Biomechanics in Soft Mechanical Sensing: From Natural Case Studies to the Artificial World
Abstract
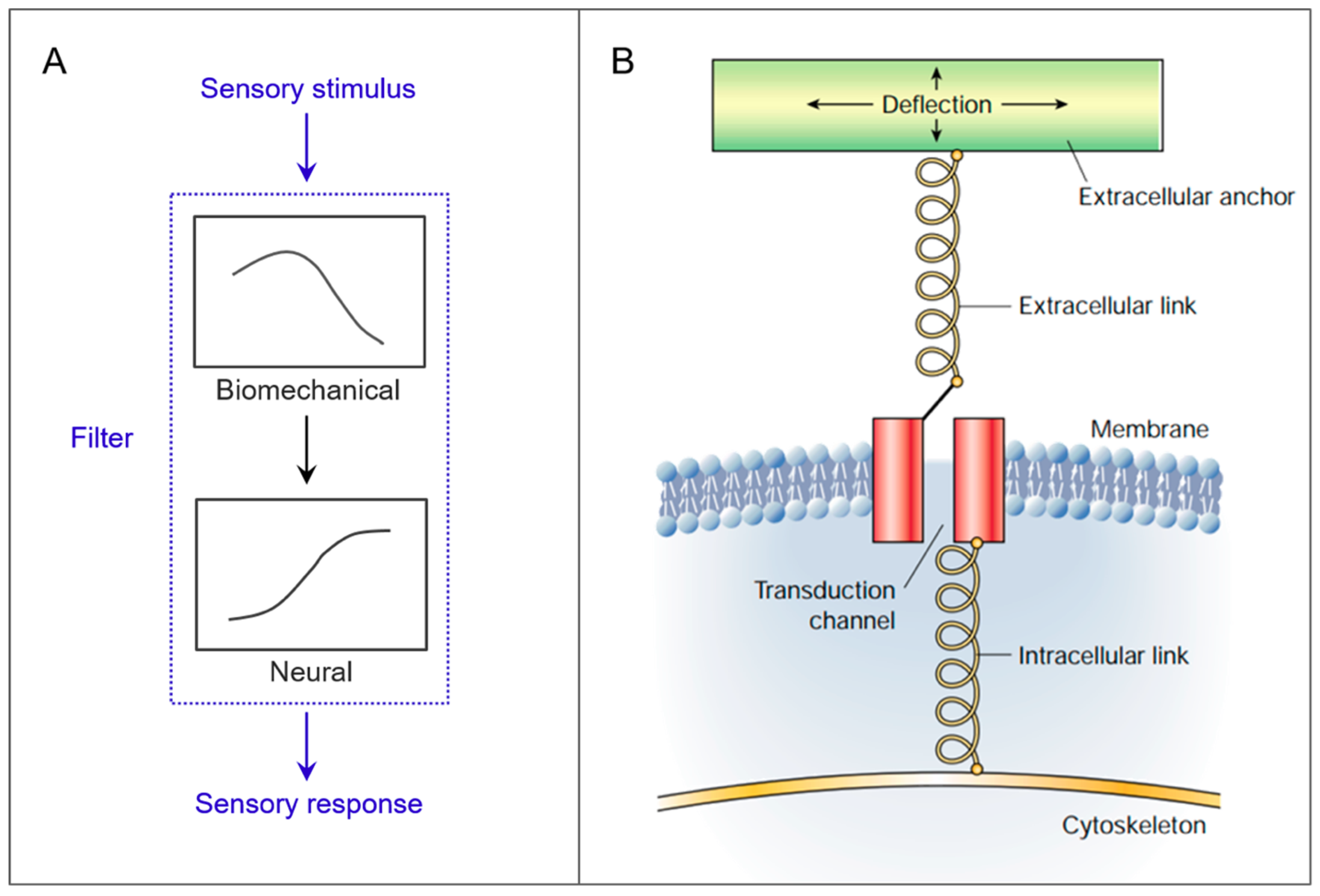
1. Introduction
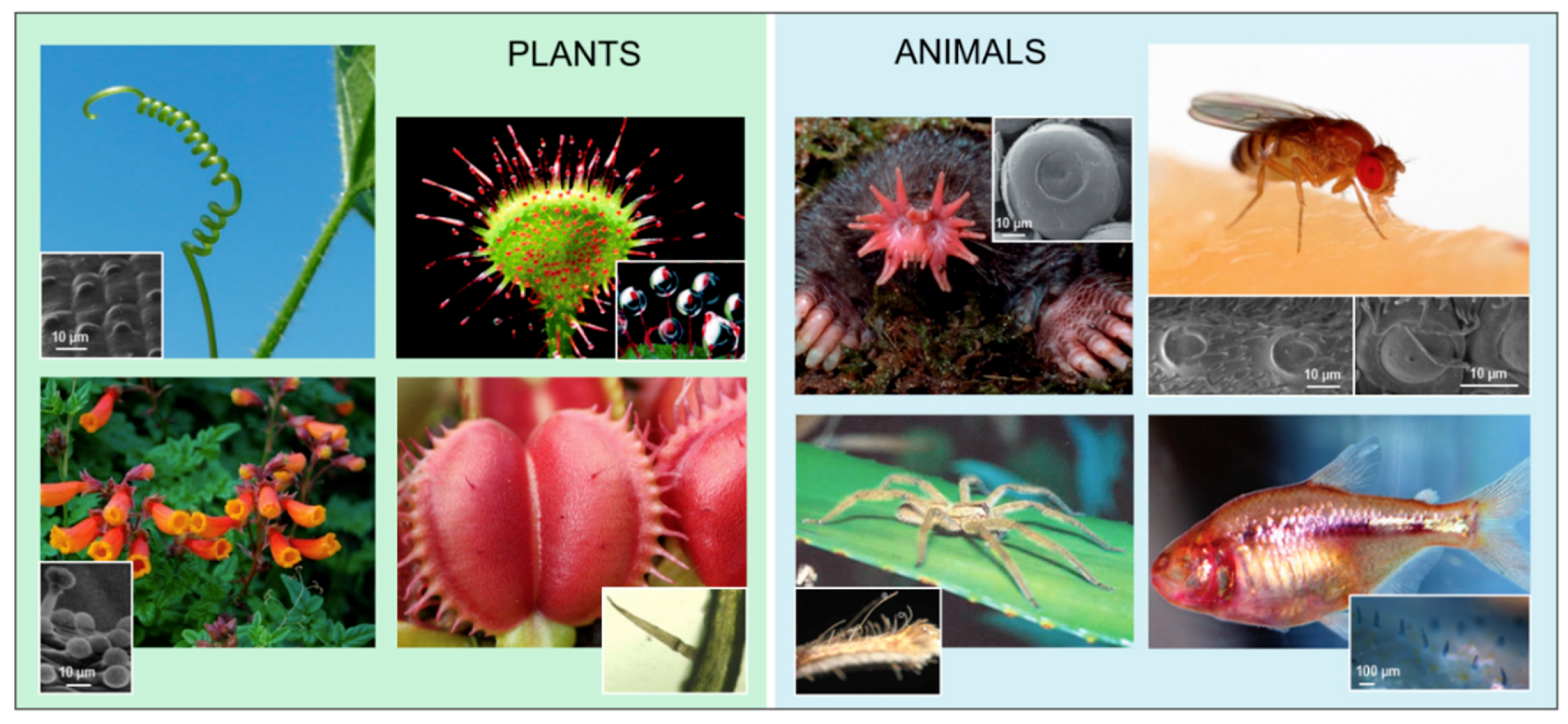
2. Case Studies
2.1. Cantilevers
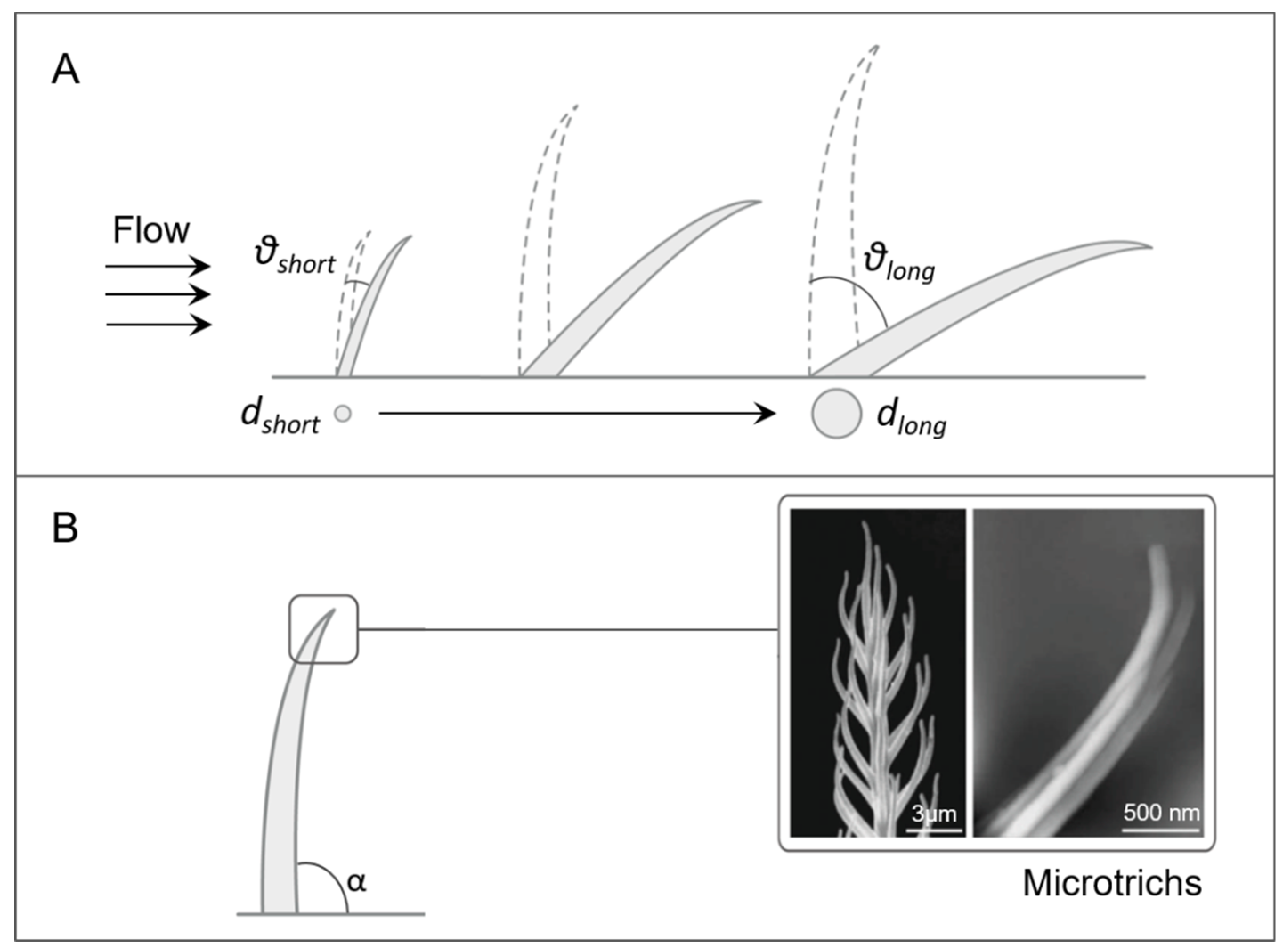
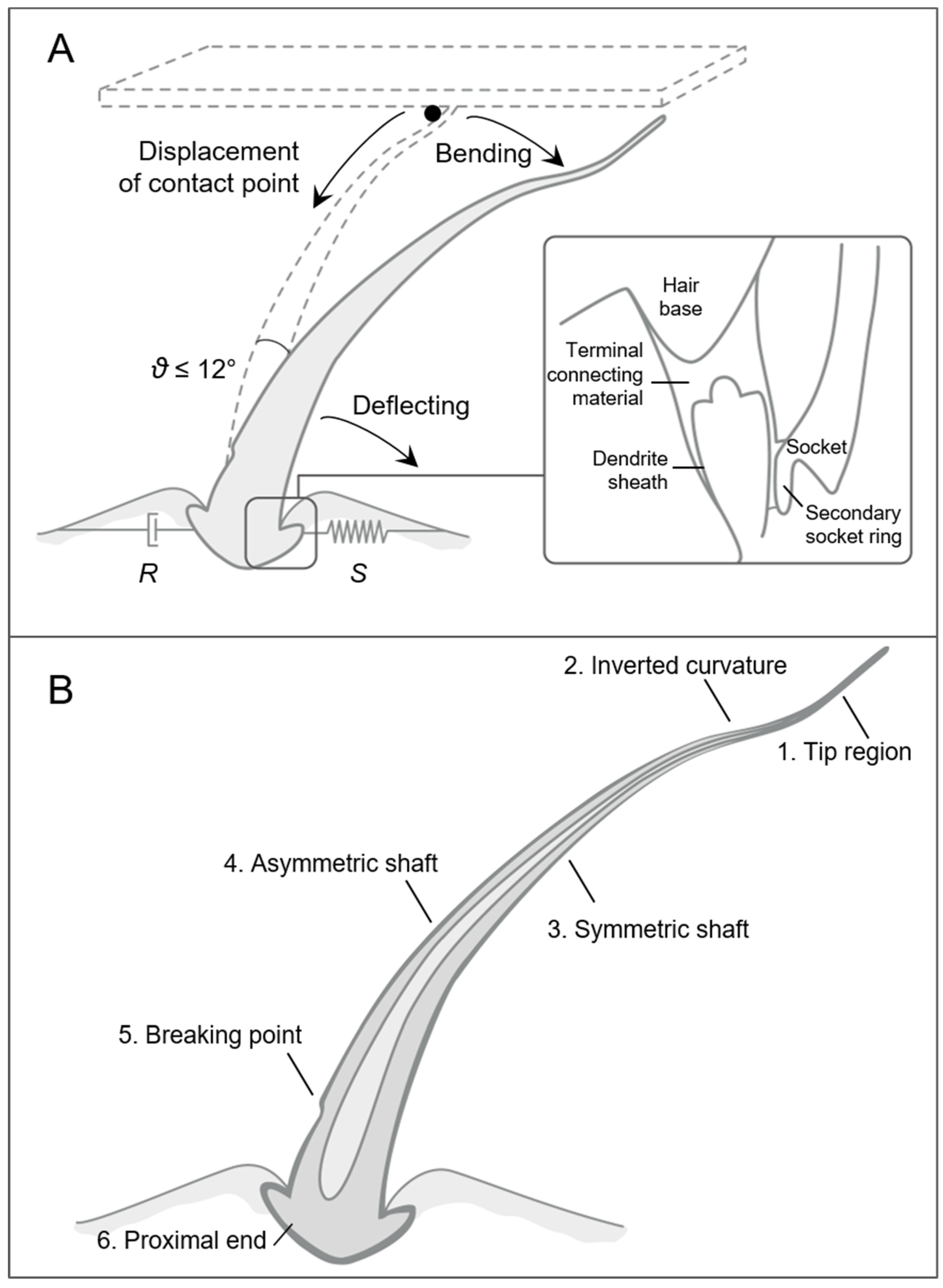
2.1.1. Arthropod Sensilla
Trichobothria
Tactile Hairs
2.1.2. Vibrissae
2.2. Cantilevers with Domes
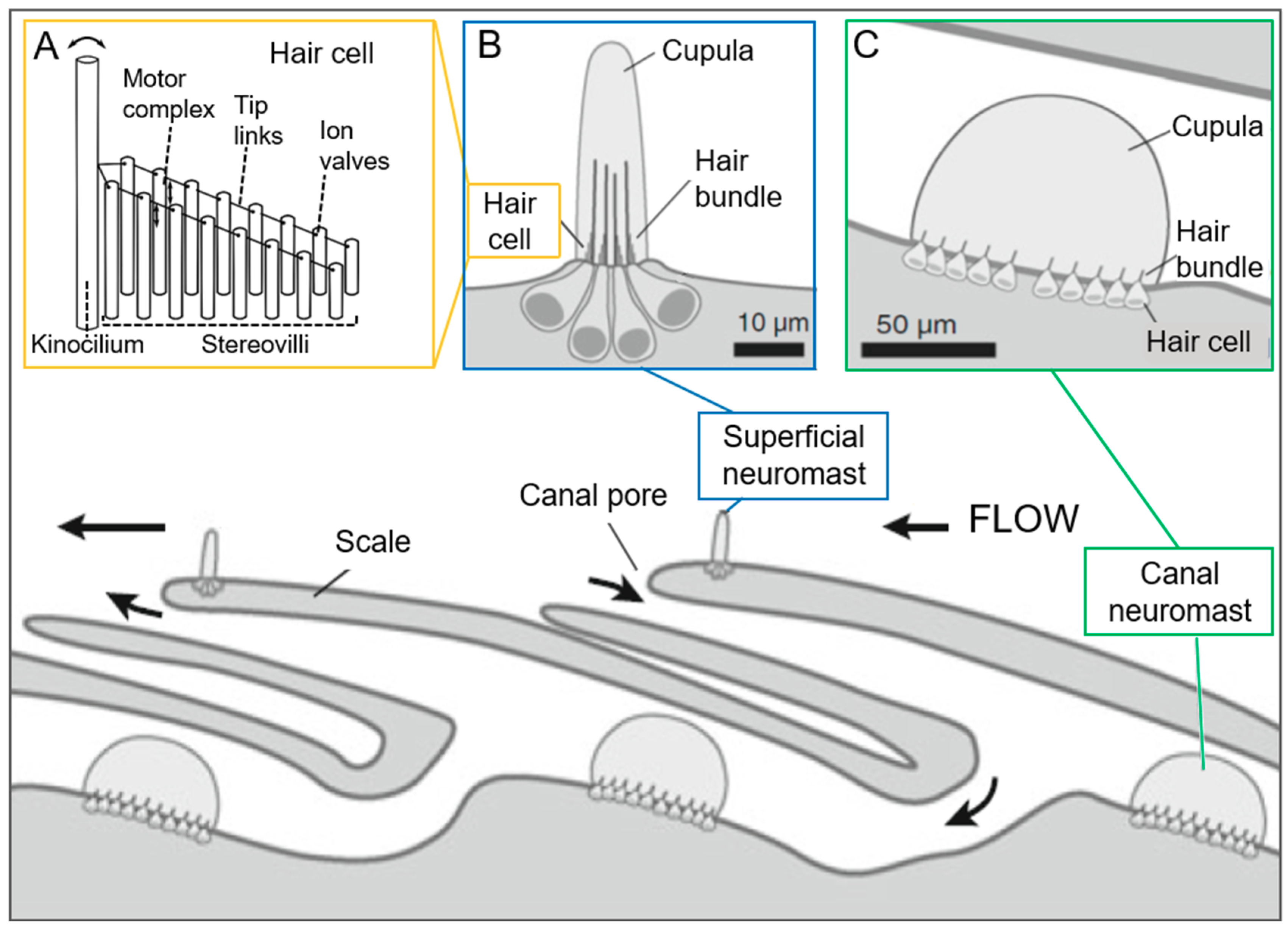
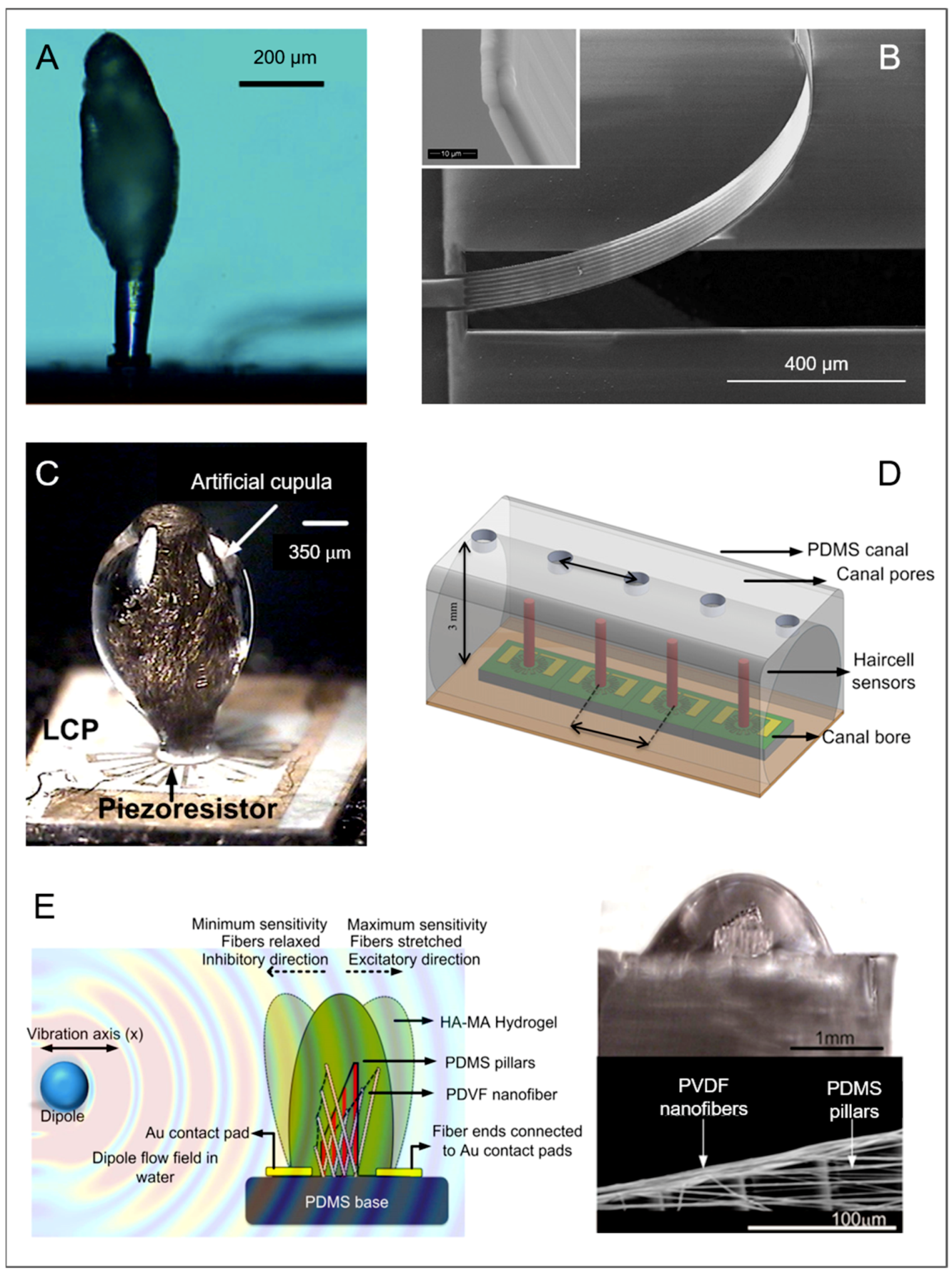
Neuromasts
2.3. Domes
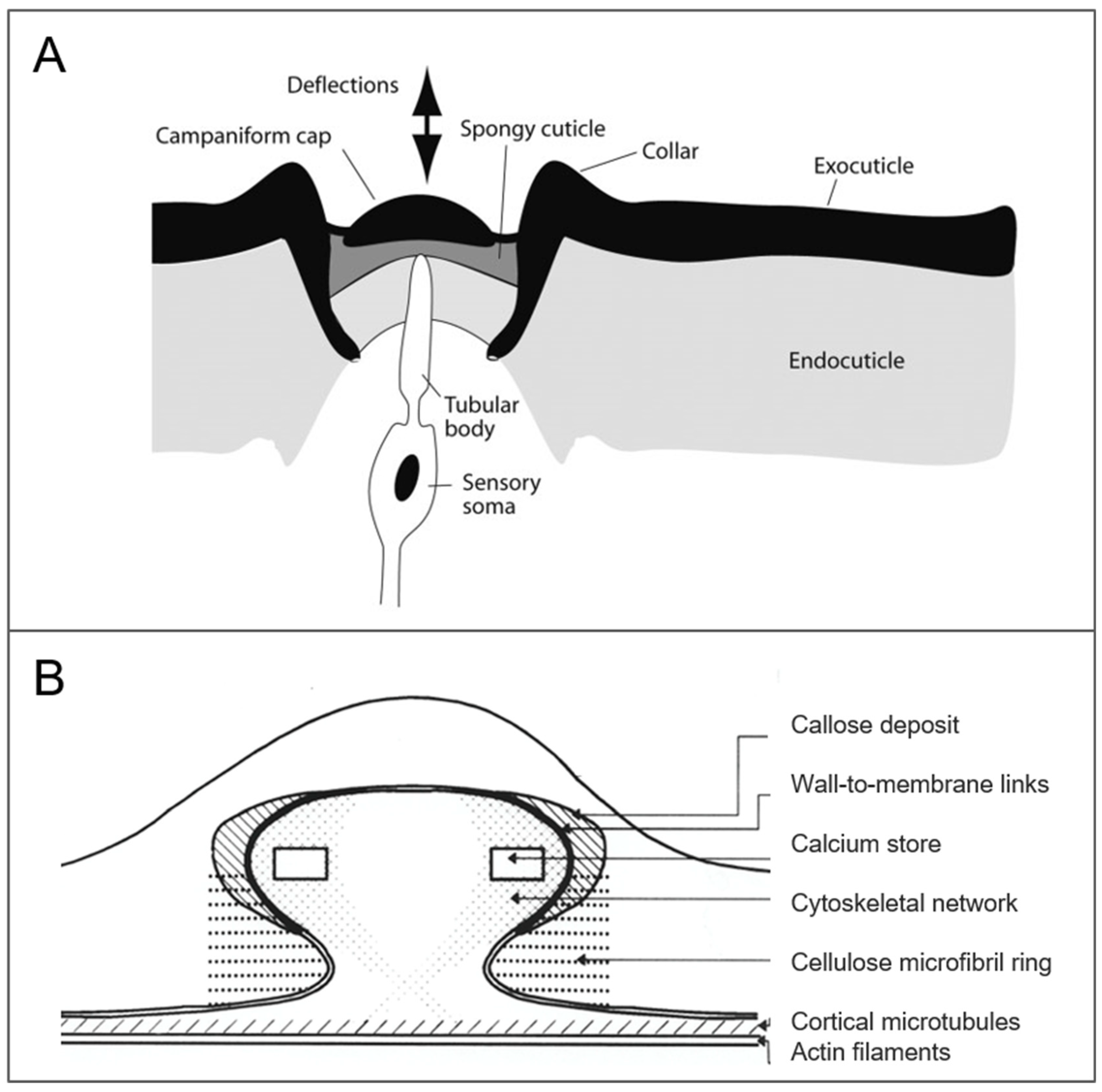
2.3.1. Campaniform Sensillum
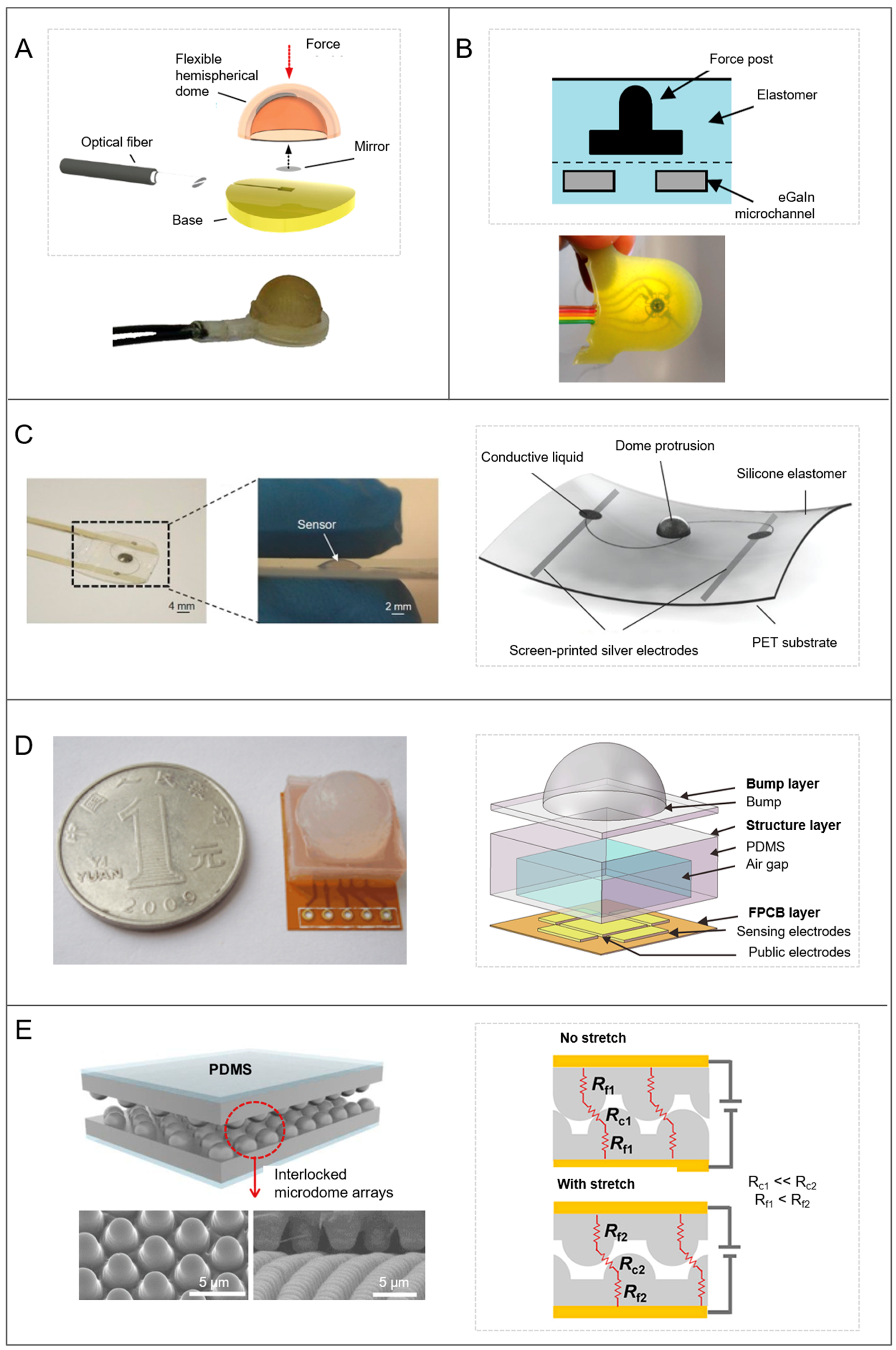
2.3.2. Tactile Blep
3. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Sane, S.P.; McHenry, M.J. The biomechanics of sensory organs. Integr. Comp. Biol. 2009, 49, i8–i23. [Google Scholar] [CrossRef]
- Paul, C. Morphological computation. Robot. Auton. Syst. 2006, 54, 619–630. [Google Scholar] [CrossRef]
- Pfeifer, R.; Lungarella, M.; Iida, F. Self-organization, embodiment, and biologically inspired robotics. Science 2007, 318, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Hauser, H.; Ijspeert, A.J.; Füchslin, R.M.; Pfeifer, R.; Maass, W. Towards a theoretical foundation for morphological computation with compliant bodies. Biol. Cybern. 2011, 105, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Müller, V.C.; Hoffmann, M. What is morphological computation? On how the body contributes to recognition and control. Artif. Life 2017, 23, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Bernth, J.E.; Ho, V.A.; Liu, H. Morphological computation in haptic sensation and interaction: From nature to robotics. Adv. Robot. 2018, 32, 340–362. [Google Scholar] [CrossRef]
- Hartmann, M.J.Z. Active touch, exploratory movements, and sensory prediction. Integr. Comp. Biol. 2009, 49, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, P.G.; Walker, R.G. Molecular basis of mechanosensory transduction. Nature 2001, 413, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Katta, S.; Krieg, M.; Goodman, M.B. Feeling force: Physical and physiological principles enabling sensory mechanotransduction. Annu. Rev. Cell Dev. Biol. 2015, 31, 347–371. [Google Scholar] [CrossRef] [PubMed]
- Laschi, C.; Mazzolai, B. Lessons from animals and plants: The symbiosis of morphological computation and soft robotics. IEEE Robot. Autom. Mag. 2016, 23, 107–114. [Google Scholar] [CrossRef]
- Boulais, N.; Misery, L. The epidermis: A sensory tissue. Eur. J. Dermatol. 2008, 18, 119–127. [Google Scholar] [PubMed]
- Ingber, D. Mechanobiology and diseases of mechanotransduction. Ann. Med. 2003, 35, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Lumpkin, E.A.; Caterina, M.J. Mechanisms of sensory transduction in the skin. Nature 2007, 445, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Montagna, W. The Structure and Function of Skin, 3rd ed.; Academic Press: New York, NY, USA, 2012. [Google Scholar]
- Orr, A.W.; Helmke, B.P.; Blackman, B.R.; Schwartz, M.A. Mechanisms of mechanotransduction. Dev. Cell 2006, 10, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Dargahi, J.; Najarian, S. Human tactile perception as a standard for artificial tactile sensing—A review. Int. J. Med. Robot. Comput. Assist. Surg. 2005, 1, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, R.S.; Metta, G.; Valle, M.; Sandini, G. Tactile sensing—From humans to humanoids. IEEE Trans. Robot. 2010, 26, 1–20. [Google Scholar] [CrossRef]
- Maynard, D.M. Simpler networks. Ann. N. Y. Acad. Sci. 2006, 193, 59–72. [Google Scholar] [CrossRef]
- Young, B.A.; Wallach, V. Description of a papillate tactile organ in the Typhlopidae. S. Afr. J. Zool. 1998, 33, 249–253. [Google Scholar] [CrossRef]
- Thurm, U. Mechanoreceptors in the cuticle of the honey bee: Fine structure and stimulus mechanism. Science 1964, 145, 1063–1065. [Google Scholar] [CrossRef] [PubMed]
- Thurm, U. Die Beziehungen zwischen mechanischen Reizgrössen und stationären Erregungszuständen bei Borstenfeld-Sensillen von Bienen. Zeitschrift für Vergleichende Physiologie 1963, 46, 351–382. [Google Scholar] [CrossRef]
- Severson, K.S.; Xu, D.; Van de Loo, M.; Bai, L.; Ginty, D.D.; O’Connor, D.H. Active touch and self-motion encoding by merkel cell-associated afferents. Neuron 2017, 94, 666–676.e9. [Google Scholar] [CrossRef] [PubMed]
- Benolken, R.M.; Jacobson, S.L. Response properties of a sensory hair excised from Venus’s flytrap. J. Gen. Physiol. 1970, 56, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Arkett, S.A.; Mackie, G.O.; Meech, R.W. Hair cell mechanoreception in the jellyfish Aglantha digitale. J. Exp. Biol. 1988, 135, 329–342. [Google Scholar]
- Pravin, S.; DeForest, M.; Berger, E.J.; Reidenbach, M.A. Effects of sensilla morphology on mechanosensory sensitivity in the crayfish. Bioinspir. Biomim. 2015, 10, 036006. [Google Scholar] [CrossRef] [PubMed]
- Mureli, S.; Fox, J.L. Haltere mechanosensory influence on tethered flight behavior in Drosophila. J. Exp. Biol. 2015, 218, 2528–2537. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.F.V.; Wegst, U.G.K. Design and mechanical properties of insect cuticle. Arthropod Struct. Dev. 2004, 33, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Blickhan, R.; Barth, F.G. Strains in the exoskeleton of spiders. J. Comp. Physiol. A 1985, 157, 115–147. [Google Scholar] [CrossRef]
- Catania, K.C. Tactile sensing in specialized predators—From behavior to the brain. Curr. Opin. Neurobiol. 2012, 22, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, J.; Wanner, G.; Groth, B.; Weiler, E. Functional anatomy of the mechanoreceptor cells in tendrils of Bryonia dioica Jacq. Planta 1995, 196. [Google Scholar] [CrossRef]
- Braam, J. In touch: Plant responses to mechanical stimuli. New Phytol. 2004, 165, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Junker, S. Ultrastructure of tactile papillae on tendrils of Eccremocarpus scaber R. et P. New Phytol. 1977, 78, 607–610. [Google Scholar] [CrossRef]
- Agrawal, S.; Grimaldi, D.; Fox, J.L. Haltere morphology and campaniform sensilla arrangement across Diptera. Arthropod Struct. Dev. 2017, 46, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Fratzl, P.; Barth, F.G. Biomaterial systems for mechanosensing and actuation. Nature 2009, 462, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Barth, F.G. Spider mechanoreceptors. Curr. Opin. Neurobiol. 2004, 14, 415–422. [Google Scholar] [CrossRef] [PubMed]
- McConney, M.E.; Chen, N.; Lu, D.; Hu, H.A.; Coombs, S.; Liu, C.; Tsukruk, V.V. Biologically inspired design of hydrogel-capped hair sensors for enhanced underwater flow detection. Soft Matter 2009, 5, 292–295. [Google Scholar] [CrossRef]
- Creative Commons—Attribution-ShareAlike 4.0 International (CC BY-SA 4.0). Available online: https://creativecommons.org/licenses/by-sa/4.0/.
- Creative Commons—Attribution-NonCommercial-NoDerivs 2.0 Generic (CC BY-NC-ND 2.0). Available online: https://creativecommons.org/licenses/by-nc-nd/2.0/.
- Creative Commons—Attribution-ShareAlike 2.5 Generic (CC BY-SA 2.5). Available online: https://creativecommons.org/licenses/by-sa/2.5/.
- Catania, K.C. The sense of touch in the star-nosed mole: From mechanoreceptors to the brain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 3016–3025. [Google Scholar] [CrossRef] [PubMed]
- Dürr, V. Stick insect antennae. In Scholarpedia of Touch; Prescott, T., Ahissar, E., Izhikevich, E., Eds.; Atlantis Press: Paris, France, 2016; pp. 45–63. ISBN 978-94-6239-133-8. [Google Scholar]
- Pringle, J.W.S. Proprioception in insects: II. The action of the campaniform sensilla on the legs. J. Exp. Biol. 1938, 15, 114–131. [Google Scholar]
- Spinola, S.M.; Chapman, K.M. Proprioceptive indentation of the campaniform sensilla of cockroach legs. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 1975, 96, 257–272. [Google Scholar]
- Soares, D. Neurology: An ancient sensory organ in crocodilians. Nature 2002, 417, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Barth, F.G. A spider’s tactile hairs. In Scholarpedia of Touch; Prescott, T.J., Ahissar, E., Izhikevich, E., Eds.; Atlantis Press: Paris, France, 2016; pp. 65–81. ISBN 978-94-6239-133-8. [Google Scholar]
- Guo, Q.; Dai, E.; Han, X.; Xie, S.; Chao, E.; Chen, Z. Fast nastic motion of plants and bioinspired structures. J. R. Soc. Interface 2015, 12, 0598. [Google Scholar] [CrossRef] [PubMed]
- Bone, Q.; Ryan, K.P. Cupular sense organs in Ciona (Tunicata: Ascidiacea). J. Zool. 1978, 186, 417–429. [Google Scholar] [CrossRef]
- Mackie, G.O.; Burighel, P. The nervous system in adult tunicates: Current research directions. Can. J. Zool. 2005, 83, 151–183. [Google Scholar] [CrossRef]
- Triantafyllou, M.S.; Weymouth, G.D.; Miao, J. Biomimetic survival hydrodynamics and flow sensing. Annu. Rev. Fluid Mech. 2016, 48, 1–24. [Google Scholar] [CrossRef]
- Montgomery, J.C.; Coombs, S.; Baker, C.F. The mechanosensory lateral line system of the hypogean form of Astyanax fasciatus. In The Biology of Hypogean Fishes; Romero, A., Ed.; Springer: Dordrecht, The Netherlands, 2001; pp. 87–96. ISBN 978-94-015-9795-1. [Google Scholar]
- Peleshanko, S.; Julian, M.D.; Ornatska, M.; McConney, M.E.; LeMieux, M.C.; Chen, N.; Tucker, C.; Yang, Y.; Liu, C.; Humphrey, J.A.C.; et al. Hydrogel-encapsulated microfabricated haircells mimicking fish cupula neuromast. Adv. Mater. 2007, 19, 2903–2909. [Google Scholar] [CrossRef]
- Sadeghi, M.M.; Peterson, R.L.; Najafi, K. Hair-based sensors for micro-autonomous systems. In Micro- and Nanotechnology Sensors, Systems, and Applications IV; George, T., Islam, M.S., Dutta, A., Eds.; SPIE: Bellingham, Washington, 2012. [Google Scholar]
- Seyfarth, E.-A.; Gnatzy, W.; Hammer, K. Coxal hair plates in spiders: Physiology, fine structure, and specific central projections. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 1990, 166, 633–642. [Google Scholar] [CrossRef]
- Schaber, C.F.; Barth, F.G. Spider joint hair sensilla: Adaptation to proprioreceptive stimulation. J. Comp. Physiol. A 2015, 201, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Seale, M.; Cummins, C.; Viola, I.M.; Mastropaolo, E.; Nakayama, N. Design principles of hair-like structures as biological machines. J. R. Soc. Interface 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Dechant, H.-E.; Rammerstorfer, F.G.; Barth, F.G. Arthropod touch reception: Stimulus transformation and finite element model of spider tactile hairs. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2001, 187, 313–322. [Google Scholar] [CrossRef]
- Albert, J.T.; Friedrich, O.C.; Dechant, H.-E.; Barth, F.G. Arthropod touch reception: Spider hair sensilla as rapid touch detectors. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2001, 187, 303–312. [Google Scholar] [CrossRef]
- McConney, M.E.; Schaber, C.F.; Julian, M.D.; Eberhardt, W.C.; Humphrey, J.A.C.; Barth, F.G.; Tsukruk, V.V. Surface force spectroscopic point load measurements and viscoelastic modelling of the micromechanical properties of air flow sensitive hairs of a spider (Cupiennius salei). J. R. Soc. Interface 2009, 6, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Barth, F.G.; Wastl, U.; Humphrey, J.A.C.; Devarakonda, R. Dynamics of arthropod filiform hairs. II. Mechanical properties of spider trichobothria (Cupiennius salei Keys.). Philos. Trans. R. Soc. Lond. B Biol. Sci. 1993, 340, 445–461. [Google Scholar] [CrossRef]
- Barth, F.G.; Höller, A. Dynamics of arthropod filiform hairs. V. The response of spider trichobothria to natural stimuli. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 183–192. [Google Scholar] [CrossRef]
- Barth, F.G. A spider’s sense of touch: What to do with myriads of tactile hairs. In The Ecology of Animal Senses: Matched Filters for Economical Sensing; von der Emde, G., Warrant, E., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 27–57. ISBN 978-3-319-25492-0. [Google Scholar]
- Devarakonda, R.; Barth, F.G.; Humphrey, J.A. Dynamics of arthropod filiform hairs. IV. Hair motion in air and water. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996, 351, 933–946. [Google Scholar] [CrossRef]
- Bathellier, B.; Barth, F.G.; Albert, J.T.; Humphrey, J.A.C. Viscosity-mediated motion coupling between pairs of trichobothria on the leg of the spider Cupiennius salei. J. Comp. Physiol. A 2005, 191, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Casas, J.; Steinmann, T.; Krijnen, G. Why do insects have such a high density of flow-sensing hairs? Insights from the hydromechanics of biomimetic MEMS sensors. J. R. Soc. Interface 2010, 7, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Lewin, G.C.; Hallam, J. A computational fluid dynamics model of viscous coupling of hairs. J. Comp. Physiol. A 2010, 196, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Barth, F.G. A Spider’s World; Springer: Berlin/Heidelberg, Germany, 2002; ISBN 978-3-642-07557-5. [Google Scholar]
- Humphrey, J.A.C.; Barth, F.G. Medium flow-sensing hairs: Biomechanics and models. In Advances in Insect Physiology; Academic Press: New York, NY, USA, 2007; Volume 34, pp. 1–80. ISBN 978-0-12-373714-4. [Google Scholar]
- Tao, J.; Yu, X. Hair flow sensors. Smart Mater. Struct. 2012, 21. [Google Scholar] [CrossRef]
- Han, Z.; Liu, L.; Wang, K.; Song, H.; Chen, D.; Wang, Z.; Niu, S.; Zhang, J.; Ren, L. Artificial hair-like sensors inspired from nature: A review. J. Bionic Eng. 2018, 15, 409–434. [Google Scholar] [CrossRef]
- Droogendijk, H.; de Boer, M.J.; Sanders, R.G.P.; Krijnen, G.J.M. Advantages of electrostatic spring hardening in biomimetic hair flow sensors. J. Microelectromech. Syst. 2015, 24, 1415–1425. [Google Scholar] [CrossRef]
- Chen, N.; Tucker, C.; Engel, J.M.; Yang, Y.; Pandya, S.; Liu, C. Design and characterization of artificial haircell sensor for flow sensing with ultrahigh velocity and angular sensitivity. J. Microelectromech. Syst. 2007, 16, 999–1014. [Google Scholar] [CrossRef]
- Liu, C. Micromachined biomimetic artificial haircell sensors. Bioinspir. Biomim. 2007, 2, S162. [Google Scholar] [CrossRef] [PubMed]
- Barbier, C.; Humphrey, J.A.C.; Paulus, J.; Appleby, M. Design, Fabrication and Testing of a Bioinspired Hybrid Hair-Like Fluid Motion Sensor Array. In Proceedings of the 2007 ASME International Mechanical Engineering Congress and Exposition IMECE2007, Seattle, WA, USA, 11–15 November 2007. [Google Scholar]
- McConney, M.E.; Anderson, K.D.; Brott, L.L.; Naik, R.R.; Tsukruk, V.V. Bioinspired material approaches to sensing. Adv. Funct. Mater. 2009, 19, 2527–2544. [Google Scholar] [CrossRef]
- Shi, X.; Cheng, C.H. Artificial Hair Cell Sensors Using Liquid Metal Alloy as Piezoresistors. In Proceedings of the 8th Annual IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Suzhou, China, 7–10 April 2013; pp. 978–981. [Google Scholar]
- Sarles, S.A.; Madden, J.D.; Leo, D.J. Hair cell inspired mechanotransduction with a gel-supported, artificial lipid membrane. Soft Matter 2011, 7, 4644–4653. [Google Scholar] [CrossRef]
- Sarlo, R.; Najem, J.S.; Leo, D.J. Flow field sensing with bio-inspired artificial hair cell arrays. Sens. Actuators B Chem. 2016, 236, 805–814. [Google Scholar] [CrossRef]
- Steinmann, T.; Casas, J.; Krijnen, G.; Dangles, O. Air-flow sensitive hairs: Boundary layers in oscillatory flows around arthropod appendages. J. Exp. Biol. 2006, 209, 4398–4408. [Google Scholar] [CrossRef] [PubMed]
- Johansson, R.S.; Flanagan, J.R. Coding and use of tactile signals from the fingertips in object manipulation tasks. Nat. Rev. Neurosci. 2009, 10, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Johansson, R.S.; Vallbo, Å.B. Tactile sensory coding in the glabrous skin of the human hand. Trends Neurosci. 1983, 6, 27–32. [Google Scholar] [CrossRef]
- Barth, F.G.; Németh, S.S.; Friedrich, O.C. Arthropod touch reception: Structure and mechanics of the basal part of a spider tactile hair. J. Comp. Physiol. A 2004, 190. [Google Scholar] [CrossRef] [PubMed]
- Keil, T.A. Functional morphology of insect mechanoreceptors. Microsc. Res. Tech. 1997, 39, 506–531. [Google Scholar] [CrossRef]
- Engel, J.M.; Chen, J.; Liu, C.; Bullen, D. Polyurethane rubber all-polymer artificial hair cell sensor. J. Microelectromech. Syst. 2006, 15, 729–736. [Google Scholar] [CrossRef]
- Alfadhel, A.; Kosel, J. Magnetic nanocomposite cilia tactile sensor. Adv. Mater. 2015, 27, 7888–7892. [Google Scholar] [CrossRef] [PubMed]
- Solomon, J.H.; Hartmann, M.J. Robotic whiskers used to sense features. Nature 2006, 443, 525. [Google Scholar] [CrossRef] [PubMed]
- Gaspard, J.C.; Bauer, G.B.; Reep, R.L.; Dziuk, K.; Read, L.; Mann, D.A. Detection of hydrodynamic stimuli by the Florida manatee (Trichechus manatus latirostris). J. Comp. Physiol. A 2013, 199, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Gläser, N.; Wieskotten, S.; Otter, C.; Dehnhardt, G.; Hanke, W. Hydrodynamic trail following in a California sea lion (Zalophus californianus). J. Comp. Physiol. A 2011, 197, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.S.W.; Graff, M.M.; Hartmann, M.J.Z. Mechanical responses of rat vibrissae to airflow. J. Exp. Biol. 2016, 219, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Pammer, L.; O’Connor, D.H.; Hires, S.A.; Clack, N.G.; Huber, D.; Myers, E.W.; Svoboda, K. The mechanical variables underlying object localization along the axis of the whisker. J. Neurosci. 2013, 33, 6726–6741. [Google Scholar] [CrossRef] [PubMed]
- Dehnhardt, G. Tactile size discrimination by a California sea lion (Zalophus californianus) using its mystacial vibrissae. J. Comp. Physiol. A 1994, 175, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Dehnhardt, G.; Dücker, G. Tactual discrimination of size and shape by a California sea lion (Zalophus californianus). Anim. Learn. Behav. 1996, 24, 366–374. [Google Scholar] [CrossRef]
- Polley, D.; Rickert, J.; Frostig, R. Whisker-based discrimination of object orientation determined with a rapid training paradigm. Neurobiol. Learn. Mem. 2005, 83, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Bauer, G.B.; Gaspard, J.C., III; Colbert, D.E.; Leach, J.B.; Stamper, S.A.; Mann, D.; Reep, R. Tactile discrimination of textures by Florida manatees (Trichechus manatus latirostris). Mar. Mammal Sci. 2012, 28, E456–E471. [Google Scholar] [CrossRef]
- Guić-Robles, E.; Valdivieso, C.; Guajardo, G. Rats can learn a roughness discrimination using only their vibrissal system. Behav. Brain Res. 1989, 31, 285–289. [Google Scholar] [CrossRef]
- Pipe, T.; Pearson, M.J. Whiskered robots. In Scholarpedia of Touch; Prescott, T.J., Ahissar, E., Izhikevich, E., Eds.; Atlantis Press: Paris, France, 2016; pp. 809–815. ISBN 978-94-6239-133-8. [Google Scholar]
- Clements, T.N.; Rahn, C.D. Three-dimensional contact imaging with an actuated whisker. IEEE Trans. Robot. 2006, 22, 844–848. [Google Scholar] [CrossRef]
- Muraoka, S. Environmental recognition using artificial active antenna system with quartz resonator force sensor. Measurement 2005, 37, 157–165. [Google Scholar] [CrossRef]
- Voges, D.; Carl, K.; Klauer, G.J.; Uhlig, R.; Schilling, C.; Behn, C.; Witte, H. Structural characterization of the whisker system of the rat. IEEE Sens. J. 2012, 12, 332–339. [Google Scholar] [CrossRef]
- Huet, L.A.; Rudnicki, J.W.; Hartmann, M.J.Z. Tactile sensing with whiskers of various shapes: Determining the three-dimensional location of object contact based on mechanical signals at the whisker base. Soft Robot. 2017, 4, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Quist, B.W.; Hartmann, M.J.Z. Mechanical signals at the base of a rat vibrissa: The effect of intrinsic vibrissa curvature and implications for tactile exploration. J. Neurophysiol. 2012, 107, 2298–2312. [Google Scholar] [CrossRef] [PubMed]
- Hanke, W.; Witte, M.; Miersch, L.; Brede, M.; Oeffner, J.; Michael, M.; Hanke, F.; Leder, A.; Dehnhardt, G. Harbor seal vibrissa morphology suppresses vortex-induced vibrations. J. Exp. Biol. 2010, 213, 2665–2672. [Google Scholar] [CrossRef] [PubMed]
- Hans, H.; Miao, J.; Weymouth, G.; Triantafyllou, M. Whisker-Like Geometries and Their Force Reduction Properties. In Proceedings of the 2013 MTS/IEEE OCEANS, Bergen, Norway, 10–14 June 2013; pp. 1–7. [Google Scholar]
- Dehnhardt, G.; Mauck, B. Mechanoreception in secondarily aquatic vertebrates. In Sensory Evolution on the Threshold: Adaptations in Secondarily Aquatic Vertebrates; University of California Press: Berkeley, CA, USA, 2008. [Google Scholar]
- Hibbeler, R.C. Mechanics of Materials, 10th ed.; Pearson: Boston, MA, USA, 2017; ISBN 978-0-13-431965-0. [Google Scholar]
- Quist, B.W.; Faruqi, R.A.; Hartmann, M.J.Z. Variation in Young’s modulus along the length of a rat vibrissa. J. Biomech. 2011, 44, 2775–2781. [Google Scholar] [CrossRef] [PubMed]
- Carl, K.; Hild, W.; Mampel, J.; Schilling, C.; Uhlig, R.; Witte, H. Characterization of statical properties of rat’s whisker system. IEEE Sens. J. 2012, 12, 340–349. [Google Scholar] [CrossRef]
- Adineh, V.R.; Liu, B.; Rajan, R.; Yan, W.; Fu, J. Multidimensional characterisation of biomechanical structures by combining atomic force microscopy and focused ion beam: A study of the rat whisker. Acta Biomater. 2015, 21, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Gul, J.Z.; Su, K.Y.; Choi, K.H. Fully 3D printed multi-material soft bio-inspired whisker sensor for underwater-induced vortex detection. Soft Robot. 2018. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.J.; Mitchinson, B.; Sullivan, J.C.; Pipe, A.G.; Prescott, T.J. Biomimetic vibrissal sensing for robots. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 3085–3096. [Google Scholar] [CrossRef] [PubMed]
- Beem, H.R.; Triantafyllou, M.S. Exquisitely sensitive seal whisker-like sensors detect wakes at large distances. arXiv, 2015; arXiv:1501.04582. [Google Scholar]
- Kroese, A.B.; Schellart, N.A. Velocity- and acceleration-sensitive units in the trunk lateral line of the trout. J. Neurophysiol. 1992, 68, 2212–2221. [Google Scholar] [CrossRef] [PubMed]
- McHenry, M.J.; Liao, J.C. The hydrodynamics of flow stimuli. In The Lateral Line System; Springer: New York, NY, USA, 2014; pp. 73–98. ISBN 978-1-4614-8851-4. [Google Scholar]
- van Netten, S.M. Hydrodynamic detection by cupulae in a lateral line canal: Functional relations between physics and physiology. Biol. Cybern. 2006, 94, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Bora, M.; Kottapalli, A.G.P.; Miao, J.; Triantafyllou, M. Sensing the flow beneath the fins. Bioinspir. Biomim. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.S. Hydrodynamic imaging of the surroundings by the lateral line of the blind cave fish Anoptichthys jordani. In The Mechanosensory Lateral Line: Neurobiology and Evolution; Coombs, S., Görner, P., Münz, H., Eds.; Springer: New York, NY, USA, 1989; pp. 217–227. ISBN 978-1-4612-3560-6. [Google Scholar]
- Crozier, W.J. On tactile responses of the de-eyed hamlet (Epinephelus striatus). J. Comp. Neurol. 1918, 29, 163–175. [Google Scholar] [CrossRef]
- Dijkgraaf, S. The functioning and significance of the lateral-line organs. Biol. Rev. 1963, 38, 51–105. [Google Scholar] [CrossRef] [PubMed]
- McHenry, M.J.; Strother, J.A.; van Netten, S.M. Mechanical filtering by the boundary layer and fluid–structure interaction in the superficial neuromast of the fish lateral line system. J. Comp. Physiol. A 2008, 194, 795. [Google Scholar] [CrossRef] [PubMed]
- McHenry, M.J.; van Netten, S.M. The flexural stiffness of superficial neuromasts in the zebrafish (Danio rerio) lateral line. J. Exp. Biol. 2007, 210, 4244–4253. [Google Scholar] [CrossRef] [PubMed]
- van Netten, S.M.; Kroese, A.B.A. Laser interferometric measurements on the dynamic behaviour of the cupula in the fish lateral line. Hear. Res. 1987, 29, 55–61. [Google Scholar] [CrossRef]
- Karavitaki, K.D.; Corey, D.P. Sliding adhesion confers coherent motion to hair cell stereocilia and parallel gating to transduction channels. J. Neurosci. 2010, 30, 9051–9063. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.D.; Lu, D.; McConney, M.E.; Han, T.; Reneker, D.H.; Tsukruk, V.V. Hydrogel microstructures combined with electrospun fibers and photopatterning for shape and modulus control. Polymer 2008, 49, 5284–5293. [Google Scholar] [CrossRef]
- Qualtieri, A.; Rizzi, F.; Epifani, G.; Ernits, A.; Kruusmaa, M.; De Vittorio, M. Parylene-coated bioinspired artificial hair cell for liquid flow sensing. Microelectron. Eng. 2012, 98, 516–519. [Google Scholar] [CrossRef]
- Asadnia, M.; Kottapalli, A.G.P.; Karavitaki, K.D.; Warkiani, M.E.; Miao, J.; Corey, D.P.; Triantafyllou, M. From biological cilia to artificial flow sensors: Biomimetic soft polymer nanosensors with high sensing performance. Sci. Rep. 2016, 6, 32955. [Google Scholar] [CrossRef] [PubMed]
- Abels, C.; Qualtieri, A.; De Vittorio, M.; Megill, W.M.; Rizzi, F. A bio-inspired real-time capable artificial lateral line system for freestream flow measurements. Bioinspir. Biomim. 2016, 11, 035006. [Google Scholar] [CrossRef] [PubMed]
- Kottapalli, A.G.P.; Asadnia, M.; Miao, J.; Triantafyllou, M. Touch at a distance sensing: Lateral-line inspired MEMS flow sensors. Bioinspir. Biomim. 2014, 9, 046011. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Klein, A.; Bleckmann, H.; Liu, C. Artificial lateral line canal for hydrodynamic detection. Appl. Phys. Lett. 2011, 99, 023701. [Google Scholar] [CrossRef]
- Nawi, M.N.M.; Manaf, A.A.; Arshad, M.R.; Sidek, O. Development of microfluidic based multidirectional flow sensor inspired from artificial cupula. Microsyst. Technol. 2015, 21, 1513–1521. [Google Scholar] [CrossRef]
- Kottapalli, A.G.P.; Bora, M.; Asadnia, M.; Miao, J.; Venkatraman, S.S.; Triantafyllou, M. Nanofibril scaffold assisted MEMS artificial hydrogel neuromasts for enhanced sensitivity flow sensing. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Creative Commons—Attribution 4.0 International (CC BY 4.0). Available online: https://creativecommons.org/licenses/by/4.0/.
- Johansson, R.S. Tactile sensibility in the human hand: Receptive field characteristics of mechanoreceptive units in the glabrous skin area. J. Physiol. 1978, 281, 101–125. [Google Scholar] [CrossRef] [PubMed]
- Leitch, D.B.; Catania, K.C. Structure, innervation and response properties of integumentary sensory organs in crocodilians. J. Exp. Biol. 2012, 215, 4217–4230. [Google Scholar] [CrossRef] [PubMed]
- Catania, K.C.; Remple, F.E. Asymptotic prey profitability drives star-nosed moles to the foraging speed limit. Nature 2005, 433, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Mazzolai, B.; Beccai, L.; Mattoli, V. Plants as model in biomimetics and biorobotics: New perspectives. Front. Bioeng. Biotechnol. 2014, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Mondini, A.; Mazzolai, B. Toward self-growing soft robots inspired by plant roots and based on additive manufacturing technologies. Soft Robot. 2017, 4, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Zill, S.N.; Schmitz, J.; Chaudhry, S.; Büschges, A. Force encoding in stick insect legs delineates a reference frame for motor control. J. Neurophysiol. 2012, 108, 1453–1472. [Google Scholar] [CrossRef] [PubMed]
- Tuthill, J.C.; Wilson, R.I. Mechanosensation and adaptive motor control in insects. Curr. Biol. 2016, 26, R1022–R1038. [Google Scholar] [CrossRef] [PubMed]
- Zill, S.N.; Moran, D.T. The exoskeleton and insect proprioception. I. Responses of tibial campaniform sensilla to external and muscle-generated forces in the American cockroach, Periplaneta americana. J. Exp. Biol. 1981, 91, 1–24. [Google Scholar]
- Krämer, K.; Markl, H. Flight-inhibition on ground contact in the American cockroach, Periplaneta americana—I. Contact receptors and a model for their central connections. J. Insect Physiol. 1978, 24, 577–586. [Google Scholar] [CrossRef]
- Vincent, J.F.V.; Clift, S.E.; Menon, C. Biomimetics of campaniform sensilla: Measuring strain from the deformation of holes. J. Bionic Eng. 2007, 4, 63–76. [Google Scholar] [CrossRef]
- Skordos, A.; Chan, P.H.; Vincent, J.F.V.; Jeronimidis, G. A novel strain sensor based on the campaniform sensillum of insects. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2002, 360, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Yusof, A.A.M.; Esa, R.; Wicaksono, D.H.B.; Ahmad, H.; Rawi, C.S.M. Strain-amplifying structural features of campaniform sensillum-inspired strain sensor: Design and simulation. Procedia Eng. 2012, 41, 710–715. [Google Scholar] [CrossRef]
- Yusof, A.A.M.; Syahrom, A.; Kadir, M.R.A.; Wicaksono, D.H. High-Gain Pre-Transduction Strain Amplification Inspired from the Dome Shape Structure of Insect Campaniform Sensillum. In Proceedings of the 2013 International Conference on Robotics, Biomimetics, Intelligent Computational Systems, Jogjakarta, Indonesia, 25–27 November 2013; pp. 228–231. [Google Scholar]
- Biddington, N.L. The effects of mechanically-induced stress in plants—A review. Plant Growth Regul. 1986, 4, 103–123. [Google Scholar] [CrossRef]
- Ding, J.P.; Pickard, B.G. Mechanosensory calcium-selective cation channels in epidermal cells. Plant J. 2008, 3, 83–110. [Google Scholar] [CrossRef]
- Jaffe, M.J. Thigmomorphogenesis: Electrical resistance and mechanical correlates of the early events of growth retardation due to mechanical stimulation in beans. Zeitschrift für Pflanzenphysiologie 1976, 78, 24–32. [Google Scholar] [CrossRef]
- Monshausen, G.B.; Haswell, E.S. A force of nature: Molecular mechanisms of mechanoperception in plants. J. Exp. Bot. 2013, 64, 4663–4680. [Google Scholar] [CrossRef] [PubMed]
- Monshausen, G.B.; Gilroy, S. Feeling green: Mechanosensing in plants. Trends Cell Biol. 2009, 19, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Peyronnet, R.; Tran, D.; Girault, T.; Frachisse, J.-M. Mechanosensitive channels: Feeling tension in a world under pressure. Front. Plant Sci. 2014, 5, 558. [Google Scholar] [CrossRef] [PubMed]
- Massa, G.D.; Gilroy, S. Touch modulates gravity sensing to regulate the growth of primary roots of Arabidopsis thaliana. Plant J. 2003, 33, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Darwin, C. Insectivorous Plants, Authorized ed.; D. Appleton: New York, NY, USA, 1897. [Google Scholar]
- Forterre, Y.; Skotheim, J.M.; Dumais, J.; Mahadevan, L. How the Venus flytrap snaps. Nature 2005, 433, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Darwin, C.; Darwin, F. The Power of Movement in Plants; J. Murray: London, UK, 1880. [Google Scholar]
- Visnovitz, T.; Világi, I.; Varró, P.; Kristóf, Z. Mechanoreceptor cells on the tertiary pulvini of Mimosa pudica L. Plant Signal. Behav. 2007, 2, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Inaguma, N.; Nejigane, K.; Tani, K.; Yamada, H. Measurement of slip, force and deformation using hybrid tactile sensor system for robot hand gripping an object. Int. J. Adv. Robot. Syst. 2013, 10, 83. [Google Scholar] [CrossRef]
- Kim, M.-S.; Ahn, H.-R.; Lee, S.; Kim, C.; Kim, Y.-J. A dome-shaped piezoelectric tactile sensor arrays fabricated by an air inflation technique. Sens. Actuators A Phys. 2014, 212, 151–158. [Google Scholar] [CrossRef]
- Fang, S.J.; Husson, S.; Fu, C.K.; Lin, C.H. Flexible Tactile Sensor Array Utilizing Microstructured PDMS Bumps with PEDOT:PSS Conductive Polymer. In Proceedings of the 2017 IEEE 30th International Conference on Micro Electro Mechanical Systems (MEMS), Las Vegas, NV, USA, 22–26 January 2017; pp. 1029–1032. [Google Scholar]
- Mastronardi, V.M.; Ceseracciu, L.; Guido, F.; Rizzi, F.; Athanassiou, A.; De Vittorio, M.; Petroni, S. Low stiffness tactile transducers based on AlN thin film and polyimide. Appl. Phys. Lett. 2015, 106, 162901. [Google Scholar] [CrossRef]
- Sareh, S.; Jiang, A.; Faragasso, A.; Noh, Y.; Nanayakkara, T.; Dasgupta, P.; Seneviratne, L.D.; Wurdemann, H.A.; Althoefer, K. Bio-Inspired Tactile Sensor Sleeve for Surgical Soft Manipulators. In Proceedings of the 2014 IEEE International Conference on Robotics and Automation (ICRA), Hong Kong, China, 31 May–7 June 2014; pp. 1454–1459. [Google Scholar]
- Paulino, T.; Ribeiro, P.; Neto, M.; Cardoso, S.; Schmitz, A.; Santos-Victor, J.; Bernardino, A.; Jamone, L. Low-Cost 3-Axis Soft Tactile Sensors for the Human-Friendly Robot Vizzy. In Proceedings of the 2017 IEEE International Conference on Robotics and Automation (ICRA), Singapore, 29 May–3 June 2017; pp. 966–971. [Google Scholar]
- Youssefian, S.; Rahbar, N.; Torres-Jara, E. Contact behavior of soft spherical tactile sensors. IEEE Sens. J. 2014, 14. [Google Scholar] [CrossRef]
- Taya, M.; Wang, J.; Xu, C.; Kuga, Y. Tactile Sensors. U.S. Patent 7,823,467, 2 November 2010. [Google Scholar]
- Wang, J.; Sato, H.; Xu, C.; Taya, M. Bioinspired design of tactile sensors based on Flemion. J. Appl. Phys. 2009, 105, 083515. [Google Scholar] [CrossRef]
- Vogt, D.M.; Park, Y.L.; Wood, R.J. Design and characterization of a soft multi-axis force sensor using embedded microfluidic channels. IEEE Sens. J. 2013, 13, 4056–4064. [Google Scholar] [CrossRef]
- Yeo, J.C.; Zhuangjian, L.; Zhang, Z.-Q.; Zhang, P.; Zhiping, W.; Lim, C.T. Wearable mechanotransduced tactile sensor for haptic perception. Adv. Mater. Technol. 2017, 2, 1700006. [Google Scholar] [CrossRef]
- Huang, Y.; Yuan, H.; Kan, W.; Guo, X.; Liu, C.; Liu, P. A flexible three-axial capacitive tactile sensor with multilayered dielectric for artificial skin applications. Microsyst. Technol. 2017, 23, 1847–1852. [Google Scholar] [CrossRef]
- Kadooka, K.; Imamura, H.; Taya, M. Tactile Sensor Integrated Dielectric Elastomer Actuator for Simultaneous Actuation and Sensing. In Proceedings of the Electroactive Polymer Actuators and Devices (EAPAD), Las Vegas, NV, USA, 21–24 March 2016; Volume 9798, p. 97982H. [Google Scholar]
- Zhang, T.; Jiang, L.; Wu, X.; Feng, W.; Zhou, D.; Liu, H. Fingertip three-axis tactile sensor for multifingered grasping. IEEE/ASME Trans. Mechatron. 2015, 1875–1885. [Google Scholar] [CrossRef]
- Chorley, C.; Melhuish, C.; Pipe, T.; Rossiter, J. Tactile Edge Detection. In Proceedings of the 2010 IEEE SENSORS, Kona, HI, USA, 1–4 November 2010; pp. 2593–2598. [Google Scholar]
- Ward-Cherrier, B.; Pestell, N.; Cramphorn, L.; Winstone, B.; Giannaccini, M.E.; Rossiter, J.; Lepora, N.F. The TacTip family: Soft optical tactile sensors with 3D-printed biomimetic orphologies. Soft Robot. 2018, 5, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, Y.; Hong, J.; Lee, Y.; Ha, M.; Jung, Y.; Lim, H.; Kim, S.Y.; Ko, H. Tactile-direction-sensitive and stretchable electronic skins based on human-skin-inspired interlocked microstructures. ACS Nano 2014, 8, 12020–12029. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, J.; Hong, J.; Lee, H.; Lee, Y.; Cho, S.; Kim, S.-W.; Kim, J.J.; Kim, S.Y.; Ko, H. Tailoring force sensitivity and selectivity by microstructure engineering of multidirectional electronic skins. NPG Asia Mater. 2018. [Google Scholar] [CrossRef]
- Astreinidi Blandin, A.; Totaro, M.; Bernardeschi, I.; Engelberth, J.; Beccai, L. Towards Plant-Inspired Tactile Sensors: Biomechanics of the Tactile Blep. In Proceedings of the MRS Fall Meeting & Exhibit, Boston, MA, USA, 26 November–1 December 2017. [Google Scholar]
- Astreinidi Blandin, A.; Totaro, M.; Bernardeschi, I.; Beccai, L. Tunable normal and shear force discrimination by a plant-inspired tactile sensor for soft robotics. In Biomimetic and Biohybrid Systems, Proceedings of the 6th International Conference, Living Machines 2017, Stanford, CA, USA, 26–28 July 2017; Mangan, M., Cutkosky, M., Mura, A., Verschure, P.F.M.J., Prescott, T.J., Lepora, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 25–34. ISBN 978-3-319-63537-8. [Google Scholar]
- Prescott, T.J.; Dürr, V. Introduction: The world of touch. In Scholarpedia of Touch; Prescott, T.J., Ahissar, E., Izhikevich, E., Eds.; Atlantis Press: Paris, France, 2016; pp. 1–28. ISBN 978-94-6239-133-8. [Google Scholar]
- Wang, H.; Totaro, M.; Beccai, L. Toward perceptive soft robots: Progress and challenges. Adv. Sci. 2018, 5, 1800541. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kim, M.; Kim, Y.-J.; Hong, N.; Ryu, S.; Kim, H.J.; Kim, S. Soft robot review. Int. J. Control Autom. Syst. 2017, 15, 3–15. [Google Scholar] [CrossRef]
- Orr, W.C.; Sohal, R.S. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science 1994, 263, 1128–1130. [Google Scholar] [CrossRef] [PubMed]
- Laschi, C.; Mazzolai, B.; Cianchetti, M. Soft robotics: Technologies and systems pushing the boundaries of robot abilities. Sci. Robot. 2016, 1, eaah3690. [Google Scholar] [CrossRef]
- Wallin, T.J.; Pikul, J.; Shepherd, R.F. 3D printing of soft robotic systems. Nat. Rev. Mater. 2018, 3, 84–100. [Google Scholar] [CrossRef]
- Guo, S.-Z.; Qiu, K.; Meng, F.; Park, S.H.; McAlpine, M.C. 3D printed stretchable tactile sensors. Adv. Mater. 2017, 29, 1701218. [Google Scholar] [CrossRef] [PubMed]
- Valentine, A.D.; Busbee, T.A.; Boley, J.W.; Raney, J.R.; Chortos, A.; Kotikian, A.; Berrigan, J.D.; Durstock, M.F.; Lewis, J.A. Hybrid 3D printing of soft electronics. Adv. Mater. 2017, 29, 1703817. [Google Scholar] [CrossRef] [PubMed]
- Truby, R.L.; Lewis, J.A. Printing soft matter in three dimensions. Nature 2016, 540, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Matsuhisa, N.; Inoue, D.; Zalar, P.; Jin, H.; Matsuba, Y.; Itoh, A.; Yokota, T.; Hashizume, D.; Someya, T. Printable elastic conductors by in situ formation of silver nanoparticles from silver flakes. Nat. Mater. 2017, 16, 834. [Google Scholar] [CrossRef] [PubMed]
- Muth, J.T.; Vogt, D.M.; Truby, R.L.; Mengüç, Y.; Kolesky, D.B.; Wood, R.J.; Lewis, J.A. Embedded 3D printing of strain sensors within highly stretchable elastomers. Adv. Mater. 2014, 26, 6307–6312. [Google Scholar] [CrossRef] [PubMed]
- Truby, R.L.; Wehner, M.; Grosskopf, A.K.; Vogt, D.M.; Uzel, S.G.M.; Wood, R.J.; Lewis, J.A. Soft somatosensitive actuators via embedded 3D printing. Adv. Mater. 2018, 30, 1706383. [Google Scholar] [CrossRef] [PubMed]
- Odent, J.; Wallin, T.J.; Pan, W.; Kruemplestaedter, K.; Shepherd, R.F.; Giannelis, E.P. Highly elastic, transparent, and conductive 3D-printed ionic composite hydrogels. Adv. Funct. Mater. 2017, 27, 1701807. [Google Scholar] [CrossRef]
- Liu, X.; Gu, H.; Wang, M.; Du, X.; Gao, B.; Elbaz, A.; Sun, L.; Liao, L.; Xiao, P.; Gu, Z. 3D printing of bioinspired liquid superrepellent structures. Adv. Mater. 2018, 30, 1800103. [Google Scholar] [CrossRef] [PubMed]
- Tricinci, O.; Terencio, T.; Pugno, N.M.; Greco, F.; Mazzolai, B.; Mattoli, V. Air trapping mechanism in artificial salvinia-like micro-hairs fabricated via direct laser lithography. Micromachines 2017, 8, 366. [Google Scholar] [CrossRef]
- Tricinci, O.; Eason, E.V.; Filippeschi, C.; Mondini, A.; Mazzolai, B.; Pugno, N.M.; Cutkosky, M.R.; Greco, F.; Mattoli, V. Dry adhesion of artificial gecko setae fabricated via direct laser lithography. In Biomimetic and Biohybrid Systems; Mangan, M., Cutkosky, M., Mura, A., Verschure, P.F.M.J., Prescott, T.J., Lepora, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 631–636. [Google Scholar]
- Tricinci, O.; Terencio, T.; Mazzolai, B.; Pugno, N.M.; Greco, F.; Mattoli, V. 3D micropatterned surface inspired by Salvinia molesta via direct laser lithography. ACS Appl. Mater. Interfaces 2015, 7, 25560–25567. [Google Scholar] [CrossRef] [PubMed]
- Bernardeschi, I.; Tricinci, O.; Mattoli, V.; Filippeschi, C.; Mazzolai, B.; Beccai, L. Three-dimensional soft material micropatterning via direct laser lithography of flexible molds. ACS Appl. Mater. Interfaces 2016, 8, 25019–25023. [Google Scholar] [CrossRef] [PubMed]
- Accardo, A.; Blatché, M.-C.; Courson, R.; Loubinoux, I.; Vieu, C.; Malaquin, L. Two-photon lithography and microscopy of 3D hydrogel scaffolds for neuronal cell growth. Biomed. Phys. Eng. Express 2018, 4, 027009. [Google Scholar] [CrossRef]
- Brigo, L.; Urciuolo, A.; Giulitti, S.; Della Giustina, G.; Tromayer, M.; Liska, R.; Elvassore, N.; Brusatin, G. 3D high-resolution two-photon crosslinked hydrogel structures for biological studies. Acta Biomater. 2017, 55, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Pennacchio, F.A.; Fedele, C.; De Martino, S.; Cavalli, S.; Vecchione, R.; Netti, P.A. Three-dimensional microstructured azobenzene-containing gelatin as a photoactuable cell confining system. ACS Appl. Mater. Interfaces 2018, 10, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Lemma, E.D.; Sergio, S.; Spagnolo, B.; Pisanello, M.; Algieri, L.; Coluccia, M.A.; Maffia, M.; De Vittorio, M.; Pisanello, F. Tunable mechanical properties of stent-like microscaffolds for studying cancer cell recognition of stiffness gradients. Microelectron. Eng. 2018, 190, 11–18. [Google Scholar] [CrossRef]
- Lemma, E.D.; Spagnolo, B.; Rizzi, F.; Corvaglia, S.; Pisanello, M.; De Vittorio, M.; Pisanello, F. Microenvironmental stiffness of 3D polymeric structures to study invasive rates of cancer cells. Adv. Healthc. Mater. 2017, 6, 1700888. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sun, X.-Z.; Parmenter, C.D.J.; Fay, M.W.; Smith, E.F.; Rance, G.A.; He, Y.; Zhang, F.; Liu, Y.; Irvine, D.; et al. Additive manufacture of complex 3D Au-containing nanocomposites by simultaneous two-photon polymerisation and photoreduction. Sci. Rep. 2017, 7, 17150. [Google Scholar] [CrossRef] [PubMed]
- Bakhtina, N.A.; MacKinnon, N.; Korvink, J.G. Advanced Two-Photon Photolithography for Patterning of Transparent, Electrically Conductive Ionic Liquid-Polymer Nanostructures. In Proceedings of the Laser 3D Manufacturing III, San Francisco, CA, USA, 15–18 February 2016; Volume 9738, p. 97380. [Google Scholar]
- Blasco, E.; Müller, J.; Müller, P.; Trouillet, V.; Schön, M.; Scherer, T.; Barner-Kowollik, C.; Wegener, M. Fabrication of conductive 3D gold-containing microstructures via direct laser writing. Adv. Mater. 2016, 28, 3592–3595. [Google Scholar] [CrossRef] [PubMed]

| Type | Mechanical Sensory Organ | Example Organism | Environment | Location/Distribution | Size |
|---|---|---|---|---|---|
| Domes | Tactile papilla | Chilean glory flower Eccremocarpus scaber | Air | Ventral and lateral side of branchlets | Base Ø 10 µm |
| Tactile papilla | Snake Rhinotyphlops | Air | Rostrum, 250 papillae | Length 110 µm; Ø 26 µm | |
| Tactile blep * | Bryony Bryonia dioica Jacq. | Air | Similar density on upper and lower side of tendrils | Base Ø 4–5 µm | |
| Eimer’s organ * | Star-nosed mole Condylura cristata | Air/Soil | Star-like nose with 22 appendages, 25,000 organs | Ø 30–50 µm | |
| Campaniform sensillum | Stick insect Carausius morosus | Air | Antenna | Base Ø 5 µm | |
| Campaniform sensillum | Fly Drosophila melanogaster | Air | Halteres, 300 sensilla/haltere | Base Ø 10 µm | |
| Campaniform sensillum | Honey bee | Air | Head, elliptical form | Length 0.9 µm | |
| Campaniform sensillum * | Cockroach Periplaneta americana | Air | Leg in groups, semi-major axis in limb direction | Length 6–24 µm | |
| Integumentary sensory organ * | Alligator Alligator mississippiensis | Air/Water | Face and mouth inner | Base Ø 200 µm | |
| Cantilevers | Hair sensillum * | Spider Cupiennius salei | Air | Legs (100 trichobothria per leg, 400 tactile hairs per mm2), joints | Length 0.1–3.2 mm; Base Ø 5–23 µm |
| Hair sensillum | Honey bee | Air | Neck, 160–180 per hair plate, spacing 6–15 µm | Length 25–150 µm; Base Ø 2–5 µm | |
| Vibrissa * | Mouse | Air | Face | Macro- and microvibrissae | |
| Hair sensillum * | Venus Dionaea muscipula | Air | On each inner lobe of leaves, 3–5 sensilla | Length 2 mm; Base Ø 200 µm | |
| Hair cell | Jellyfish Aglantha digitale | Water | Velum and tentacle bases | Cilium length up to 30 µm, surrounded by graded microvilli | |
| Hair sensillum | Crayfish Procambarus clarkii | Water | Lateral antennular flagellum | Length 80–200 µm; Base Ø 5–15 µm | |
| Cantilevers with Domes | Cupular organ * | Sea squirt Ciona intestinalis | Water | Siphons, 75–100 organs | Cupula length 250 µm; Macula base Ø 100 µm |
| Cupular organ | Sea squirt Corella eumyota | Water | Branchial sac on atrial side, 34 organs | Cupula length 100–130 µm; Macula base Ø 80–100 µm | |
| Cupular strand | Sea squirt Corella inflata | Water | Dorsal fold of the branchial sac on atrial side, 1 organ | Length 7–8 mm; Width 20–30 µm | |
| Neuromast * | Fish Astyanax fasciatus | Water | Lateral line system, 'superficial' on skin surface, 'canal' in lateral line canals | Superficial: height 50–400 µm; Canal: order of magnitude higher |
| Type | Mechanical Sensory Organ | Example Organism | Material | Exteroception | Proprioception | Detected Stimuli | Reference(s) |
|---|---|---|---|---|---|---|---|
| Domes | Tactile papilla | Chilean glory flower Eccremocarpus scaber | - | X | Touch ** | [32] | |
| Tactile papilla | Snake Rhinotyphlops | - | X | Touch ** | [19] | ||
| Tactile blep * | Bryony Bryonia dioica Jacq. | Multiple (callose, cellulose, cell wall, cytoplasm) | X | Shear forces ** | [30] | ||
| Eimer's organ * | Star-nosed mole Condylura cristata | - | X | Touch | [40] | ||
| Campaniform sensillum | Stick insect Carausius morosus | - | X | X | Shear forces for bending | [41] | |
| Campaniform sensillum | Fly Drosophila melanogaster | - | X | X | Strain | [26] | |
| Campaniform sensillum | Honey bee | Resilin, E = 1 MPa | X | X | Position/Inertia | [20,27] | |
| Campaniform sensillum * | Cockroach Periplaneta americana | Multiple | X | X | Strain | [42,43] | |
| Integumentary sensory organ * | Alligator Alligator mississippiensis | - | X | Flow/Touch | [44] | ||
| Cantilevers | Hair sensillum * | Spider Cupiennius salei | Cuticle, E= 18 GPa | X | X | Flow/Touch/Position | [28,45] |
| Hair sensillum | Honey bee | Resilin (joint membrane), E= 1 MPa | X | X | Position/Inertia | [20,21,27] | |
| Vibrissa * | Mouse | - | X | X | Active touch/Self | [22] | |
| Hair sensillum * | Venus Dionaea muscipula | Multicellular, transversal sensory layer | X | Location | [23,31,46] | ||
| Hair cell | Jellyfish Aglantha digitale | - | X | Flow | [24] | ||
| Hair sensillum | Crayfish Procambarus clarkii | Torsional stiffness 10−12 Nm/° | X | Flow/Chemical | [25] | ||
| Cantilevers with Domes | Cupular organ * | Sea squirt Ciona intestinalis | Irregular folding of gelatinous proteinaceous cupula | X | Flow ** | [47] | |
| Cupular organ | Sea squirt Corella eumyota | Irregular folding of gelatinous proteinaceous cupula | X | Flow ** | [48] | ||
| Cupular strand | Sea squirt Corella inflata | Finely fibrous proteinaceous cupula | X | Flow ** | [48] | ||
| Neuromast * | Fish Astyanax fasciatus | Gelatinous cupula, 10 kPa (superficial, blind cave fish) | X | Flow velocity/Acceleration | [49,50,51] |
| Mechanical Aspects | Details |
|---|---|
| 1. Orientation | Of the sensory organ on the supporting body (e.g., for active touch) |
| 2. Shape | Specific geometries according to the environment, length, diameter, coating (e.g., cupula), surface aspect (e.g., undulations) |
| 3. Distribution | Often increased in areas of major importance or of multiple input types, redundancy |
| 4. Materials | Mechanical characteristics, homo- or heterogeneity, proportions |
| 5. Micromechanics | Shape and/or materials. Attachment site with the rest of the body (e.g., hair base), microstructures (e.g., microtrichs), shape (e.g., variation of diameter) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astreinidi Blandin, A.; Bernardeschi, I.; Beccai, L. Biomechanics in Soft Mechanical Sensing: From Natural Case Studies to the Artificial World. Biomimetics 2018, 3, 32. https://doi.org/10.3390/biomimetics3040032
Astreinidi Blandin A, Bernardeschi I, Beccai L. Biomechanics in Soft Mechanical Sensing: From Natural Case Studies to the Artificial World. Biomimetics. 2018; 3(4):32. https://doi.org/10.3390/biomimetics3040032
Chicago/Turabian StyleAstreinidi Blandin, Afroditi, Irene Bernardeschi, and Lucia Beccai. 2018. "Biomechanics in Soft Mechanical Sensing: From Natural Case Studies to the Artificial World" Biomimetics 3, no. 4: 32. https://doi.org/10.3390/biomimetics3040032
APA StyleAstreinidi Blandin, A., Bernardeschi, I., & Beccai, L. (2018). Biomechanics in Soft Mechanical Sensing: From Natural Case Studies to the Artificial World. Biomimetics, 3(4), 32. https://doi.org/10.3390/biomimetics3040032







