Real-Time Monitoring of Interactions between Solid-Supported Lipid Vesicle Layers and Short- and Medium-Chain Length Alcohols: Ethanol and 1-Pentanol
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Lipid Vesicle Preparation
2.3. Quartz Crystal Microbalance with Dissipation Monitoring Measurements
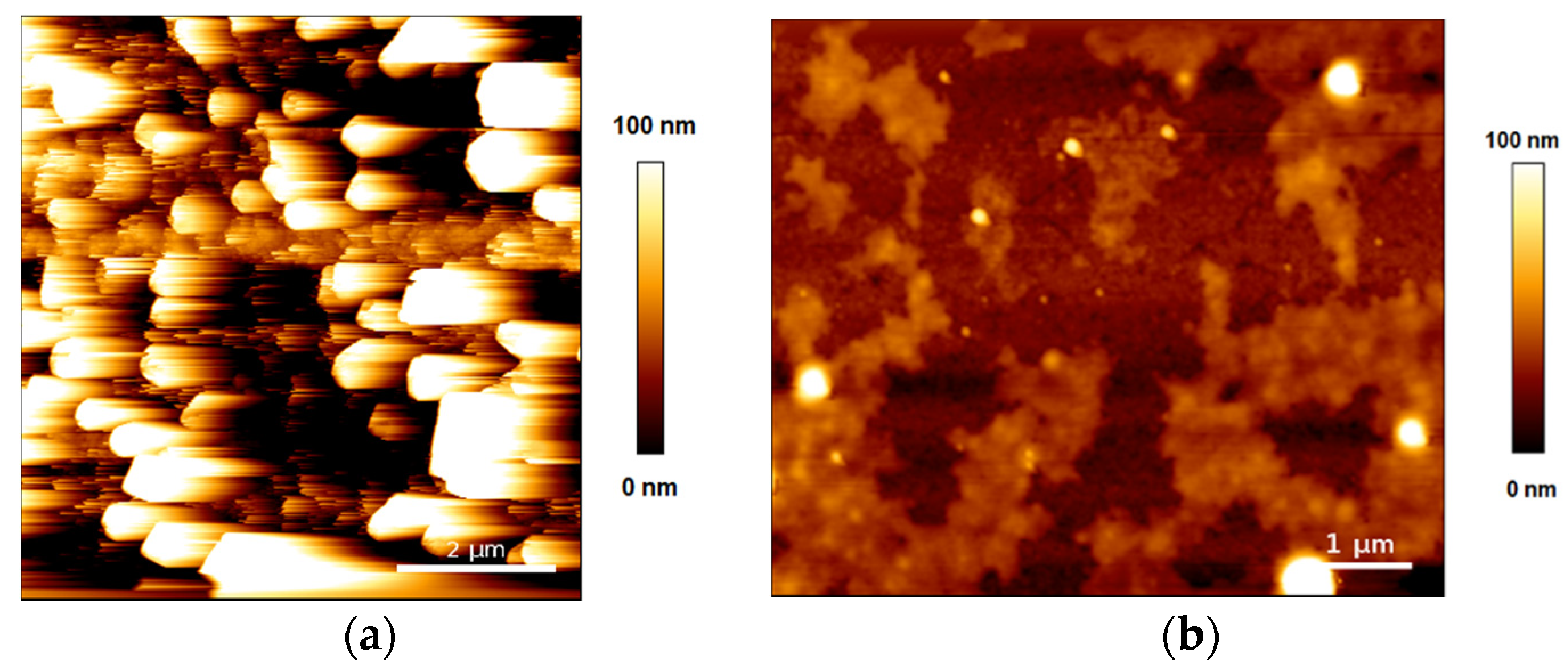
2.4. Atomic Force Microscopy Measurements
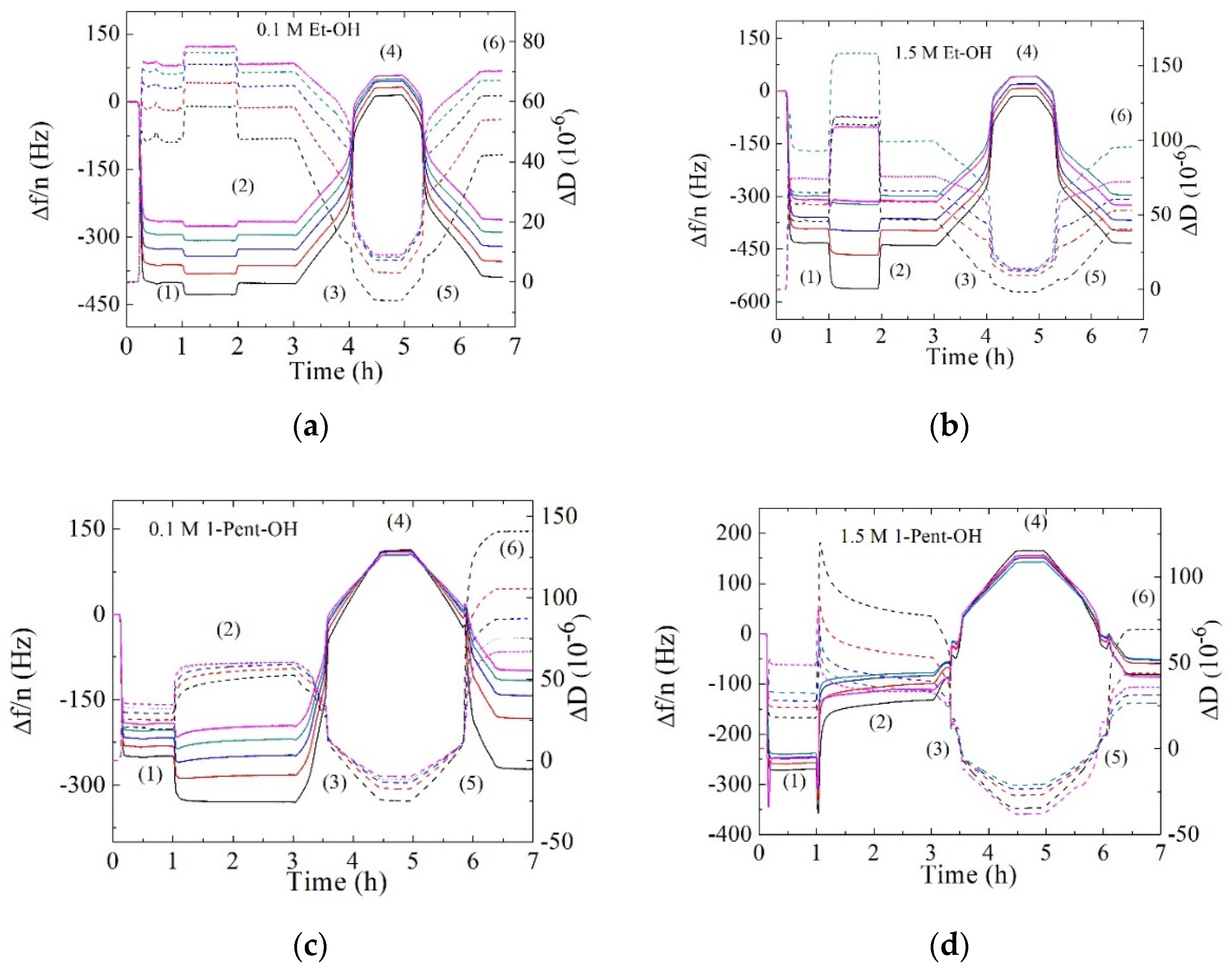
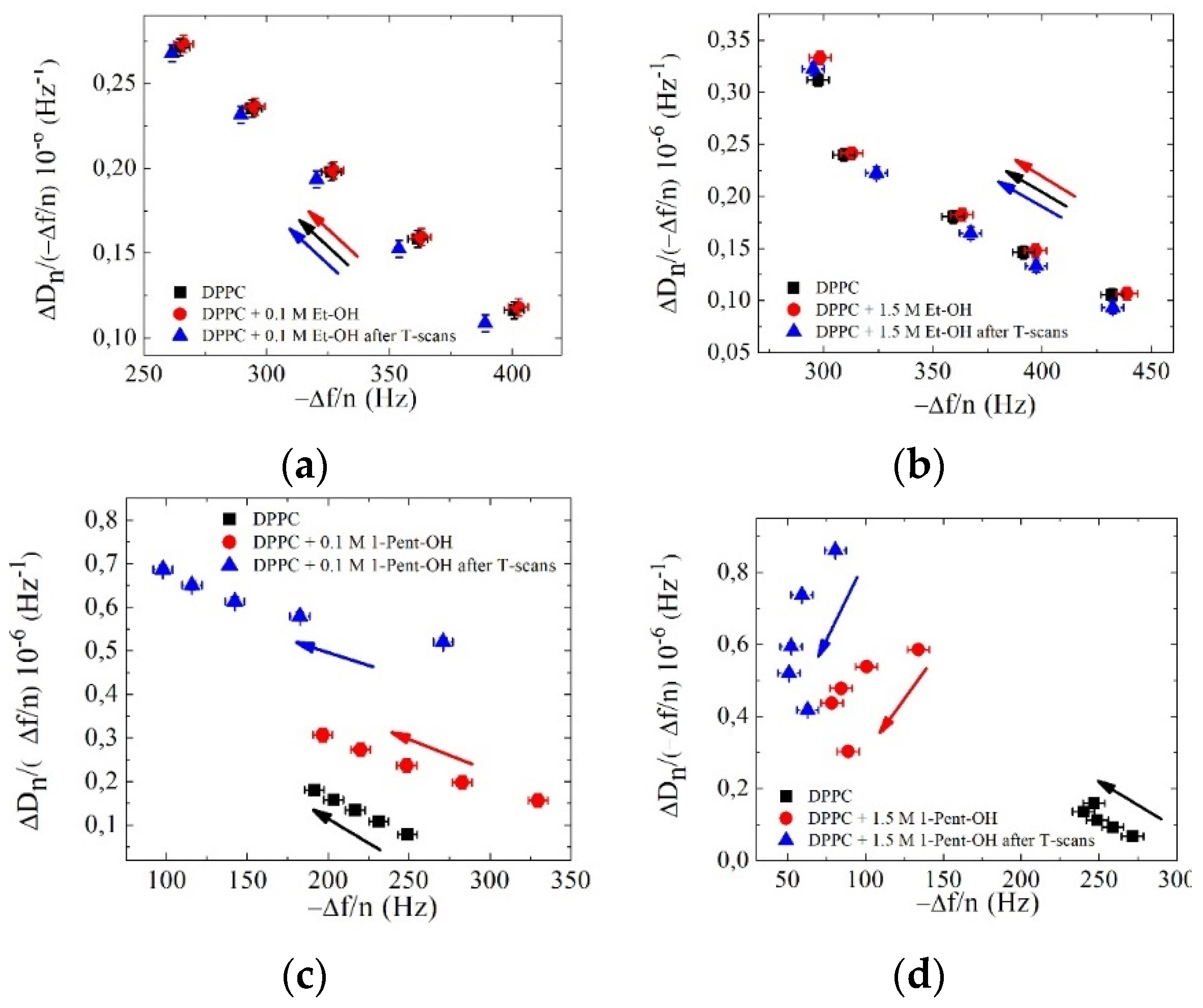
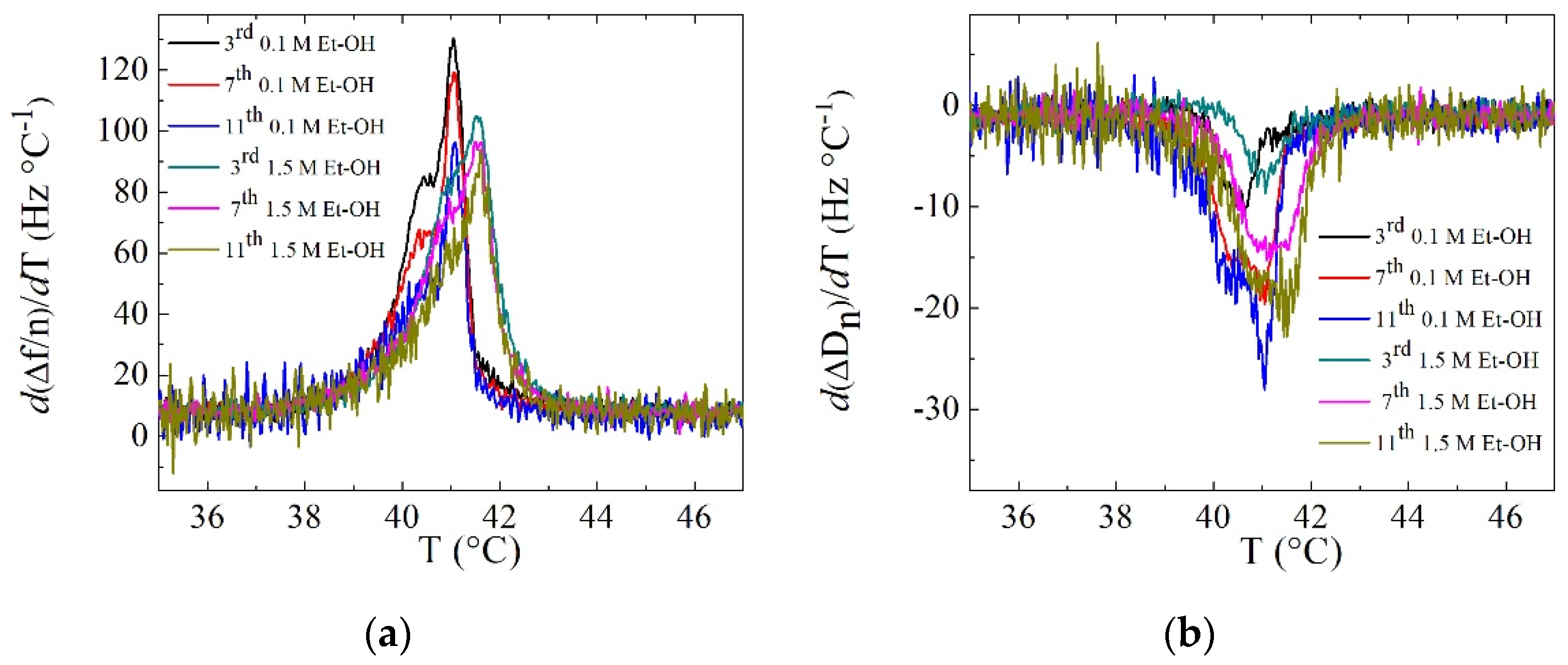
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sozio, M.; Crabb, D.W. Alcohol and lipid metabolism. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E10–E16. [Google Scholar] [CrossRef] [PubMed]
- Paxman, J.; Hunt, B.; Hallan, D.; Zarbock, S.R.; Woodbury, D.J. Drunken membranes: Short-chain alcohols alter fusion of liposomes to planar lipid bilayers. Biophys. J. 2017, 112, 121–132. [Google Scholar] [CrossRef] [PubMed]
- You, K.M.; Rosenfield, C.M.; Knipple, D.C. Ethanol tolerance in yeast Saccharomyces cerevisiae is dependent on cellular oleic acid content. App. Environ. Microbiol. 2003, 69, 1499–1503. [Google Scholar] [CrossRef]
- Seeman, P. The membrane actions of anesthetics and tranquilizers. Pharmacol. Rev. 1972, 24, 583–655. [Google Scholar] [PubMed]
- Pang, K.Y.; Braswell, L.M.; Chang, L.; Sommer, T.J.; Miller, M.W. The perturbation of lipid bilayers by general anesthetics: A quantitative test of the disordered lipid hypothesis. Mol. Pharmacol. 1980, 18, 84–90. [Google Scholar] [PubMed]
- Ly, H.V.; Longo, M.L. The influence of short-chain alcohols on interfacial tension, mechanical properties, area/molecule, and permeability of fluid lipid bilayers. Biophys. J. 2004, 1013–1033. [Google Scholar] [CrossRef] [PubMed]
- Aagard, T.H.; Kristensen, M.N.; Westh, P. Packing properties of 1-alkanols and alkanes in a phospholipid membrane. Biophys. Chem. 2006, 119, 61–68. [Google Scholar] [CrossRef]
- Pang, K.Y.; Chang, T.L.; Miller, M.W. On the coupling between anesthetic induced membrane fluidization and cation permeability in lipid vesicles. Mol. Pharmacol. 1979, 15, 729–738. [Google Scholar]
- Barry, J.A.; Gawrisch, K. Direct NMR evidence for ethanol binding to the lipid–water interface of phospholipid bilayers. Biochemistry 1994, 33, 8082–8088. [Google Scholar] [CrossRef]
- Franks, N.P.; Lieb, W.R. Partitioning of long-chain alcohols into lipid bilayers: Implications for mechanisms of general anesthesia. Proc. Natl. Acad. Sci. USA 1986, 83, 5116–5120. [Google Scholar] [CrossRef]
- McIntosh, T.J.; McDaniel, R.V.; Simon, S.A. Induction of an interdigitated gel phase in fully hydrated phosphatidylcholine bilayers. Biochim. Biophys. Acta 1983, 731, 109–114. [Google Scholar] [CrossRef]
- Löbbecke, L.; Cevc, G. Effects of short-chain alcohols on the phase behavior and interdigitation of phosphatidylcholine bilayer membranes. Biochim. Biophys. Acta 1995, 1237, 59–69. [Google Scholar] [CrossRef]
- Lohner, K. Effect of small organic molecules on phospholipid phase transitions. Chem. Phys. Lipids 1991, 57, 341–364. [Google Scholar] [CrossRef]
- Zana, R. Effect of medium chain-length alcohols on the micelles of tetradecyltrimethylammonium bromide. J. Colloid Interface Sci. 1984, 101, 587–590. [Google Scholar] [CrossRef]
- Heimburg, T.; Jackson, A.D. The thermodynamics of general anesthesia. Biophys. J. 2007, 92, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dea, P. Interaction of 1-propanol and 2-propanol with dipalmitoylphosphatidylcholine bilayer: A calorimetric study. J. Chem. Eng. Data 2009, 54, 1447–1451. [Google Scholar] [CrossRef]
- Reeves, M.D.; Schawel, A.K.; Wang, W.; Dea, P. Effect of butanol isomers on dipalmitoylphosphatidylcholine bilayer membranes. Biophys. Chem. 2007, 128, 13–18. [Google Scholar] [CrossRef]
- Rowe, E.S. Lipid chain length and temperature dependence on ethanol-phosphatidylcholine interactions. Biochemistry 1983, 22, 3299–3305. [Google Scholar] [CrossRef]
- Lee, A.G. Interactions between anesthetics and lipid mixtures. Normal alcohols. Biochemistry 1976, 15, 2448–2454. [Google Scholar] [CrossRef]
- Westerman, P.W.; Pope, J.M.; Phonphok, N.; Doane, J.W.; Dubro, D.W. The interaction of n-alkanols with lipid bilayer membranes: A 2H-NMR study. Biochim. Biophys. Acta 1988, 939, 64–78. [Google Scholar] [CrossRef]
- Simon, S.A.; McIntosh, T.J. Interdigitated hydrocarbon chain packing causes the biphasic transition behavior in lipid/alcohol suspensions. Biochim. Biophys. Acta 1984, 773, 169–172. [Google Scholar] [CrossRef]
- Angelova, M.I.; Mutafchieva, R.; Dimova, R.; Tenchov, B. Shape transformations of giant unilamellar vesicles induced by ethanol and temperature variations. Colloids Surf. A Physicochem. Eng. Asp. 1999, 149, 201–205. [Google Scholar] [CrossRef]
- Cho, N.J.; Frank, C.W.; Kasemo, B.; Höök, F. Quartz crystal microbalance with dissipation monitoring of supported lipid bilayers on various substrates. Nat. Protoc. 2010, 5, 1096–1106. [Google Scholar] [CrossRef]
- Neupane, S.; De Smet, Y.; Renner, F.U.; Losada-Pérez, P. Quartz crystal microbalance with dissipation monitoring: A versatile tool to monitor phase transitions in biomimetic membranes. Front. Mater. 2018, 5, 46. [Google Scholar] [CrossRef]
- Filipovic-Gric, J.; Skalko-Basnet, N.; Jalsenjak, I. Mucoadhesive chitosan-coated liposomes: Characteristics and stability. J. Microencapsul. 2001, 18, 3–12. [Google Scholar] [CrossRef]
- Hianik, T.; Snejdarkova, M.; Sokolikova, L.; Meszar, E.; Krivanek, R.; Tvarozek, V.; Novotny, L.; Wang, J. Immunosensors based on supported lipid membranes, protein films and liposomes modified by antibodies. Sens. Actuators B 1999, 57, 201–212. [Google Scholar] [CrossRef]
- Smith, E.A.; Dea, P.K. Differential scanning calorimetry studies of phospholipid membranes: The interdigitated gel phase. In Applications of Calorimetry in a Wide Context; Elkordy, A.A., Ed.; IntechOpen: London, UK, 2013; pp. 407–444. [Google Scholar]
- Hsiang, Y.; Chen, L.J. Viscosity and density of dilute aqueous solutions of 1-pentanol and 2-methyl-2-butanol. J. Chem. Eng. Data 1998, 43, 665–667. [Google Scholar]
- Khattab, I.S.; Bandakar, F.; Fakhree, M.A.A.; Jouyban, A. Density, viscosity and surface tension of water + ethanol mixtures from 293 to 323 K. Korean J. Chem. Eng. 2012, 29, 812–817. [Google Scholar] [CrossRef]
- Losada-Pérez, P.; Jiménez-Monroy, K.L.; van Grinsven, B.; Leys, J.; Janssens, S.D.; Peeters, M.; Glorieux, C.; Thoen, J.; Haenen, K.; De Ceuninck, W.; et al. Phase transitions in lipid vesicles detected by a complementary set of methods: Heat-transfer measurements, adiabatic scanning calorimetry and dissipation-mode quartz crystal microbalance. Phys. Status Solidi A 2014, 211, 1377–1388. [Google Scholar] [CrossRef]
- Cho, N.J.; Hwang, L.Y.; Solandt, J.J.R.; Frank, C.W. Comparison of extruded and sonicated vesicles for planar bilayer self-assembly. Materials 2013, 6, 3294–3308. [Google Scholar] [CrossRef]
- Keller, C.A.; Kasemo, B. Surface specific kinetics of lipid vesicle adsorption measured with a quartz crystal microbalance. Biophys. J. 1998, 75, 1397–1402. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Seneca, S.; Ethirajan, A.; Neupane, S.; Renner, F.U.; Losada-Pérez, P. Ionic strength dependent vesicle adsorption and phase behaviour of anionic phospholipids on a gold substrate. Biointerphases 2016, 11, 019006. [Google Scholar] [CrossRef]
- Reviakine, I.; Gallego, M.; Johannsmann, D.; Tellechea, E. Adsorbed liposome deformation studied with quartz crystal microbalance. J. Chem. Phys. 2012, 136, 084702. [Google Scholar] [CrossRef]
- Jackmann, J.A.; Avsar, S.Y.; Ferham, A.R.; Li, D.; Park, J.H.; Zhdanov, V.P.; Cho, N.J. Quantitative profiling of nanoscale liposome deformation by a localized surface plasmon resonance sensor. Anal. Chem. 2017, 89, 1102–1109. [Google Scholar] [CrossRef]
- Lu, N.; Yang, K.; Yuan, B.; Ma, Y. Molecular response and cooperative behaviour during the interactions of melittin with a membrane: Dissipative quartz crystal microbalance experiments and simulations. J. Phys. Chem. B 2012, 116, 9432–9438. [Google Scholar] [CrossRef]
- Losada-Pérez, P.; Khorshid, M.; Hermans, C.; Robijns, T.; Peeters, M.; Jiménez-Monroy, K.L.; Truong, L.T.N.; Wagner, P. Melittin disruption of raft and non-raft forming biomimetic membranes: A study by quartz crystal microbalance with dissipation monitoring. Colloids Surf. B 2014, 123, 938–944. [Google Scholar] [CrossRef]
- Losada-Pérez, P.; Khorshid, M.; Renner, F.U. Interactions of aqueous imidazolium-based ionic liquid mixtures with solid-supported phospholipid membranes. PLoS ONE 2016, 11, e0163518. [Google Scholar] [CrossRef]
- Fan, X.; Korytowsky, A.; Makky, A.; Tanaka, M.; Wink, M. Ib-AMP4 insertion causes rearrangement in the phospholipid bilayer of biomembranes: Implications from quartz crystal microbalance with dissipation. Biochim. Biophys. Acta Biomembr. 2018, 1860, 617–623. [Google Scholar] [CrossRef]
- Tellechea, E.; Johannsmann, D.; Steinmetz, N.F.; Richter, R.P.; Reviakine, I. Model-independent analysis of QCM data on colloidal particle adsorption. Langmuir 2009, 25, 5177–5184. [Google Scholar] [CrossRef]
- Olsson, A.L.J.; Quevedo, I.R.; He, D.; Basnet, M.; Tufenkji, N. Using the quartz crystal microbalance with dissipation monitoring to evaluate the size of nanoparticles deposited on surfaces. ACS Nano 2013, 7, 7833–7843. [Google Scholar] [CrossRef]
- Hunt, G.R.A.; Kaszuba, M. The effect of n-alcohols on vesicular permeability induced at the lipid phase transition temperature: A 1H-NMR study. Chem. Phys. Lipids 1989, 51, 55–65. [Google Scholar] [CrossRef]
- Ohlsson, G.; Tigerstrom, A.; Höök, F.; Kasemo, B. Phase transitions in adsorbed lipid vesicles measured using quartz crystal microbalance with dissipation monitoring. Soft Matter 2011, 7, 10749–10755. [Google Scholar] [CrossRef]
- Losada-Pérez, P.; Mertens, N.; De Medio-Vasconcelos, B.; Slenders, E.; Leys, J.; Peeters, M.; van Grinsven, B.; Gruber, J.; Glorieux, C.; Pfeiffer, H.; et al. Phase transitions of binary liquid mixtures: A combined study by adiabatic scanning calorimetry and quartz crystal microbalance with dissipation monitoring. Adv. Cond. Mater. Phys. 2015, 2015, 479318. [Google Scholar]
- Losada-Pérez, P.; Khorshid, M.; Yongabi, D.; Wagner, P. Effect of cholesterol on the phase behavior of solid-supported lipid vesicle layers. J. Phys. Chem. B 2015, 119, 4985–4992. [Google Scholar] [CrossRef]
- Rosser, M.F.N.; Lue, H.M.; Dea, P. Effects of alcohols on lipid bilayers with and without cholesterol: The dipalmitoylphosphatidylcholine system. Biophys. Chem. 1999, 81, 33–44. [Google Scholar] [CrossRef]
- Griffin, K.L.; Cheng, C.Y.; Smith, E.A.; Dea, P.K. Effects of Pentanol Isomers on the Phase Behavior of Phospholipid Bilayer Membranes. Biophys. Chem. 2010, 152, 178–183. [Google Scholar] [CrossRef]
- Peschel, A.; Langhoff, A.; Uhl, E.; Dathathreyan, A.; Haindl, S.; Johannsmann, D.; Reviakine, I. Lipid phase behaviour studied with a quartz crystal microbalance: A technique for biophysical studies with applications in screening. J. Chem. Phys. 2016, 145, 204904. [Google Scholar] [CrossRef]
- Jing, Y.; Trefna, H.; Persson, M.; Kasemo, B.; Svedhem, S. Formation of supported lipid bilayers on silica: Relation to lipid phase transition temperature and liposome size. Soft Matter 2014, 10, 187–195. [Google Scholar] [CrossRef]


| Vesicle Dispersion | Diameter (nm) 1 | Polydispersity Index |
|---|---|---|
| Pure DPPC SUVs used without alcohol addition | 114 ± 40 | 0.14 |
| Pure DPPC SUVs used before ethanol addition | 139 ± 55 | 0.19 |
| Pure DPPC SUVs used before 1-pentanol addition | 114 ± 30 | 0.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neupane, S.; Cordoyiannis, G.; Renner, F.U.; Losada-Pérez, P. Real-Time Monitoring of Interactions between Solid-Supported Lipid Vesicle Layers and Short- and Medium-Chain Length Alcohols: Ethanol and 1-Pentanol. Biomimetics 2019, 4, 8. https://doi.org/10.3390/biomimetics4010008
Neupane S, Cordoyiannis G, Renner FU, Losada-Pérez P. Real-Time Monitoring of Interactions between Solid-Supported Lipid Vesicle Layers and Short- and Medium-Chain Length Alcohols: Ethanol and 1-Pentanol. Biomimetics. 2019; 4(1):8. https://doi.org/10.3390/biomimetics4010008
Chicago/Turabian StyleNeupane, Shova, George Cordoyiannis, Frank Uwe Renner, and Patricia Losada-Pérez. 2019. "Real-Time Monitoring of Interactions between Solid-Supported Lipid Vesicle Layers and Short- and Medium-Chain Length Alcohols: Ethanol and 1-Pentanol" Biomimetics 4, no. 1: 8. https://doi.org/10.3390/biomimetics4010008
APA StyleNeupane, S., Cordoyiannis, G., Renner, F. U., & Losada-Pérez, P. (2019). Real-Time Monitoring of Interactions between Solid-Supported Lipid Vesicle Layers and Short- and Medium-Chain Length Alcohols: Ethanol and 1-Pentanol. Biomimetics, 4(1), 8. https://doi.org/10.3390/biomimetics4010008





