Photothermal Porous Material with Gradient Hydrophobicity for Fast and Highly Selective Oil/Water Separation and Crude Oil Recovery
Abstract
1. Introduction
2. Materials and Methods
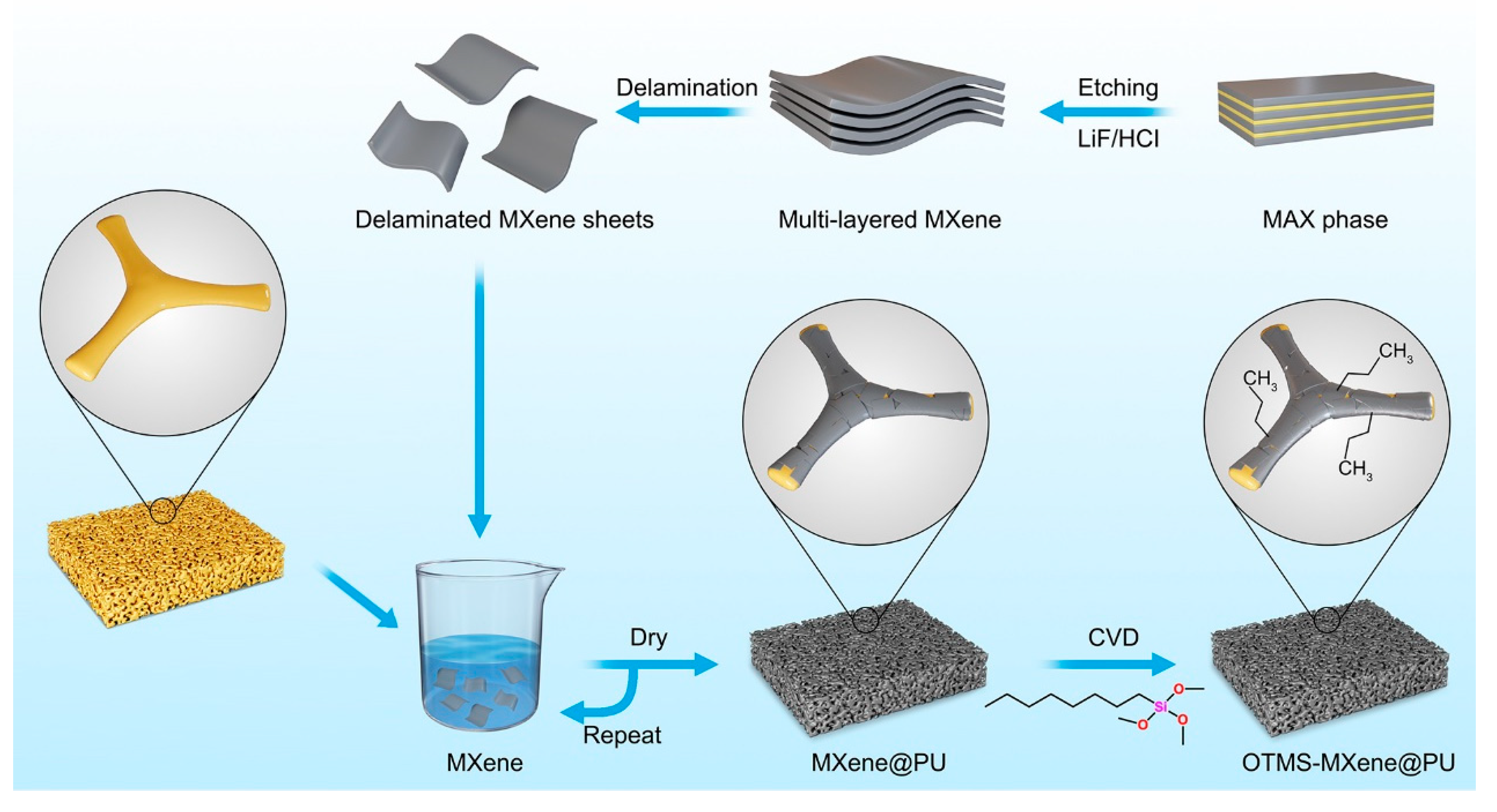
2.1. Preparation of the MXene Nanosheets
2.2. The Coating of MXene Nanosheets on PU Sponge
2.3. Gradient Modification of Silane onto the MXene@PU
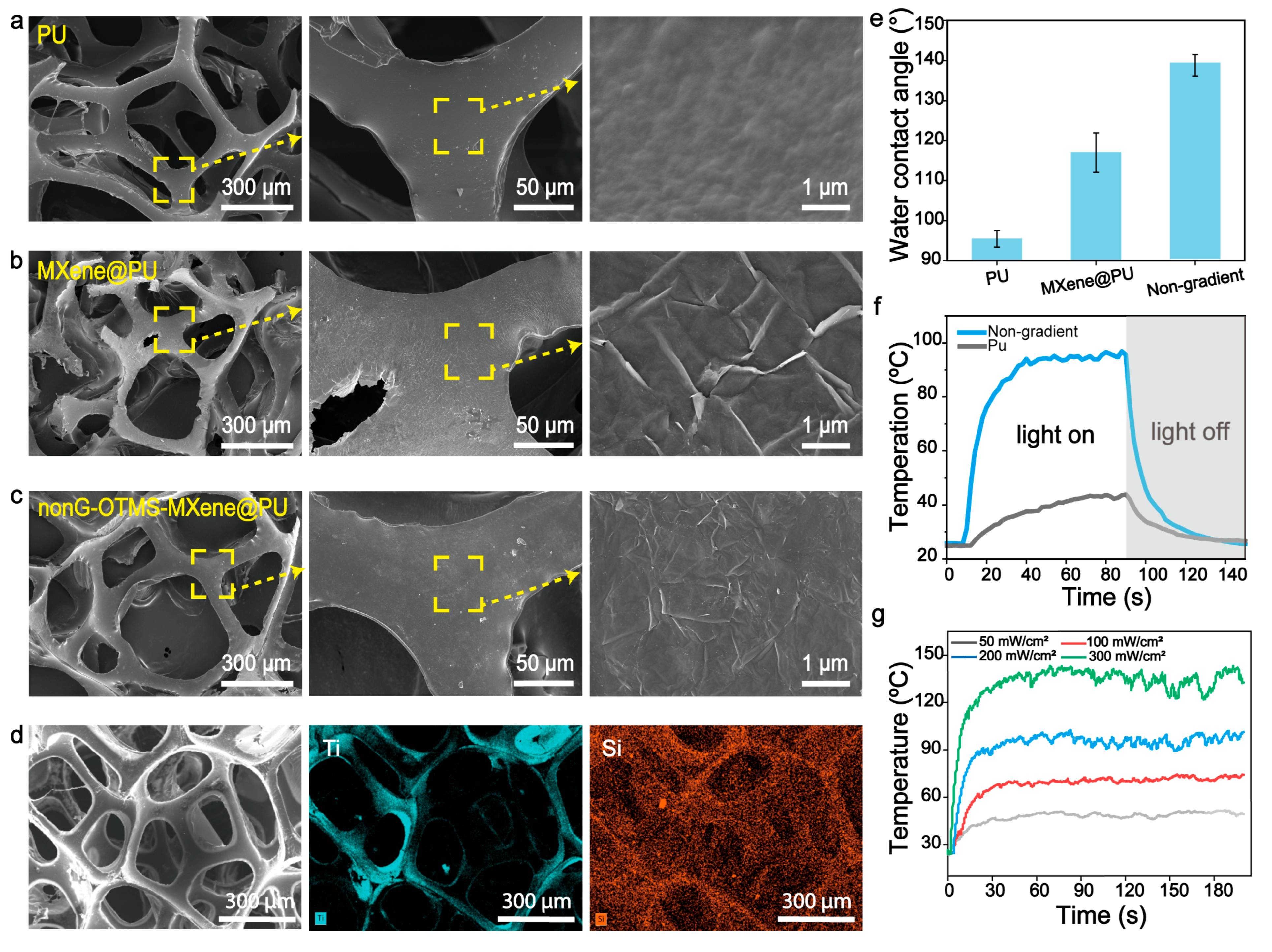
2.4. Characterizations
2.5. Characterization of the Absorption Properties
2.6. Photothermal Heating Assisted Crude Oil Absorption
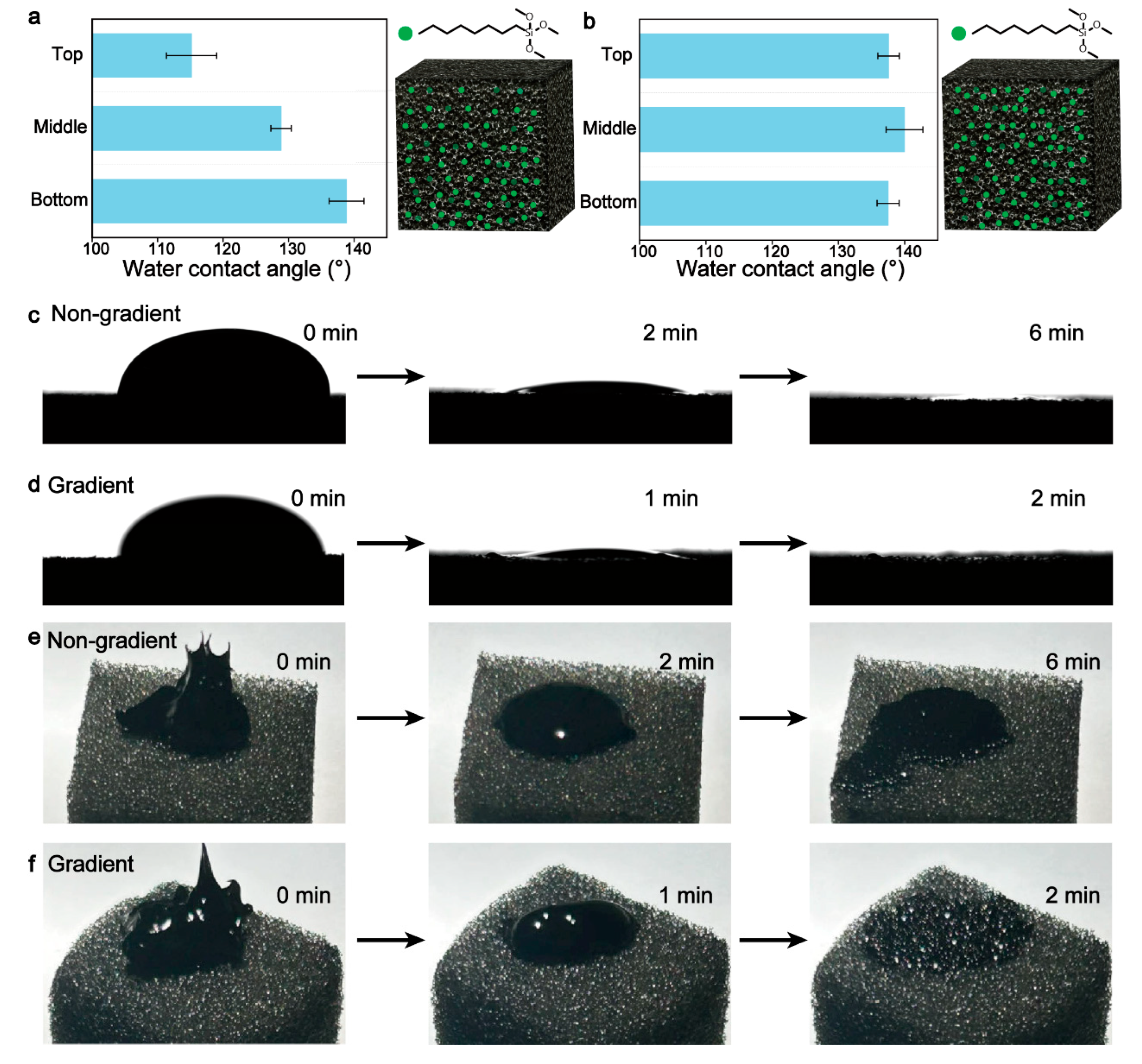
2.7. Characterization of the Influence of Gradient Hydrophobicity on Absorption Rate
2.8. Anti-Oxidation Test of the MXene with or Without Silane Modification
3. Results
3.1. Preparation of the Silane-Modified Photothermal Sponge Absorbent
3.2. Gradient Hydrophobicity Enhanced Oil Absorption Rate
3.3. Versatility and Repeatability of the G-OTMS-MXene@PU Sponges for Highly Selective Oil Absorption
3.4. Anti-Aging Property of the Silane-Modified MXene
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PU | Polyurethane |
| OTMS | Octyltrimethoxysilane |
| DI | Deionized |
| CVD | Chemical vapor deposition |
References
- Peterson, C.H.; Rice, S.D.; Short, J.W.; Esler, D.; Bodkin, J.L.; Ballachey, B.E.; Irons, D.B. Long-Term Ecosystem Response to the Exxon Valdez Oil Spill. Science 2003, 302, 2082–2086. [Google Scholar] [CrossRef]
- Brette, F.; Machado, B.; Cros, C.; Incardona, J.P.; Scholz, N.L.; Block, B.A. Crude Oil Impairs Cardiac Excitation-Contraction Coupling in Fish. Science 2014, 343, 772–776. [Google Scholar] [CrossRef]
- Passow, U.; Overton, E.B. The Complexity of Spills: The Fate of the Deepwater Horizon Oil. Annu. Rev. Mar. Sci. 2021, 13, 109–136. [Google Scholar] [CrossRef]
- Boufadel, M.C.; Özgökmen, T.; Socolofsky, S.A.; Kourafalou, V.H.; Liu, R.; Lee, K. Oil Transport Following the Deepwater Horizon Blowout. Annu. Rev. Mar. Sci. 2023, 15, 67–93. [Google Scholar] [CrossRef] [PubMed]
- Brock, M.L.; Tavares-Reager, J.F.; Dong, J.; Larkin, A.A.; Lam, T.; Pineda, N.; Olivares, C.I.; Mackey, K.R.M.; Martiny, A.C. Bacterial Response to the 2021 Orange County, California, Oil Spill Was Episodic but Subtle Relative to Natural Fluctuations. Microbiol. Spectr. 2024, 12, e02267-24. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Zhuang, L.; Shi, L.; Zhang, S. Study on the Critical Factors and Hot Spots of Crude Oil Tanker Accidents. Ocean. Coast. Manag. 2022, 217, 106010. [Google Scholar] [CrossRef]
- Tanudjaja, H.J.; Hejase, C.A.; Tarabara, V.V.; Fane, A.G.; Chew, J.W. Membrane-Based Separation for Oily Wastewater: A Practical Perspective. Water Res. 2019, 156, 347–365. [Google Scholar] [CrossRef]
- Chu, Z.; Feng, Y.; Seeger, S. Oil/Water Separation with Selective Superantiwetting/Superwetting Surface Materials. Angew. Chem. Int. Ed. 2015, 54, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, N.; Cao, Y.; Lin, X.; Liu, Y.; Feng, L. Superwetting Porous Materials for Wastewater Treatment: From Immiscible Oil/Water Mixture to Emulsion Separation. Adv. Mater. Interfaces 2017, 4, 1600029. [Google Scholar] [CrossRef]
- Kujawinski, E.B.; Kido Soule, M.C.; Valentine, D.L.; Boysen, A.K.; Longnecker, K.; Redmond, M.C. Fate of Dispersants Associated with the Deepwater Horizon Oil Spill. Environ. Sci. Technol. 2011, 45, 1298–1306. [Google Scholar] [CrossRef]
- Dou, Y.; Tian, D.; Sun, Z.; Liu, Q.; Zhang, N.; Kim, J.H.; Jiang, L.; Dou, S.X. Fish Gill Inspired Crossflow for Efficient and Continuous Collection of Spilled Oil. ACS Nano 2017, 11, 2477–2485. [Google Scholar] [CrossRef]
- Prendergast, D.P.; Gschwend, P.M. Assessing the Performance and Cost of Oil Spill Remediation Technologies. J. Clean. Prod. 2014, 78, 233–242. [Google Scholar] [CrossRef]
- Gelderen, L.V.; Brogaard, N.L.; Sørensen, M.X.; Fritt-Rasmussen, J.; Rangwala, A.S.; Jomaas, G. Importance of the Slick Thickness for Effective In-Situ Burning of Crude Oil. Fire Saf. J. 2015, 78, 1–9. [Google Scholar] [CrossRef]
- Fritt-Rasmussen, J.; Linnebjerg, J.F.; Sørensen, M.X.; Brogaard, N.L.; Riget, F.F.; Kristensen, P.; Jomaas, G.; Boertmann, D.M.; Wegeberg, S.; Gustavson, K. Effects of Oil and Oil Burn Residues on Seabird Feathers. Mar. Pollut. Bull. 2016, 109, 446–452. [Google Scholar] [CrossRef]
- Liu, L.; Wang, W.; Hong, Y. A cost-effective and high efficient Janus membrane for the treatment of oily brine using membrane distillation. Nanotechnology 2024, 35, 305703. [Google Scholar] [CrossRef]
- Song, P.; Cui, J.; Di, J.; Liu, D.; Xu, M.; Tang, B.; Zeng, Q.; Xiong, J.; Wang, C.; He, Q.; et al. Carbon Microtube Aerogel Derived from Kapok Fiber: An Efficient and Recyclable Sorbent for Oils and Organic Solvents. ACS Nano 2020, 14, 595–602. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, C.; Hu, Y.; Cheng, H.; Shi, G.; Qu, L. A Versatile, Ultralight, Nitrogen-Doped Graphene Framework. Angew. Chem. Int. Ed. 2012, 51, 11371–11375. [Google Scholar] [CrossRef]
- Luo, Z.; Huang, S.; Kong, N.; Zhang, J.; Tao, J.; Li, J.; Li, S. Hydrophobic dual-polymer–reinforced graphene composite aerogel for efficient water–oil separation. RSC Adv. 2025, 15, 1–13. [Google Scholar] [CrossRef]
- Jiang, S.; Agarwal, S.; Greiner, A. Low-Density Open Cellular Sponges as Functional Materials. Angew. Chem. Int. Ed. 2017, 56, 15520–15525. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, M.; Azizian, S. Synthesis of a Novel Highly Oleophilic and Highly Hydrophobic Sponge for Rapid Oil Spill Cleanup. ACS Appl. Mater. Interfaces 2015, 7, 25326–25333. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; Ai, K.; Li, X.; Lu, L. A Superhydrophobic Sponge with Excellent Absorbency and Flame Retardancy. Angew. Chem. Int. Ed. 2014, 53, 5556–5560. [Google Scholar] [CrossRef]
- Guan, H.; Cheng, Z.; Wang, X. Highly Compressible Wood Sponges with a Spring-like Lamellar Structure as Effective and Reusable Oil Absorbents. ACS Nano 2018, 12, 10365–10373. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Q.; Zhan, X.; Chen, F. Renewable, Biomass-Derived, Honeycomb-Like Aerogel as a Robust Oil Absorbent with Two-Way Reusability. ACS Sustain. Chem. Eng. 2017, 5, 10307–10316. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Liu, K.; Jiang, L. Bioinspired Multifunctional Foam with Self-Cleaning and Oil/Water Separation. Adv. Funct. Mater. 2013, 23, 2881–2886. [Google Scholar] [CrossRef]
- Bandura, L.; Woszuk, A.; Kołodynska, D.; Franus, W. Application of Mineral Sorbents for Removal of Petroleum Substances: A Review. Minerals 2017, 7, 37. [Google Scholar] [CrossRef]
- Ge, J.; Shi, L.A.; Wang, Y.C.; Zhao, H.Y.; Yao, H.B.; Zhu, Y.B.; Zhang, Y.; Zhu, H.W.; Wu, H.A.; Yu, S.H. Joule-Heated Graphene-Wrapped Sponge Enables Fast Clean-Up of Viscous Crude-Oil Spill. Nat. Nanotechnol. 2017, 12, 434–440. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; Wu, X.; You, H.; Yang, G.; Zhang, K.; Sun, C.; Liu, S. A Degradable Photo-Thermal Aerogel with Biomimetic Structure and Selective Wettability for High-Throughput Recovery of Viscous Crude Oil. Adv. Funct. Mater. 2025, 35, 2425886. [Google Scholar] [CrossRef]
- Kuang, Y.; Chen, C.; Chen, G.; Pei, Y.; Pastel, G.; Jia, C.; Song, J.; Mi, R.; Yang, B.; Das, S.; et al. Bioinspired Solar-Heated Carbon Absorbent for Efficient Cleanup of Highly Viscous Crude Oil. Adv. Funct. Mater. 2019, 29, 1900162. [Google Scholar] [CrossRef]
- Gong, C.; Lao, J.; Wang, B.; Li, X.; Li, G.; Gao, J.; Wan, Y.; Sun, X.; Guo, R.; Luo, J. Fast and All-Weather Cleanup of Viscous Crude-Oil Spills with Ti3C2Tx MXene Wrapped Sponge. J. Mater. Chem. A 2020, 8, 20162–20167. [Google Scholar] [CrossRef]
- Khalilifard, M.; Javadian, S. Magnetic Superhydrophobic Polyurethane Sponge Loaded with Fe3O4@Oleic Acid@Graphene Oxide as High-Performance Absorbent Oil from Water. Chem. Eng. J. 2021, 402, 127369. [Google Scholar] [CrossRef]
- Wu, N.; Yang, Y.; Wang, C.; Wu, Q.; Pan, F.; Zhang, R.; Liu, J.; Zeng, Z. Ultrathin Cellulose Nanofiber Assisted Ambient-Pressure-Dried, Ultralight, Mechanically Robust, Multifunctional MXene Aerogels. Adv. Mater. 2022, 34, 2207969. [Google Scholar] [CrossRef]
- Qiao, A.; Huang, R.; Penkova, A.; Qi, W.; He, Z.; Su, R. Superhydrophobic, Elastic and Anisotropic Cellulose Nanofiber Aerogels for Highly Effective Oil/Water Separation. Sep. Purif. Technol. 2022, 301, 121266. [Google Scholar] [CrossRef]
- Parker, A.R.; Lawrence, C.R. Water Capture by a Desert Beetle. Nature 2001, 414, 33–34. [Google Scholar] [CrossRef]
- Yu, Z.; Yun, F.F.; Wang, Y.; Yao, L.; Dou, S.; Liu, K.; Jiang, L.; Wang, X. Desert Beetle-Inspired Superwettable Patterned Surfaces for Water Harvesting. Small 2017, 13, 1701403. [Google Scholar] [CrossRef]
- Hou, L.; Liu, X.; Ge, X.; Hu, R.; Cui, Z.; Wang, N.; Zhao, Y. Designing of Anisotropic Gradient Surfaces for Directional Liquid Transport: Fundamentals, Construction, and Applications. Innovation 2023, 4, 100508. [Google Scholar] [CrossRef]
- Zhao, N.; Li, M.; Gong, H.; Bai, H. Controlling Ice Formation on Gradient Wettability Surface for High-Performance Bioinspired Materials. Sci. Adv. 2020, 6, eabb4712. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Luo, Y.-Q.; Liu, L.; Li, Y.; Yao, X.; Ju, J.; Jiang, L. Ultra-Fast, Unidirectional Water Absorption on Wood Ear. Adv. Mater. 2025, 37, 2413364. [Google Scholar] [CrossRef]
- Zhao, X.; Vashisth, A.; Blivin, J.W.; Tan, Z.; Holta, D.E.; Kotasthane, V.; Shah, S.A.; Habib, T.; Liu, S.; Lutkenhaus, J.L.; et al. pH, Nanosheet Concentration, and Antioxidant Affect the Oxidation of Ti3C2Tx and Ti2CTx MXene Dispersions. Adv. Mater. Interfaces 2020, 7, 2000845. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, L.; Lao, J.; Luo, K.; Liu, X.; Sui, K.; Gao, J.; Jiang, L. Reliable and Low Temperature Actuation of Water and Oil Slugs in Janus Photothermal Slippery Tube. ACS Appl. Mater. Interfaces 2022, 14, 17968–17974. [Google Scholar] [CrossRef]
- Cao, J.; Liu, L.; Liu, C.; He, C. Phase transition mechanisms of paraffin in waxy crude oil in the absence and presence of pour point depressant. J. Mol. Liq. 2022, 345, 116989. [Google Scholar] [CrossRef]
- Kanaveli, I.-P.; Atzemi, M.; Lois, E. Predicting the Viscosity of Diesel/Biodiesel Blends. Fuel 2017, 199, 248–263. [Google Scholar] [CrossRef]
- Kumar, S.; Kumari, N.; Singh, T.; Seo, Y. Shielding 2D MXenes against Oxidative Degradation: Recent Advances, Factors and Preventive Measures. J. Mater. Chem. C 2024, 12, 8243–8281. [Google Scholar] [CrossRef]
- Soomro, R.A.; Zhang, P.; Fan, B.; Wei, Y.; Xu, B. Progression in the oxidation stability of MXenes. Nano-Micro Lett. 2023, 15, 106. [Google Scholar] [CrossRef]
- Iqbal, A.; Hong, J.; Ko, T.Y.; Koo, C.M. Improving oxidation stability of 2D MXenes: Synthesis, storage media, and conditions. Nano Converg. 2021, 8, 9. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Song, S.; Bao, S.; Gong, Y.; Wang, Y.; Wang, C.; Ma, W.; Liu, N.; Sui, K.; Gao, J.; et al. Photothermal Porous Material with Gradient Hydrophobicity for Fast and Highly Selective Oil/Water Separation and Crude Oil Recovery. Biomimetics 2025, 10, 585. https://doi.org/10.3390/biomimetics10090585
Wang T, Song S, Bao S, Gong Y, Wang Y, Wang C, Ma W, Liu N, Sui K, Gao J, et al. Photothermal Porous Material with Gradient Hydrophobicity for Fast and Highly Selective Oil/Water Separation and Crude Oil Recovery. Biomimetics. 2025; 10(9):585. https://doi.org/10.3390/biomimetics10090585
Chicago/Turabian StyleWang, Tianwen, Song Song, Shiwen Bao, Yanfeng Gong, Yujue Wang, Chuncai Wang, Wenshao Ma, Nuo Liu, Kunyan Sui, Jun Gao, and et al. 2025. "Photothermal Porous Material with Gradient Hydrophobicity for Fast and Highly Selective Oil/Water Separation and Crude Oil Recovery" Biomimetics 10, no. 9: 585. https://doi.org/10.3390/biomimetics10090585
APA StyleWang, T., Song, S., Bao, S., Gong, Y., Wang, Y., Wang, C., Ma, W., Liu, N., Sui, K., Gao, J., & Liu, X. (2025). Photothermal Porous Material with Gradient Hydrophobicity for Fast and Highly Selective Oil/Water Separation and Crude Oil Recovery. Biomimetics, 10(9), 585. https://doi.org/10.3390/biomimetics10090585





