Suture Materials: Conventional and Stimulatory-Responsive Absorbable Polymers with Biomimetic Function
Abstract
1. Introduction
2. Understanding the Relationship Between the Properties of Suture Materials and Their Performance
2.1. Mechanics
- ♦
- Tensile Strength
- ♦
- Tissue Assimilation
- ♦
- Friction force
- ♦
- Cross-sectional size
- ♦
- Elasticity
- ♦
- Knot Security and strength
- ♦
- Memory
- ♦
- Plasticity
2.2. Histology
- ♦
- Tissue Reactivity
2.3. Specific Properties of Suture Materials
- ♦
- Origin and Structural Design
- ♦
- Capillarity and surface adhesion due to intermolecular forces are critical components
- ♦
- Fluid Uptake
3. Sutures: An Overview
Resorbable Suture Materials
- ♦
- Polydioxanone
- ♦
- Polyglactin 910 (coated Vicryl, Vicryl Rapide, and coated Vicryl Plus)
- ♦
- Poliglecaprone 25 (Monocryl)
- ♦
- Polyglycolic acid (Dexon, Dexon II)
- ♦
- Polyglycolide–trimethylene carbosnate (Maxon)
- ♦
- Caprosyn
- ♦
- Polyhydroxyalkanoates (PHAs)
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, R.H.; Bell, R.J.; Dowling, B.A.; Dart, A.J. Suture materials: Composition and applications in veternary wound repair. Aust. Vet. J. 2003, 81, 140–145. [Google Scholar] [CrossRef]
- Khalid, G.M.; Billa, N. Drug-Eluting Sutures by Hot-Melt Extrusion: Current Trends and Future Potentials. Materials 2023, 16, 7245. [Google Scholar] [CrossRef]
- Sriyai, M.; Tasati, J.; Molloy, R.; Meepowpan, P.; Somsunan, R.; Worajittiphon, P.; Daranarong, D.; Meerak, J.; Punyodom, W. Development of an Antimicrobial-Coated Absorbable Monofilament Suture from a Medical-Grade Poly(l-lactide-co-ε-caprolactone) Copolymer. ACS Omega 2021, 6, 28788–28803. [Google Scholar] [CrossRef] [PubMed]
- Jummaat, F.; Yahya, E.B.; Khalil, H.P.S.A.; Adnan, A.S.; Alqadhi, A.M.; Abdullah, C.K.; A.K., A.S.; Olaiya, N.G.; Abdat, M. The Role of Biopolymer-Based Materials in Obstetrics and Gynecology Applications: A Review. Polymers 2021, 13, 633. [Google Scholar] [CrossRef]
- Tajirian, A.L.; Goldberg, D.J. A review of sutures and other skin closure materials. J. Cosmet. Laser Ther. 2010, 12, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.C.; Bachmann, E.; Lädermann, A.; Lajtai, G.; Jentzsch, T. The best knot and suture configurations for high-strength suture material. An in vitro biomechanical study. Orthop. Traumatol. Surg Res. 2018, 104, 1277–1282. [Google Scholar] [CrossRef]
- Chen, X.; Hou, D.; Tang, X.; Wang, L. Quantitative physical and handling characteristics of novel antibacterial braided silk suture materials. J. Mech. Behav. Biomed. Mater. 2015, 50, 160–170. [Google Scholar] [CrossRef]
- Levy, T.; Lerman, I.; Waibel, J.; Gauglitz, G.G.; Clementoni, M.T.; Friedmann, D.P.; Duplechain, K.; Peng, P.; Lim, D.; Al-Niaimi, F.; et al. Expert Consensus on Clinical Recommendations for Fractional Ablative CO2 Laser, in Facial Skin Rejuvenation Treatment. Lasers Surg. Med. 2025, 57, 15–26. [Google Scholar] [CrossRef]
- Zitelli, J.A.; Moy, R.L. Buried vertical mattress suture. J. Dermatol. Surg. Oncol. 1989, 15, 17–19. [Google Scholar] [CrossRef]
- Moy, R.L.; Lee, A.; Zalka, A. Commonly used suture materials in skin surgery. Am. Fam. Physician 1991, 44, 2123–2128. [Google Scholar]
- Viinikainen, A.; Göransson, H.; Huovinen, K.; Kellomäki, M.; Törmälä, P.; Rokkanen, P. Material and knot properties of braided polyester (Ticron) and bioabsorbable poly-L/D-lactide (PLDLA) 96/4 sutures. J. Mater. Sci. Mater. Med. 2006, 17, 169–177. [Google Scholar] [CrossRef]
- Tera, H.; Aberg, C. Tensile strengths of twelve types of knot employed in surgery, using different suture materials. Acta Chir. Scand. 1976, 142, 1–7. [Google Scholar]
- Edlich, R.F.; Becker, D.G.; Thacker, J.G.; Rodeheaver, G.T. Scientific basis for selecting staple and tape skin closures. Clin. Plast. Surg. 1990, 17, 571–578. [Google Scholar] [CrossRef]
- Bennett, R.G. Selection of wound closure materials. J. Am. Acad. Dermatol. 1988, 18 Pt 1, 619–637. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.P., III; Siegle, R.J.; Abbott, C.; Bennett, R.G. Surgical pearl: The self-removable suture. J. Am. Acad. Dermatol. 1997, 36 Pt 1, 1009–1010. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.G. The meaning and significance of tissue margins. Adv. Dermatol. 1989, 4, 343–355; discussion 356–357. [Google Scholar] [PubMed]
- Rodeheaver, G.T.; Thacker, J.G.; Edlich, R.F. Mechanical performance of polyglycolic acid and polyglactin 910 synthetic absorbable sutures. Surg. Gynecol. Obstet. 1981, 153, 835–841. [Google Scholar]
- Rodeheaver, G.T.; Powell, T.A.; Thacker, J.G.; Edlich, R.F. Mechanical performance of monofilament synthetic absorbable sutures. Am. J. Surg. 1987, 154, 544–547. [Google Scholar] [CrossRef]
- Outlaw, K.K.; Vela, A.R.; O’Leary, J.P. Breaking strength and diameter of absorbable sutures after in vivo exposure in the rat. Am. Surg. 1998, 64, 348–354. [Google Scholar]
- Hochberg, J.; Meyer, K.M.; Marion, M.D. Suture choice and other methods of skin closure. Surg. Clin. N. Am. 2009, 89, 627–641. [Google Scholar] [CrossRef]
- Hochberg, J.; Murray, G.F. Textbook of Surgery, 15th ed.; Sabiston, D.C., Ed.; Saunders: Philadelphia, PA, USA, 1992; pp. 253–263. [Google Scholar]
- Iavazzo, C.; Gkegkes, I.D.; Vouloumanou, E.K.; Mamais, I.; Peppas, G.; Falagas, M.E. Sutures versus staples for the management of surgical wounds: A meta-analysis of randomized controlled trials. Am. Surg. 2011, 77, 1206–1221. [Google Scholar] [CrossRef]
- Zeplin, P.H.; Schmidt, K.; Laske, M.; Ziegler, U.E. Comparison of various methods and materials for treatment of skin laceration by a 3-dimensional measuring technique in a pig experiment. Ann. Plast. Surg. 2007, 58, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, C.T. Advances in Dermatology; James, W.D., Ed.; Mosby: St. Louis, MO, USA, 2003; Volume 19, pp. 313–318. [Google Scholar]
- Ammirati, C.T. Advances in wound closure materials. Adv. Dermatol. 2002, 18, 313–338. [Google Scholar]
- Zhu, X.; Hall, D.; Ridenour, G.; Boo, S.; Jennings, T.; Hochberg, J.; Cilento, E.; Reilly, F.D. A mouse model for studying rapid intraoperative methods of skin closure and wound healing. Med. Sci. Monit. 2003, 9, BR109–BR115. [Google Scholar] [PubMed]
- Aukerman, D.F.; Sebastianelli, W.J.; Nashelsky, J. Clinical inquiries. How does tissue adhesive compare with suturing for superficial lacerations? J. Fam. Pract. 2005, 54, 378. [Google Scholar]
- Tritle, N.M.; Haller, J.R.; Gray, S.D. Aesthetic comparison of wound closure techniques in a porcine model. Laryngoscope 2001, 111 Pt 1, 1949–1951. [Google Scholar] [CrossRef]
- Kawecki, F. The potential of cell-assembled extracellular matrix for biological sutures: A promising innovation. Sci. Prog. 2023, 106, 368504231219180. [Google Scholar] [CrossRef]
- Tandon, S.; Ensor, N.D.; Pacilli, M.; Laird, A.J.; Bortagaray, J.I.; Stunden, R.J.; Nataraja, R.M. Tissue adhesive, adhesive tape, and sutures for skin closure of paediatric surgical wounds: Prospective randomized clinical trial. Br. J. Surg. 2022, 109, 1087–1095. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Castilho, H.T.; Hochberg, J.; Ardenghy, M.; Toledo, S.R.; Cruz, R.G.; Tardelli, H. Triangular mattress suture in abdominal diastasis to prevent epigastric bulging. Ann. Plast. Surg. 2001, 46, 130–134. [Google Scholar] [CrossRef]
- Graeber, G.M.; Collins, J.J., Jr.; DeShong, J.L.; Murray, G.F. Are sutures better than staples for closing bronchi and pulmonary vessels? Ann. Thorac. Surg. 1991, 51, 901–904; discussion 904–905. [Google Scholar] [CrossRef]
- Ge, M.; Zheng, W.; Yao, P.; Gao, L.; Ge, X.; Zhang, Q.; Wang, X.; Guo, C. Progress in tension-relieving suturing surgery: Revolutionary surgical techniques and patient prognosis evaluation methods. Front. Surg. 2025, 12, 1587582. [Google Scholar]
- Trimbos, J.B.; Brohim, R.; van Rijssel, E.J. Factors relating to the volume of surgical knots. Int. J. Gynaecol. Obstet. 1989, 30, 355–359. [Google Scholar] [CrossRef]
- van Rijssel, E.J.; Brand, R.; Admiraal, C.; Smit, I.; Trimbos, J.B. Tissue reaction and surgical knots: The effect of suture size, knot configuration, and knot volume. Obstet. Gynecol. 1989, 74, 64–68. [Google Scholar] [PubMed]
- Trimbos, J.B.; Van Rijssel, E.J.; Klopper, P.J. Performance of sliding knots in monofilament and multifilament suture material. Obstet. Gynecol. 1986, 68, 425–430. [Google Scholar] [CrossRef]
- Avoine, X.; Lussier, B.; Brailovski, V.; Inaekyan, K.; Beauchamp, G. Evaluation of the effect of 4 types of knots on the mechanical properties of 4 types of suture material used in small animal practice. Can. J. Vet. Res. 2016, 80, 162–170. [Google Scholar]
- Silver, E.; Wu, R.; Grady, J.; Song, L. Knot Security—How is it Affected by Suture Technique, Material, Size, and Number of Throws? J. Oral. Maxillofac. Surg. 2016, 74, 1304–1312. [Google Scholar] [CrossRef]
- Byrne, M.; Aly, A. The Surgical Suture. Aesthet. Surg. J. 2019, 39 (Suppl. 2), S67–S72. [Google Scholar] [CrossRef]
- Bariol, S.V.; Stewart, G.D.; Tolley, D.A. Laparoscopic suturing: Effect of instrument handling on suture strength. J. Endourol. 2005, 19, 1127–1133. [Google Scholar] [CrossRef]
- Alves, R.S.; Tanaka, L.Y.; Carvalho, V.B.; Lopes, L.C.; Santos, S.B.D.; Dsouki, N.A.; Pereira, B.F.; Sato, M.A. Comparative Evaluation of Skin Suture in Rats with Polyglycaprone 25 and Nylon. Acta Ortop. Bras. 2023, 31, e266635. [Google Scholar] [CrossRef]
- Petillo, A.; Di Rosa, A.; Burgio, C.; Di Leonardo, S.; Burriesci, G.; Bosco, F.; Lucenti, L.; Camarda, L. Double-loop suture repair of radial meniscal tears provides favourable biomechanical performance compared to conventional repair techniques: A biomechanical study. J. Exp. Orthop. 2025, 12, e70366. [Google Scholar] [CrossRef]
- Dalos, D.; Grisolia Seifert, E.; Fiedler, I.; Frerichs, M.; Busse, B.; Dust, T.; Klatte, T.O. Effects of different cortical all-suture anchor (Y-Knot RC) insertion points and deep vs. flat insertion on its biomechanical properties. J. Shoulder Elb. Surg. 2025, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.P.; Noronha, J.C.; Completo, A. Biomechanical study of the Nice knot as an alternative fixation for anterior cruciate ligament reconstruction. J. Orthop. Sci. 2025, S0949-2658(25)00131-9. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, V.G.J.; Ammo, T.; Leypold, S.; Praster, M.; Jonigk, D.; Beier, J.P.; Leypold, T. Comparison of Biomechanical and Histopathological Properties of Robot-Assisted Anastomoses Using the Symani Surgical System versus Conventional Anastomoses in a Preclinical Microsurgical Model. J. Reconstr. Microsurg. 2025. [Google Scholar] [CrossRef]
- Greenberg, J.A.; Clark, R.M. Advances in suture material for obstetric and gynecologic surgery. Rev. Obstet. Gynecol. 2009, 2, 146–158. [Google Scholar]
- Greenberg, J.A. The use of barbed sutures in obstetrics and gynecology. Rev. Obstet. Gynecol. 2010, 3, 82–91. [Google Scholar]
- Shah, M.R.; Strauss, E.J.; Kaplan, K.; Jazrawi, L.; Rosen, J. Initial loop and knot security of arthroscopic knots using high-strength sutures. Arthroscopy 2007, 23, 884–888. [Google Scholar] [CrossRef]
- Stott, P.M.; Ripley, L.G.; Lavelle, M.A. The ultimate Aberdeen knot. Ann. R. Coll. Surg. Engl. 2007, 89, 713–717. [Google Scholar] [CrossRef]
- Mellado Sanz, M.; Alba Moratilla, C.; Martínez Delgado, M.; Gerónimo Pardo, M.; Painceira-Villar, R.; Trevissón-Redondo, B. Evaluation of the Biophysical Signs of Healing in the Protocolized Use of the High Capillarity Dressing: A Pilot Study. Int. Wound J. 2025, 22, e70332. [Google Scholar] [CrossRef]
- Meloni, M.; Colboc, H.; Armstrong, D.G.; Dissemond, J.; Rayman, G.; Lázaro-Martínez, J.L.; Rial, R.; Hartemann, A.; Atkin, L.; Swanson, T.; et al. TLC-NOSF dressings as a first-line local treatment of chronic wounds: A systematic review of clinical evidence. J. Wound Care 2024, 33, 756–770. [Google Scholar] [CrossRef]
- Nair, H.; Venkateshwaran, N.; Seetharaman S, S.; Deng, W.; Uthaipaisanwong, A.; Galea, E. Benefits of sucrose octasulfate (TLC-NOSF) dressings in the treatment of chronic wounds: A systematic review. J. Wound Care 2021, 30 (Suppl. 4), S42–S52. [Google Scholar] [CrossRef]
- Rousseau, T.; Plomion, C.; Sandy-Hodgetts, K. An advanced transparent hydropolymer wound dressing for undisturbed post-op management of surgical wounds following hip and knee replacement: A prospective observational series. Int. Wound J. 2022, 19, 1456–1462. [Google Scholar] [CrossRef]
- Neligan, P.C.; Plastic Surgery Educational Foundation Technology Assessment Committee. Bioactive sutures. Plast. Reconstr. Surg. 2006, 118, 1645–1647. [Google Scholar] [CrossRef] [PubMed]
- Markel, D.C.; Bergum, C.; Wu, B.; Bou-Akl, T.; Ren, W. Does Suture Type Influence Bacterial Retention and Biofilm Formation After Irrigation in a Mouse Model? Clin. Orthop. Relat. Res. 2019, 477, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.R.; Bergum, C.; Jackson, N.; Markel, D.C. Decreased Bacterial Adherence, Biofilm Formation, and Tissue Reactivity of Barbed Monofilament Suture in an In Vivo Contaminated Wound Model. J. Arthroplast. 2017, 32, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Bucci, M.; Borgonovo, A.; Bianchi, A.; Zanellato, A.; Re, D. Microbiological analysis of bacterial plaque on three different threads in oral surgery. Minerva Stomatol. 2017, 66, 28–34. [Google Scholar] [CrossRef]
- Fowler, J.R.; Perkins, T.A.; Buttaro, B.A.; Truant, A.L. Bacteria adhere less to barbed monofilament than braided sutures in a contaminated wound model. Clin. Orthop. Relat. Res. 2013, 471, 665–671. [Google Scholar] [CrossRef]
- Fawi, H.M.T.; Papastergiou, P.; Khan, F.; Hart, A.; Coleman, N.P. Use of monofilament sutures and triclosan coating to protect against surgical site infections in spinal surgery: A laboratory-based study. Eur. J. Orthop. Surg. Traumatol. 2023, 33, 3051–3058. [Google Scholar] [CrossRef]
- Swilley, S.N.; Wu, H.; Tomasina, C.; Moroni, L.; Wieringa, P.; Baker, M.B.; Matson, J.B. Electrospun Polymer Fiber Mats for Persulfide Prodrug Delivery. Biomacromolecules 2025. [Google Scholar] [CrossRef]
- Asher, R.; Chacartchi, T.; Tandlich, M.; Shapira, L.; Polak, D. Microbial accumulation on different suture materials following oral surgery: A randomized controlled study. Clin. Oral. Investig. 2019, 23, 559–565. [Google Scholar] [CrossRef]
- Nadafpour, N.; Montazeri, M.; Moradi, M.; Ahmadzadeh, S.; Etemadi, A. Bacterial Colonization on Different Suture Materials Used in Oral Implantology: A Randomized Clinical Trial. Front. Dent. 2021, 18, 25. [Google Scholar] [CrossRef]
- Makrygiannis, I.H.; Nikolaidis, A.K.; Tilaveridis, I.; Kouvelas, A.D.; Lykakis, I.Ν.; Venetis, G. Coated sutures for use in oral surgery: A comprehensive review. Clin. Oral. Investig. 2025, 29, 109. [Google Scholar] [CrossRef] [PubMed]
- Dhom, J.; Bloes, D.A.; Peschel, A.; Hofmann, U.K. Bacterial adhesion to suture material in a contaminated wound model: Comparison of monofilament, braided, and barbed sutures. J. Orthop. Res. 2017, 35, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Amecke, B.; Bendix, D.; Entenmann, G. Resorbable polyesters: Composition, properties, applications. Clin. Mater. 1992, 10, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Nenna, A.; Lemmo, F.; Chello, M.; Chachques, J.C.; Acar, C.; Larobina, D. Finite Element Analysis Investigate Pulmonary Autograft Root and Leaflet Stresses to Understand Late Durability of Ross Operation. Biomimetics 2020, 5, 37. [Google Scholar] [CrossRef]
- Schonebaum, D.I.; Garbaccio, N.; Escobar-Domingo, M.J.; Wood, S.; Smith, J.E.; Foster, L.; Mehdizadeh, M.; Cordero, J.J.; Foppiani, J.A.; Choudry, U.; et al. Comparing Biomechanical Properties of Bioabsorbable Suture Anchors: A Comprehensive Review. Biomimetics 2025, 10, 175. [Google Scholar] [CrossRef]
- Wheeler, B.P.; Medd, K.; Woodworth, K.E.; Davenport Huyer, L. Molecular and Macroscopic Considerations for Degradable Aliphatic Polyester Biomaterial Design. Biomacromolecules 2025, 26, 4784–4811. [Google Scholar] [CrossRef]
- Seger, E.W.; McClure, S.P.; Neill, B.C.; Jibbe, A. Fast absorbing gut sutures in dermatologic surgery: A systematic review and meta-analysis. Arch. Dermatol. Res. 2024, 316, 351. [Google Scholar] [CrossRef]
- Seppälä, J.V.; Helminen, A.O.; Korhonen, H. Degradable polyesters through chain linking for packaging and biomedical applications. Macromol. Biosci. 2004, 4, 208–217. [Google Scholar] [CrossRef]
- Jo, Y.Y.; Kweon, H.; Kim, D.W.; Kim, M.K.; Kim, S.G.; Kim, J.Y.; Chae, W.S.; Hong, S.P.; Park, Y.H.; Lee, S.Y.; et al. Accelerated biodegradation of silk sutures through matrix metalloproteinase activation by incorporating 4-hexylresorcinol. Sci. Rep. 2017, 7, 42441. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Goh, J.C.; Teoh, S.H. An introduction to biodegradable materials for tissue engineering applications. Ann. Acad. Med. Singap. 2001, 30, 183–191. [Google Scholar]
- Cartmill, B.T.; Parham, D.M.; Strike, P.W.; Griffiths, L.; Parkin, B. How do absorbable sutures absorb? A prospective double-blind randomized clinical study of tissue reaction to polyglactin 910 sutures in human skin. Orbit 2014, 33, 437–443. [Google Scholar] [CrossRef]
- Zhu, Y.; Reinach, P.S.; Ge, C.; Li, Y.; Wu, B.; Xie, Q.; Tong, L.; Chen, W. Corneal Collagen Cross-Linking Pretreatment Mitigates Injury-Induced Inflammation, Hemangiogenesis and Lymphangiogenesis In Vivo. Transl. Vis. Sci. Technol. 2021, 10, 11. [Google Scholar] [CrossRef]
- Furtado, T.A.; Carvalho, Á.C.; Furtado, M.L.; Alberti, L.R.; Garcia, D.P.C. Biomechanical evaluation of prophylactic reinforcement of the linea alba with a novel method of positioning and fixation of polypropylene mesh, in an animal model. Acta Cir. Bras. 2025, 40, e405325. [Google Scholar] [CrossRef]
- Saini, I.; Prajapati, K.S.; Nayak, J.; Devi, K.; Kumar, S.; Kumar, R. Arginine-Conjugated Bioinspired Polydopamine Surface for the Enhanced Antibacterial and Antifouling Performance of Surgical Sutures. Langmuir 2025, 41, 17849–17860. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Fu, M.; Yang, H.; Fang, K.; Wang, Y.; Wu, T. Bioactive microfiber yarns serving as sutures with antibacterial and pro-tissue repair capabilities. Chem. Commun. 2025, 61, 9250–9253. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Spadaccio, C.; Castaldo, C.; Di Meglio, F.; Nurzynska, D.; Montagnani, S.; Chello, M.; Acar, C. Reinforcement of the pulmonary artery autograft with a polyglactin and polydioxanone mesh in the Ross operation: Experimental study in growing lamb. J. Heart Valve Dis. 2014, 23, 145–148. [Google Scholar] [PubMed]
- Spadaccio, C.; Montagnani, S.; Acar, C.; Nappi, F. Introducing bioresorbable scaffolds into the show. A potential adjunct to resuscitate Ross procedure. Int. J. Cardiol. 2015, 190, 50–52. [Google Scholar] [CrossRef]
- Nappi, F.; Spadaccio, C.; Fouret, P.; Hammoudi, N.; Chachques, J.C.; Chello, M.; Acar, C. An experimental model of the Ross operation: Development of resorbable reinforcements for pulmonary autografts. J. Thorac. Cardiovasc. Surg. 2015, 149, 1134–1142. [Google Scholar] [CrossRef]
- Nappi, F.; Spadaccio, C.; Fraldi, M.; Montagnani, S.; Fouret, P.; Chachques, J.C.; Acar, C. A composite semiresorbable armoured scaffold stabilizes pulmonary autograft after the Ross operation: Mr Ross’s dream fulfilled. J. Thorac. Cardiovasc. Surg. 2016, 151, 155–164.e1. [Google Scholar] [CrossRef]
- Nappi, F.; Carotenuto, A.R.; Di Vito, D.; Spadaccio, C.; Acar, C.; Fraldi, M. Stress-shielding, growth and remodeling of pulmonary artery reinforced with copolymer scaffold and transposed into aortic position. Biomech. Model. Mechanobiol. 2016, 15, 1141–1157. [Google Scholar] [CrossRef]
- Nappi, F.; Carotenuto, A.R.; Cutolo, A.; Fouret, P.; Acar, C.; Chachques, J.C.; Fraldi, M. Compliance mismatch and compressive wall stresses drive anomalous remodelling of pulmonary trunks reinforced with Dacron grafts. J. Mech. Behav. Biomed. Mater. 2016, 63, 287–302. [Google Scholar] [CrossRef]
- Nappi, F.; Fraldi, M.; Spadaccio, C.; Carotenuto, A.R.; Montagnani, S.; Castaldo, C.; Chachques, J.C.; Acar, C. Biomechanics drive histological wall remodeling of neoaortic root: A mathematical model to study the expression levels of ki 67, metalloprotease, and apoptosis transition. J. Biomed. Mater. Res. A 2016, 104, 2785–2793. [Google Scholar] [CrossRef]
- Nappi, F.; Nassif, A.; Schoell, T. External Scaffold for Strengthening the Pulmonary Autograft in the Ross Procedure. Biomimetics 2024, 9, 674. [Google Scholar] [CrossRef] [PubMed]
- Tomihari, M.; Yamanokuchi, K.; Yanagawa, M.; Tagawa, M.; Tanaka, T.; Hasegawa, T. A basic study on the mechanical properties of synthetic absorbable sutures under various pH conditions in vitro. J. Vet. Med. Sci. 2025, 87, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Stehlik, L.; Guha, D.; Anandakumar, S.; Taskova, A.; Vasakova, M.K. Biodegradable tracheal stents: Our ten-year experience with adult patients. BMC Pulm. Med. 2024, 24, 238. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Y.; Zeng, J.; Ma, Y.; Cui, Z.; Zhou, Y.; Ruan, Z. Study onin vivoandin vitrodegradation of polydioxanone weaving tracheal stents. Biomed. Mater. 2024, 19, 055032. [Google Scholar] [CrossRef]
- Larsen, R.F. Polyglycolic acid sutures in general practice. N. Z. Vet. J. 1978, 26, 258–259. [Google Scholar] [CrossRef]
- Odijk, R.; Hennipman, B.; Rousian, M.; Madani, K.; Dijksterhuis, M.; de Leeuw, J.W.; van Hof, A. The MOVE-trial: Monocryl® vs. Vicryl Rapide™ for skin repair in mediolateral episiotomies: A randomized controlled trial. BMC Pregnancy Childbirth 2017, 17, 355. [Google Scholar] [CrossRef]
- Hohenleutner, U.; Egner, N.; Hohenleutner, S.; Landthaler, M. Intradermal buried vertical mattress suture as sole skin closure: Evaluation of 149 cases. Acta Derm. Venereol. 2000, 80, 344–347. [Google Scholar] [CrossRef][Green Version]
- DeNardo, G.A.; Brown, N.O.; Trenka-Benthin, S.; Marretta, S.M. Comparison of seven different suture materials in the feline oral cavity. J. Am. Anim. Hosp. Assoc. 1996, 32, 164–172. [Google Scholar] [CrossRef]
- Katz, A.R.; Mukherjee, D.P.; Kaganov, A.L.; Gordon, S. A new synthetic monofilament absorbable suture made from polytrimethylene carbonate. Surg. Gynecol. Obstet. 1985, 161, 213–222. [Google Scholar]
- Cagici, C.A.; Cakmak, O.; Bal, N.; Yavuz, H.; Tuncer, I. Effects of different suture materials on cartilage reshaping. Arch. Facial Plast. Surg. 2008, 10, 124–129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ishizaki, T.; Mazaki, J.; Kasahara, K.; Udo, R.; Tago, T.; Nagakawa, Y. Stapled versus handsewn closure of enterotomy for intracorporeal overlap anastomosis in laparoscopic colectomy: In vitro study. Tech. Coloproctol. 2025, 29, 148. [Google Scholar] [CrossRef] [PubMed]
- Neutzling, C.B.; Lustosa, S.A.; Proenca, I.M.; da Silva, E.M.; Matos, D. Stapled versus handsewn methods for colorectal anastomosis surgery. Cochrane Database Syst. Rev. 2012, 2012, CD003144. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Toritani, K.; Kunisaki, R.; Goto, K.; Kuroki, H.; Tatsumi, K.; Koganei, K.; Sugita, A.; Endo, I. Current status of fertility rates and modes of delivery after restorative proctocolectomy with ileal pouch-anal anastomosis. Int. J. Colorectal Dis. 2025, 40, 145. [Google Scholar] [CrossRef]
- Juszkiewicz, M. [Experiments on new synthetic and non-absorbable surgical threads]. Polim. Med. 1998, 28, 33–58. [Google Scholar]
- de Werra, C.; Rendano, F.; D’Armiento, F.; Somma, P.; Forestieri, P. [Comparison of five synthetic absorbable suture materials in intestinal anastomosis: Experimental study in rats]. Chir. Ital. 2003, 55, 227–233. [Google Scholar]
- Wei, M.; Yang, Y.; Yang, J.; Zhang, S. Pregnancy outcomes with the reuse of the same suture in the history-indicated modified transvaginal cervicoisthmic cerclage. Medicine 2025, 104, e42687. [Google Scholar] [CrossRef]
- Alfirevic, Z.; Stampalija, T.; Medley, N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst. Rev. 2017, 6, CD00899. [Google Scholar]
- Metz, S.A.; Chegini, N.; Masterson, B.J. In vivo and in vitro degradation of monofilament absorbable sutures, PDS and Maxon. Biomaterials 1990, 11, 41–45. [Google Scholar] [CrossRef]
- Sanz, L.E.; Patterson, J.A.; Kamath, R.; Willett, G.; Ahmed, S.W.; Butterfield, A.B. Comparison of Maxon suture with Vicryl, chromic catgut, and PDS sutures in fascial closure in rats. Obstet. Gynecol. 1988, 71 Pt 1, 418–422. [Google Scholar] [PubMed]
- Obiweluozor, F.O.; Emechebe, G.A.; Tiwari, A.P.; Kim, J.Y.; Park, C.H.; Kim, C.S. Short duration cancer treatment: Inspired by a fast bio-resorbable smart nano-fiber device containing NIR lethal polydopamine nanospheres for effective chemo-photothermal cancer therapy. Int. J. Nanomedicine 2018, 13, 6375–6390. [Google Scholar] [CrossRef] [PubMed]
- Adler, D.M.T.; Østergaard, S.; Jørgensen, E.; Jacobsen, S. Bidirectional knotless barbed versus conventional smooth suture for closure of surgical wounds in inguinal castration in horses. BMC Vet. Res. 2020, 16, 250. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Andjelić, S.; Zhou, J.; Xu, Y.; Vailhe, C.; Vetrecin, R. Thermal stability and melt rheology of poly(p-dioxanone). J. Mater. Sci. Mater. Med. 2008, 19, 3481–3487. [Google Scholar] [CrossRef]
- Pineros-Fernandez, A.; Drake, D.B.; Rodeheaver, P.A.; Moody, D.L.; Edlich, R.F.; Rodeheaver, G.T. CAPROSYN*, another major advance in synthetic monofilament absorbable suture. J. Long Term Eff. Med. Implant. 2004, 14, 359–368. [Google Scholar] [CrossRef]
- Rodeheaver, G.T.; Beltran, K.A.; Green, C.W.; Faulkner, B.C.; Stiles, B.M.; Stanimir, G.W.; Traeland, H.; Fried, G.M.; Brown, H.C.; Edlich, R.F. Biomechanical and clinical performance of a new synthetic monofilament absorbable suture. J. Long Term Eff. Med. Implant. 1996, 6, 181–198. [Google Scholar]
- Chiriac, S.; Facca, S.; Diaconu, M.; Gouzou, S.; Liverneaux, P. Experience of using the bioresorbable copolyester poly(DL-lactideε-caprolactone) nerve conduit guide Neurolac™ for nerve repair in peripheral nerve defects: Report on a series of 28 lesions. J. Hand Surg. Eur. 2012, 37, 342–349. [Google Scholar] [CrossRef]
- Utsunomia, C.; Ren, Q.; Zinn, M. Poly(4-hydroxybutyrate): Current state and perspectives. Front. Bioeng. Biotechnol. 2020, 8, 257. [Google Scholar] [CrossRef]
- Doi, Y.; Segawa, A.; Kunioka, M. Biosynthesis and characterization of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) in Alcaligenes eutrophus. Int. J. Biol. Macromol. 1990, 12, 106–111. [Google Scholar] [CrossRef]
- Huong, K.H.; The, C.H.; Amirul, A.A. Microbial-based synthesis of highly elastomeric biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate) thermoplastic. Int. J. Biol. Macromol. 2017, 101, 983–995. [Google Scholar] [CrossRef]
- Murayama, A.; Yoneda, H.; Maehara, A.; Shiomi, N.; Hirata, H. A highly elastic absorbable monofilament suture fabricated from poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Sci. Rep. 2023, 13, 3275. [Google Scholar] [CrossRef] [PubMed]
- Carrel, T.; Schwerzmann, M.; Eckstein, F.; Aymard, T.; Kadner, A. Preliminary results following reinforcement of the pulmonary autograft to prevent dilatation after the Ross procedure. J. Thorac. Cardiovasc. Surg. 2008, 136, 472–475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carrel, T. The autograft inclusion: An obligatory step to avoid late failurefollowing the Ross procedure? J. Thorac. Cardiovasc. Surg. 2015, 149, S53–S54. [Google Scholar] [CrossRef] [PubMed]
- Al Rashidi, F.; Bhat, M.; Hoglund, P.; Meurling, C.; Roijer, A.; Koul, B. The modified Ross operation using a Dacron prosthetic vascular jacket does prevent pulmonary autograft dilatation at 4.5-year follow-up. Eur. J. Cardiothorac. Surg. 2010, 37, 928–933. [Google Scholar] [CrossRef][Green Version]
- Koul, B.; Al-Rashidi, F.; Bhat, M.; Meurling, C. A modified Ross operation to prevent pulmonary autograft dilatation. Eur. J. Cardiothorac. Surg. 2007, 31, 127–128. [Google Scholar] [CrossRef]
- Slater, M.; Shen, I.; Welke, K.; Komanapalli, C.; Ungerleider, R. Modification to the Ross procedure to prevent autograft dilatation. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2005, 8, 181–184. [Google Scholar] [CrossRef]
- Husain, S.A. The continued evolution of a transformational operation: New options for wrapping the Ross autograft? J. Thorac. Cardiovasc. Surg. 2015, 149, 1142–1143. [Google Scholar] [CrossRef][Green Version]
- Spadaccio, C.; Rainer, A.; Barbato, R.; Chello, M.; Meyns, B. The fate of largediameter Dacron® vascular grafts in surgical practice: Are we really satisfied? Int. J. Cardiol. 2013, 168, 5028–5029. [Google Scholar] [CrossRef]
- Gonzales, V.; Rightsell, C.; Morales Betancourt, A.; Nash, K.L. Non-enzymatic glucose detection via ordered 2D arrays of nickel and nickel chitosan nanowires. Sens. Actuators Rep. 2024, 8, 100249. [Google Scholar] [CrossRef]
- Bi, J.; Wang, H.; Luo, H.; Qian, C.; Zhou, J.; Li, X.; Qi, X.; Shen, S.; Cao, J. Self-healing hydrogels loaded with selenium nanoparticles/chitosan/cellulose nanofibers as carriers of mesenchymal stem cells for diabetic wound healing. Int. J. Biol. Macromol. 2025, 322, 146905. [Google Scholar] [CrossRef]
- Kuten Pella, O.; Hornyák, I.; Horváthy, D.; Fodor, E.; Nehrer, S.; Lacza, Z. Albumin as a Biomaterial and Therapeutic Agent in Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 10557. [Google Scholar] [CrossRef]
- Erten, E.; Arslan, Y.E. The Great Harmony in Translational Medicine: Biomaterials and Stem Cells. Adv. Exp. Med. Biol. 2018, 1119, 21–39. [Google Scholar]
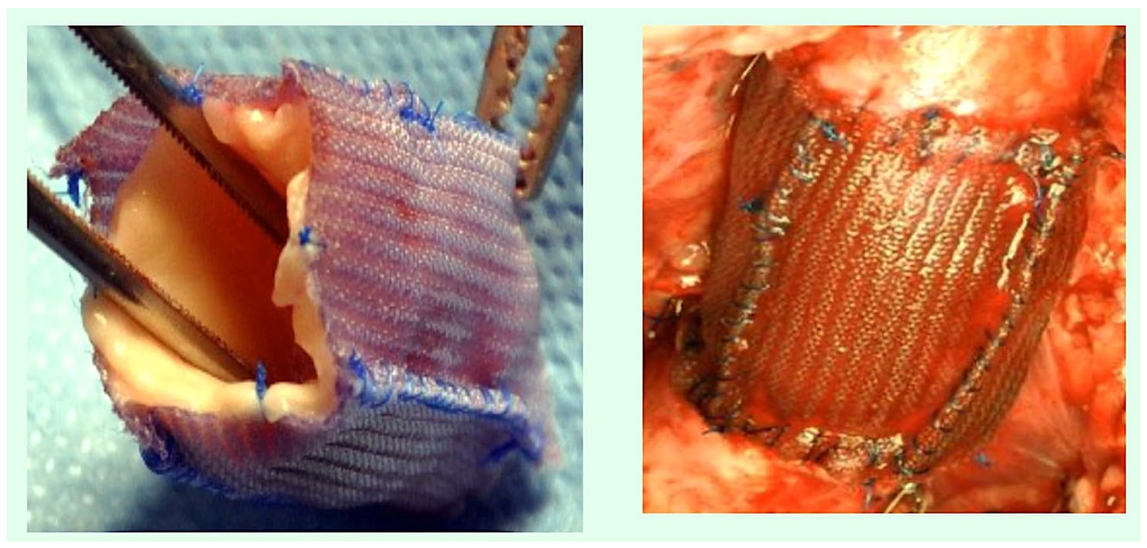
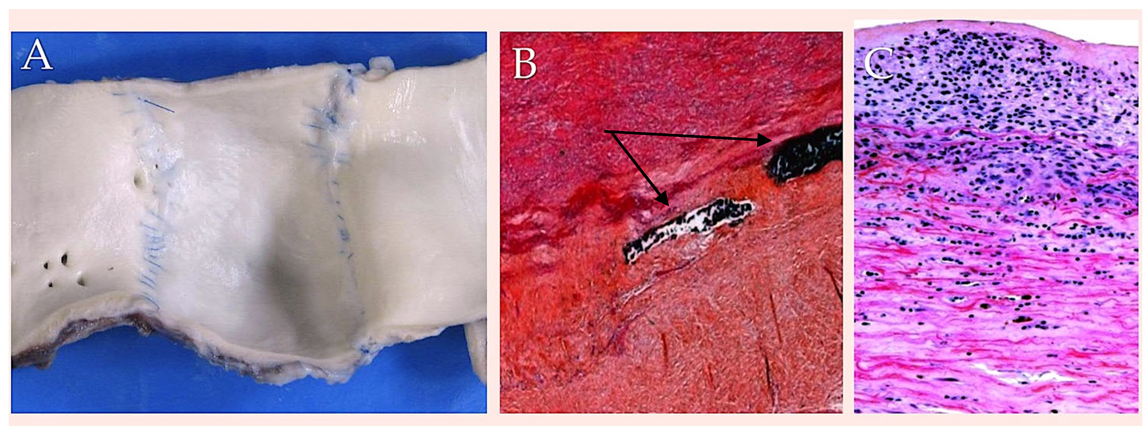

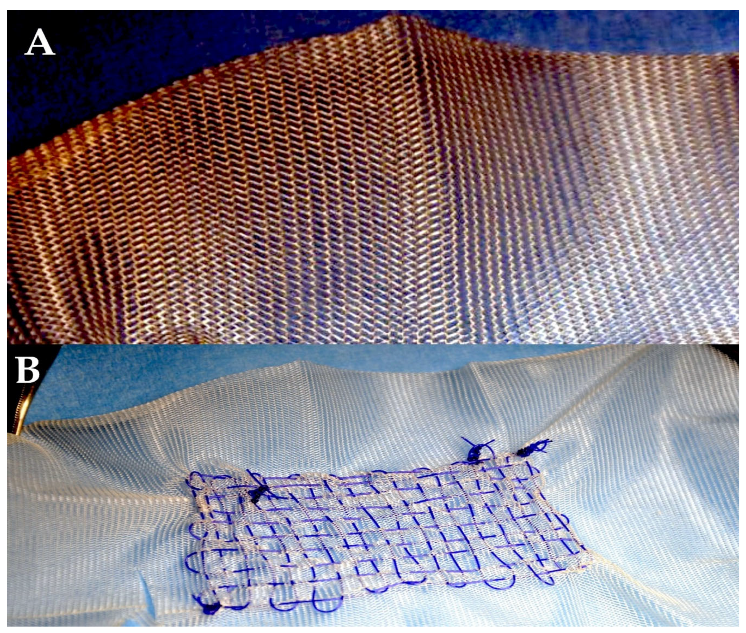
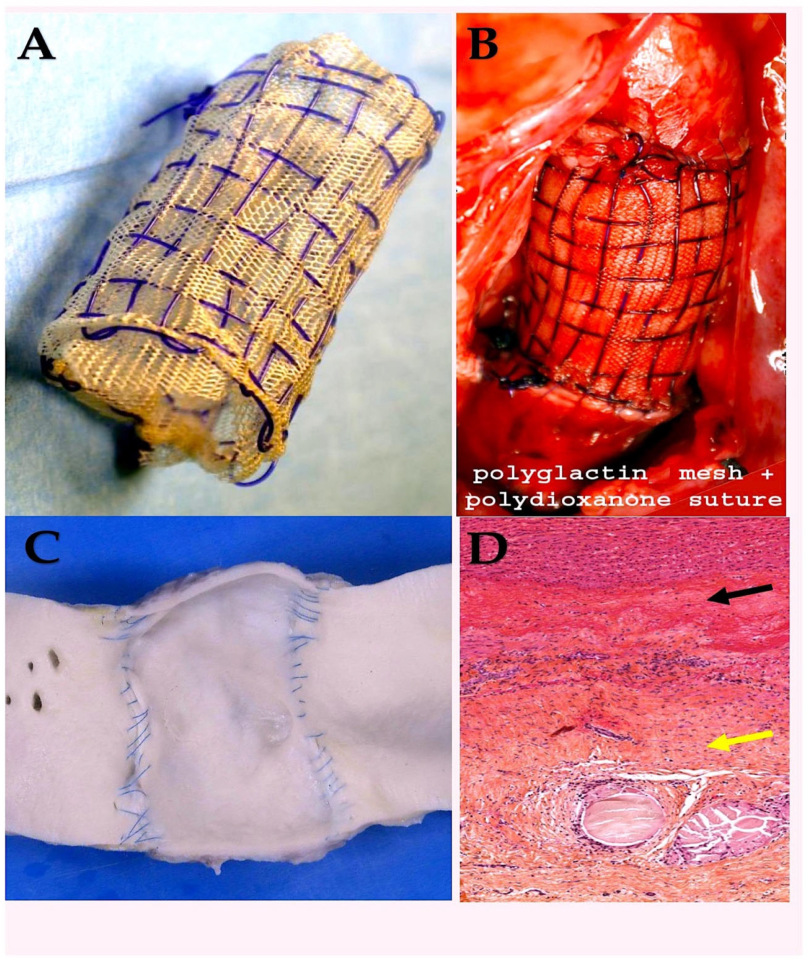


| Suture Type | Strand | Unprocessed Material | Employment | Properties | Drawbacks |
|---|---|---|---|---|---|
| Polydioxanone (PDS II) | Monofilament | Polymer of paradioxanone | Used to approximate tissues over extended periods. Useful in infected tissues. | It maintains 74% of its tensile strength after two weeks and 25% after six weeks. It is absorbed by hydrolysis at 6–7 months. | The knot is not securely fastened and its handling characteristics are poor due to its stiffness and memory. |
| Polyglactin 910 (Vicryl) | Braided multifilament | A copolymer of glycolic and lactic acid that is coated with calcium stearate. | Less complicated to use than gut Causes less tissue reaction. More robust Used in infected wounds. | Strength is lost at the two-week mark, with 65% of strength being gone by the three-week point. | It has the potential to make a clean cut through tissue that may be friable. |
| Poliglecaprone 25 (Monocryl) | Monofilament | Copolymer of glycolide and epsiloncaprolacto | It is easy to handle, and it secures knots well. Minimal tissue reaction. General tissue approximation | The substance loses 30–40% of its strength after 14 days, and there is no strength remaining after 3 weeks. It is absorbed by idrolysis after 90–120 days. | The use of this product is contraindicated in cases of delayed healing. |
| Polyglycolic acid (Dexon, Dexon II) | Braided multifilament | A synthetic homopolymer of glycolic acid. The Dexon II has been coated with a polycarbonate coating. | This product is comparable to Vicryl. This product has a wide variety of uses, including applications in both normal and contaminated tissues. This product is ideal for use in C-sections and intestinal anastomosis. | Maintains 89% of its effectiveness after seven days, 63% after 14 days and 17% after 21 days; Is absorbed by hydrolysis within 90–120 days. | The process of breakdown is intensified in the urine and oral cavity. The uncoated form exhibits a high coefficient of friction. |
| Polyglycolide–trimethylene carbosnate (Maxon) | Monofilament | This product is a copolymer of glycolic acid and trimethylene. | This product has been shown to be more secure than PDS II and comparable applications, while also offering similar functionality. | The tensile strength of the material is maintained for 42–92 days. In comparison to the 64–80 days required for PDS. The product is fully absorbed by hydrolysis after six months. | In comparison to the PDSII, this product exhibits inferior handling characteristics. |
| Caprosyn | Monofilament | The product comprises a blend of glycolide, caprolactone, trimethylene carbonate and lactide. | This product boasts superior handling and tensile strength, along with greater knot security when compared with gut. In addition, it offers increased resistance to infection for optimal reliability. | The material displays 50–60% tensile strength at 5 days, and 0% at 3 weeks. It should be noted that the process was fully absorbed within 56 days. | Due to its short retention time, the use of this substance is restricted to plastic surgery, obstetrics and gynaecology, where rapid absorption and minimal scarring are of the essence. |
| Polyhydroxyalkanoates (PHAs) | Biodegradable polymers of microbial origin | 4-hydroxybutyrate (4HB) polymers | Polyhydroxyalkanoates are a great choice for making stretchy medical devices in the field of biomedical engineering. They are mostly used in stitches and heart stents. | Decrease in the initial linear tensile strength of the P(3HB-co-4HB) sutures from 161 to 104.6 MPa (i.e., by 35.0%). The fractional weight-average molecular weight (Mw) exhibits a decrease. The P(3HB-co-4HB) suture showed increased elasticity and extensibility when it was broken (160% compared to the first measurement of 240%). | The suture’s circumference is smaller than PDS II’s, its knot area is smaller too and its knot area to thread diameter ratio is lower. Nevertheless, the former possesses a greater thread size and as a consequence, a considerably reduced knot diameter |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nappi, F. Suture Materials: Conventional and Stimulatory-Responsive Absorbable Polymers with Biomimetic Function. Biomimetics 2025, 10, 590. https://doi.org/10.3390/biomimetics10090590
Nappi F. Suture Materials: Conventional and Stimulatory-Responsive Absorbable Polymers with Biomimetic Function. Biomimetics. 2025; 10(9):590. https://doi.org/10.3390/biomimetics10090590
Chicago/Turabian StyleNappi, Francesco. 2025. "Suture Materials: Conventional and Stimulatory-Responsive Absorbable Polymers with Biomimetic Function" Biomimetics 10, no. 9: 590. https://doi.org/10.3390/biomimetics10090590
APA StyleNappi, F. (2025). Suture Materials: Conventional and Stimulatory-Responsive Absorbable Polymers with Biomimetic Function. Biomimetics, 10(9), 590. https://doi.org/10.3390/biomimetics10090590







