Scalable Fabrication of Biomimetic Antibacterial Nanospikes on PMMA Films Using Atmospheric-Pressure Low-Temperature Plasma
Abstract
1. Introduction
2. Materials and Methods
2.1. PMMA Film Preparation
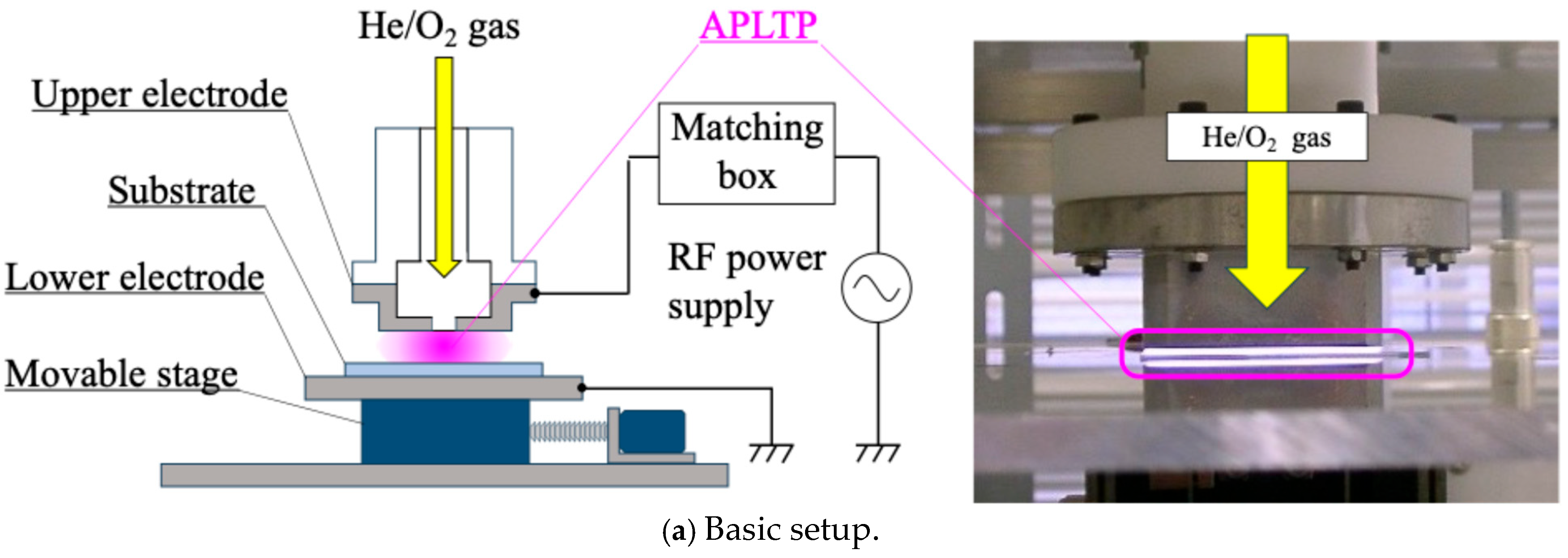
2.2. Plasma Treatment
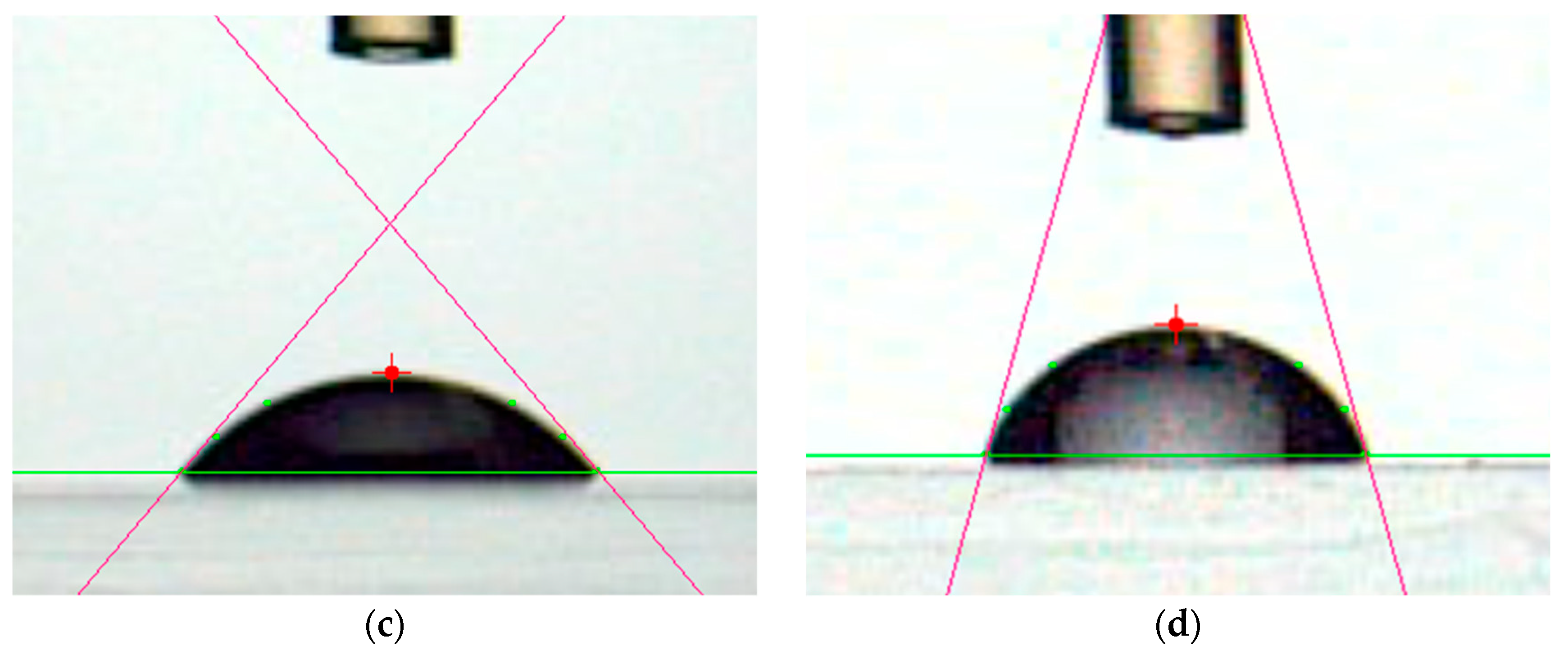
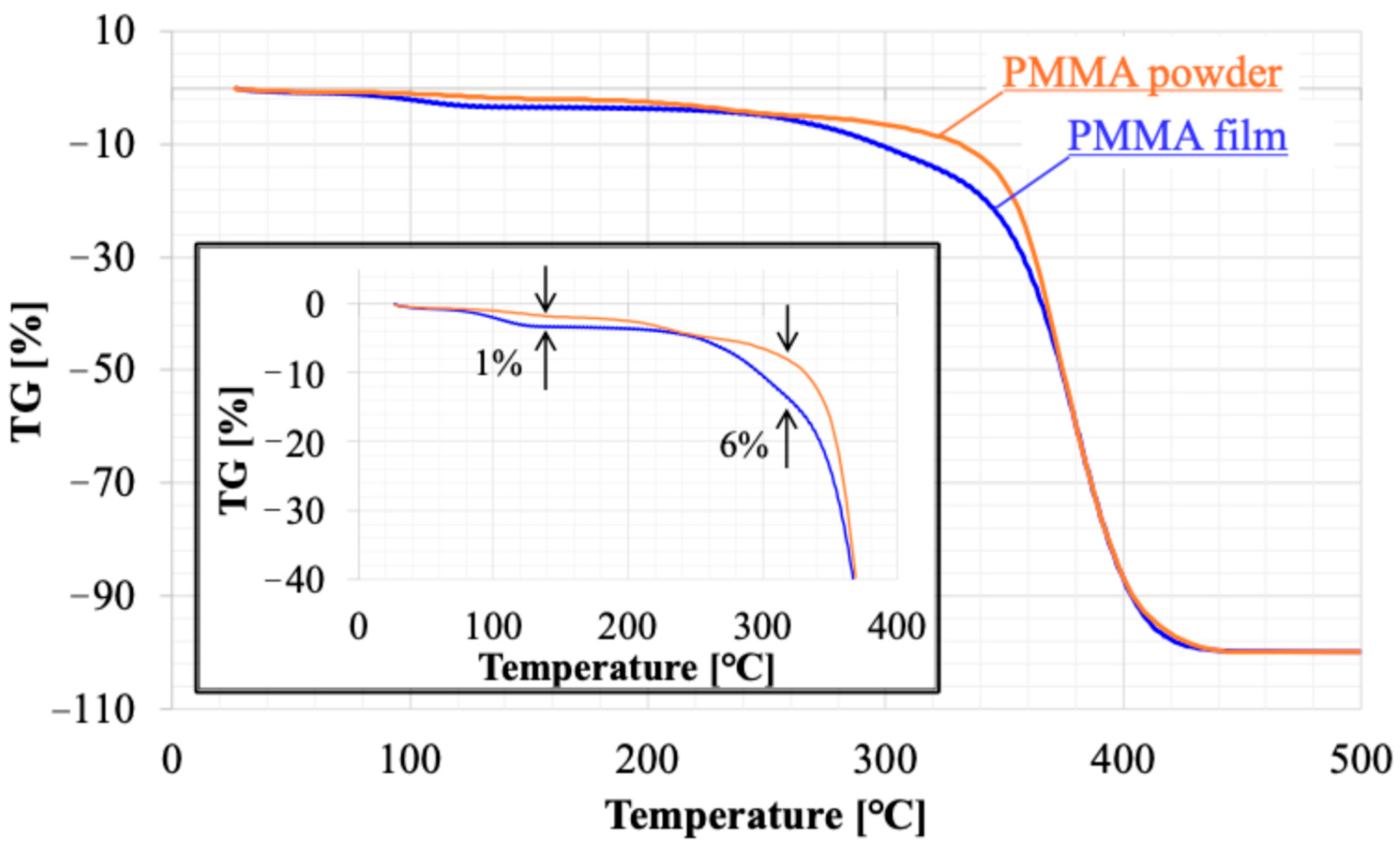
2.3. Evaluation of PMMA Film
3. Results
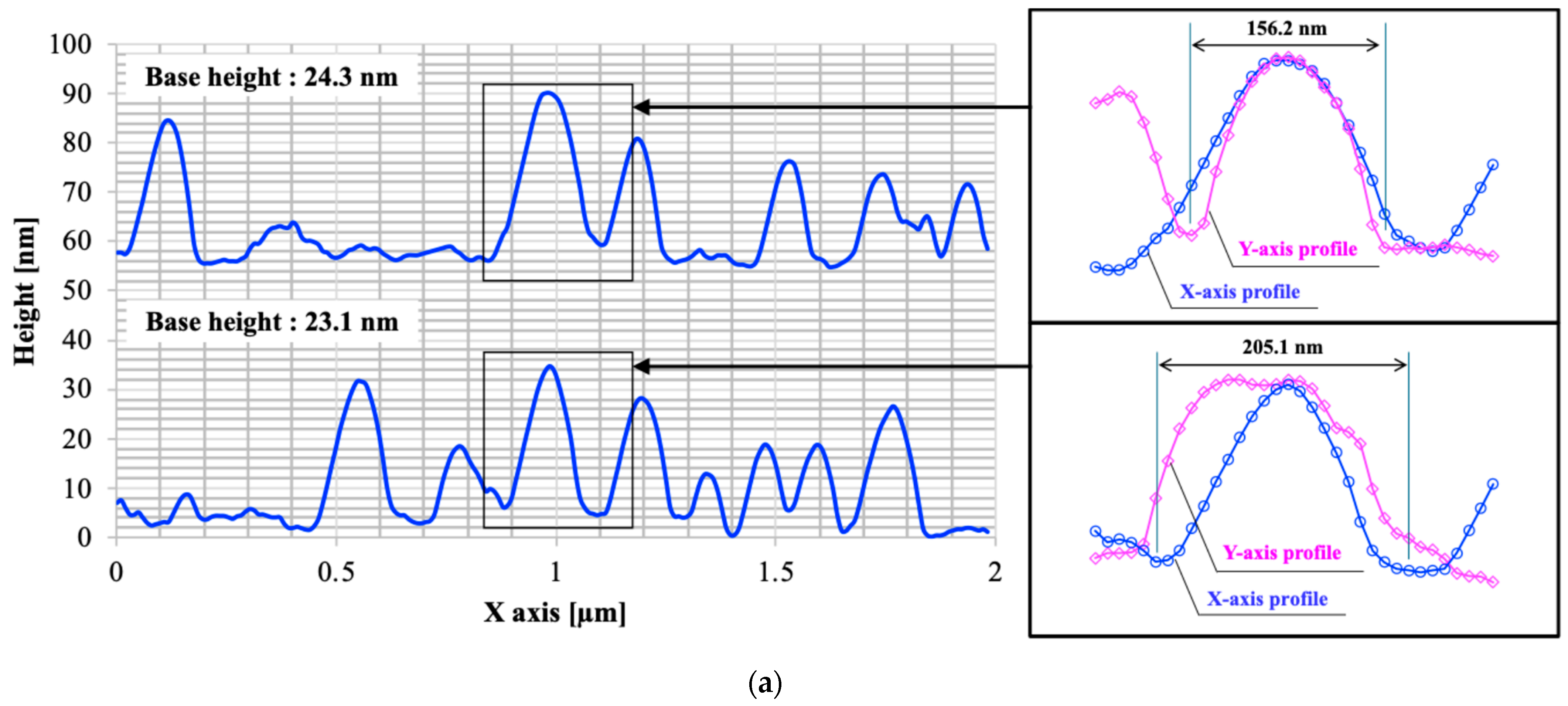
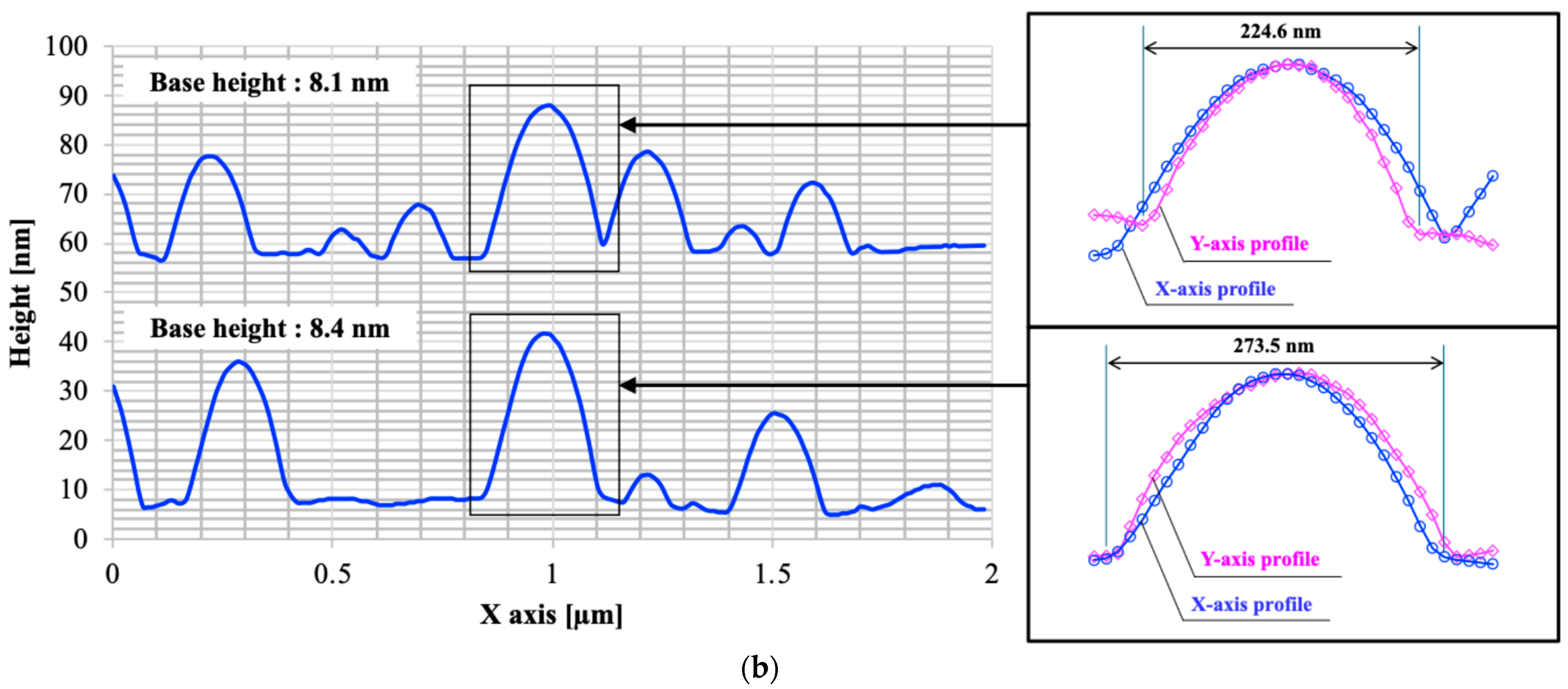
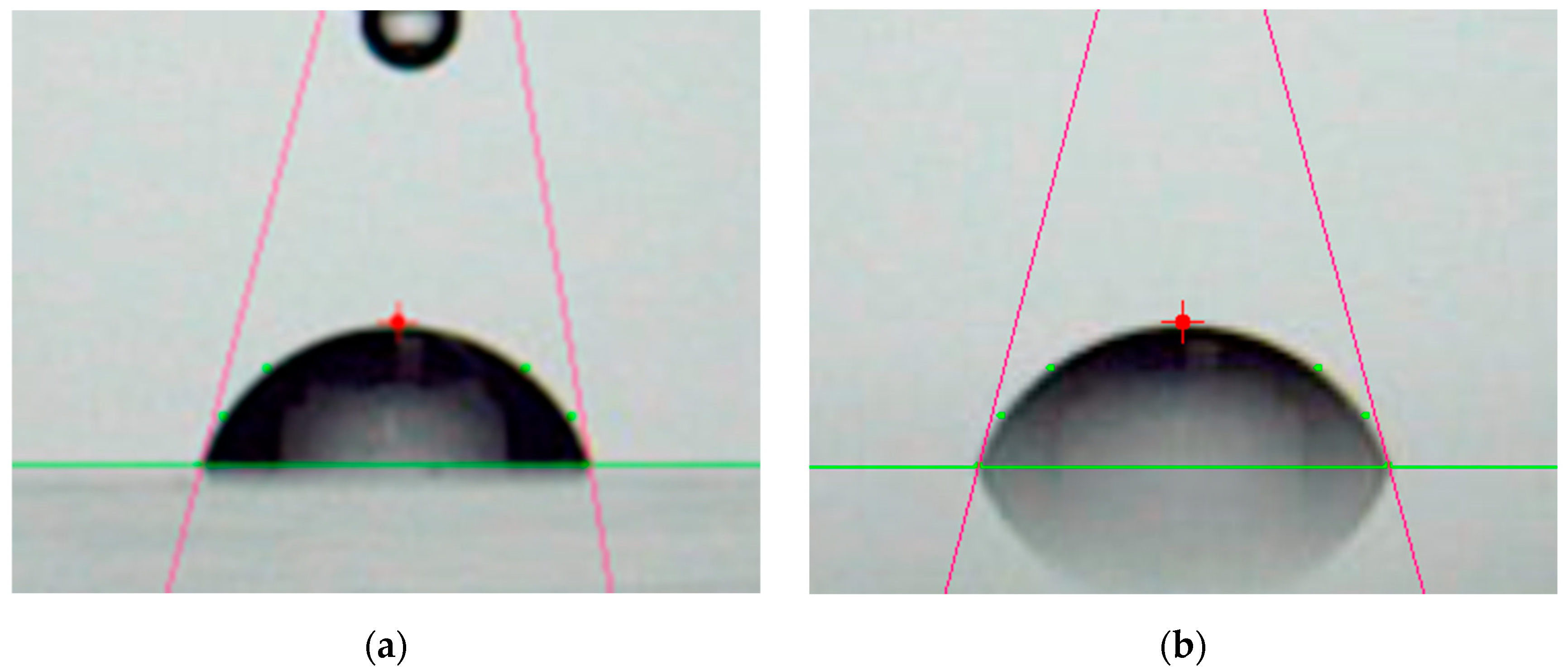
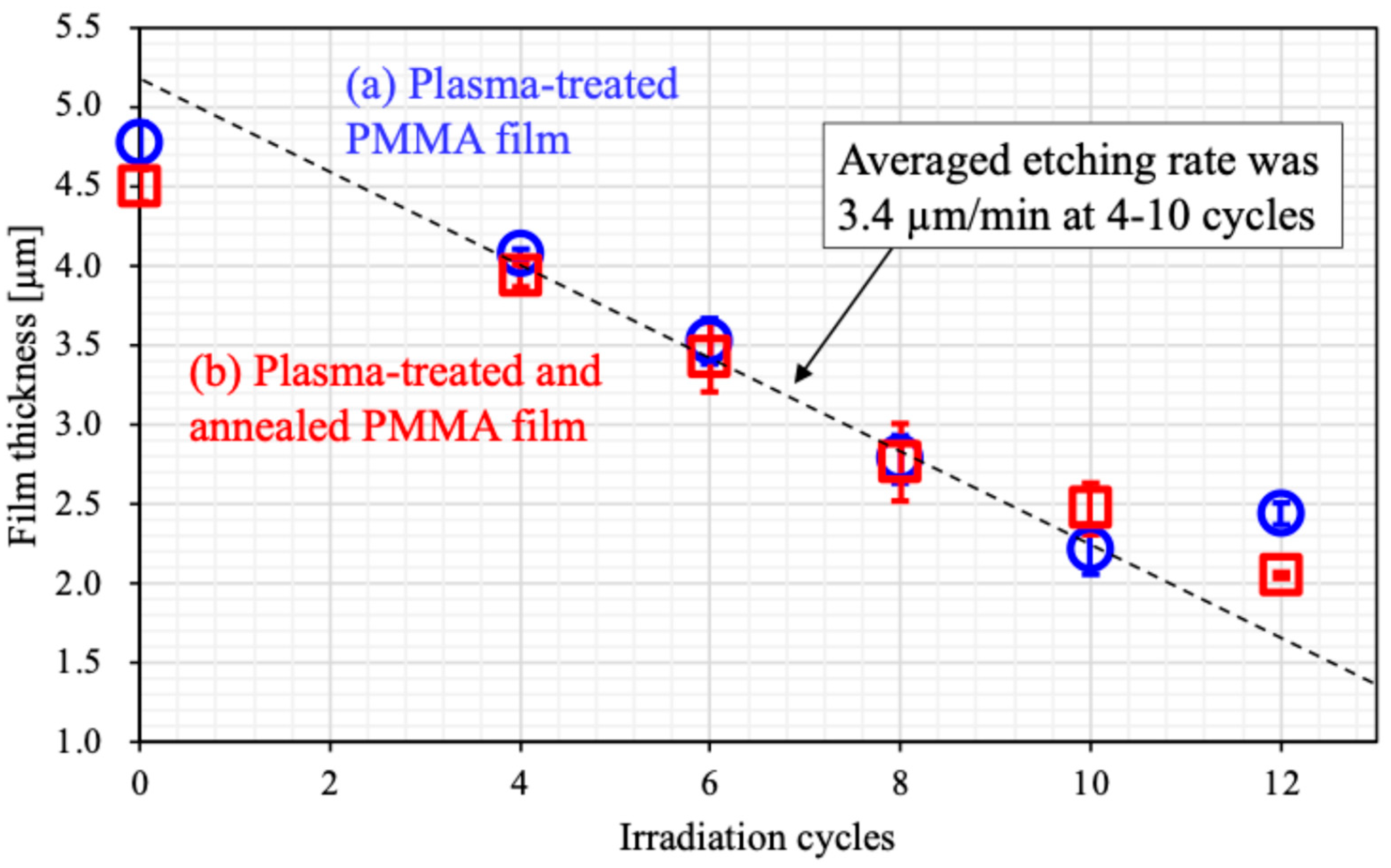
3.1. Surface Morphology
3.2. Wettability
3.3. Antibacterial Test
4. Discussion
4.1. Thermal Shrinking
4.2. Recovery of Wettability
4.3. Antimicrobial Performance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Baulin, V.A.; Pogodin, S.; Wang, J.Y.; Tobin, M.J.; et al. Natural bactericidal surfaces: Mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Nguyen, S.H.; Webb, H.K.; Hasan, J.; Truong, V.K.; Lamb, R.N.; Duan, X.; Tobin, M.J.; Mahon, P.J.; Crawford, R.J. Molecular organization of the nanoscale surface structures of the dragonfly Hemianax papuensis wing epicuticle. PLoS ONE 2013, 8, e67893. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.F.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Nguyen, S.H.; Guo, Y.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Wandiyanto, J.V.; Garvey, C.J.; Mahon, P.J.; Mainwaring, D.E.; et al. Bactericidal activity of self-assembled palmitic and stearic fatty acid crystals on highly ordered pyrolytic graphite. Acta Biomater. 2017, 59, 148–157. [Google Scholar] [CrossRef]
- Román-Kustas, J.; Hoffman, J.B.; Reed, J.H.; Gonsalves, A.E.; Oh, J.; Li, L.; Hong, S.; Jo, K.D.; Dana, C.E.; Miljkovic, N.; et al. Molecular and topographical organization: Influence on cicada wing wettability and bactericidal properties. Adv. Mater. Interfaces 2020, 7, 2000112. [Google Scholar] [CrossRef]
- Pogodin, S.; Hasan, J.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Nguyen, T.H.P.; Boshkovikj, V.; Fluke, C.J.; Watson, G.S.; Watson, J.A.; et al. Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophys. J. 2013, 104, 835. [Google Scholar] [CrossRef]
- Hasan, J.; Webb, H.K.; Truong, V.K.; Pogodin, S.; Baulin, V.A.; Watson, G.S.; Watson, J.A.; Crawford, R.J.; Ivanova, E.P. Selective bactericidal activity of nanopatterned superhydrophobic cicada Psaltoda claripennis wing surfaces. Appl. Microbiol. Biotechnol. 2013, 97, 9257–9262. [Google Scholar] [CrossRef]
- Kelleher, S.M.; Habimana, O.; Lawler, J.; O’Reilly, B.; Daniels, S.; Casey, E.; Cowley, A. Cicada Wing Surface Topography: An investigation into the bactericidal properties of nanostructural features. ACS Appl. Mater. Interfaces 2016, 8, 14966–14974. [Google Scholar] [CrossRef]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and bioinspired nanostructured bactericidal surfaces. Adv. Colloid Interface Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef]
- Bandara, D.; Singh, S.; Afara, I.O.; Wolff, A.; Tesfamichael, T.; Ostrikov, K.; Oloyede, A. Bactericidal effects of natural nanotopography of dragonfly wing on Escherichia coli. ACS Appl. Mater. Interfaces 2017, 9, 6746–6760. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546. [Google Scholar] [CrossRef]
- Oh, J.; Dana, C.E.; Hong, S.; Román, J.K.; Jo, K.D.; Hong, J.W.; Nguyen, J.; Cropek, D.M.; Alleyne, M.; Miljkovic, N. Exploring the role of habitat on the wettability of cicada wings. ACS Appl. Mater. Interfaces 2017, 9, 27173–27184. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.S.; Watson, J.A.; Hu, S.; Brown, C.L.; Cribb, B.W.; Myhra, S. Micro and nanostructures found on insect wings—Designs for minimising adhesion and friction. Int. J. Nanomanuf. 2010, 5, 112–128. [Google Scholar] [CrossRef]
- Watson, G.S.; Myhra, S.; Cribb, B.W.; Watson, J.A. Putative functions and functional efficiency of ordered cuticular nanoarrays on insect wings. Biophys. J. 2008, 94, 3352–3360. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.M.; Watson, J.A.; Cribb, B.W.; Watson, G.S. Fouling of nanostructured insect cuticle: Adhesion of natural and artificial contaminants. Biofouling 2011, 27, 1125–1137. [Google Scholar] [CrossRef]
- Linklater, D.P.; Saita, S.; Murata, T.; Yanagishita, T.; Dekiwadia, C.; Crawford, R.J.; Masuda, H.; Kusaka, H.; Ivanova, E.P. Nanopillar polymer films as antibacterial packaging materials. ACS Appl. Nano Mater. 2022, 5, 2578–2591. [Google Scholar] [CrossRef]
- Wu, S.; Zuber, F.; Maniura-Weber, K.; Brugger, J.; Ren, Q. Nanostructured surface topographies have an effect on bactericidal activity. J. Nanobiotechnol. 2018, 16, 20. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, D.; Yang, Q.; Wang, X.; Dai, B.; Li, X.; Hao, X.; Ding, Y.; Si, J.; Hou, X. Anisotropic wetting on microstrips surface fabricated by femtosecond laser. Langmuir 2011, 27, 359–365. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, W.; Su, B.L. Superhydrophobic surfaces: From natural to biomimetic to functional. J. Colloid Interface Sci. 2011, 353, 335–355. [Google Scholar] [CrossRef]
- Mundo, R.D.; Nardulli, M.; Milella, A.; Favia, P.; d’Agostino, R.; Gristina, R. Cell adhesion on nanotextured slippery superhydrophobic substrates. Langmuir 2011, 27, 4914–4921. [Google Scholar] [CrossRef]
- Truong, V.K.; Webb, H.K.; Fadeeva, E.; Chichkov, B.N.; Wu, A.H.F.; Lamb, R.; Wang, J.Y.; Crawford, R.J.; Ivanova, E.P. Air-directed attachment of coccoid bacteria to the surface of superhydrophobic lotus-like titanium. Biofouling 2012, 28, 539–550. [Google Scholar] [CrossRef]
- Nguyen, S.H.; Webb, H.K.; Hasan, J.; Tobin, M.J.; Crawford, R.J.; Ivanova, E.P. Dual role of outer epicuticular lipids in determining the wettability of dragonfly wings. Colloids Surf. B Biointerfaces 2013, 106, 126–134. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Z.; Zhang, J.; Zhang, G.; Xie, G. Precise replication of antireflective nanostructures from biotemplates. Appl. Phys. Lett. 2007, 90, 123115. [Google Scholar] [CrossRef]
- Ito, S.; Kasuya, M.; Kawasaki, K.; Washiya, R.; Shimazaki, Y.; Miyauchi, A.; Kurihara, K.; Nakagawa, M. Selection of diacrylate monomers for sub-15 nm ultraviolet nanoimprinting by resonance shear measurement. Langmuir 2018, 34, 9366–9375. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Zou, J.; Zdyrko, B.; Iyer, K.S.; Luzinov, I. Capillary force lithography: The versatility of this facile approach in developing nanoscale applications. Nanoscale 2015, 7, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Matschuk, M.; Larsen, N.B. Injection molding of high aspect ratio sub-100 nm nanostructures. J. Micromech. Microeng. 2013, 23, 025003. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Jafari, R.; Momen, G.; Farzaneh, M. Micro-nanostructured polymer surfaces using injection molding: A review. Mater. Today Commun. 2017, 13, 126–143. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Wang, Z.L. Controlled replication of butterfly wings for achieving tunable photonic properties. Nano Lett. 2006, 6, 2325–2331. [Google Scholar] [CrossRef]
- Seol, M.L.; Woo, J.H.; Lee, D.I.; Im, H.; Hur, J.; Choi, Y.K. Nature-replicated nano-in-micro structures for triboelectric energy harvesting. Small 2014, 10, 3887–3894. [Google Scholar] [CrossRef]
- Zada, W.; Zhang, W.; Zheng, W.; Zhu, Y.; Zhang, Z.; Zhang, J.; Imtiaz, M.; Abbas, W.; Zhang, D. Ror2 signaling regulates Golgi structure and transport through IFT20 for tumor invasiveness. Sci. Rep. 2017, 7, 1. [Google Scholar] [CrossRef]
- Weng, F.; Wang, M.; Zhou, D.; Yang, B.; Jiang, B. Fabrication of hierarchical polymer surfaces with superhydrophobicity by injection molding from nature and function-oriented design. Appl. Surf. Sci. 2018, 436, 224–233. [Google Scholar] [CrossRef]
- Weng, J.; Yang, J.; Wang, F.; Ding, T.; Zhai, Z. Thermodynamic analysis and injection molding of hierarchical superhydrophobic polypropylene surfaces. J. Polym. Eng. 2020, 40, 86–97. [Google Scholar] [CrossRef]
- Chen, J.-K.; Qui, J.-Q.; Fan, S.-K.; Kuo, S.-W.; Ko, F.-H.; Chu, C.-W.; Chang, F.-C. Using colloid lithography to fabricate silicon nanopillar arrays on silicon substrates. J. Colloid Interface Sci. 2012, 367, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Du, K.; Wathuthanthri, I.; Choi, C.-H.; Fisher, F.T.; Yang, E.-H. Transfer patterning of large-area graphene nanomesh via holographic lithography and plasma etching. J. Vac. Sci. Technol. B 2014, 32, 06FF01. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Xie, G.; Liu, Z.; Shao, H. Cicada wings: A stamp from nature for nanoimprint lithography. Small 2006, 2, 1440–1443. [Google Scholar] [CrossRef]
- Hong, S.-H.; Hwang, J.; Lee, H. Replication of cicada wing’s nano-patterns by hot embossing and UV nanoimprinting. Nanotechnology. Nanotechnology 2009, 20, 385303. [Google Scholar] [CrossRef]
- Yasuda, H.; Gazicki, M. Biomedical applications of plasma polymerization and plasma treatment of polymer surfaces. Biomaterials 1982, 3, 68–77. [Google Scholar] [CrossRef]
- Poncin-Epaillard, F.; Chevet, B.; Brosse, J.-C. Plasma-induced surface grafting on polymer films. Eur. Polym. J. 1990, 26, 333. [Google Scholar] [CrossRef]
- Olde Riekerink, M.B.; Terlingen, J.G.A.; Engbers, G.H.M.; Feijen, J. Selective etching of semicrystalline polymers: CF4 gas Plasma Treatment of Poly(ethylene). Langmuir 1999, 15, 4847–4856. [Google Scholar] [CrossRef]
- Wang, B.; An, W.; Wang, L.; Jiao, L.; Zhang, H.; Song, H.; Liu, S. Superhydrophobic and antibacterial hierarchical surface fabricated by femtosecond laser. Sustainability 2022, 14, 12412. [Google Scholar] [CrossRef]
- Hawi, S.; Goel, S.; Kumar, V.; Giusca, C.; Pearce, O.; Nishio, W. Precision laser manufacturing and metrology of nature-inspired bioactive surfaces for antibacterial medical implants. Surf. Interfaces 2025, 62, 106267. [Google Scholar] [CrossRef]
- Halappa, C.; Park, S.-J. Pattern formation using polystyrene benzaldimine self-assembled monolayer by soft X-ray. Surf. Interface Anal. 2018, 51, 408–412. [Google Scholar] [CrossRef]
- Liu, H.; Wang, B.; Ke, L.; Deng, J.; Chum, C.C.; Teo, S.L.; Shen, L.; Maier, S.A.; Teng, J. High aspect subdiffraction-limit photolithography via a silver superlens. Nano Lett. 2012, 12, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Satyanarayana, V.S.V.; Singh, V.; Kalyani, V.; Parameswaran, P.C.; Sharma, S.; Ghosh, S.; Gonsalves, K.E. A hybrid polymeric material bearing a ferrocene-based pendant organometallic functionality: Synthesis and applications in nanopatterning using EUV lithography. RSC Adv. 2014, 4, 59817–59820. [Google Scholar] [CrossRef]
- Yang, X.; Xiao, S.; Hu, W.; Hwu, J.; Van De Veerdonk, R.; Wago, K.; Lee, K.; Kuo, D. Integration of nanoimprint lithography with block copolymer directed self-assembly for fabrication of a sub-20 nm template for bit-patterned media. Nanotechnology 2014, 25, 395301. [Google Scholar] [CrossRef]
- Kim, J.K.; Cho, H.S.; Jung, H.S.; Lim, K.; Kim, K.B.; Choi, D.G.; Jeong, J.H.; Suh, K.Y. P Effect of surface tension and coefficient of thermal expansion in 30 nm scale nanoimprinting with two flexible polymer molds. Nanotechnology 2012, 23, 235303. [Google Scholar] [CrossRef]
- Krauss, P.R.; Chou, S.Y. Sub-10 nm imprint lithography and applications. J. Vac. Sci. Technol. B 1997, 15, 2897–2904. [Google Scholar]
- von Keudell, A.; Corbella, C. Review Article: Unraveling synergistic effects in plasma-surface processes by means of beam experiments. J. Vac. Sci. Technol. A 2017, 35, 050801. [Google Scholar] [CrossRef]
- Borghi, F.; Rider, A.E.; Kumar, S.; Han, Z.J.; Haylock, D.; Ostrikov, K. Emerging stem cell controls: Nanomaterials and plasma effects. J. Nanomater. 2013, 2013, 329139. [Google Scholar] [CrossRef]
- Xu, S.; Levchenko, I.; Huang, S.Y.; Ostrikov, K. Self-organized vertically aligned single-crystal silicon nanostructures with controlled shape and aspect ratio by reactive plasma etching. Appl. Phys. Lett. 2009, 95, 111505. [Google Scholar] [CrossRef]
- Mariotti, D.; Ostrikov, K. Tailoring microplasma nanofabrication: From nanostructures to nanoarchitectures. J. Phys. D Appl. Phys. 2009, 42, 092002. [Google Scholar] [CrossRef]
- Mariotti, D.; Bose, A.C.; Ostrikov, K. Atmospheric-microplasma-assisted nanofabrication: Metaland metal-oxide nanostructures and nanoarchitectures. IEEE Trans. Plasma Sci. 2009, 37, 1027–1033. [Google Scholar] [CrossRef]
- Yamamoto, M.; Mori, Y.; Kumagai, T.; Sekiguchi, A.; Minami, H.; Horibe, H. Microstructure formation on poly (methyl methacrylate) film using atmospheric pressure low-temperature plasma. J. Photopolym. Sci. Technol. 2021, 34, 385–392. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takada, A.; Fujii, N.; Sekiguchi, A.; Horibe, H. Formation of double roughness structure on PVDF/PMMA blended film using atmospheric pressure low-temperature plasma. J. Photopolym. Sci. Technol. 2024, 37, 355–362. [Google Scholar] [CrossRef]
- Sekiguchi, A.; Yamamoto, M.; Kumagai, T.; Mori, Y.; Minami, H.; Aikawa, M.; Horibe, H. Development of Bile direct stent having antifouling properties by atmospheric pressure low-temperature plasma. J. Photopolym. Sci. Technol. 2021, 34, 401–410. [Google Scholar] [CrossRef]
- Yamamoto, M.; Hamasaki, T.; Sekiguchi, A.; Minami, H.; Aikawa, M.; Horibe, H. Development of bile duct stent with antifouling property using atmospheric pressure and low temperature plasma. J. Photopolym. Sci. Technol. 2022, 35, 233–239. [Google Scholar] [CrossRef]
- Sekiguchi, A.; Yamamoto, M.; Aikawa, M.; Yasuda, M.; Hirai, Y. The development of bile duct stent having antifouling properties by using atmosphere pressure cold plasma(2). J. Photopolym. Sci. Technol. 2024, 37, 371–378. [Google Scholar] [CrossRef]
- Reed, J.H.; Gonsalves, A.E.; Román, J.K.; Oh, J.; Cha, H.; Dana, C.E.; Toc, M.; Hong, S.; Hoffman, J.B.; Andrade, J.E.; et al. Ultrascalable multifunctional nanoengineered copper and aluminum for antiadhesion and bactericidal applications. ACS Appl. Bio Mater. 2019, 2, 2726–2737. [Google Scholar] [CrossRef]
- Horibe, H.; Taniyama, M. Poly(vinylidene fluoride) crystal structure of poly(vinylidene fluoride) and poly(methyl methacrylate) blend after annealing. J. Electrochem. Soc. 2006, 153, G119–G124. [Google Scholar] [CrossRef]
- Oshiro, H.; Kono, A.; Danno, T.; Horibe, H. Crystal structure control of poly(vinylidene fluoride) (PVDF) in the blend films of PVDF and poly(methyl methacrylate) (PMMA) prepared by solvent casting. Kobunshi Ronbunshu 2012, 69, 135–141. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Gotoh, K.; Kobayashi, Y.; Yasukawa, A.; Ishigami, Y. Surface modification of PET films by atmospheric pressure plasma exposure with three reactive gas sources. Colloid Polym. Sci. 2012, 290, 1005–1014. [Google Scholar] [CrossRef]
- Her, K.; Chung, H.S.; Moon, M.W.; Oh, K.H. An angled nano-tunnel fabricated on poly(methyl methacrylate) by a focused ion beam. Nanotechnology 2009, 20, 285301. [Google Scholar] [CrossRef]
- Mayama, H. Secret of lotus leaf: Importance of double roughness structures for super water-repellency. J. Photopolym. Sci. Technol. 2018, 31, 705–710. [Google Scholar] [CrossRef]
- Roach, P.; Shirtcliffe, N.J.; Newton, M.I. Progess in superhydrophobic surface development. Soft Matter 2008, 4, 224–240. [Google Scholar] [CrossRef]
- Bonn, D.; Eggers, J.; Indekeu, J.; Meunier, J.; Rolley, E. Wetting and spreading. Rev. Mod. Phys. 2009, 81, 739–805. [Google Scholar] [CrossRef]
- Singh, N.S.; Zhang, J.; Stafford, J.; Anthony, C.; Gao, N. Implementing superhydrophobic surfaces within various condensation environments: A review. Adv. Mater. Interfaces 2021, 8, 2001442. [Google Scholar] [CrossRef]
- Pellegrino, P.; Farella, I.; Matteis, V.D.; Cascione, M.; Calcagnile, M.; Villani, S.; Vincenti, L.; Alifano, P.; Quaranta, F.; Rinaldi, R. Morphological and mechanical variations in E. coli induced by PMMA nanostructures patterned via electron beam lithography: An atomic force microscopy study. Surf. Interfaces 2025, 56, 105707. [Google Scholar]
- Hawi, S.; Goel, S.; Kumar, V.; Pearce, O.; Ayre, W.N.; Ivanova, E.P. Critical review of nanopillar-based mechanobactericidal systems. ACS Appl. Nano Mater. 2022, 5, 1–17. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Linklater, D.P.; Werner, M.; Baulin, V.A.; Xu, X.; Vrancken, N.; Rubanov, S.; Hanssen, E.; Wandiyanto, J.; Truong, V.K.; et al. The multi-faceted mechano-bactericidal mechanism of nanostructured surfaces. Proc. Natl. Acad. Sci. USA 2020, 117, 12598–12605. [Google Scholar] [CrossRef]
- Jindai, K.; Nakade, K.; Masuda, K.; Sagawa, T.; Kojima, H.; Shimizu, T.; Shingubara, S.; Ito, T. Adhesion and bactericidal properties of nanostructured surfaces dependent on bacterial motility. RSC Adv. 2020, 10, 5673–5680. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, M.; Tada, K.; Takada, A.; Sekiguchi, A. Scalable Fabrication of Biomimetic Antibacterial Nanospikes on PMMA Films Using Atmospheric-Pressure Low-Temperature Plasma. Biomimetics 2025, 10, 601. https://doi.org/10.3390/biomimetics10090601
Yamamoto M, Tada K, Takada A, Sekiguchi A. Scalable Fabrication of Biomimetic Antibacterial Nanospikes on PMMA Films Using Atmospheric-Pressure Low-Temperature Plasma. Biomimetics. 2025; 10(9):601. https://doi.org/10.3390/biomimetics10090601
Chicago/Turabian StyleYamamoto, Masashi, Kentaro Tada, Ayumu Takada, and Atsushi Sekiguchi. 2025. "Scalable Fabrication of Biomimetic Antibacterial Nanospikes on PMMA Films Using Atmospheric-Pressure Low-Temperature Plasma" Biomimetics 10, no. 9: 601. https://doi.org/10.3390/biomimetics10090601
APA StyleYamamoto, M., Tada, K., Takada, A., & Sekiguchi, A. (2025). Scalable Fabrication of Biomimetic Antibacterial Nanospikes on PMMA Films Using Atmospheric-Pressure Low-Temperature Plasma. Biomimetics, 10(9), 601. https://doi.org/10.3390/biomimetics10090601




