Zhao, N.; Zhang, X. Preparation and Characterization of Nano-Hydroxyapatite/Chitosan Composite Scaffolds. J. Biomed. Mater. Res. Part A 2005, 75A, 275–282. [Google Scholar] [CrossRef]
- Rogina, A.; Ivanković, M.; Ivanković, H. Preparation and Characterization of Nano-Hydroxyapatite within Chitosan Matrix. Mater. Sci. Eng. C 2013, 33, 4539–4544. [Google Scholar] [CrossRef] [PubMed]
- Rogina, A.; Rico, P.; Gallego Ferrer, G.; Ivanković, M.; Ivanković, H. Effect of in Situ Formed Hydroxyapatite on Microstructure of Freeze-Gelled Chitosan-Based Biocomposite Scaffolds. Eur. Polym. J. 2015, 68, 278–287. [Google Scholar] [CrossRef]
- Danilchenko, S.N. Chitosan–Hydroxyapatite Composite Biomaterials Made by a One Step Co-Precipitation Method: Preparation, Characterization and in Vivo Tests. JBPC 2009, 9, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Redepenning, J.; Venkataraman, G.; Chen, J.; Stafford, N. Electrochemical Preparation of Chitosan/Hydroxyapatite Composite Coatings on Titanium Substrates. J. Biomed. Mater. Res. Part A 2003, 66A, 411–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silant’ev, V.E.; Egorkin, V.S.; Zemskova, L.A.; Sinebryukhov, S.L.; Gnedenkov, S.V. Synthesis of Phosphate Phases on Polysaccharide Template. Solid State Phenom. 2020, 312, 314–318. [Google Scholar] [CrossRef]
- Mavis, B. Homogeneous Precipitation of Nickel Hydroxide Powders; IS-T 2111; Ames Lab.: Ames, IA, USA, 2003. Available online: https://www.osti.gov/biblio/822049 (accessed on 30 May 2021).
- Zhitomirsky, I. Electrophoretic Deposition of Organic–Inorganic Nanocomposites. J. Mater. Sci. 2006, 41, 8186–8195. [Google Scholar] [CrossRef]
- Gerente, C.; Lee, V.K.C.; Cloirec, P.L.; McKay, G. Application of Chitosan for the Removal of Metals From Wastewaters by Adsorption—Mechanisms and Models Review. Crit. Rev. Environ. Sci. Technol. 2007, 37, 41–127. [Google Scholar] [CrossRef]
- Lokhande, P.E.; Pawar, K.; Chavan, U.S. Chemically Deposited Ultrathin α-Ni(OH)2 Nanosheet Using Surfactant on Ni Foam for High Performance Supercapacitor Application. Mater. Sci. Energy Technol. 2018, 1, 166–170. [Google Scholar] [CrossRef]
- Lurie, J. Handbook of Analytical Chemistry; Mir Publishers: Moscow, Russia, 1975. [Google Scholar]
- Zhitomirsky, I. Cathodic Electrodeposition of Ceramic and Organoceramic Materials. Fundamental Aspects. Adv. Colloid Interface Sci. 2002, 97, 279–317. [Google Scholar] [CrossRef]
- Egorin, A.; Tokar, E.; Zemskova, L.; Didenko, N.; Portnyagin, A.; Azarova, Y.; Palamarchuk, M.; Tananaev, I.; Avramenko, V. Chitosan-Ferrocyanide Sorbents for Concentrating Cs-137 from Seawater. Sep. Sci. Technol. 2017, 52, 1983–1991. [Google Scholar] [CrossRef]
- Vincent, T.; Vincent, C.; Barré, Y.; Guari, Y.; Saout, G.L.; Guibal, E. Immobilization of Metal Hexacyanoferrates in Chitin Beads for Cesium Sorption: Synthesis and Characterization. J. Mater. Chem. A 2014, 2, 10007–10021. [Google Scholar] [CrossRef]
- Vincent, C.; Hertz, A.; Vincent, T.; Barré, Y.; Guibal, E. Immobilization of Inorganic Ion-Exchanger into Biopolymer Foams—Application to Cesium Sorption. Chem. Eng. J. 2014, 236, 202–211. [Google Scholar] [CrossRef]


| Composite Material | Application Target Product | Synthesis Conditions | References |
|---|---|---|---|
| Fe3O4/CS | Sorbent for Sr | Mixture of solutions of Fe (III) and Fe (II) salts (molar ratio 1:2) was added into a 1% CS solution (in 0.1 M HCl), stirred and then NH4OH added until neutral reaction, washed, filtered, air-dried, heated at 100 °C, crushed, and sieved. | [18,19] |
| Fe(OH)3/CS | Sorbent for Sr | Similar procedure, solution of Fe(III) salt was added into the CS solution. | [18] |
| Ni(OH)2/CS | Fabrication of nanomaterials | A chitosan solution (2 wt.%) was mixed with nickel nitrate solution at various volumetric ratios of chitosan to nickel nitrate solution of 1:0.5, 1:1, 1:1.5, and 1:2. The chitosan/nickel nitrate mixtures were dripped vertically via a needle into a precipitation bath consisting of 1.5 M NaOH solution using a syringe pump. The dried beads were annealed at 500 and 600 °C. | [20] |
| Al(OH)3/CS Si(OH)4/CS | Porous ceramics | The aluminum nitrate aqueous solution was added into chitosan solution under stirring, and then this Al-chitosan solution was added into a NH4OH solution (50% v/v) under stirring to form of drops with a syringe. The gel spheres dried at ambient temperature. CS solution mixed with tetraethylorthosilicate (TEOS) and ethanol as a solvent was added into a NH4OH solution. The spherical metal oxides (Al and Si) samples were obtained for calcinations of hybrid spheres at 350, 550, and 700 °C. | [21] |
| Ni(OH)2/CS | Electrode material, sorbent | Homogeneous hydrolysis of the NiCl2 precursor in the presence of urea CO(NH2)2 (at a molar ratio of 0.07: 0.5) and CS solution 0.1 wt.% in 0.01 M HCl at 90 ° C for 9 h. The cooled gel was filtered, dried, and heated at 100 °C. | Present study |
| Ni(OH)2/CS/ACF | Electrode material, sorbent | Similar procedure in the presence of ACF as a substrate. | Present study |
| Al(OH)3/CS γ–Fe2O3 Zr(OH)4/CS Ag/CS | Composite films—biomedical implants, antimicrobial coatings, biosensors | Electrodeposition from solutions of ZrO(NO3)2, Al(NO3)2, FeCl3, and AgNO3 water or aqueous-alcoholic solvents containing 0–0.6 g/L CS in galvanostatic mode on Pt or stainless steel foil. | [22] |
| Ni(OH)2/CS/ACF | Electrode material | Electrodeposition from a NiCl2 and CS solution in the background electrolyte NaCl in a potentiostatic mode onto an ACF electrode at a potential of −700 (−900) mV related to Ag/AgCl. | [23] |
| Cu(OH)2/CS/ACF | Catalyst, antibacterial coatings | Electrodeposition from a CuCl2 and CS solution in the background electrolyte NaCl in a potentiostatic mode onto an ACF electrode at a potential of −700 (−940) mV rel. Ag/AgCl. | [24] |
| MnO2/CS/ACF | Electrode material, sorbent | Electrodeposition from a solution of MnCl2 and CS in the background electrolyte NH4Cl in a potentiostatic mode onto an ACF electrode at a potential of −700 mV rel. Ag/AgCl with air purging of the electrolyte. | [25] |
| CFS—chitosan ferrocyanide sorbent K-Cu | Sorbent for Cs | Chitosan granules with a water content of 92–96 wt.% were formed from a solution of chitosan in acetic acid. Then it was saturated with an aqueous solution of Cu(II) sulfate until the copper sorption tank is filled. Then it is treated with a K4[Fe(CN)6] salt solution. | [26] |
| CFS—chitosan ferrocyanide sorbent K-Ni, K-Cu, K-Zn (CS/FOC K-Ni, CS/FOC K-Cu, CS/FOC K-Zn) | Sorbent for Cs | The chitosan acidic solution was combined with transition metal salt (Ni, Cu, or Zn), then the obtained mixture was dispersed to the alkaline solution of potassium ferrocyanide. Otherwise (vice versa), the alkaline solution of potassium ferrocyanide was dispersed to the chitosan acidic solution containing a Ni(II) salt. The molar ratio M2+/[Fe(CN)6]4− = 3:1. The precipitate was filtered and heated at 100 °C. | Present study [27,28] |
| HA/CS | Composites, films, biomedical coatings, membranes | The solution of Ca(NO3)2 and CaCl2 salts or the suspension of Ca(OH)2 and CaCO3 in chitosan solution were added with phosphates: (NH4)2HPO4, NaH2PO4, K2HPO4, H3PO4, or urea-phosphate. Then alkalization with NH4OH or NaOH. Drying in air or lyophilization. | [29,30,31,32,33,34,35] |
| HA/CS | Coatings | Electrochemical deposition from the CS solution containing brushite. Conversion of brushite into HA by treatment with alkali 0.1 M NaOH (24 h at 95–100 °C; 72 h at room temperature). | [36] |
| HA/CS | Films, biomedical coatings, membranes | The CS solution was combined with salts CaCl2 and K2HPO4 at the molar ratio Ca/P = 1.67. The mixture was placed into NH3 atmosphere and held there for 1 h until pH ~10. Then the mixture was heated at 100 °C for 12 h. Conversion of the film to by treatment with alkali 0.1 M NaOH (24 h at 95–100 °C; 72 h at room temperature). | Present study [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zemskova, L.; Silant’ev, V.; Tokar, E.; Egorin, A. Synthesis of Inorganic Compounds in the Matrix of Polysaccharide Chitosan. Biomimetics 2021, 6, 45. https://doi.org/10.3390/biomimetics6030045
Zemskova L, Silant’ev V, Tokar E, Egorin A. Synthesis of Inorganic Compounds in the Matrix of Polysaccharide Chitosan. Biomimetics. 2021; 6(3):45. https://doi.org/10.3390/biomimetics6030045
Chicago/Turabian StyleZemskova, Larisa, Vladimir Silant’ev, Eduard Tokar, and Andrei Egorin. 2021. "Synthesis of Inorganic Compounds in the Matrix of Polysaccharide Chitosan" Biomimetics 6, no. 3: 45. https://doi.org/10.3390/biomimetics6030045
APA StyleZemskova, L., Silant’ev, V., Tokar, E., & Egorin, A. (2021). Synthesis of Inorganic Compounds in the Matrix of Polysaccharide Chitosan. Biomimetics, 6(3), 45. https://doi.org/10.3390/biomimetics6030045






