Exploring the Rheological Properties of 3D Bioprinted Alginate-Based Hydrogels for Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Hydrogels
2.2. Characterization of the Bioinks
- Initial stage—A low shear rate (approximately 0.011 s−1) was applied for 120 s, simulating the hydrogel’s initial state before printing.
- High shear rate stage—The shear rate was significantly increased to around 100 s−1, disrupting the internal gel structure and temporarily lowering the viscosity for 120 s. This step simulates the behavior of the hydrogel during extrusion.
- Recovery stage—The shear rate was reduced to a low value for another 120 s, allowing the bioink to regain stability and return to its original viscosity, which simulates the final state of the hydrogel after it has been printed.
2.3. Characterization of the Hydrogels
2.3.1. Fourier Transform Infrared (FTIR) Spectroscopy
2.3.2. Rheology
- Stage 1—A twist of 10−8 rad was applied, followed by a 900 s relaxation period. This stage was intended for stabilization, and no data were collected for characterization.
- Stage 2—A twist of 0.02 rad was applied, followed by a 1200 s relaxation period, with data being recorded every 0.2 s.
- Stage 3—A dynamic frequency sweep was conducted under strain-controlled conditions, ranging from 0.01 Hz to the maximum frequency allowed by the test conditions, with a maximum strain of 1%. Data were collected at five points per decade.
3. Results and Discussion
3.1. Properties of the Bioinks
3.2. FTIR Evaluation of the Hydrogels
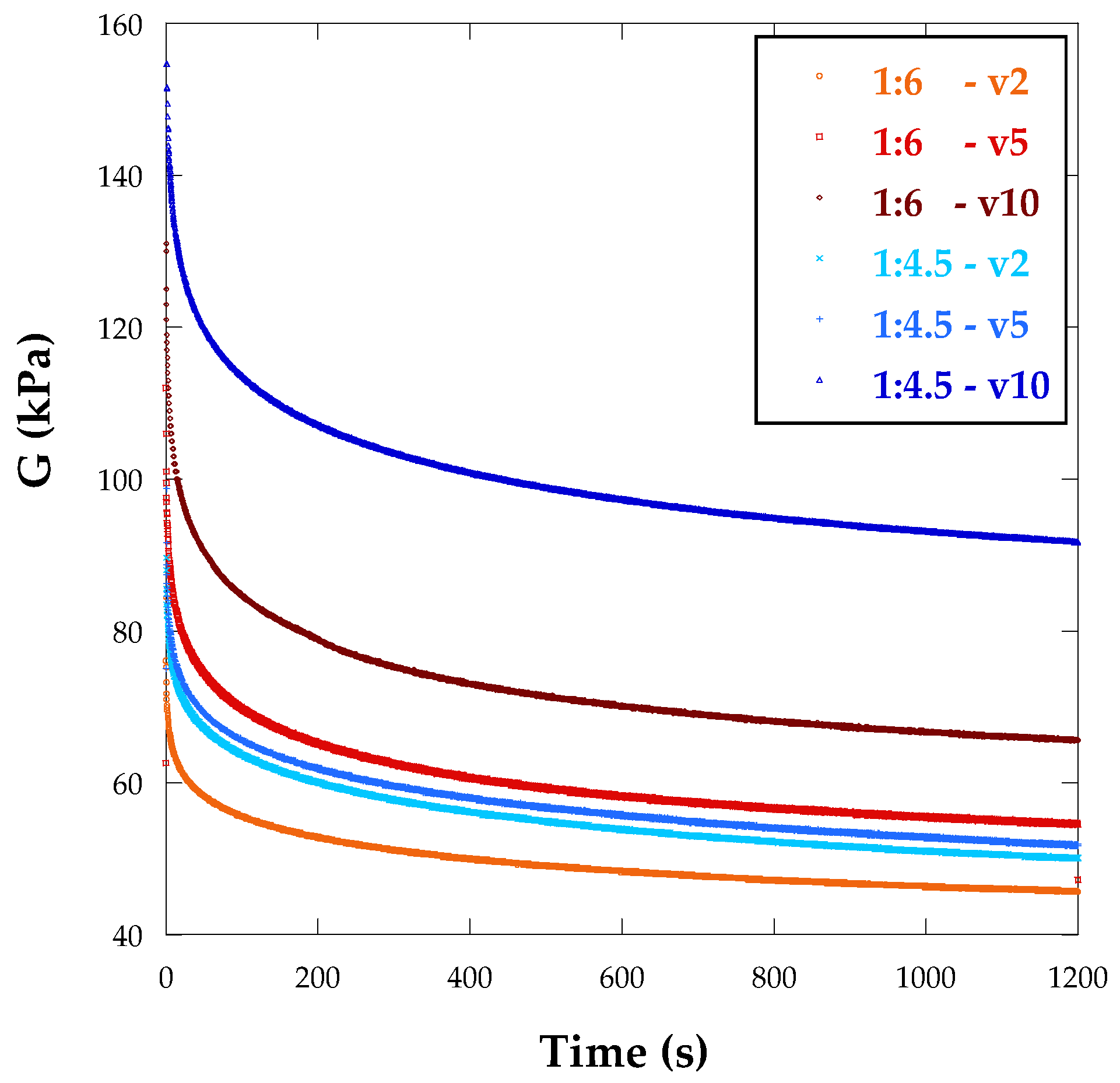
3.3. Viscoelastic Properties of the Hydrogels
4. Conclusions
- Alginate/polyacrylamide hydrogels with varying alginate contents were successfully fabricated using 3D printing with photopolymerization, and their printability was evaluated.
- Rheological tests proved to be effective in analyzing the viscoelastic behavior of alginate/polyacrylamide hydrogels. Since torsional tests mainly involve pure shear stress, they allowed for the study of the viscoelastic behavior without interference from other phenomena, such as poroelasticity.
- The relaxation curves of the hydrogels were modeled using a two-term Prony series equation, providing a comprehensive understanding of their viscoelastic characteristics.
- The manufacturing process significantly impacts the mechanical properties of alginate/polyacrylamide hydrogels in two main ways. First, 3D printing notably enhances the stiffness of the hydrogels. Second, both viscoelasticity and relaxation times are increased in hydrogels produced via 3D printing. However, the velocity of the UV lamp used for curing does not seem to influence these behaviors to a significant extent.
- Differences were observed among hydrogels produced with the same method but differing cross-linker concentrations. For 3D printed hydrogels, the shear modulus increased as the alginate content decreased.
- The experimental results indicated that the cross-linking of alginate in these hydrogels was irreversible. The elastic component dominated across all hydrogels and frequencies studied, confirming that the hydrogels primarily exhibited elastic behavior.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fox, C.F.; Skalak, R. Tissue Engineering: Proceedings of a Workshop, Held at Granlibakken, Lake Tahoe, California, February 26–29, 1988; Liss: New York, NY, USA, 1988. [Google Scholar]
- Oberpenning, F.; Meng, J.; Yoo, J.J.; Atala, A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat. Biotechnol. 1999, 17, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Chidume, T. Promoting older adult fall prevention education and awareness in a community setting: A nurse-led intervention. Appl. Nurs. Res. 2021, 57, 151392. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.L.; Wang, S.; Yeong, W.Y.; Naing, M.W. Skin Bioprinting: Impending Reality or Fantasy? Trends Biotechnol. 2016, 34, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Gao, L.; Ma, L.; Luo, Y.; Yang, H.; Cui, Z. 3D Bioprinting: A Novel Avenue for Manufacturing Tissues and Organs. Engineering 2019, 5, 777–794. [Google Scholar] [CrossRef]
- Fang, Y.; Guo, Y.; Liu, T.; Xu, R.; Mao, S.; Mo, X.; Zhang, T.; Ouyang, L.; Xiong, Z.; Sun, W. Advances in 3D Bioprinting. Chin. J. Mech. Eng. Addit. Manuf. Front. 2022, 1, 100011. [Google Scholar] [CrossRef]
- Jor, J.W.Y.; Parker, M.D.; Taberner, A.J.; Nash, M.P.; Nielsen, P.M.F. Computational and experimental characterization of skin mechanics: Identifying current challenges and future directions. WIREs Mech. Dis. 2013, 5, 539–556. [Google Scholar] [CrossRef] [PubMed]
- Huzaira, M.; Rius, F.; Rajadhyaksha, M.; Anderson, R.R.; González, S. Topographic Variations in Normal Skin, as Viewed by In Vivo Reflectance Confocal Microscopy. J. Investig. Dermatol. 2001, 116, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, G.L.; Brown, I.A.; Wildnauer, R.H. The biomechanical properties of skin. CRC Crit. Rev. Bioeng. 1973, 1, 453–495. [Google Scholar] [PubMed]
- Sahana, T.G.; Rekha, P.D. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Bio. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Stramer, B.M.; Mori, R.; Martin, P. The Inflammation–Fibrosis Link? A Jekyll and Hyde Role for Blood Cells during Wound Repair. J. Investig. Dermatol. 2007, 127, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Enoch, S.; Grey, J.E.; Harding, K.G. Non-surgical and drug treatments. BMJ 2006, 332, 900–903. [Google Scholar] [CrossRef] [PubMed]
- Chua, A.W.C.; Khoo, Y.C.; Tan, B.K.; Tan, K.C.; Foo, C.L.; Chong, S.J. Skin tissue engineering advances in severe burns: Review and therapeutic applications. Burn. Trauma 2016, 4, s41038-016-0027-y. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Midha, S.; Giri, S.; Mohanty, S. Functional Skin Grafts: Where Biomaterials Meet Stem Cells. Stem Cells Int. 2019, 2019, 1286054. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.L.; Qi, J.T.Z.; Yeong, W.Y.; Naing, M.W. Proof-of-concept: 3D bioprinting of pigmented human skin constructs. Biofabrication 2018, 10, 025005. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, N.; Chen, L.; Alépée, N.; Cai, Z. A ready-to-use integrated in vitro skin corrosion and irritation testing strategy using EpiSkinTM model in China. Toxicol. In Vitro 2020, 65, 104778. [Google Scholar] [CrossRef] [PubMed]
- Géniès, C.; Jacques-Jamin, C.; Duplan, H.; Rothe, H.; Ellison, C.; Cubberley, R.; Schepky, A.; Lange, D.; Klaric, M.; Hewitt, N.J.; et al. Comparison of the metabolism of 10 cosmetics-relevant chemicals in EpiSkinTM S9 subcellular fractions and in vitro human skin explants. J. Appl. Toxicol. 2020, 40, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Krishna, D.V.; Sankar, M.R. Extrusion based bioprinting of alginate based multicomponent hydrogels for tissue regeneration applications: State of the art. Mater. Today Commun. 2023, 35, 105696. [Google Scholar] [CrossRef]
- Mehta, P.; Sharma, M.; Devi, M. Hydrogels: An overview of its classifications, properties, and applications. J. Mech. Behav. Biomed. Mater. 2023, 147, 106145. [Google Scholar] [CrossRef] [PubMed]
- Ersumo, N.; Witherel, C.E.; Spiller, K.L. Differences in time-dependent mechanical properties between extruded and molded hydrogels. Biofabrication 2016, 8, 035012. [Google Scholar] [CrossRef] [PubMed]
- Naghieh, S.; Chen, X. Printability–A key issue in extrusion-based bioprinting. J. Pharm. Anal. 2021, 11, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Kirchmajer, D.M.; Gorkin, R., III; Panhuis, M.I.H. An overview of the suitability of hydrogel-forming polymers for extrusion-based 3D-printing. J. Mater. Chem. B 2015, 3, 4105–4117. [Google Scholar] [CrossRef] [PubMed]
- Jungst, T.; Smolan, W.; Schacht, K.; Scheibel, T.; Groll, J. Strategies and Molecular Design Criteria for 3D Printable Hydrogels. Chem. Rev. 2016, 116, 1496–1539. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.X.B. Extrusion Bioprinting of Scaffolds for Tissue Engineering Applications; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Bakarich, S.E.; Panhuis, M.I.H.; Beirne, S.; Wallace, G.G.; Spinks, G.M. Extrusion printing of ionic–covalent entanglement hydrogels with high toughness. J. Mater. Chem. B 2013, 1, 4939. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Niu, X.; Shao, L.; Zhou, L.; Lin, Z.; Sun, A.; Fu, J.; Chen, Z.; Hu, J.; Liu, Y.; et al. 3D printing of complex GelMA-based scaffolds with nanoclay. Biofabrication 2019, 11, 035006. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Yang, L.; Wei, D.; Liang, M.; Xu, L.; Zhang, T.; Hu, W.; Zhang, Z.; Zhang, Q. Alginate/polyacrylamide host-guest supramolecular hydrogels with enhanced adhesion. Int. J. Biol. Macromol. 2023, 242, 124885. [Google Scholar] [CrossRef] [PubMed]
- Axpe, E.; Oyen, M. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef] [PubMed]
- Pragya, A.; Mutalik, S.; Younas, M.W.; Pang, S.-K.; So, P.-K.; Wang, F.; Zheng, Z.; Noor, N. Dynamic cross-linking of an alginate–acrylamide tough hydrogel system: Time-resolved in situ mapping of gel self-assembly. RSC Adv. 2021, 11, 10710–10726. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Illeperuma, W.R.K.; Suo, Z.; Vlassak, J.J. Hybrid Hydrogels with Extremely High Stiffness and Toughness. ACS Macro Lett. 2014, 3, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Subramani, R.; Izquierdo-Alvarez, A.; Bhattacharya, P.; Meerts, M.; Moldenaers, P.; Ramon, H.; Van Oosterwyck, H. The Influence of Swelling on Elastic Properties of Polyacrylamide Hydrogels. Front. Mater. 2020, 7, 212. [Google Scholar] [CrossRef]
- Christensen, K.; Davis, B.; Jin, Y.; Huang, Y. Effects of printing-induced interfaces on localized strain within 3D printed hydrogel structures. Mater. Sci. Eng. C 2018, 89, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yan, K.C.; Ouyang, L.; Sun, W. Mechanical characterization of bioprinted in vitro soft tissue models. Biofabrication 2013, 5, 045010. [Google Scholar] [CrossRef] [PubMed]
- Farzadi, A.; Solati-Hashjin, M.; Asadi-Eydivand, M.; Osman, N.A.A. Effect of Layer Thickness and Printing Orientation on Mechanical Properties and Dimensional Accuracy of 3D Printed Porous Samples for Bone Tissue Engineering. PLoS ONE 2014, 9, e108252. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Caccavo, D.; Cascone, S.; Lamberti, G.; Barba, A.A. Hydrogels: Experimental characterization and mathematical modelling of their mechanical and diffusive behaviour. Chem. Soc. Rev. 2018, 47, 2357–2373. [Google Scholar] [CrossRef] [PubMed]
- Reinhards, C.; Rico, A.; Rodríguez, J. Crosslinker concentration effect on the poroviscoelastic relaxation of polyacrylamide hydrogels using depth-sensing indentation. Polym. Test. 2021, 100, 107265. [Google Scholar] [CrossRef]
- Yan, C.; Pochan, D.J. Rheological properties of peptide-based hydrogels for biomedical and other applications. Chem. Soc. Rev. 2010, 39, 3528. [Google Scholar] [CrossRef] [PubMed]
- Stieger, M. The Rheology Handbook—For users of rotational and oscillatory rheometers. Appl. Rheol. 2002, 12, 232. [Google Scholar] [CrossRef]
- Caccavo, D.; Lamberti, G. PoroViscoElastic model to describe hydrogels’ behavior. Mater. Sci. Eng. C 2017, 76, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Cacopardo, L.; Guazzelli, N.; Nossa, R.; Mattei, G.; Ahluwalia, A. Engineering hydrogel viscoelasticity. J. Mech. Behav. Biomed. Mater. 2019, 89, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Suo, Z. Viscoelasticity and poroelasticity in elastomeric gels. Acta Mech. Solida Sin. 2012, 25, 441–458. [Google Scholar] [CrossRef]
- Fitzgerald, M.M.; Bootsma, K.; Berberich, J.A.; Sparks, J.L. Tunable Stress Relaxation Behavior of an Alginate-Polyacrylamide Hydrogel: Comparison with Muscle Tissue. Biomacromolecules 2015, 16, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Strange, D.G.T.; Fletcher, T.L.; Tonsomboon, K.; Brawn, H.; Zhao, X.; Oyen, M.L. Separating poroviscoelastic deformation mechanisms in hydrogels. Appl. Phys. Lett. 2013, 102, 031913. [Google Scholar] [CrossRef]
- Wang, Q.-M.; Mohan, A.C.; Oyen, M.L.; Zhao, X.-H. Separating viscoelasticity and poroelasticity of gels with different length and time scales. Acta Mech. Sin. 2014, 30, 20–27. [Google Scholar] [CrossRef]
- Bai, R.; Yang, Q.; Tang, J.; Morelle, X.P.; Vlassak, J.; Suo, Z. Fatigue fracture of tough hydrogels. Extrem. Mech. Lett. 2017, 15, 91–96. [Google Scholar] [CrossRef]
- Chirani, N.; L’Hocine, Y.; Lukas, G.; Federico, L.M.; Soumia, C.; Silvia, F. History and Applications of Hydrogels. J. Biomed. Sci. 2015, 4, 13. [Google Scholar] [CrossRef]
- Denisin, A.K.; Pruitt, B.L. Tuning the Range of Polyacrylamide Gel Stiffness for Mechanobiology Applications. ACS Appl. Mater. Interfaces 2016, 8, 21893–21902. [Google Scholar] [CrossRef] [PubMed]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jin, Y.; Yin, J.; Xu, C.; Xiong, R.; Christensen, K.; Ringeisen, B.R.; Chrisey, D.B.; Huang, Y. Evaluation of bioink printability for bioprinting applications. Appl. Phys. Rev. 2018, 5, 041304. [Google Scholar] [CrossRef]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioprinting of embryonic stem cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef] [PubMed]
- Bom, S.; Ribeiro, R.; Ribeiro, H.M.; Santos, C.; Marto, J. On the progress of hydrogel-based 3D printing: Correlating rheological properties with printing behaviour. Int. J. Pharm. 2022, 615, 121506. [Google Scholar] [CrossRef] [PubMed]
- Pettinelli, N.; Sabando, C.; Rodríguez-Llamazares, S.; Bouza, R.; Castaño, J.; Valverde, J.C.; Rubilar, R.; Frizzo, M.; Recio-Sánchez, G. Sodium alginate-g-polyacrylamide hydrogel for water retention and plant growth promotion in water-deficient soils. Ind. Crops Prod. 2024, 222, 119759. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopoly-mannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef]
- Alizadeh Behbahani, B.; Jooyandeh, H.; Taki, M.; Falah, F. Evaluation of the probiotic, anti-bacterial, anti-biofilm, and safety properties of Lacticaseibacillus paracasei B31-2. LWT 2024, 207, 116676. [Google Scholar] [CrossRef]
- Ying, X.; Wang, H.; Liu, J.; Li, X. Polyacrylamide-grafted calcium alginate microspheres as protein-imprinting materials. Polym. Bull. 2018, 75, 2139–2150. [Google Scholar] [CrossRef]
- Jamburidze, A.; De Corato, M.; Huerre, A.; Pommella, A.; Garbin, V. High-frequency linear rheology of hydrogels probed by ultrasound-driven microbubble dynamics. Soft Matter 2017, 13, 3946–3953. [Google Scholar] [CrossRef] [PubMed]
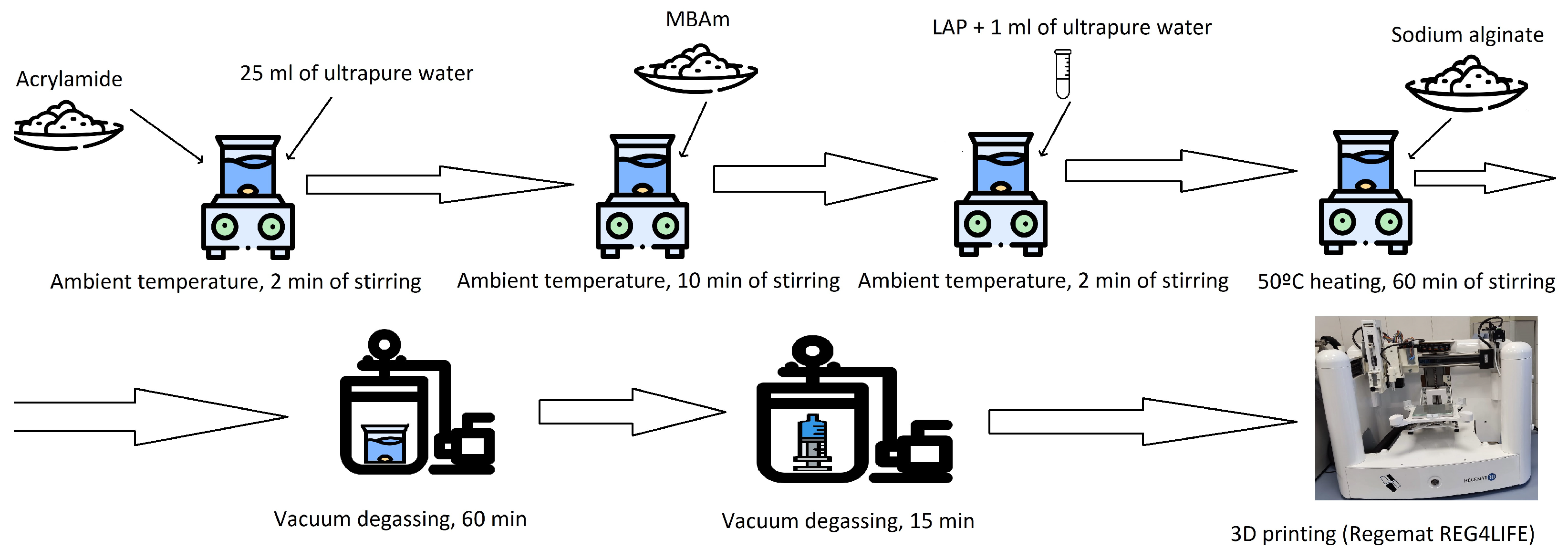
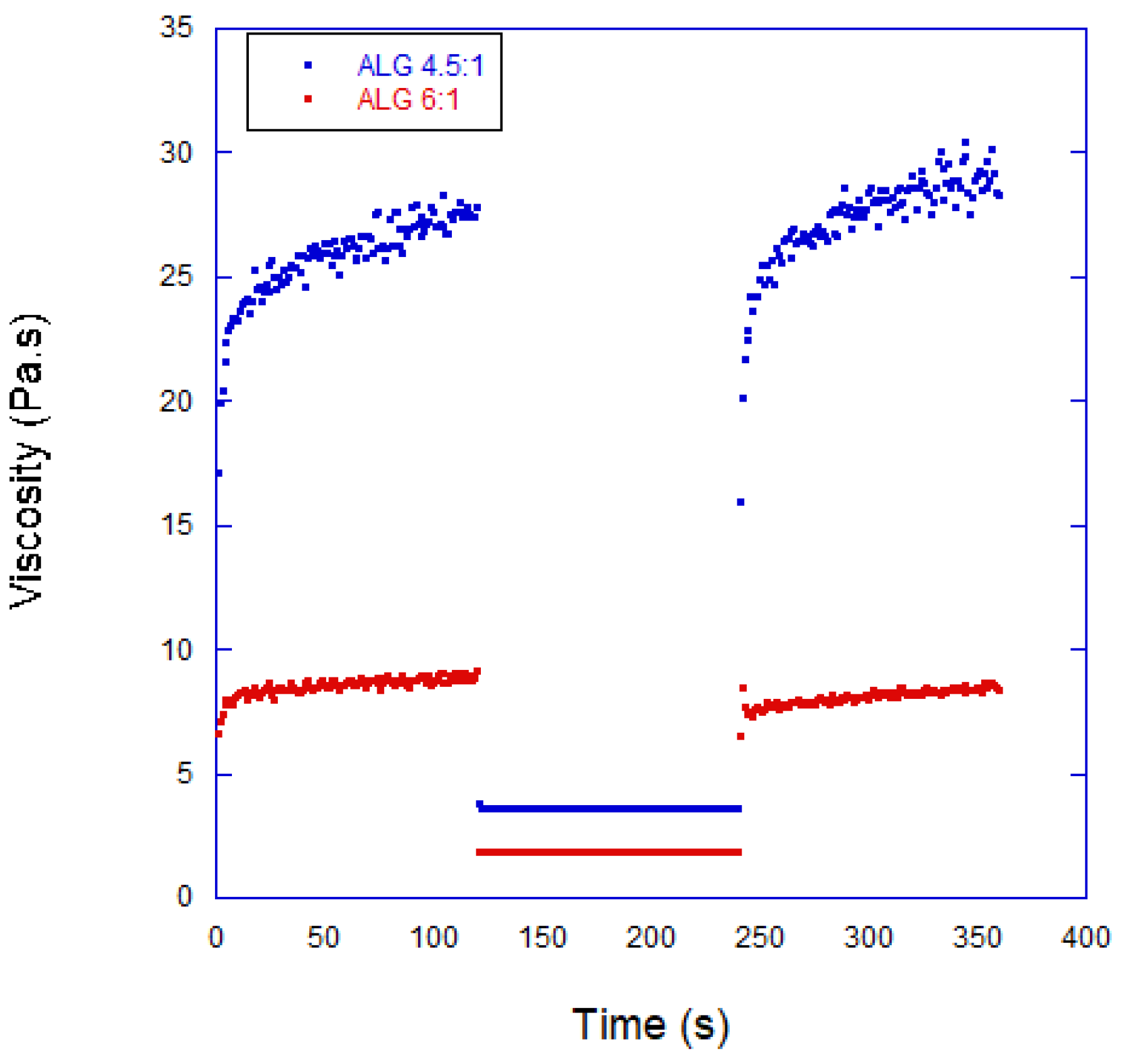
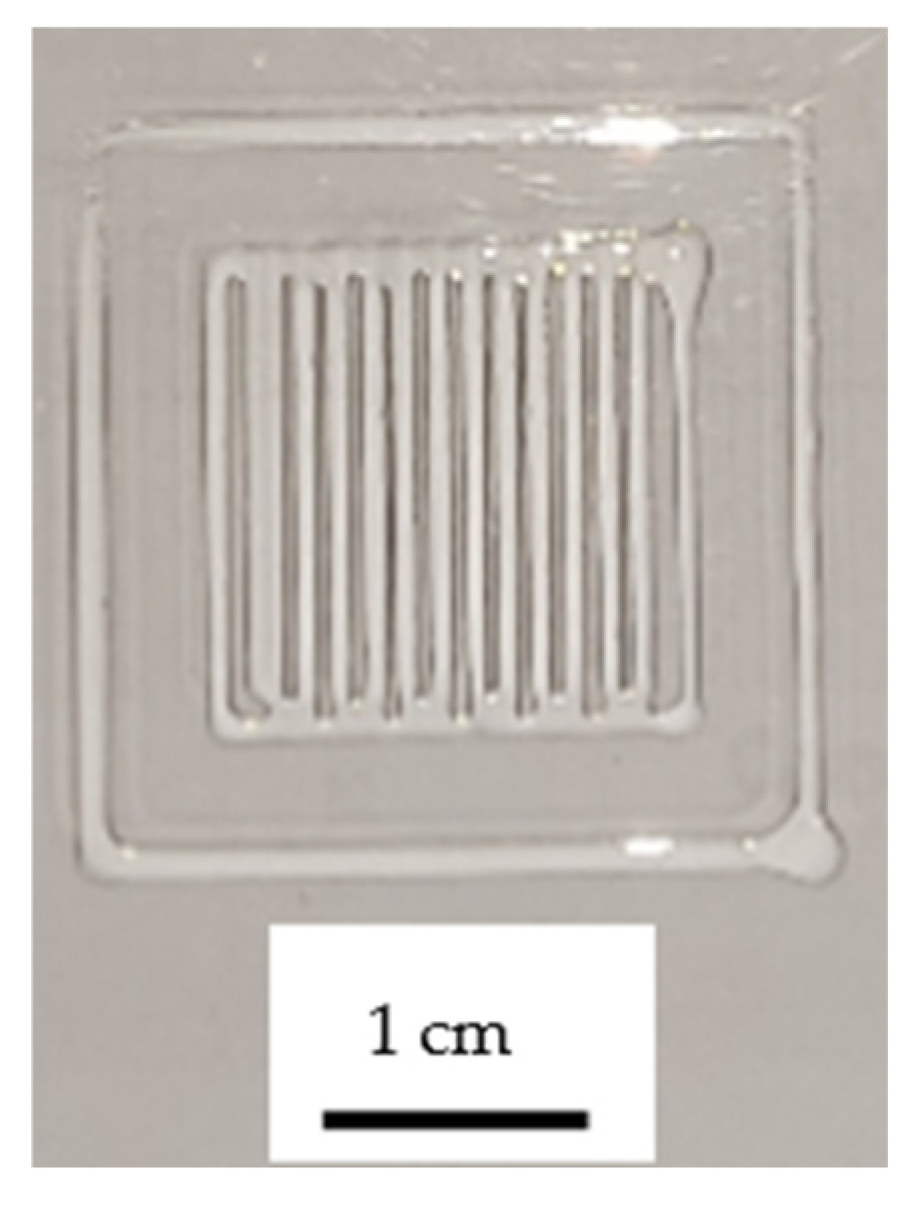
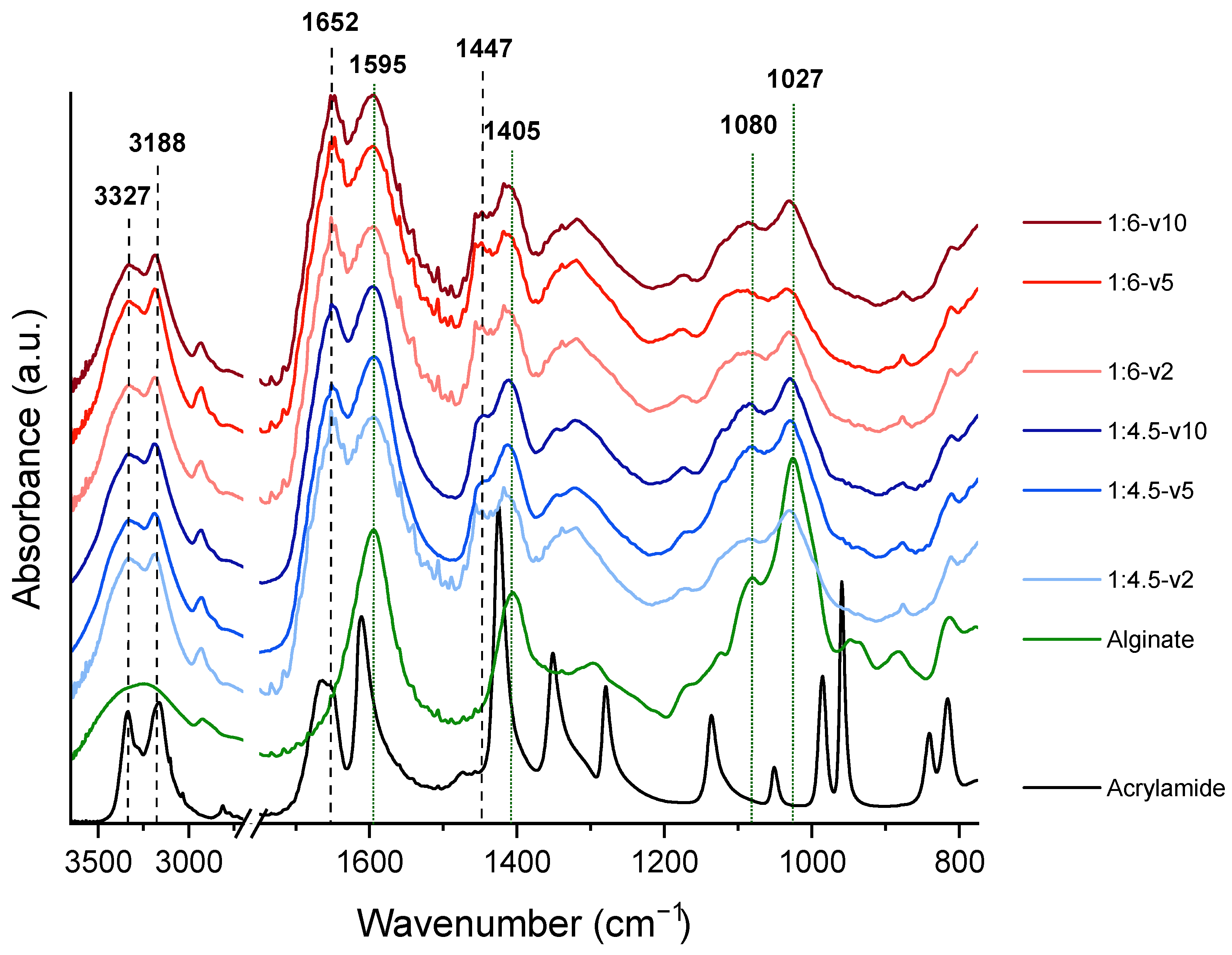
| Composition of Alginate/Acrylamide | Acrylamide (g) | Cross-Linker (g) | Sodium Alginate (g) | LAP (g) |
|---|---|---|---|---|
| 1:4.5 | 3.525 | 0.0225 | 0.7833 | 0.01 |
| 1:6 | 3.525 | 0.0225 | 0.5875 | 0.01 |
| Sample Name | Polyacrylamide–Alginate Proportion | UV Lamp Velocity (m/s) | Frequency Range (Hz) | Holding Time (s) |
|---|---|---|---|---|
| 1:4.5 v2 | 1:4.5 | 2 | 0.01–100 | 1200 |
| 1:4.5 v5 | 1:4.5 | 5 | 0.01–100 | 1200 |
| 1:4.5 v10 | 1:4.5 | 10 | 0.01–100 | 1200 |
| 1:6 v2 | 1:6 | 2 | 0.01–100 | 1200 |
| 1:6 v5 | 1:6 | 5 | 0.01–100 | 1200 |
| 1:6 v10 | 1:6 | 10 | 0.01–100 | 1200 |
| Composition | UV Lamp Velocity | Ratio with C=O Stretching [1652 cm−1] of Polyacrylamide | ||
| COO Asymmetric [1595 cm−1] | COO Symmetric [1405 cm−1] | C-O-C [1027 cm−1] | ||
| 1:4.5 | v2 | 0.98 | 0.76 | 0.67 |
| v5 | 1.10 | 0.78 | 0.88 | |
| v10 | 1.06 | 0.74 | 0.76 | |
| 1:6 | v2 | 0.97 | 0.72 | 0.64 |
| v5 | 1.00 | 0.73 | 0.54 | |
| v10 | 1.00 | 0.72 | 0.67 | |
| Composition | UV Lamp Velocity | Ratio with CH2 Bending [1447 cm−1] of Polyacrylamide | ||
| COO Asymmetric [1595 cm−1] | COO Symmetric [1405 cm−1] | C-O-C [1027 cm−1] | ||
| 1:4.5 | v2 | 1.43 | 1.10 | 1.00 |
| v5 | 1.65 | 1.18 | 1.32 | |
| v10 | 1.69 | 1.17 | 1.20 | |
| 1:6 | v2 | 1.47 | 1.10 | 1.00 |
| v5 | 1.45 | 1.05 | 0.78 | |
| v10 | 1.58 | 1.14 | 1.06 | |
| Polyacrylamide–Alginate Proportion | Manufacturing Process | Fitting Parameters | ||||
|---|---|---|---|---|---|---|
| Short Relaxation Time (s) | Long Relaxation Time (s) | Pre-Exponential Factor, A (-) | (kPa) | (kPa) | ||
| 1:4.5 | 3D printing/v10 | 21 | 380 | 0.51 | 149 | 91 |
| 1:4.5 | 3D printing/v5 | 26 | 390 | 0.50 | 91 | 52 |
| 1:4.5 | 3D printing/v2 | 29 | 460 | 0.44 | 80 | 49 |
| 1:6 | 3D printing/v10 | 24 | 340 | 0.49 | 114 | 65 |
| 1:6 | 3D printing/v5 | 25 | 363 | 0.50 | 92 | 54 |
| 1:6 | 3D printing/v2 | 27 | 430 | 0.46 | 68 | 45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacín-García, R.; Goñi, L.; Río, T.G.-d. Exploring the Rheological Properties of 3D Bioprinted Alginate-Based Hydrogels for Tissue Engineering. Biomimetics 2025, 10, 491. https://doi.org/10.3390/biomimetics10080491
Palacín-García R, Goñi L, Río TG-d. Exploring the Rheological Properties of 3D Bioprinted Alginate-Based Hydrogels for Tissue Engineering. Biomimetics. 2025; 10(8):491. https://doi.org/10.3390/biomimetics10080491
Chicago/Turabian StylePalacín-García, R., L. Goñi, and T. Gómez-del Río. 2025. "Exploring the Rheological Properties of 3D Bioprinted Alginate-Based Hydrogels for Tissue Engineering" Biomimetics 10, no. 8: 491. https://doi.org/10.3390/biomimetics10080491
APA StylePalacín-García, R., Goñi, L., & Río, T. G.-d. (2025). Exploring the Rheological Properties of 3D Bioprinted Alginate-Based Hydrogels for Tissue Engineering. Biomimetics, 10(8), 491. https://doi.org/10.3390/biomimetics10080491






