NDs@PDA@ICG Conjugates for Photothermal Therapy of Glioblastoma Multiforme
Abstract
1. Introduction
2. Materials and Methods
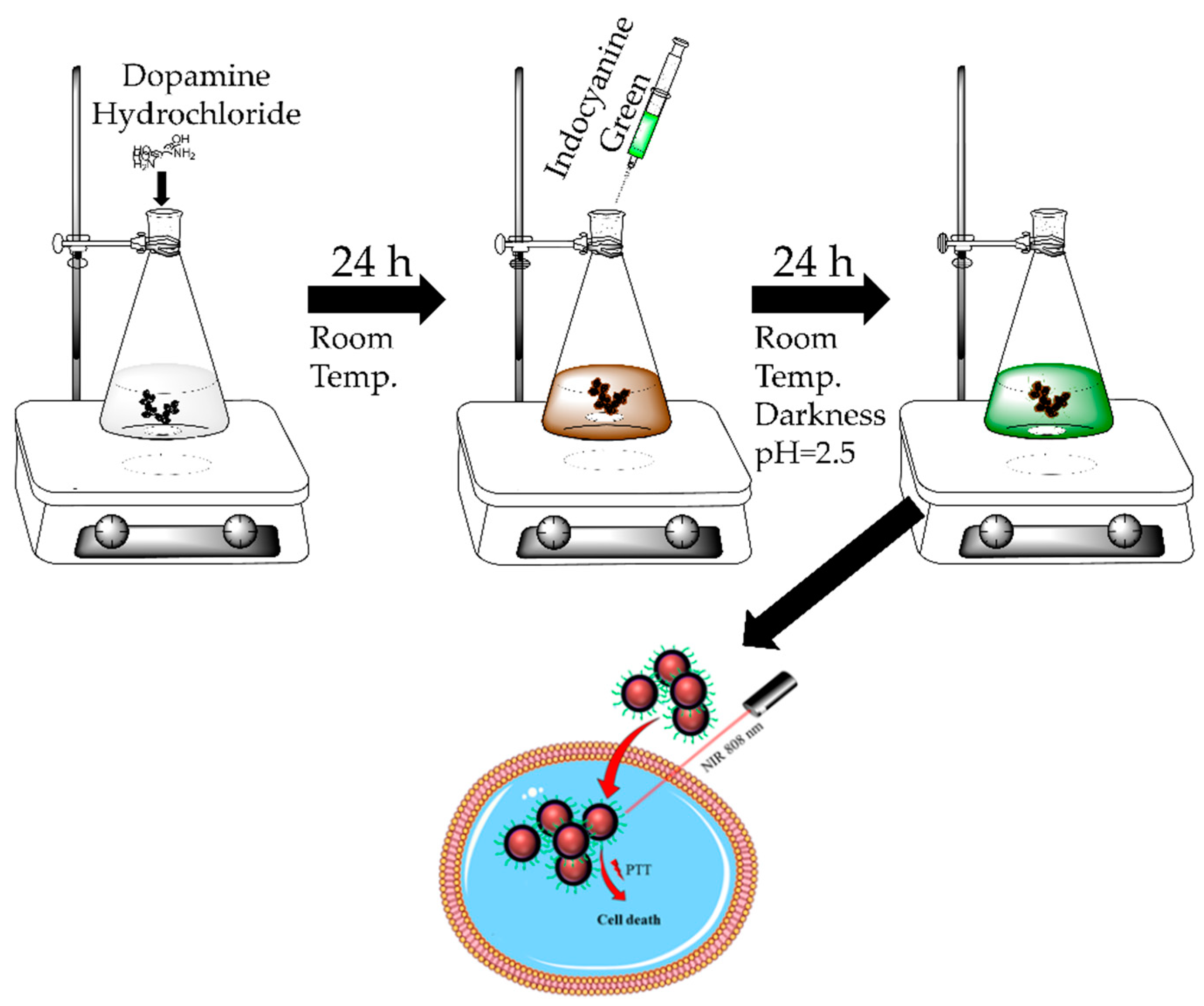
2.1. Preparation of NDs@PDA
2.2. Indocyanine Green Conjugation
2.3. Spectroscopy Measurements
2.4. Size Determination of NDs-Based Materials and Zeta Potential
2.5. Photothermal Measurements
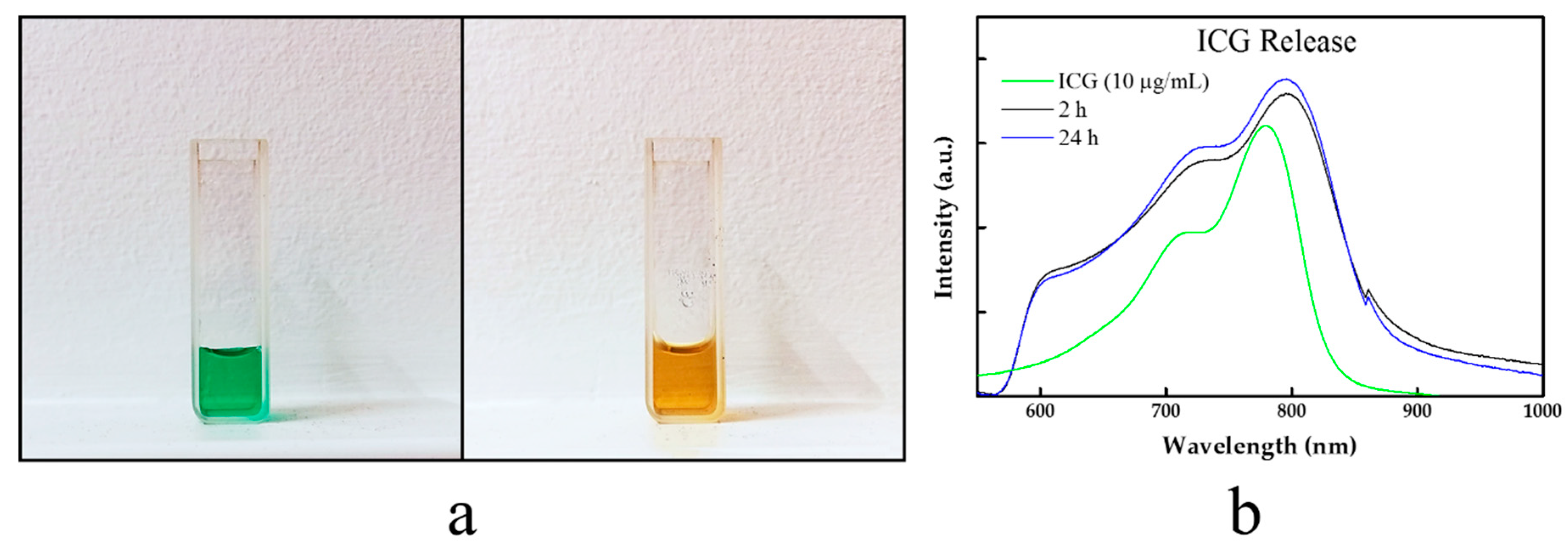
2.6. ICG Release
2.7. Cytotoxicity of NDs-Based Clusters
2.8. Glioblastoma Multiforme Cells Irradiation
2.9. Statistical Analysis
3. Results
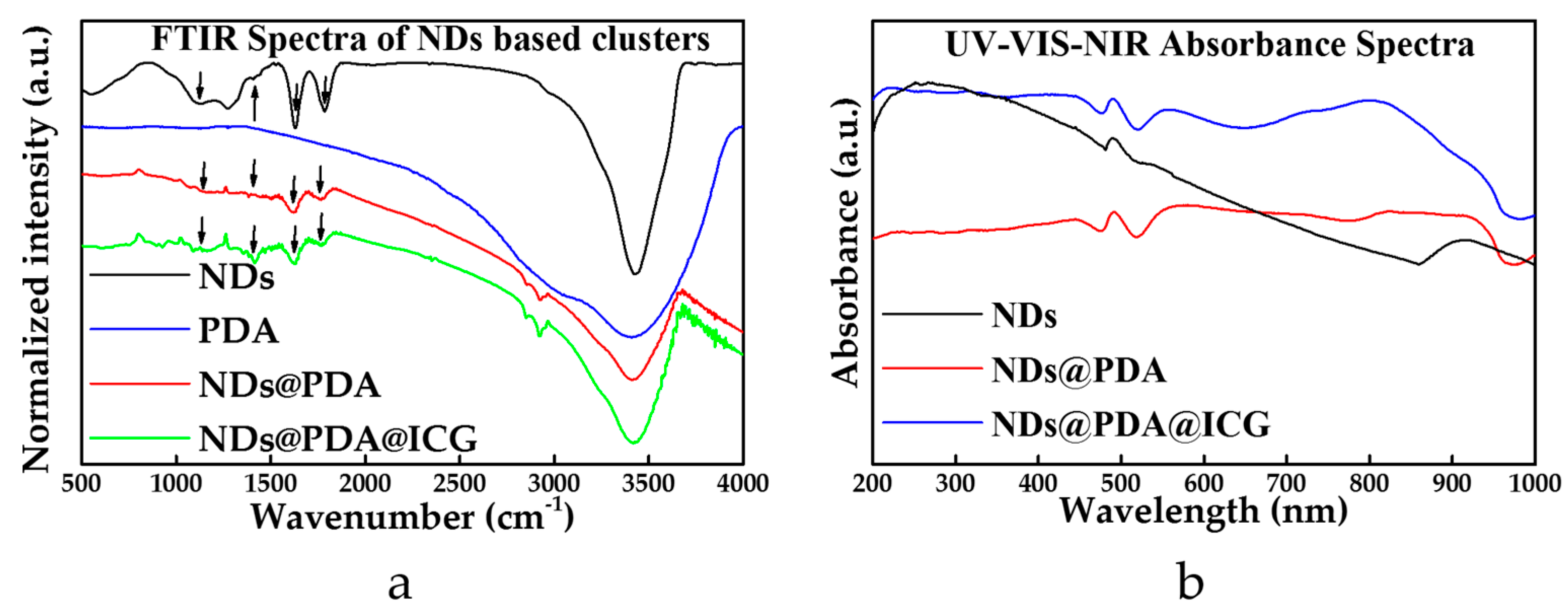
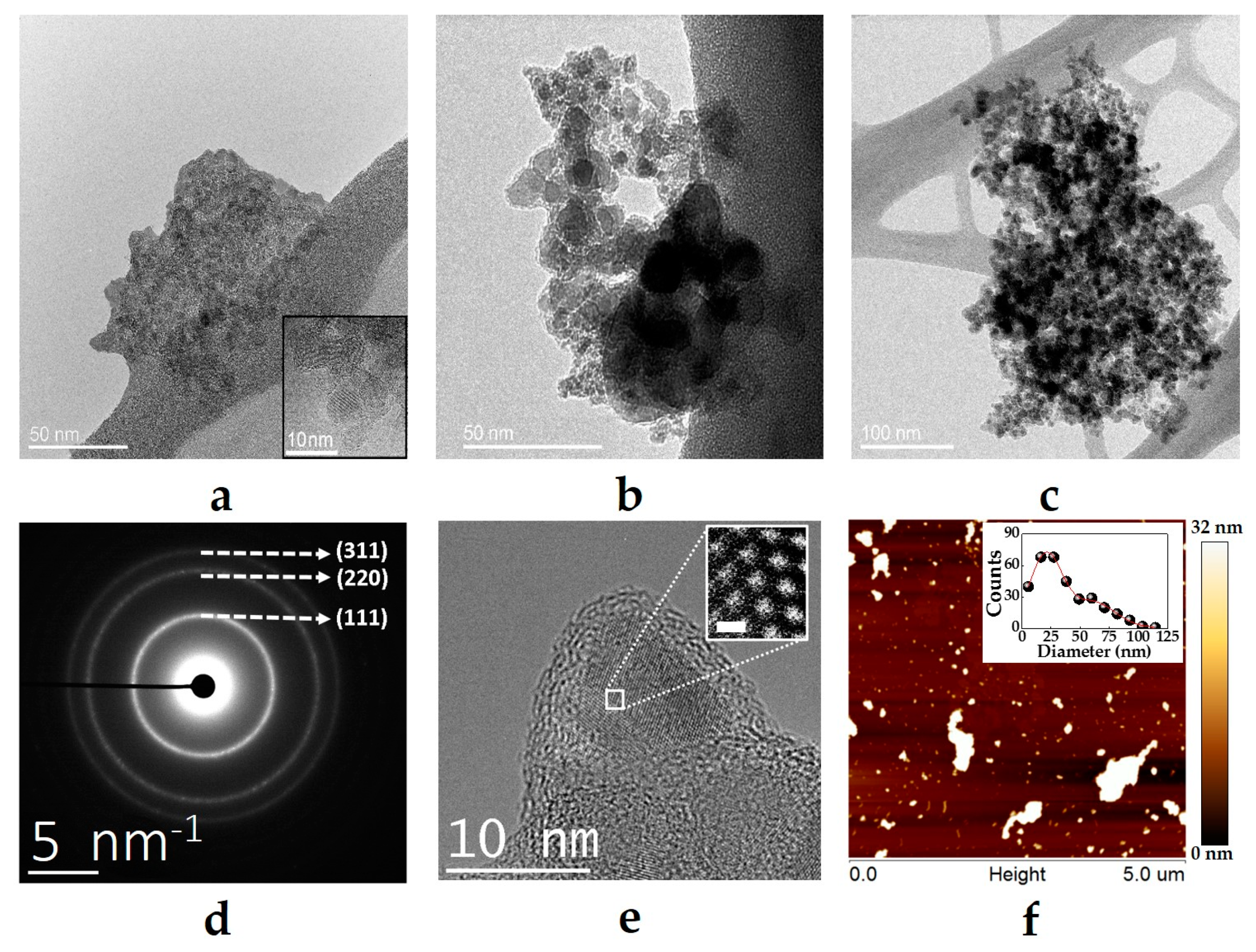
3.1. Characterization of NDs, NDs@PDA, and NDs@PDA@ICG
3.2. Photothermal Properties
3.3. Cell Viability and In Vitro PTT of Glioblastoma Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gaffet, E. Nanomaterials: A review of the definitions, applications, health effects. How to implement secure development. 2011; <hal-00598817>. [Google Scholar]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological interactions of graphene-family nanomaterials—An interdisciplinary review. Chem. Res. Toxicol. 2012, 15–34. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Wang, W.; Liao, S.; Liu, M.; Zhao, Q.; Zhu, Y. Polymer composites reinforced by nanotubes as scaffolds for tissue engineering. Int. J. Polym. Sci. 2014, 2014. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Ryu, T.K.; Baek, S.W.; Lee, G.J.; Rhee, C.K.; Choi, S.W. Targeted tumor therapy based on nanodiamonds decorated with doxorubicin and folic acid. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef]
- Ushizawa, K.; Sato, Y.; Mitsumori, T. Covalent immobilization of DNA on diamond and its verification by diffuse reflectance infrared spectroscopy. 2002, 351, 105–108. [Google Scholar]
- Mehta, A.I.; Linninger, A.; Lesniak, M.S.; Engelhard, H.H. Current status of intratumoral therapy for glioblastoma. J. Neurooncol. 2015, 125, 1–7. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, D.; Zeng, Y.; Wu, L.; Liu, X.; Liu, J. Nanocluster of superparamagnetic iron oxide nanoparticles coated with poly (dopamine) for magnetic field-targeting, highly sensitive MRI and photothermal cancer therapy. Nanotechnology 2015, 26. [Google Scholar] [CrossRef]
- Mrówczyński, R.; Jędrzak, A.; Szutkowski, K.; Grześkowiak, B.; Coy, E.; Markiewicz, R.; Jesionowski, T.; Jurga, S. Cyclodextrin-based magnetic nanoparticles for cancer therapy. Nanomaterials 2018, 8, 170. [Google Scholar] [CrossRef]
- Siegel, R.; Miller, K.D.; Ahmedin, J. Cáncer statistics. Ca Cáncer J. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Urruticoechea, A.; Alemany, R.; Balart, J.; Villanueva, A.; Vinals, F.; Capella, G. recent advances in cancer therapy: An overview. Curr. Pharm. Des. 2010, 16, 3–10. [Google Scholar] [CrossRef]
- Gatzka, M.V. Targeted tumor therapy remixed—An update on the use of small-molecule drugs in combination therapies. Cancers (Basel) 2018, 10. [Google Scholar] [CrossRef]
- Johannsen, M.; Gneveckow, U.; Eckelt, L.; Feussner, A.; WaldÖFner, N.; Scholz, R.; Deger, S.; Wust, P.; Loening, S.A.; Jordan, A. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: Presentation of a new interstitial technique. Int. J. Hyperth. 2005, 21, 637–647. [Google Scholar] [CrossRef]
- Alhaddad, A.; Adam, M.P.; Botsoa, J.; Dantelle, G.; Perruchas, S.; Gacoin, T.; Mansuy, C.; Lavielle, S.; Malvy, C.; Treussart, F.; et al. Nanodiamond as a vector for siRNA delivery to Ewing sarcoma cells. Small 2011, 7, 3087–3095. [Google Scholar] [CrossRef]
- Chow, E.K.-H.; Ho, D. Cancer nanomedicine: From drug delivery to imaging. Sci. Transl. Med. 2013, 5, 216rv4. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Ryu, T.K.; Baek, S.W.; Kang, R.H.; Choi, S.W. Selective photothermal tumor therapy using nanodiamond-based nanoclusters with folic acid. Adv. Funct. Mater. 2016, 26, 6428–6436. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, D.Y. Photothermal therapy with gold nanoparticles as an anticancer medication. J. Pharm. Investig. 2017, 47, 19–26. [Google Scholar] [CrossRef]
- Eldridge, B.N.; Bernish, B.W.; Fahrenholtz, C.D.; Singh, R. Photothermal therapy of glioblastoma multiforme using multiwalled carbon nanotubes optimized for diffusion in extracellular space. ACS Biomater. Sci. Eng. 2016, 2, 963–976. [Google Scholar] [CrossRef]
- Huang, H.; Pierstorff, E.; Osawa, E.; Ho, D. Active nanodiamond hydrogels for chemotherapeutic delivery. Nano Lett. 2007, 7, 3305–3314. [Google Scholar] [CrossRef]
- Markovic, Z.M.; Harhaji-Trajkovic, L.M.; Todorovic-Markovic, B.M.; Kepić, D.P.; Arsikin, K.M.; Jovanović, S.P.; Pantovic, A.C.; Dramićanin, M.D.; Trajkovic, V.S. In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes. Biomaterials 2011, 32, 1121–1129. [Google Scholar] [CrossRef]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 206–212. [Google Scholar] [CrossRef]
- Tran, S.; DeGiovanni, P.-J.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 44. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Murawska, M.; Wiatr, M.; Nowakowski, P.; Szutkowski, K.; Skrzypczak, A.; Kozak, M. The structure and morphology of gold nanoparticles produced in cationic gemini surfactant systems. Radiat. Phys. Chem. 2013, 93, 160–167. [Google Scholar] [CrossRef]
- Zhao, H.; Chao, Y.; Liu, J.; Huang, J.; Pan, J.; Guo, W.; Wu, J.; Sheng, M.; Yang, K.; Wang, J.; et al. Polydopamine coated single-walled carbon nanotubes as a versatile platform with radionuclide labeling for multimodal tumor imaging and therapy. Theranostics 2016, 6, 1833–1843. [Google Scholar] [CrossRef]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, X.J. Nano-carbons as theranostics. Theranostics 2012, 2, 235–237. [Google Scholar] [CrossRef]
- Smart, S.K.; Cassady, A.I.; Lu, G.Q.; Martin, D.J. The biocompatibility of carbon nanotubes. Carbon N. Y. 2006, 44, 1034–1047. [Google Scholar] [CrossRef]
- Sajid, M.I.; Jamshaid, U.; Jamshaid, T.; Zafar, N.; Fessi, H.; Elaissari, A. Carbon nanotubes from synthesis to in vivo biomedical applications. Int. J. Pharm. 2016, 501, 278–299. [Google Scholar] [CrossRef]
- Danilenko, V.V. On the history of the discovery of nanodiamond synthesis. Phys. Solid State 2004, 46, 595–599. [Google Scholar] [CrossRef]
- Nunn, N.; Torelli, M.; McGuire, G.; Shenderova, O. Nanodiamond: A high impact nanomaterial. Curr. Opin. Solid State Mater. Sci. 2017, 21, 1–9. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Mochalin, V.N. Biomedical applications of nanodiamond (Review). Nanotechnology 2017, 252001, 1–28. [Google Scholar] [CrossRef]
- Kazi, S. A review article on nanodiamonds discussing their properties and applications. Int. J. Pharm. Sci. Invent. ISSN (Online) 2014, 3, 2319–6718. [Google Scholar]
- Liu, K.-K.; Cheng, C.-L.; Chang, C.-C.; Chao, J.-I. Biocompatible and detectable carboxylated nanodiamond on human cell. Nanotechnology 2007, 18, 325102. [Google Scholar] [CrossRef]
- Paget, V.; Sergent, J.A.; Grall, R.; Altmeyer-Morel, S.; Girard, H.A.; Petit, T.; Gesset, C.; Mermoux, M.; Bergonzo, P.; Arnault, J.C.; et al. Carboxylated nanodiamonds are neither cytotoxic nor genotoxic on liver, kidney, intestine and lung human cell lines. Nanotoxicology 2014, 8, 46–56. [Google Scholar] [CrossRef]
- Taylor, A.C.; Vagaska, B.; Edgington, R.; Hébert, C.; Ferretti, P.; Bergonzo, P.; Jackman, R.B. Biocompatibility of nanostructured boron doped diamond for the attachment and proliferation of human neural stem cells. J. Neural Eng. 2015, 12. [Google Scholar] [CrossRef]
- Taylor, A.C.; González, C.H.; Miller, B.S.; Edgington, R.J.; Ferretti, P.; Jackman, R.B. Surface functionalisation of nanodiamonds for human neural stem cell adhesion and proliferation. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Kong, X.L.; Huang, L.C.L.; Hsu, C.M.; Chen, W.H.; Han, C.C.; Chang, H.C. High-affinity capture of proteins by diamond nanoparticles for mass spectrometric analysis. Anal. Chem. 2005, 77, 259–265. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, X.Q.; Man, H.B.; Lam, R.; Chow, E.K.; Ho, D. Nanodiamond vectors functionalized with polyethylenimine for siRNA delivery. J. Phys. Chem. Lett. 2010, 1, 3167–3171. [Google Scholar] [CrossRef]
- Li, X.; Shao, J.; Qin, Y.; Shao, C.; Zheng, T.; Ye, L. TAT-conjugated nanodiamond for the enhanced delivery of doxorubicin. J. Mater. Chem. 2011, 21, 7966. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Petran, A.; Mrówczyński, R.; Filip, C.; Turcu, R.; Liebscher, J. Melanin-like polydopa amides-synthesis and application in functionalization of magnetic nanoparticles. Polym. Chem. 2015, 6, 2139–2149. [Google Scholar] [CrossRef]
- Shi, C.; Deng, C.; Zhang, X.; Yang, P. Synthesis of highly water-dispersible polydopamine-modified multiwalled carbon nanotubes for matrix-assisted laser desorption/ionization mass spectrometry analysis. ACS Appl. Mater. Interfaces 2013, 5, 7770–7776. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, W.; Wang, Z.; Singamaneni, S.; Wang, R. Multifunctional surface modification of nanodiamonds based on dopamine polymerization. Langmuir 2018, 34, 4036–4042. [Google Scholar] [CrossRef]
- Mrówczyński, R.; Jurga-Stopa, J.; Markiewicz, R.; Coy, E.L.; Jurga, S.; Woźniak, A. Assessment of polydopamine coated magnetic nanoparticles in doxorubicin delivery. RSC Adv. 2016, 6, 5936–5943. [Google Scholar] [CrossRef]
- Mrówczyński, R. Polydopamine-based multifunctional (nano)materials for cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 7541–7561. [Google Scholar] [CrossRef]
- Jaque, D.; Martínez Maestro, L.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Martín Rodríguez, E.; García Solé, J. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef]
- Zhu, Z.; Su, M. Polydopamine nanoparticles for combined chemo- and photothermal cancer therapy. Nanomaterials 2017, 7, 160. [Google Scholar] [CrossRef]
- Mrówczyński, R.; Coy, L.E.; Scheibe, B.; Czechowski, T.; Augustyniak-Jabłokow, M.; Jurga, S.; Tadyszak, K. Electron paramagnetic resonance imaging and spectroscopy of polydopamine radicals. J. Phys. Chem. B 2015, 119, 10341–10347. [Google Scholar] [CrossRef]
- Ishizawa, T.; Fukushima, N.; Shibahara, J.; Masuda, K.; Tamura, S.; Aoki, T.; Hasegawa, K.; Beck, Y.; Fukayama, M.; Kokudo, N. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 2009, 115, 2491–2504. [Google Scholar] [CrossRef]
- Gotoh, K.; Yamada, T.; Ishikawa, O.; Takahashi, H.; Eguchi, H.; Yano, M.; Ohigashi, H.; Tomita, Y.; Miyamoto, Y.; Imaoka, S. A novel image-guided surgery of hepatocellular carcinoma by indocyanine green fluorescence imaging navigation. J. Surg. Oncol. 2009, 100, 75–79. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Tisler, J.; Balasubramanian, G.; Naydenov, B.; Kolesov, R.; Grotz, B.; Reuter, R.; Boudou, J.P.; Curmi, P.A.; Sennour, M.; Thorel, A.; et al. Fluorescence and spin properties of defects in single digit nanodiamonds. ACS Nano 2009, 3, 1959–1965. [Google Scholar] [CrossRef]
- Galli, G. Computer-based modeling of novel carbon systems and their properties. In Computer-Based Modeling of Novel Carbon Systems and Their Properties; Springer Netherlands: Dordrecht, The Netherlands, 2010; Volume 3, pp. 37–57. ISBN 978-1-4020-9717-1. [Google Scholar]
- Hu, D.; Zhang, J.; Gao, G.; Sheng, Z.; Cui, H.; Cai, L. Indocyanine green-loaded polydopamine-reduced graphene oxide nanocomposites with amplifying photoacoustic and photothermal effects for cancer theranostics. Theranostics 2016, 6, 1043–1052. [Google Scholar] [CrossRef]
- Paci, J.T.; Man, H.B.; Saha, B.; Ho, D.; Schatz, G.C. Understanding the surfaces of nanodiamonds. J. Phys. Chem. C 2013, 117, 17256–17267. [Google Scholar] [CrossRef]
- Sharma, S.; Shukla, P.; Misra, A.; Mishra, P.R. Interfacial and Colloidal Properties of Emulsified Systems: Pharmaceutical and Biological Perspective. Pharmaceutical and Biological Perspective.; Elsevier B.V.: Amsterdam, The Netherlands, 2014; ISBN 9780444626080. [Google Scholar]
- Portnoy, E.; Vakruk, N.; Bishara, A.; Shmuel, M.; Magdassi, S.; Golenser, J.; Eyal, S. Indocyanine green liposomes for diagnosis and therapeutic monitoring of cerebral malaria. Theranostics 2016, 6, 167–176. [Google Scholar] [CrossRef]
- Hu, D.; Liu, C.; Song, L.; Cui, H.; Gao, G.; Liu, P.; Sheng, Z.; Cai, L. Indocyanine green–loaded polydopamine–iron ions coordination nanoparticles for photoacoustic/magnetic resonance dual-modal imaging-guided cancer photothermal therapy. Nanoscale 2016, 8, 17150–17158. [Google Scholar] [CrossRef]
- Xu, F.; Liu, M.; Li, X.; Xiong, Z.; Cao, X.; Shi, X.; Guo, R. Loading of indocyanine green within polydopamine-coated laponite nanodisks for targeted cancer photothermal and photodynamic therapy. Nanomaterials 2018, 8. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef]
- Battigelli, A.; Ménard-Moyon, C.; Da Ros, T.; Prato, M.; Bianco, A. Endowing carbon nanotubes with biological and biomedical properties by chemical modifications. Adv. Drug Deliv. Rev. 2013, 65, 1899–1920. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Ren, K.; Chen, T.; Chen, X.; Li, B.; Chang, H.; Ji, J. Polydopamine nanocoating for effective photothermal killing of bacteria and fungus upon near-infrared irradiation. Adv. Mater. Interfaces 2016, 3, 1–6. [Google Scholar] [CrossRef]
- Wick, P.; Manser, P.; Limbach, L.K.; Dettlaff-Weglikowska, U.; Krumeich, F.; Roth, S.; Stark, W.J.; Bruinink, A. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol. Lett. 2007, 168, 121–131. [Google Scholar] [CrossRef]
- Shilo, M.; Sharon, A.; Baranes, K.; Motiei, M.; Lellouche, J.-P.M.; Popovtzer, R. The effect of nanoparticle size on the probability to cross the blood-brain barrier: An in-vitro endothelial cell model. J. Nanobiotechnol. 2015, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Ceña, V.; Játiva, P. Nanoparticle crossing of blood–brain barrier: A road to new therapeutic approaches to central nervous system diseases. Nanomedicine 2018, 13, 1513–1516. [Google Scholar] [CrossRef]
- Betzer, O.; Shilo, M.; Opochinsky, R.; Barnoy, E.; Motiei, M.; Okun, E.; Yadid, G.; Popovtzer, R. The effect of nanoparticle size on the ability to cross the blood–brain barrier: An in vivo study. Nanomedicine 2017, 12, 1533–1546. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Tse, B.W.-C.; Yang, H.; Thorling, C.A.; Liu, Y.; Touraud, M.; Chouane, J.B.; Liu, X.; Roberts, M.S.; et al. Indocyanine green-incorporating nanoparticles for cancer theranostics. Theranostics 2018, 8, 1227–1242. [Google Scholar] [CrossRef]

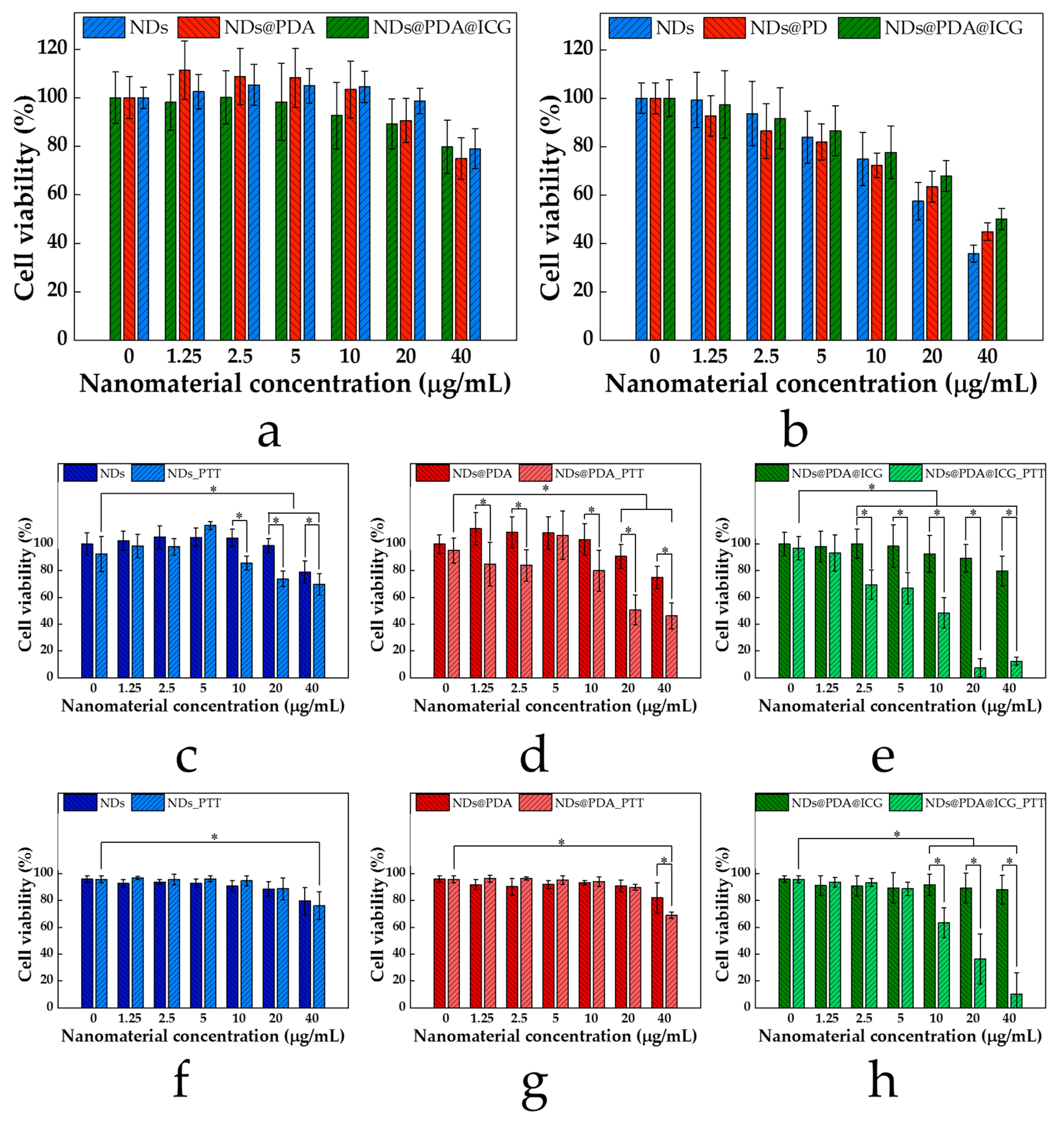
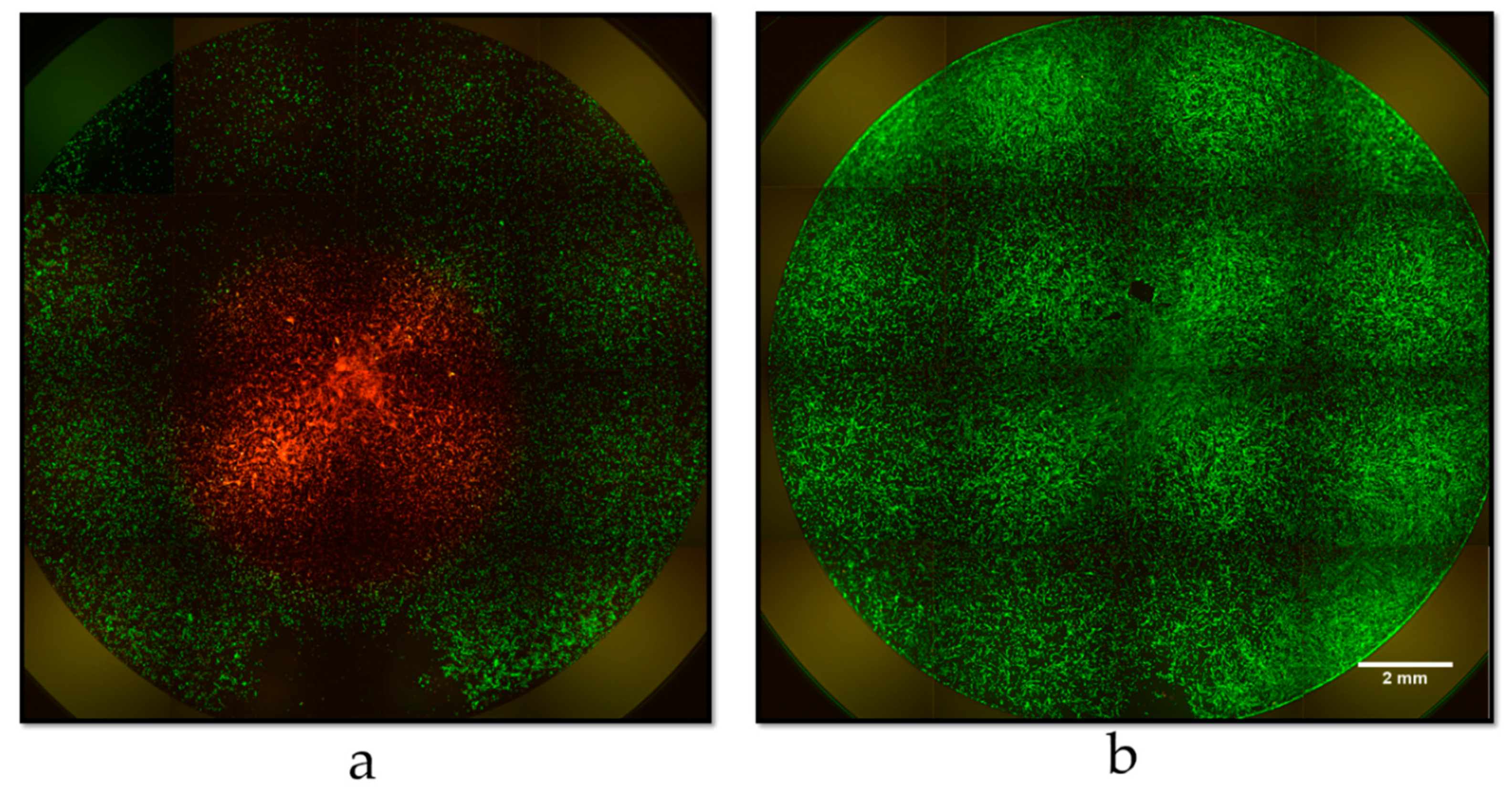
| Sample | Size (nm) 1 | Polydisperisty Index 1 | Zeta potential (mV) |
|---|---|---|---|
| NDs | 255.47 ± 8.19 | 0.36 ± 0.01 | −38.02 |
| NDs@PDA | 228.27 ± 3.76 | 0.24 ± 0.01 | −28.06 |
| NDs@PDA@ICG | 357.57 ± 8.63 | 0.25 ± 0.02 | - |
| Sample | ΔTmax | η |
|---|---|---|
| NDs | 3.9 | 8.7% |
| NDs@PDA | 17.1 | 36.5% |
| NDs@PDA@ICG | 38.7 | 44.5% |
| NDs-BM | 5.4 | 5.4% |
| NDs-AL | 8.7 | 5.7% |
| ICG | 56.6 | 56.6% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maziukiewicz, D.; Grześkowiak, B.F.; Coy, E.; Jurga, S.; Mrówczyński, R. NDs@PDA@ICG Conjugates for Photothermal Therapy of Glioblastoma Multiforme. Biomimetics 2019, 4, 3. https://doi.org/10.3390/biomimetics4010003
Maziukiewicz D, Grześkowiak BF, Coy E, Jurga S, Mrówczyński R. NDs@PDA@ICG Conjugates for Photothermal Therapy of Glioblastoma Multiforme. Biomimetics. 2019; 4(1):3. https://doi.org/10.3390/biomimetics4010003
Chicago/Turabian StyleMaziukiewicz, Damian, Bartosz F. Grześkowiak, Emerson Coy, Stefan Jurga, and Radosław Mrówczyński. 2019. "NDs@PDA@ICG Conjugates for Photothermal Therapy of Glioblastoma Multiforme" Biomimetics 4, no. 1: 3. https://doi.org/10.3390/biomimetics4010003
APA StyleMaziukiewicz, D., Grześkowiak, B. F., Coy, E., Jurga, S., & Mrówczyński, R. (2019). NDs@PDA@ICG Conjugates for Photothermal Therapy of Glioblastoma Multiforme. Biomimetics, 4(1), 3. https://doi.org/10.3390/biomimetics4010003







