Optimizing Epicardial Restraint and Reinforcement Following Myocardial Infarction: Moving Towards Localized, Biomimetic, and Multitherapeutic Options
Abstract
1. Introduction and Rationale for Mechanical Reinforcement of the Left Ventricle Post-Myocardial Infarction
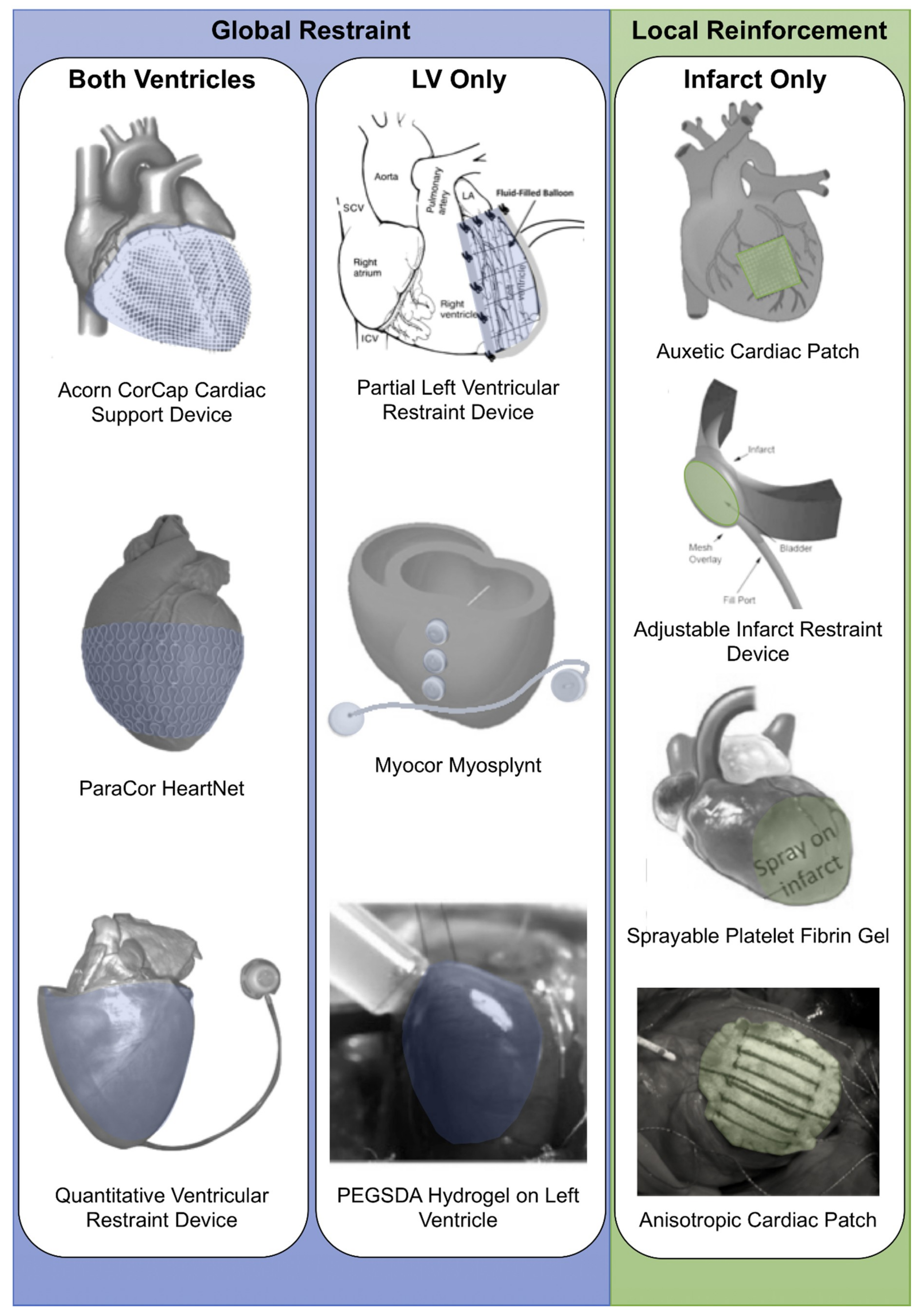
2. Optimizing Biventricular Restraint Devices
2.1. Adjustability
2.2. Adding Functionality
3. Optimizing Left Ventricle Restraint Devices
3.1. Adjustability
3.2. Adding Functionality
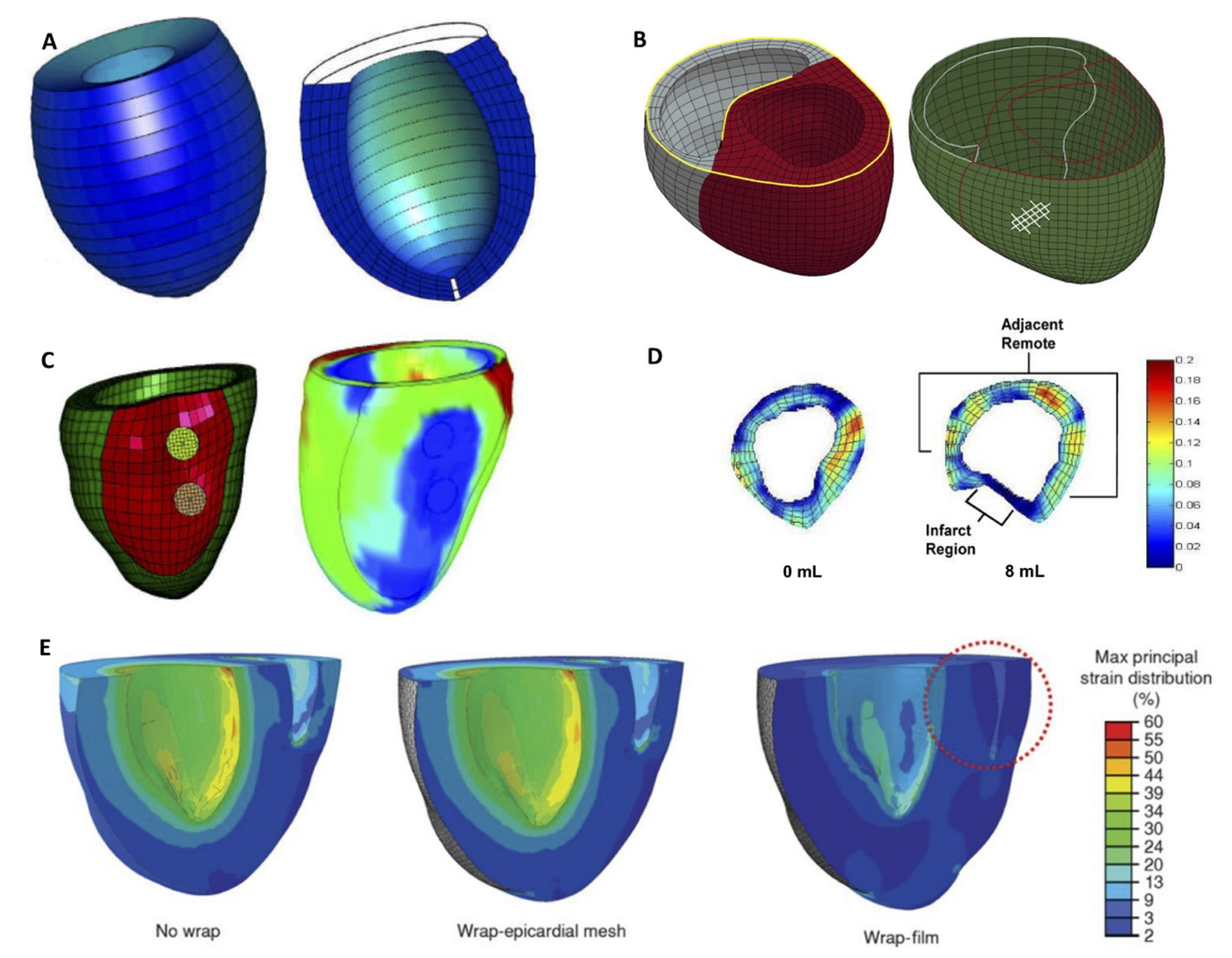
4. Computational Modeling
5. Optimizing Local Reinforcement: Multifunctional, Biomimetic and Adjustable Devices
5.1. Overview and Timing of Intervention
5.2. Adding Functionality
5.3. Adjustability
6. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart disease and stroke statistics—2018 Update: A report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.A.; Braunwald, E. Ventricular remodeling after myocardial infarction: Experimental observations and clinical implications. Circulation 1990, 81, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.H.; Cevasco, M.; Schmitto, J.D.; Chen, F.Y. Ventricular restraint therapy for heart failure: A review, summary of state of the art, and future directions. J. Thorac. Cardiovasc. Surg. 2012, 144, 771–777. [Google Scholar] [CrossRef]
- Chachques, J.C.; Jegaden, O.; Mesana, T.; Glock, Y.; Grandjean, P.A.; Carpentier, A.F. Cardiac bioassist: Results of the French multicenter cardiomyoplasty study. Asian Cardiovasc. Thorac. Ann. 2009, 17, 573–580. [Google Scholar] [CrossRef]
- Clarke, S.A.; Ghanta, R.K.; Ailawadi, G.; Holmes, J.W. Cardiac restraint and support following myocardial infarction. In Stud Mechanobiol Tissue Eng Biomater; Springer-Verlag: Berlin/Heidelberg, Germany, 2013/2014; pp. 15 and 169–206.
- Lee, L.S.; Ghanta, R.K.; Mokashi, S.A.; Coelho-Filho, O.; Kwong, R.Y.; Kwon, M.; Guan, J.; Liao, R.; Chen, F.Y. Optimized ventricular restraint therapy: Adjustable restraint is superior to standard restraint in an ovine model of ischemic cardiomyopathy. J. Thorac. Cardiovasc. Surg. 2013, 145, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Fomovsky, G.M.; MacAdangdang, J.R.; Ailawadi, G.; Holmes, J.W. Model-based design of mechanical therapies for myocardial infarction. J. Cardiovasc. Transl. Res. 2011, 4, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Fomovsky, G.M.; Clark, S.A.; Parker, K.M.; Ailawadi, G.; Holmes, J.W. Anisotropic reinforcement of acute anteroapical infarcts improves pump function. Circ. Hear. Fail. 2012, 5, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, T.; Schurer, R.A.J.; Al Ali, L.; van Den Heuvel, A.F.M.; van der Harst, P. Left ventricular restoration devices post myocardial infarction. Heart Fail. Rev. 2018. [Google Scholar] [CrossRef]
- Zhu, Y.; Matsumura, Y.; Wagner, W.R. Ventricular wall biomaterial injection therapy after myocardial infarction: Advances in material design, mechanistic insight and early clinical experiences. Biomaterials 2017, 129, 37–53. [Google Scholar] [CrossRef]
- Sabbah, H.N. The cardiac support device and the Myosplint: Treating heart failure by targeting left ventricular size and shape. Ann. Thorac. Surg. 2003, 75, S13–S19. [Google Scholar] [CrossRef]
- Ghanta, R.K.; Rangaraj, A.; Umakanthan, R.; Lee, L.; Laurence, R.G.; Fox, J.A.; Bolman, R.M.; Cohn, L.H.; Chen, F.Y. Adjustable, physiological ventricular restraint improves left ventricular mechanics and reduces dilatation in an ovine model of chronic heart failure. Circulation 2007, 115, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Klodell, C.T.; Aranda, J.M.; McGiffin, D.C.; Rayburn, B.K.; Sun, B.; Abraham, W.T.; Pae, W.E.; Boehmer, J.P.; Klein, H.; Huth, C. Worldwide surgical experience with the Paracor HeartNet cardiac restraint device. J. Thorac. Cardiovasc. Surg. 2008, 135, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Mokashi, S.A.; Lee, L.S.; Schmitto, J.D.; Ghanta, R.K.; McGurk, S.; Laurence, R.G.; Bolman, R.M.; Cohn, L.H.; Chen, F.Y. Restraint to the left ventricle alone is superior to standard restraint. J. Thorac. Cardiovasc. Surg. 2013, 146, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Raman, J. Management of Heart Failure, 2nd ed.; Volume 2: Surgical; Springer-Verlag: London, UK, 2016; p. 284. [Google Scholar]
- Vilaeti, A.D.; Dimos, K.; Lampri, E.S.; Mantzouratou, P.; Tsitou, N.; Mourouzis, I.; Oikonomidis, D.L.; Papalois, A.; Pantos, C.; Malamou-Mitsi, V.; et al. Short-term ventricular restraint attenuates post-infarction remodeling in rats. Int. J. Cardiol. 2013, 165, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Kapnisi, M.; Mansfield, C.; Marijon, C.; Guex, A.G.; Perbellini, F.; Bardi, I.; Humphrey, E.J.; Puetzer, J.L.; Mawad, D.; Koutsogeorgis, D.C.; et al. Auxetic cardiac patches with tunable mechanical and conductive properties toward treating myocardial infarction. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef] [PubMed]
- Koomalsingh, K.J.; Witschey, W.R.T.; McGarvey, J.R.; Shuto, T.; Kondo, N.; Xu, C.; Jackson, B.M.; Gorman, J.H.; Gorman, R.C.; Pilla, J.J. Optimized local infarct restraint improves left ventricular function and limits remodeling. Ann. Thorac. Surg. 2013, 95, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Vandergriff, A.; Wang, Z.; Hensley, M.T.; Cores, J.; Allen, T.A.; Dinh, P.-U.; Zhang, J.; Caranasos, T.G.; Cheng, K. A regenerative cardiac patch formed by spray painting of biomaterials onto the heart. Tissue Eng. Part C Methods 2017, 23, 146–155. [Google Scholar] [CrossRef]
- Oz, M.C.; Konertz, W.F.; Kleber, F.X.; Mohr, F.W.; Gummert, J.F.; Ostermeyer, J.; Lass, M.; Raman, J.; Acker, M.A.; Smedira, N. Global surgical experience with the Acorn cardiac support device. J. Thorac. Cardiovasc. Surg. 2003, 126, 983–991. [Google Scholar] [CrossRef]
- Power, J.M.; Raman, J.; Dornom, A.; Farish, S.J.; Burrell, L.M.; Tonkin, A.M.; Buxton, B.; Alferness, C.A. Passive ventricular constraint amends the course of heart failure: A study in an ovine model of dilated cardiomyopathy. Cardiovasc. Res. 1999, 44, 549–555. [Google Scholar] [CrossRef]
- Power, J.M.; Raman, J.; Byrne, M.J.; Alferness, C.A. Efficacy of the Acorn cardiac support device in animals with heart failure secondary to high rate pacing. Heart Fail. Rev. 2005, 10, 117–123. [Google Scholar] [CrossRef]
- Chaudhry, P.A.; Mishima, T.; Sharov, V.G.; Hawkins, J.; Alferness, C.; Paone, G.; Sabbah, H.N. Passive epicardial containment prevents ventricular remodeling in heart failure. Ann. Thorac. Surg. 2000, 70, 1275–1280. [Google Scholar] [CrossRef]
- Blom, A.S.; Mukherjee, R.; Pilla, J.J.; Lowry, A.S.; Yarbrough, W.M.; Mingoia, J.T.; Hendrick, J.W.; Stroud, R.E.; McLean, J.E.; Affuso, J.; et al. Cardiac support device modifies left ventricular geometry and myocardial structure after myocardial infarction. Circulation 2005, 112, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- Blom, A.S.; Pilla, J.J.; Arkles, J.; Dougherty, L.; Ryan, L.P.; Gorman, J.H.; Acker, M.A.; Gorman, R.C. Ventricular Restraint Prevents Infarct Expansion and Improves Borderzone Function After Myocardial Infarction: A study using magnetic resonance imaging, three-dimensional surface modeling, and myocardial tagging. Ann. Thorac. Surg. 2007, 84, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Konertz, W.F.; Shapland, J.E.; Hotz, H.; Dushe, S.; Braun, J.P.; Stantke, K.; Kleber, F.X. Passive containment and reverse remodeling by a novel textile cardiac support device. Circulation 2001, 104 (Suppl. 1), I270–I275. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.C.; Konertz, W.F.; Raman, J.; Kleber, F.X. Reverse remodeling of the failing ventricle: Surgical intervention with the Acorn cardiac support device. Congest. Hear. Fail. 2004, 10, 96–104. [Google Scholar] [CrossRef]
- Speziale, G.; Nasso, G.; Piancone, F.; Generali, K.; Paterno, C.; Miccoli, A.; Fiore, F.; Del Prete, A.; Del Prete, G.; Lopriore, V.; et al. One-year results after implantation of the CorCap for dilated cardiomyopathy and heart failure. Ann. Thorac. Surg. 2011, 91, 1356–1362. [Google Scholar] [CrossRef]
- Acker, M.A.; Jessup, M.; Bolling, S.F.; Oh, J.; Starling, R.C.; Mann, D.L.; Sabbah, H.N.; Shemin, R.; Kirklin, J.; Kubo, S.H. Mitral valve repair in heart failure: Five-year follow-up from the mitral valve replacement stratum of the Acorn randomized trial. J. Thorac. Cardiovasc. Surg. 2011, 142, 569–574. [Google Scholar] [CrossRef]
- Yue, S.; Naveed, M.; Gang, W.; Chen, D.; Wang, Z.; Yu, F.; Zhou, X. Cardiac support device (ASD) delivers bone marrow stem cells repetitively to epicardium has promising curative effects in advanced heart failure. Biomed. Microdevices 2018, 20. [Google Scholar] [CrossRef]
- Naveed, M.; Wenhua, L.; Gang, W.; Mohammad, I.S.; Abbas, M.; Liao, X.; Yang, M.; Zhang, L.; Liu, X.; Qi, X.; et al. A novel ventricular restraint device (ASD) repetitively deliver Salvia miltiorrhiza to epicardium have good curative effects in heart failure management. Biomed. Pharmacother. 2017, 95, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, M.; Miyagawa, S.; Fukushima, S. Biodegradable versus non-biodegradable cardiac support device for treating ischemic cardiomyopathy in a canine heart. Semin. Thorac. Cardiovasc. Surg. 2017, 29, 51–61. [Google Scholar] [CrossRef]
- Okada, M.; Akita, T.; Mizuno, F.; Nakayama, A.; Morioka, K. Beneficial effects of a cardiac support device on left ventricular remodeling after posterior myocardial infarction: An evaluation by echocardiography, pressure–volume curves and ventricular histology. Surg. Today 2016, 46, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Magovern, J.A.; Teekell-Taylor, L.; Mankad, S.; Dasika, U.; McGregor, W.; Biederman, R.W.W.; Yamrozik, J.; Trumble, D.R. Effect of a flexible ventricular restraint device on cardiac remodeling after acute myocardial infarction. ASAIO J. 2006, 52, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Ghanta, R.K.; Lee, L.S.; Umakanthan, R.; Laurence, R.G.; Fox, J.A.; Bolman, R.M.; Cohn, L.H.; Chen, F.Y. Real-time adjustment of ventricular restraint therapy in heart failure. Eur. J. Cardio-Thoracic. Surg. 2008, 34, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Snowden, T.; Biswas, S.; Criscione, J. Modulation of diastolic filling using an epicardial diastolic recoil device. J. Med. Devices 2013, 7, 034503. [Google Scholar] [CrossRef]
- Park, J.; Choi, S.; Janardhan, A.H.; Lee, S.Y.; Raut, S.; Soares, J.; Shin, K.; Yang, S.; Lee, C.; Kang, K.W.; et al. Electromechanical cardioplasty using a wrapped elasto-conductive epicardial mesh. Sci. Transl. Med. 2016, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X. Active Hydraulic Ventricular Attaching Support System. U.S. Patent Application No. 9,089,425, 8 May 2009. [Google Scholar]
- Roche, E.T.; Horvath, M.A.; Wamala, I.; Song, S.E.; Whyte, W.; Machaidze, Z.; Vasilyev, N.V.; Mooney, D.J.; Pigula, F.A.; Walsh, C.J. Soft robotic sleeve restores heart function. Sci. Transl. Med. 2017, 9, 3925. [Google Scholar] [CrossRef] [PubMed]
- Payne, C.J.; Wamala, I.; Bautista-Salinas, D.; Saeed, M.; Van Story, D.; Thalhofer, T.; Horvath, M.A.; Abah, C.; del Nido, P.J.; Walsh, C.J.; et al. Soft robotic ventricular assist device with septal bracing for therapy of heart failure. Sci. Robot. 2017, 2, 6736. [Google Scholar] [CrossRef]
- Payne, C.J.; Wamala, I.; Abah, C.; Thalhofer, T.; Saeed, M.; Bautista-Salinas, D.; Horvath, M.A.; Vasilyev, N.V.; Roche, E.T.; Pigula, F.A.; et al. An Implantable Extracardiac Soft Robotic Device for the Failing Heart: Mechanical coupling and synchronization. Soft Robot. 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Horvath, M.A.; Varela, C.E.; Dolan, E.B.; Whyte, W.; Monahan, D.S.; Payne, C.J.; Wamala, I.A.; Vasilyev, N.V.; Pigula, F.A.; Mooney, D.J.; et al. Towards alternative approaches for coupling of a soft robotic sleeve to the heart. Ann. Biomed. Eng. 2018, 46, 1534–1547. [Google Scholar] [CrossRef]
- McCarthy, P.M.; Takagaki, M.; Ochiai, Y.; Young, J.B.; Tabata, T.; Shiota, T.; Qin, J.X.; Thomas, J.D.; Mortier, T.J.; Schroeder, R.F.; et al. Device-based change in left ventricular shape: A new concept for the treatment of dilated cardiomyopathy. J. Thorac. Cardiovasc. Surg. 2001, 122, 482–490. [Google Scholar] [CrossRef]
- Kalogerakos, P.D.; Hassoulas, J.; Ladopoulos, V.S. Beyond heart transplantation: Potentials and problems of the shape memory alloy fibers in the treatment of heart failure. ASAIO J. 2014, 60, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Grossi, E.A.; Patel, N.; Woo, Y.J.; Goldberg, J.D.; Schwartz, C.F.; Subramanian, V.; Feldman, T.; Bourge, R.; Baumgartner, N.; Genco, C.; et al. Outcomes of the RESTOR-MV trial (Randomized Evaluation of a Surgical Treatment for Off-Pump Repair of the Mitral Valve). J. Am. Coll. Cardiol. 2010, 56, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Mishra, Y.K.; Mittal, S.; Jaguri, P.; Trehan, N. Coapsys mitral annuloplasty for chronic functional ischemic mitral regurgitation: 1-Year results. Ann. Thorac. Surg. 2006, 81, 42–46. [Google Scholar] [CrossRef]
- Kashem, A.; Santamore, W.P.; Hassan, S.; Crabbe, D.L.; Margulies, K.B.; Melvin, D.B. CardioClasp: A new passive device to reshape cardiac enlargement. ASAIO J. 2002. [Google Scholar] [CrossRef]
- Kashem, A.; Hassan, S.; Crabbe, D.L.; Melvin, D.B.; Santamore, W.P.; Chitwood, W.R. Left ventricular reshaping: Effects on the pressure–volume relationship. J. Thorac. Cardiovasc. Surg. 2003, 125, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Kashem, A.; Santamore, W.P.; Hassan, S.; Melvin, D.B.; Crabbe, D.L.; Margulies, K.B.; Goldman, B.I.; Llort, F.; Krieger, C.; Lesniak, J. CardioClasp changes left ventricular shape acutely in enlarged canine heart. J. Card. Surg. 2003, 18, S49–S60. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.S.; Ghanta, R.K.; Mokashi, S.A.; Coelho-Filho, O.; Kwong, R.Y.; Bolman, R.M.; Chen, F.Y. Ventricular restraint therapy for heart failure: The right ventricle is different from the left ventricle. J. Thorac. Cardiovasc. Surg. 2010, 139, 1012–1018. [Google Scholar] [CrossRef]
- Jhun, C.-S.; Wenk, J.F.; Zhang, Z.; Wall, S.T.; Sun, K.; Sabbah, H.N.; Ratcliffe, M.B.; Guccione, J.M. Effect of adjustable passive constraint on the failing left ventricle: A finite-element model study. Ann. Thorac. Surg. 2010, 89, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Wenk, J.F.; Ge, L.; Zhang, Z.; Mojsejenko, D.; Potter, D.D.; Tseng, E.E.; Guccione, J.M.; Ratcliffe, M.B. Biventricular finite element modeling of the Acorn CorCap cardiac support device on a failing heart. Ann. Thorac. Surg. 2013, 95, 2022–2027. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Zhang, Z.; Suzuki, T.; Wenk, J.F.; Stander, N.; Einstein, D.R.; Saloner, D.A.; Wallace, A.W.; Guccione, J.M.; Ratcliffe, M.B. Dor procedure for dyskinetic anteroapical myocardial infarction fails to improve contractility in the border zone. J. Thorac. Cardiovasc. Surg. 2010, 140, 233–239. [Google Scholar] [CrossRef]
- Pantoja, J.L.; Zhang, Z.; Tartibi, M.; Sun, K.; Macmillan, W.; Guccione, J.M.; Ge, L.; Ratcliffe, M.B. Residual stress impairs pump function after surgical ventricular remodeling: A finite element analysis. Ann. Thorac. Surg. 2015, 100, 2198–2205. [Google Scholar] [CrossRef]
- Carrick, R.; Ge, L.; Lee, L.C.; Zhang, Z.; Mishra, R.; Axel, L.; Guccione, J.M.; Grossi, E.A.; Ratcliffe, M.B. Patient-specific finite element-based analysis of ventricular myofiber stress after Coapsys: Importance of residual stress. Ann. Thorac. Surg. 2012, 93, 1964–1971. [Google Scholar] [CrossRef] [PubMed]
- Sack, K.L.; Baillargeon, B.; Acevedo-Bolton, G.; Genet, M.; Rebelo, N.; Kuhl, E.; Klein, L.; Weiselthaler, G.M.; Burkhoff, D.; Franz, T.; et al. Partial LVAD restores ventricular outputs and normalizes LV but not RV stress distributions in the acutely failing heart in silico. Int. J. Artif. Organs 2016, 39, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Kelley, S.T.; Malekan, R.; Gorman, J.H.; Jackson, B.M.; Gorman, R.C.; Suzuki, Y.; Plappert, T.; Bogen, D.K.; St. John Sutton, M.G.; Edmunds, L.H. Restraining infarct expansion preserves left ventricular geometry and function after acute anteroapical infarction. Circulation 1999, 99, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Chi, N.H.; Yang, M.C.; Chung, T.W.; Chou, N.K.; Wang, S.S. Cardiac repair using chitosan-hyaluronan/silk fibroin patches in a rat heart model with myocardial infarction. Carbohydr. Polym. 2013, 92, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.A.; Goodman, N.C.; Ailawadi, G.; Holmes, J.W. Effect of scar compaction on the therapeutic efficacy of anisotropic reinforcement following myocardial infarction in the dog. J. Cardiovasc. Transl. Res. 2015, 8, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Mewhort, H.E.M.; Turnbull, J.D.; Satriano, A.; Chow, K.; Flewitt, J.A.; Andrei, A.C.; Guzzardi, D.G.; Svystonyuk, D.A.; White, J.A.; Fedak, P.W.M. Epicardial infarct repair with bioinductive extracellular matrix promotes vasculogenesis and myocardial recovery. J. Hear. Lung Transplant. 2016, 35, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Whyte, W.; Roche, E.T.; Varela, C.E.; Mendez, K.; Islam, S.; O’Neill, H.; Weafer, F.; Shirazi, R.N.; Weaver, J.C.; Vasilyev, N.V.; et al. Sustained release of targeted cardiac therapy with a replenishable implanted epicardial reservoir /692/4019/2773 /639/301/54/152 /14/5 /14/35 /14/63 /59/5 /96/106 /96/100 /96/34 article. Nat. Biomed. Eng. 2018, 2, 416–428. [Google Scholar] [CrossRef]
- Caggiano, L.R.; Lee, J.-J.; Holmes, J.W. surgical reinforcement alters collagen alignment and turnover in healing myocardial infarcts. Am. J. Physiol. Circ. Physiol. 2018, 315, H1041–H1050. [Google Scholar] [CrossRef]
- Rodness, J.; Mihic, A.; Miyagi, Y.; Wu, J.; Weisel, R.D.; Li, R.K. VEGF-loaded microsphere patch for local protein delivery to the ischemic heart. Acta Biomater. 2016, 45, 169–181. [Google Scholar] [CrossRef]
- Kai, D.; Wang, Q.L.; Wang, H.J.; Prabhakaran, M.P.; Zhang, Y.; Tan, Y.Z.; Ramakrishna, S. Stem cell-loaded nanofibrous patch promotes the regeneration of infarcted myocardium with functional improvement in rat model. Acta Biomater. 2014, 10, 2727–2738. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, J.; Sun, H.; Qiu, X.; Mou, Y.; Liu, Z.; Zhao, Y.; Li, X.; Han, Y.; Duan, C.; et al. Engineering the heart: Evaluation of conductive nanomaterials for improving implant integration and cardiac function. Sci. Rep. 2014, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, A.; Yoshizumi, T.; Luketich, S.K.; Wolf, M.T.; Gu, X.; Cammarata, M.; Hoff, R.; Badylak, S.F.; Wagner, W.R. Bi-layered polyurethane—Extracellular matrix cardiac patch improves ischemic ventricular wall remodeling in a rat model. Biomaterials 2016, 107, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mewhort, H.E.M.; Turnbull, J.D.; Meijndert, H.C.; Ngu, J.M.C.; Fedak, P.W.M. Epicardial infarct repair with basic fibroblast growth factor-enhanced CorMatrix-ECM biomaterial attenuates postischemic cardiac remodeling. J. Thorac. Cardiovasc. Surg. 2014, 147, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Spadaccio, C.; Nappi, F.; De Marco, F.; Sedati, P.; Taffon, C.; Nenna, A.; Crescenzi, A.; Chello, M.; Trombetta, M.; Gambardella, I.; et al. Implantation of a poly-l-lactide GCSF-functionalized scaffold in a model of chronic myocardial infarction. J. Cardiovasc. Transl. Res. 2017, 10, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, A.; Zeng, X.; Guerrero, J.L.; Kozak, A.; Braithwaite, G.; Levine, R.A.; Vlahakes, G.J.; Hung, J. Application of polymer-mesh device to remodel left ventricular–mitral valve apparatus in ischemic mitral regurgitation. J. Thorac. Cardiovasc. Surg. 2018, 155, 1485–1493. [Google Scholar] [CrossRef]
- McGarvey, J.R.; Shimaoka, T.; Takebayashi, S.; Aoki, C.; Kondo, N.; Takebe, M.; Zsido, G.A.; Jassar, A.; Gorman, J.H.; Pilla, J.J.; et al. Minimally invasive delivery of a novel direct epicardial assist device in a porcine heart failure model. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2014, 9, 16–21. [Google Scholar] [CrossRef]
- Konstam, M.A.; Kramer, D.G.; Patel, A.R.; Maron, M.S.; Udelson, J.E. Left ventricular remodeling in heart failure: Current concepts in clinical significance and assessment. JACC Cardiovasc. Imaging 2011, 4, 98–108. [Google Scholar] [CrossRef]
- Mewhort, H.E.M.; Svystonyuk, D.A.; Turnbull, J.D.; Teng, G.; Belke, D.D.; Guzzardi, D.G.; Park, D.S.; Kang, S.; Hollenberg, M.D.; Fedak, P.W.M. Bioactive extracellular matrix scaffold promotes adaptive cardiac remodeling and repair. JACC Basic Transl. Sci. 2017, 2, 450–464. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Dawkins, J.; Bi, X.; Marbán, E.; Li, D. Diffusion tensor cardiac magnetic resonance reveals exosomes from cardiosphere-derived cells preserve myocardial fiber architecture after myocardial infarction. JACC Basic to Transl. Sci. 2018, 3, 97–109. [Google Scholar] [CrossRef]
- Wu, M.-T.; Su, M.-Y.M.; Huang, Y.-L.; Chiou, K.-R.; Yang, P.; Pan, H.-B.; Reese, T.G.; Wedeen, V.J.; Tseng, W.-Y.I. Sequential changes of myocardial microstructure in patients postmyocardial infarction by diffusion-tensor cardiac MR correlation with left ventricular structure and function. Circ. Cardiovasc. Imaging 2009, 2, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.X.; Wu, Y.; Nicholls, J.M.; Wang, J.; Liao, S.; Zhu, S.; Lau, C.-P.; Tse, H.-F. MR diffusion tensor imaging study of postinfarct myocardium structural remodeling in a porcine model. Magn. Reson. Med. 2007, 58, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.T.; Tseng, W.Y.; Su, M.Y.; Liu, C.P.; Chiou, K.R.; Wedeen, V.J.; Reese, T.G.; Yang, C.F. Diffusion tensor magnetic resonance imaging mapping the fiber architecture remodeling in human myocardium after infarction correlation with viability and wall motion. Circulation 2006, 114, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Sack, K.L.; Aliotta, E.; Choy, J.S.; Ennis, D.B.; Davies, N.H.; Franz, T.; Kassab, G.S.; Guccione, J.M. Effect of intra-myocardial Algisyl-LVRTM injectates on fibre structure in porcine heart failure. J. Mech. Behav. Biomed. Mater. 2018, 87, 172–179. [Google Scholar] [CrossRef] [PubMed]

| Type of Restraint | Animal | Infarct/HF Model | Follow-Up Time Post-MI | Restraint Device or Patch Material | EDV | ESV | EF | SV | CO | FS/FAS/WT | dP/dt | ESPVR | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Global (both ventricles) | Male Sprague Dawley rats | LAD ligation | 30 days | ASD device (biocompatible silicone) + BMSC | - | - | - | - | - | - | ↑ | - | [30] |
| Global (both ventricles) | Male Sprague Dawley rats | LAD ligation | 30 days | ASD device (silicone) + Salvia miltiorrhiza | - | - | - | - | - | ↑ | - | [31] | |
| Global (both ventricles) | Beagle dogs | LAD diagonal ligation | 12 weeks | Biodegradable polyglycolic acid suture knitted support device | ↔ | ↔, ↓ ΔLVESV | ↑ LVEF | - | - | ↔ | - | [32] | |
| Nonbiodegradable polyethylene terephthalate suture knitted support device | ↔, ↓ ΔLVEDV | ↔, ↓ ΔLVESV | ↔ LVEF | - | - | ↔ | - | ||||||
| Global (both ventricles) | Beagle dogs | Posterior wall infarction by ligation of proximal/distal branches of left diagonal, obtuse marginal and posterior coronary arteries | 3 months | CSD knitted dog mesh (polyester sutures) | ↔ | ↔ | ↑ | - | - | - | Emax ↑ | [33] | |
| Global (both ventricles) | Wistar rats | LAD ligation | 15 days | PEGSDA-coated polyanhydroglucuronic-acid scaffold | - | - | ↑ | - | - | - | - | [16] | |
| Global (LV only) | Wistar rats | LAD ligation | PEGSDA hydrogel | - | - | ↑ | - | - | - | - |
| Animal | Infarct/HF Model | Follow-Up Time Post-MI | Restraint Device or Patch Material | EDV | ESV | EF | SV | CO | FS/FAS/WT | dP/dt | ESPVR | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male C57BL mice | LAD ligation | 14 days | Medical polyester mesh or silicone patch | [42] | ||||||||
| Female Sprague Dawley rats | LAD ligation | 28 days | Therepi (TPU and polycarbonate membrane) + rMSC paracrine factors | ↑ | ↑ FS | [61] | ||||||
| Sheep | LCx ligation/ischemic mitral regurgitation | 16 weeks | Poly-mesh (polyester mesh containing polyacrylamide granules with outer border of polyester fabric) | ↓ | ↓ | ↔ LVEF | ↔ | ↑ Emax | [69] | |||
| Male Sprague Dawley rats | LAD ligation | 6 weeks | PDMS-coated Dacron patch | ↓ infarct WT | [62] | |||||||
| Male Sprague Dawley rats | LAD ligation | 2 weeks | Polyaniline and phytic acid grown on micropatterned chitosan films | ↔ | ↔ | ↔ FS | [72] | |||||
| Male New Zealand white rabbits | Left posterolateral/lateral coronary artery ligation | 6 weeks | PLLA patch + GCSF | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ FS | [68] | ||
| Male CD1 mice | LAD ligation | 21 days | Platelet fibrin patch | ↑ | ↑ FS | [19] | ||||||
| Rat | LAD ligation | 14 weeks | Inactivated SIS-ECM patch | ↓ | ↑ | ↑ WT | (dP/dT)/LVEDV ↑ | ↑ | [73] | |||
| Female Lewis rats | Proximal Left Coronary Artery ligation | 10 weeks | Biodegradable PECUU + isotropic ECM enriched layer | ↑ FAC | [66] | |||||||
| Female Sprague Dawley rats | LAD ligation | 1 month | Ca–alginate microsphere patch covered in chitosan sheet | ↑ FS | [63] | |||||||
| Male Landrace pigs | Ischemia reperfusion | 6 weeks | SIS-ECM patch | ↔ | ↔ | ↔ | [60] | |||||
| Male Mongrel dogs | LAD ligation | 8 weeks | Longitudinally inextensible knitted polyester and bovine collagen patch | ↔ | ↔ | ↔ infarct WT | [59] | |||||
| Male Fischer CDF rats | LAD ligation | 16 weeks | bFGF-enhanced SIS-ECM patch | ↓ | ↑ | ↔ ESPVR and EDPVR | [67] | |||||
| Male Sprague Dawley rats | LAD ligation | 6 weeks | SWNT/gelatin hydrogel patch + neonatal rat cardiomyocytes | ↑ | FS ↑ | ↑ | [65] | |||||
| Gelatin hydrogel patch + neonatal rat cardiomyocytes | ↑ | FS ↑ | ||||||||||
| Gelatin hydrogel patch + neonatal rat cardiac fibroblasts | ↑ | FS ↑ | ||||||||||
| Female Sprague Dawley rats | LAD ligation | 5 weeks | Biodegradable PG nanofibrous patch + rMSC | ↓ | ↓ | ↑ | FS ↑ | [64] | ||||
| Pigs | LCx ligation | 4 weeks | Polypropylene mesh covering balloon catheter | ↑ | ↑ | ↑ | [18] | |||||
| Female Wistar rats | Cryo-injury of LV | 8 weeks | Chitosan–HYA/SF patches | ↑ WT, ↑ FS | [58] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varela, C.E.; Fan, Y.; Roche, E.T. Optimizing Epicardial Restraint and Reinforcement Following Myocardial Infarction: Moving Towards Localized, Biomimetic, and Multitherapeutic Options. Biomimetics 2019, 4, 7. https://doi.org/10.3390/biomimetics4010007
Varela CE, Fan Y, Roche ET. Optimizing Epicardial Restraint and Reinforcement Following Myocardial Infarction: Moving Towards Localized, Biomimetic, and Multitherapeutic Options. Biomimetics. 2019; 4(1):7. https://doi.org/10.3390/biomimetics4010007
Chicago/Turabian StyleVarela, Claudia E., Yiling Fan, and Ellen T. Roche. 2019. "Optimizing Epicardial Restraint and Reinforcement Following Myocardial Infarction: Moving Towards Localized, Biomimetic, and Multitherapeutic Options" Biomimetics 4, no. 1: 7. https://doi.org/10.3390/biomimetics4010007
APA StyleVarela, C. E., Fan, Y., & Roche, E. T. (2019). Optimizing Epicardial Restraint and Reinforcement Following Myocardial Infarction: Moving Towards Localized, Biomimetic, and Multitherapeutic Options. Biomimetics, 4(1), 7. https://doi.org/10.3390/biomimetics4010007





