Deep Learning-Based Evaluation of Postural Control Impairments Caused by Stroke Under Altered Sensory Conditions
Abstract
1. Introduction
- A:
- Achieving rapid and accurate classification of postural signals under different sensory conditions using deep learning techniques, enabling real-time or near-real-time assessment in clinical settings.
- B:
- Demonstrating the advantages of deep learning-based models over traditional statistical approaches in analyzing complex physiological signals with high-dimensional and nonlinear characteristics.
- C:
- Eliminating the need for manual feature selection by leveraging automated feature extraction capabilities of convolutional neural networks.
- D:
- Evaluating the performance of the proposed hybrid model (CNN + Type-2 Fuzzy Logic) under diverse uncertainties including sensor noise and inter-subject variability, which commonly occur in real-world clinical data.
- E:
- Providing a practical modeling approach for the classification of balance impairments based on raw or minimally pre-processed center of pressure (CoP) signals, thereby simplifying the processing pipeline.
- F:
- Integrating a deep CNN with Type-2 fuzzy logic to enhance robustness and algorithmic stability in postural classification tasks, particularly under noisy or uncertain measurement conditions.
2. Related Works
2.1. Clinical Studies and Prior Applications
2.2. AI-Based Approaches for Postural Assessment
2.2.1. CNN-Based Approaches
2.2.2. Hybrid CNN-RNN Models
2.2.3. Fuzzy Logic and Type-1 Systems
2.2.4. Hybrid Deep Fuzzy Models
2.2.5. Gap and Motivation for This Study
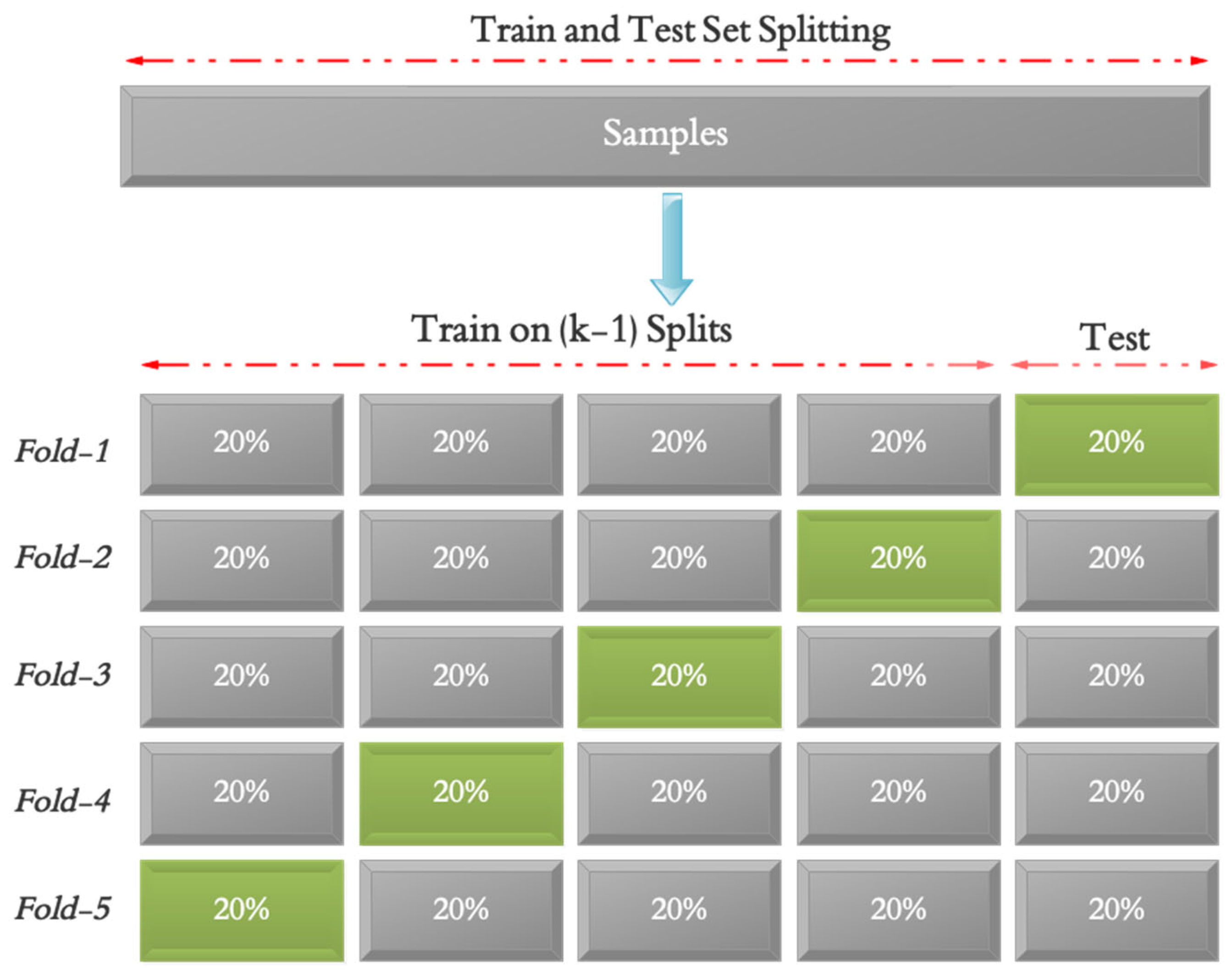
3. Methodology
3.1. Problem Statement
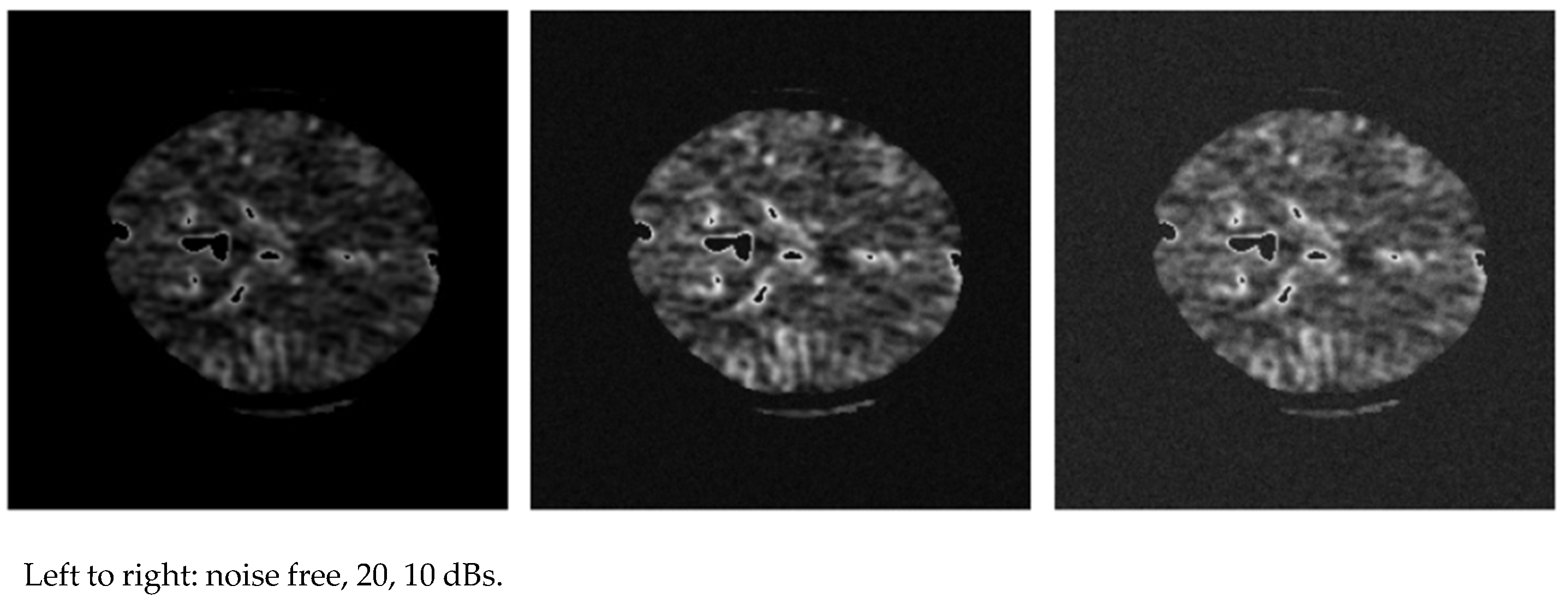
- Mathematical formulation: Let X ∈ RN×T×C denote the multichannel time-series data of CoP signals, where N is the number of samples, T is the number of time steps, and C is the number of sensor channels. Each Xi corresponds to one of the six standardized Sensory Organization Test (SOT) scenarios, recorded via the EquiTest dynamic posturography system from either a healthy control or a stroke patient. The task is to learn a function: fθ:Xi ↦ yi where yi ∈ {0,1} represents the binary class label: 0 for healthy postural control and 1 for impaired postural control. The objective is to optimize the parameters θ of an Artificial Neural Network (ANN)-based model to minimize classification error while achieving high generalization across all sensory conditions and participant variability. Model performance is assessed using accuracy, precision, sensitivity, and specificity, with robustness further validated under Gaussian noise at SNR levels from 20 dB to 1 dB.
3.2. Overview of the Proposed Approach
3.3. Data Collection
3.4. Algorithms Employed in the Proposed Model
3.4.1. Deep Learning Networks
3.4.2. Convolutional Neural Networks
3.4.3. Fuzzy Sets
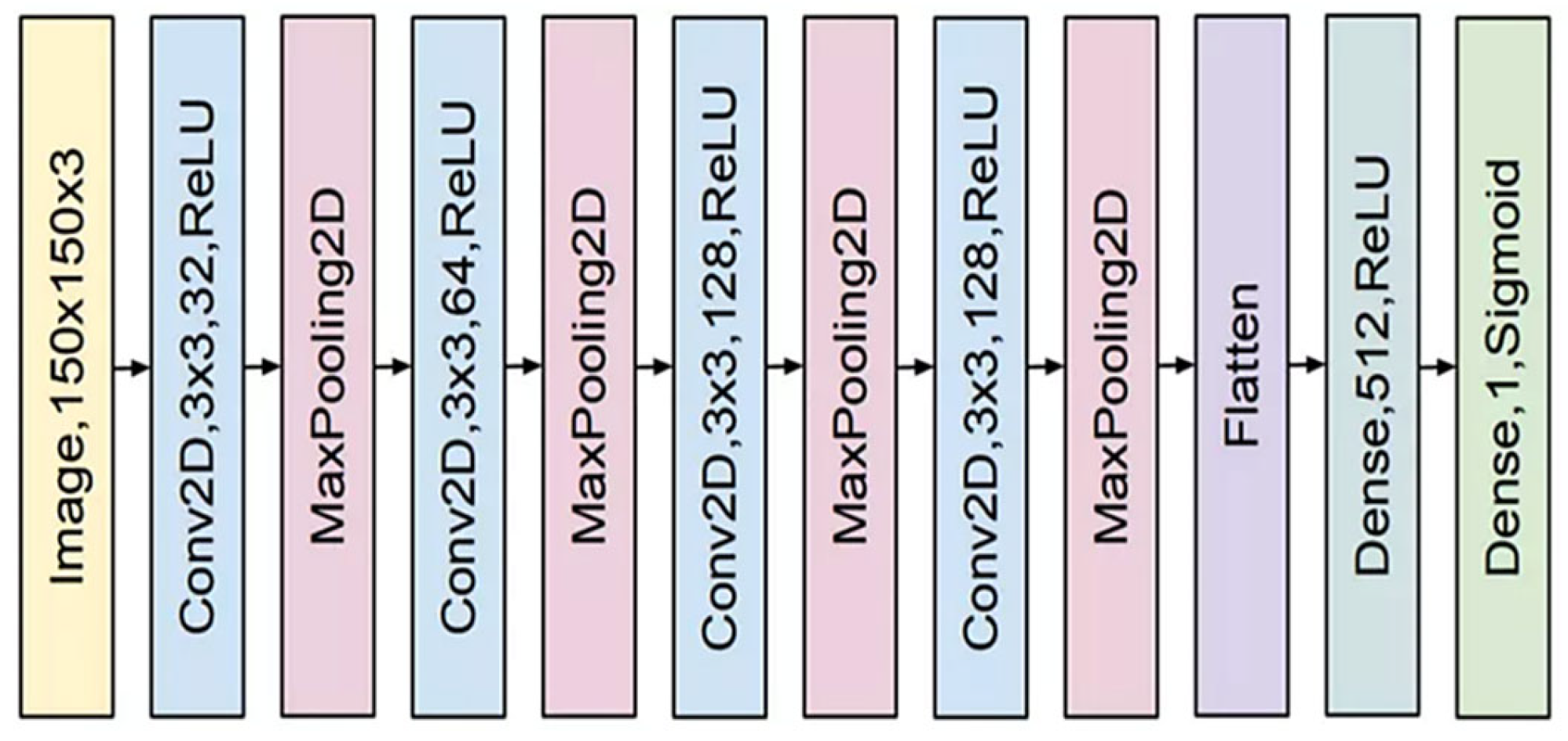
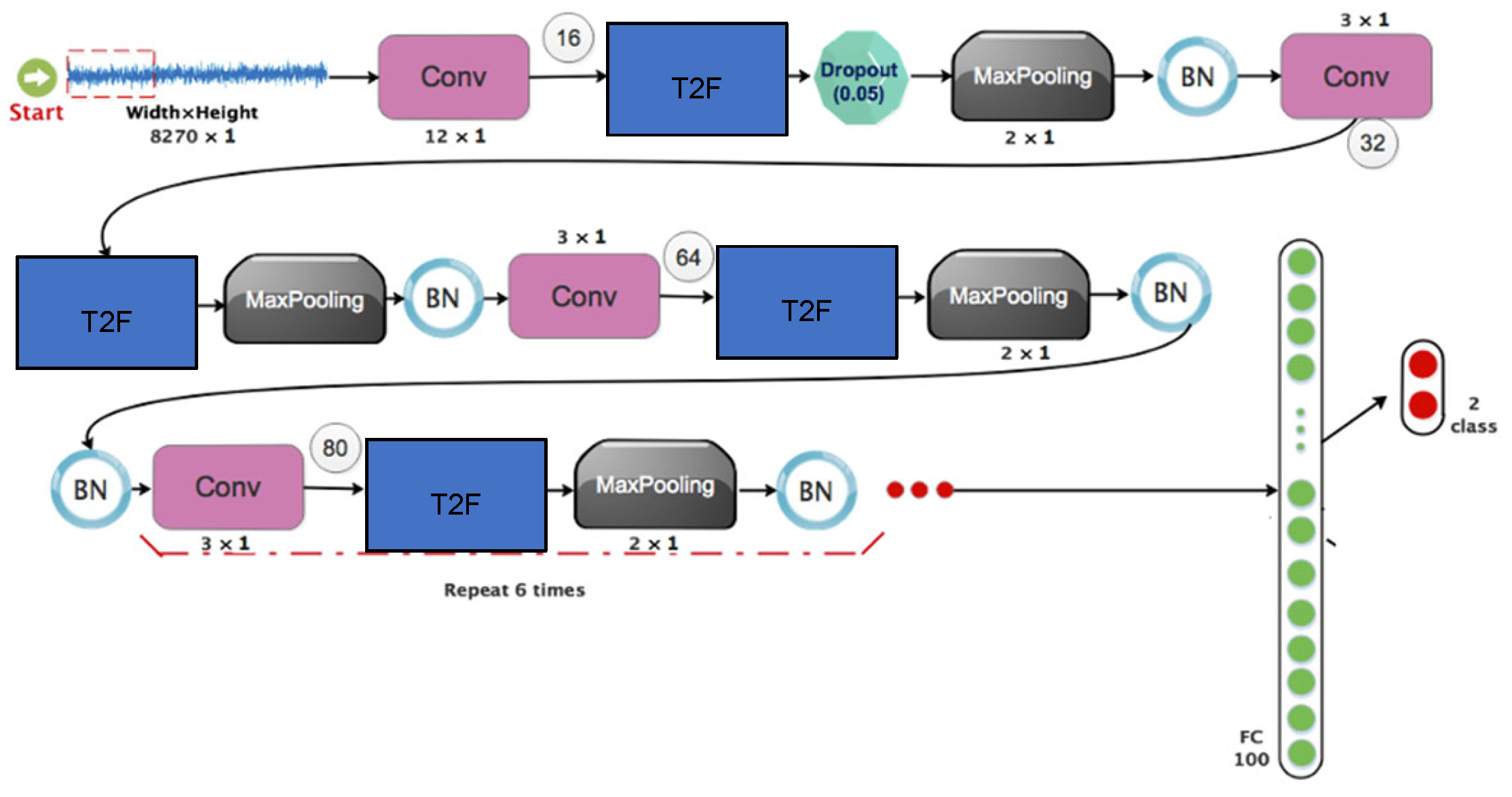
4. Proposed Method
- (a)
- A convolutional layer that combines a batch normalizer layer, a maximum merging layer, a dropout layer, and a Leakey-ReLU function.
- (b)
- In the preceding stage, the architecture is replicated nine more times.
- (c)
- Two 2D matrices are linked to the outcomes of the last iteration.
- (d)
- Each output is categorized and given a score using two completely linked layers.
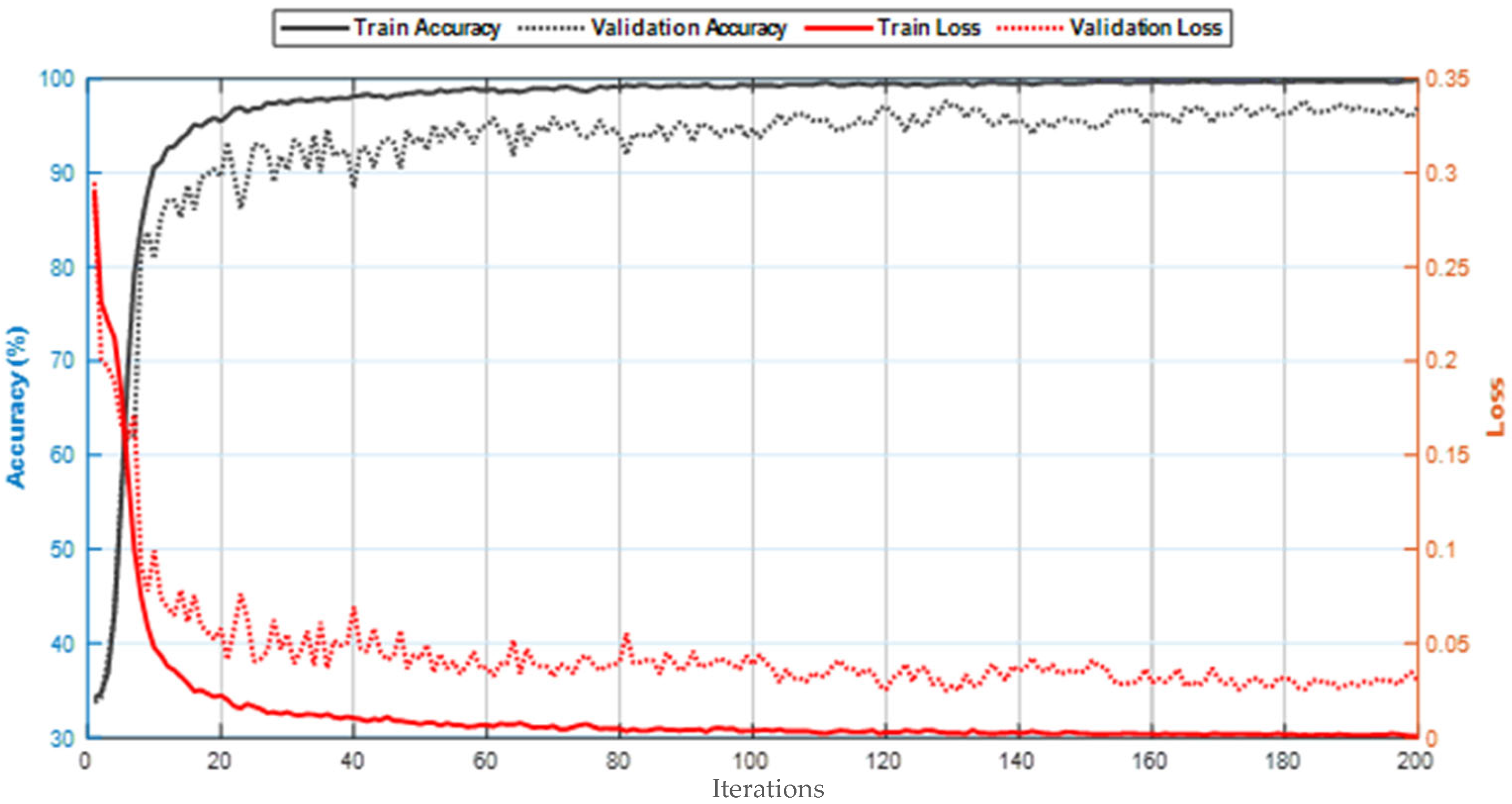
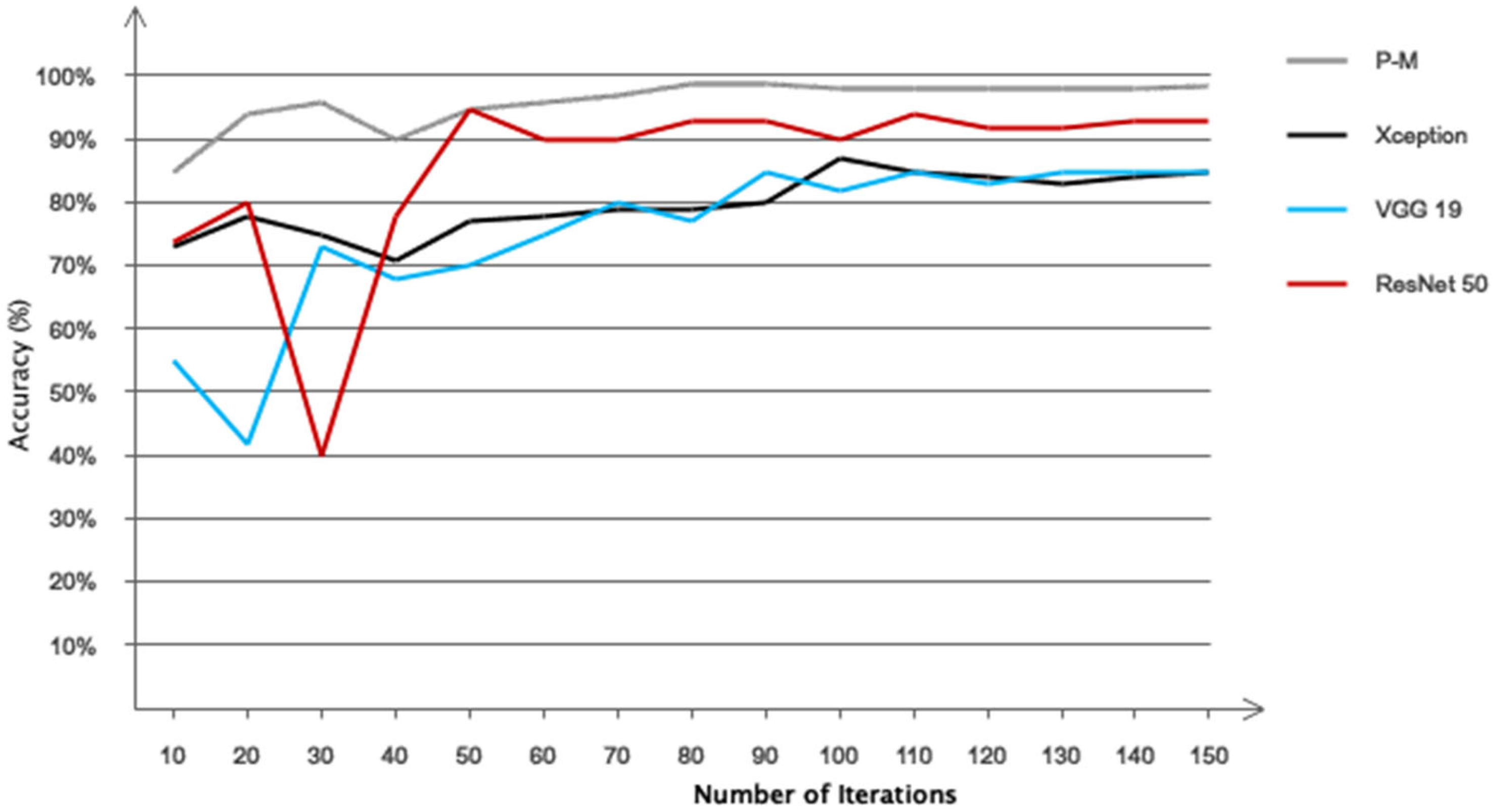
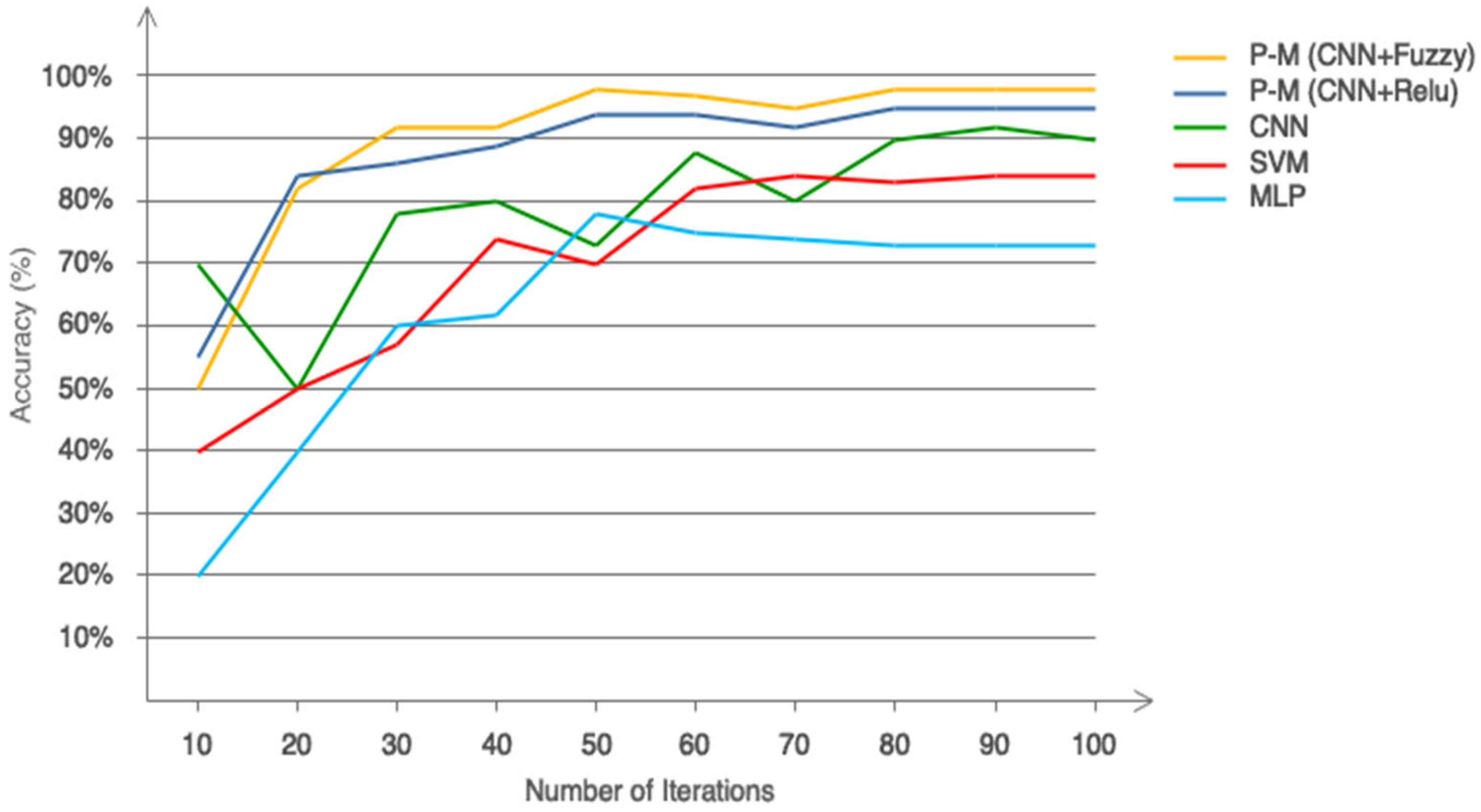
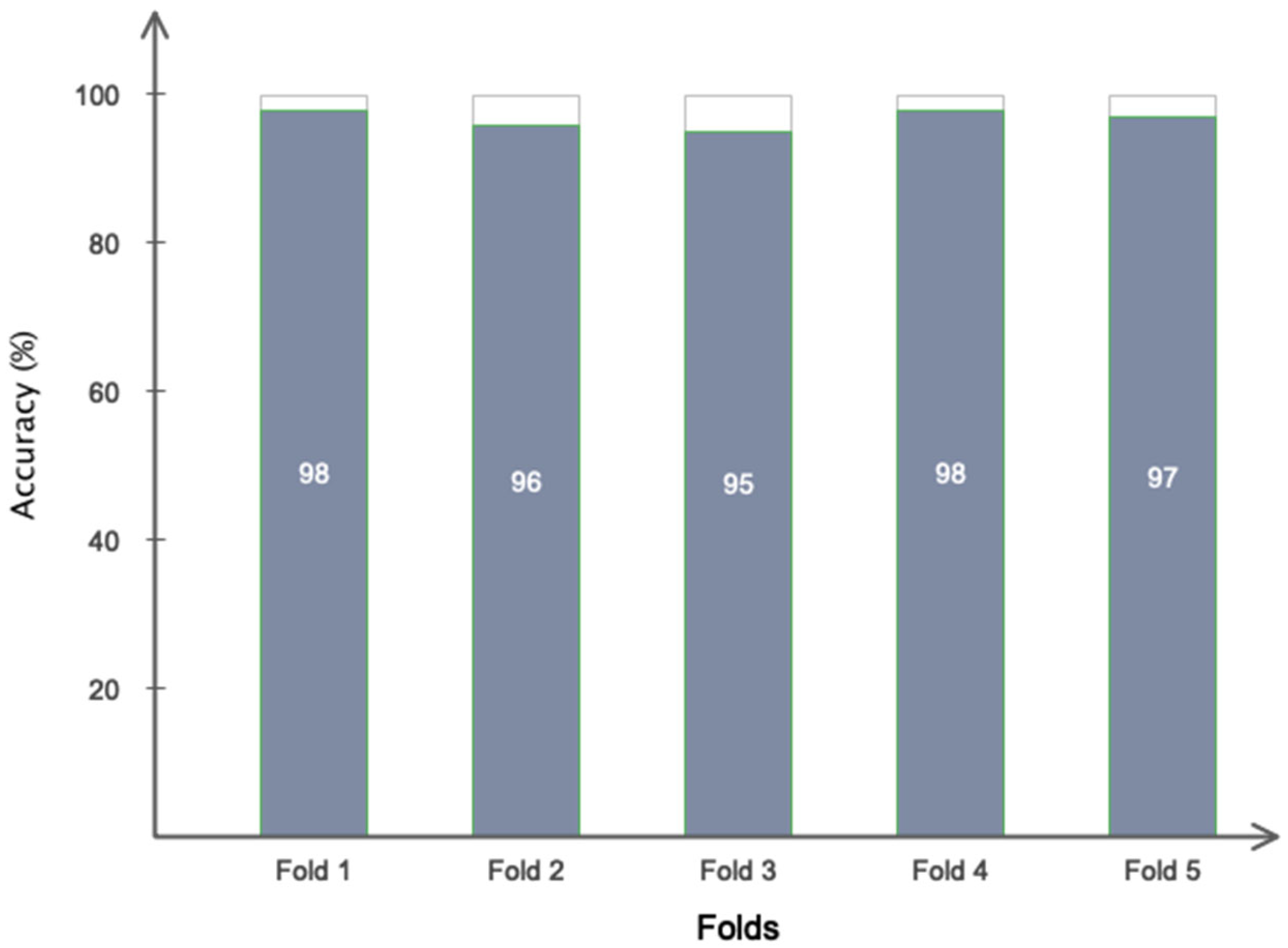
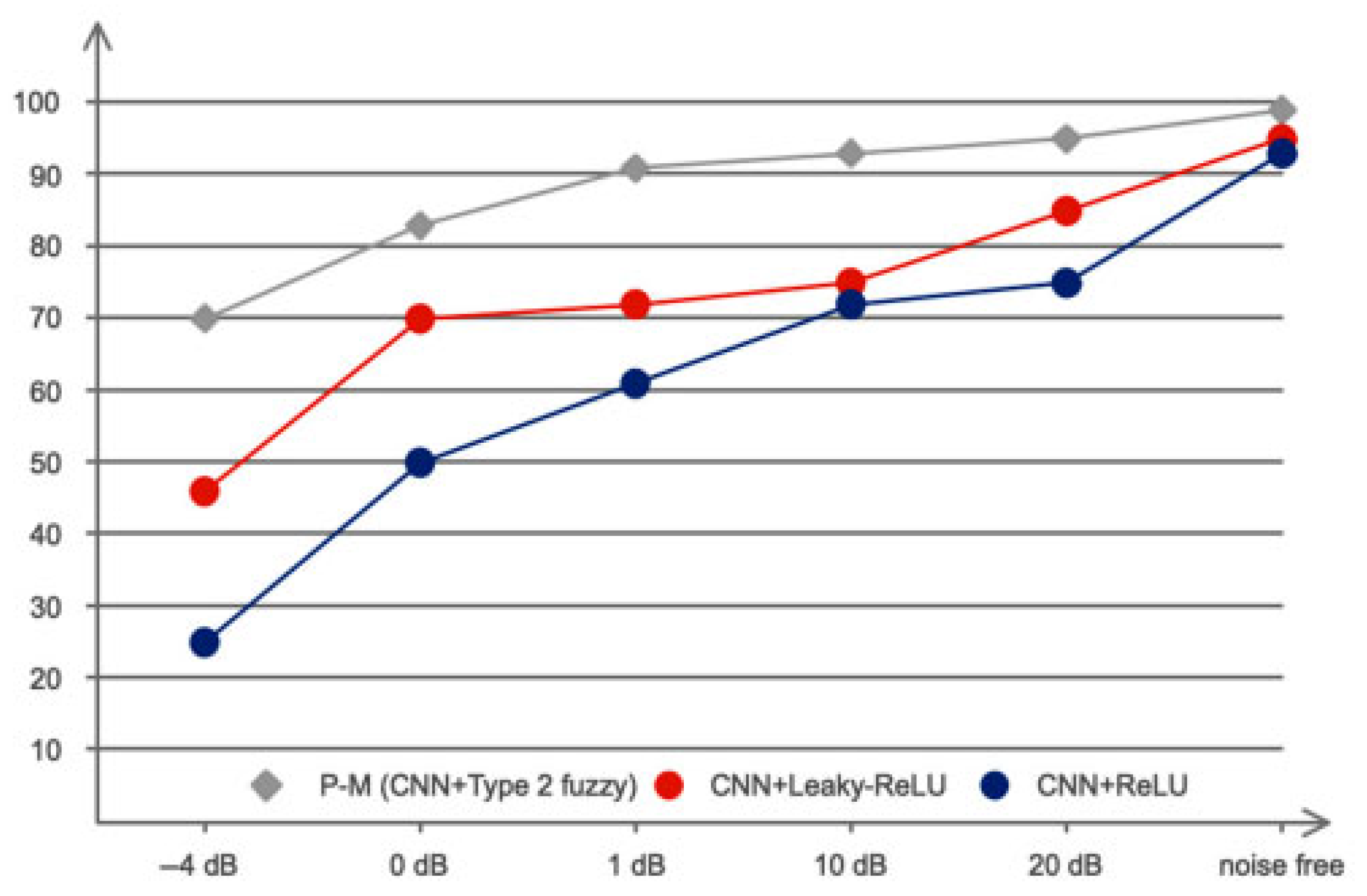
5. Results
6. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Symbol | Definition |
| X | Input matrix or multichannel time-series data of CoP signals |
| X(i,j) | Element of the input matrix at row i and column j |
| y | Output label (binary classification: 0 = healthy, 1 = impaired) |
| ŷᵢ | Predicted class label for sample i |
| K | Convolution kernel (filter)—a small matrix of learnable weights (e.g., 3 × 3) |
| K(m,n) | Element of the convolution kernel at the m-th row and n-th column |
| S(i,j) | Value in the feature map at position (i,j), representing activation of a learned feature |
| θ | Trainable parameters of the ANN model |
| pᵢ | Probability assigned by the model to class i |
| μA(x) | Membership function value of element x in fuzzy set A (Type-1) |
| Ã | Type-2 fuzzy set |
| μÃ(x,u) | Membership function of a Type-2 fuzzy set, where u is the secondary grade |
| J | Objective or cost function used for training the model |
| E | Error term (e.g., classification error) |
| L | Loss function applied during optimization |
| T | Number of time steps in the time-series input |
| C | Number of channels in the input data |
| N | Number of samples in the dataset |
| fθ | ANN model parameterized by θ |
| ⊗ | Convolution operation |
References
- Arienti, C.; Lazzarini, S.G.; Pollock, A.; Negrini, S. Rehabilitation interventions for improving balance following stroke: An overview of systematic reviews. PLoS ONE 2019, 14, e021978. [Google Scholar] [CrossRef]
- Seyedkhamoushi, F.; Sassani Asl, M.; Seyedkhamooshi, R.; Abdollahi, H.R.; Sharifnia, M. Multimodal Smart Eyeglasses for Adaptive Vision, Predictive Ocular and Hemodynamic Monitoring, and Emergency Response. World Intellectual Property Organization Patent WO2025163631A1, 2025. Available online: https://patents.google.com/patent/WO2025163631A1/en/ (accessed on 18 August 2025).
- Blaszczyk, J.W.; Prince, F.; Raiche, M.; Hébert, R. Effect of ageing and vision on limb load asymmetry during quiet stance. J. Biomech. 2000, 33, 1243–1248. [Google Scholar] [CrossRef]
- Ghnemat, R.; Khalil, A.; Abu Al-Haija, Q. Ischemic stroke lesion segmentation using mutation model and generative adversarial network. Electronics 2023, 12, 590. [Google Scholar] [CrossRef]
- Soltanpour, M.; Greiner, R.; Boulanger, P.; Buck, B. Improvement of automatic ischemic stroke lesion segmentation in CT perfusion maps using a learned deep neural network. Comput. Biol. Med. 2021, 137, 104849. [Google Scholar] [CrossRef] [PubMed]
- Sadura-Sieklucka, T.; Czerwosz, L.T.; Kądalska, E.; Kożuchowski, M.; Księżopolska-Orłowska, K.; Targowski, T. Is balance training using biofeedback effective in the prophylaxis of falls in women over the age of 65? Brain Sci. 2023, 13, 629. [Google Scholar] [CrossRef] [PubMed]
- Talis, V.; Kazennikov, O. Effects of body turn on postural sway during symmetrical and asymmetrical standing. Exp. Brain Res. 2019, 237, 2231–2237. [Google Scholar] [CrossRef]
- Vouriot, A.; Gauchard, G.C.; Chau, N.; Benamghar, L.; Lepori, M.-L.; Mur, J.-M.; Perrin, P.P. Sensorial organisation favouring higher visual contribution is a risk factor of falls in an occupational setting. Neurosci. Res. 2004, 48, 239–247. [Google Scholar] [CrossRef]
- Gray, R.; Kouhy, R.; Lavers, S. Corporate social and environmental reporting: A review of the literature and a longitudinal study of UK disclosure. Account. Audit. Account. J. 1995, 8, 47–77. [Google Scholar] [CrossRef]
- Kim, S.; Moon, J. The effect of visual biofeedback balance training on time to stabilization and kinetic variables in patients with chronic ankle instability. Korean J. Sport Sci. 2022, 33, 359–368. [Google Scholar] [CrossRef]
- Chamberlin, C.; Marmelat, V.; Rosen, A.B.; Burcal, C.J. The effects of visual biofeedback and visual biofeedback scale size on single limb balance. J. Bodyw. Mov. Ther. 2021, 26, 268–272. [Google Scholar] [CrossRef]
- Nichols, D.S. Balance retraining after stroke using force platform biofeedback. Phys. Ther. 2002, 77, 553–558. [Google Scholar] [CrossRef]
- Chiari, L.; Rocchi, L.; Cappello, A. Stabilometric parameters are affected by anthropometry and foot placement. Clin. Biomech. 2002, 17, 666–677. [Google Scholar] [CrossRef]
- Norris, J.A.; Marsh, A.P.; Smith, I.J.; Kohut, R.I.; Miller, M.E. Ability of static and statistical mechanics posturographic measures to distinguish between age and fall risk. J. Biomech. 2004, 38, 1263–1272. [Google Scholar] [CrossRef]
- Li, L.; Zhang, S.; Dobson, J. The contribution of small and large sensory afferents to postural control in patients with peripheral neuropathy. J. Sport Health Sci. 2019, 8, 218–227. [Google Scholar] [CrossRef]
- Keyvanara, M.; Sadigh, M.J.; Meijer, K.; Esfahanian, M. A model of human postural control inspired by separated human sensory systems. Biocybern. Biomed. Eng. 2021, 41, 255–264. [Google Scholar] [CrossRef]
- Richmond, S.B.; Whittier, T.T.; Peterson, D.S.; Fling, B.W. Advanced characterization of static postural control dysfunction in persons with multiple sclerosis and associated neural mechanisms. Gait Posture 2021, 83, 114–120. [Google Scholar] [CrossRef]
- De Oliveira, M.R.; Fabrin, L.F.; de Oliveira Gil, A.W.; Benassi, G.H.; Camargo, M.Z.; da Silva, R.A.; de Lima, R.R. Acute effect of core stability and sensory-motor exercises on postural control during sitting and standing positions in young adults. J. Bodyw. Mov. Ther. 2021, 28, 98–103. [Google Scholar] [CrossRef]
- Tuncer, D.; Gurses, H.N.; Senaran, H.; Uzer, G.; Tuncay, I. Evaluation of postural control in children with increased femoral anteversion. Gait Posture 2022, 95, 109–114. [Google Scholar] [CrossRef]
- Seifi, N.; Ghoodjani, E.; Majd, S.S.; Maleki, A.; Khamoushi, S. Evaluation and Prioritization of Artificial Intelligence Integrated Block Chain Factors in Healthcare Supply Chain: A Hybrid Decision Making Approach. Comput. Decis. Mak. Int. J. 2025, 2, 374–405. [Google Scholar] [CrossRef]
- Xu, Y.; Zeng, Z.; Oliveria, C.; Munoz, R.; de Almeida, R.; Quezada, A.; de Albuquerque, V.H.C. Postural evaluation based on body movement and mapping sensors. Measurement 2022, 190, 110538. [Google Scholar] [CrossRef]
- Novikov, D.S.; Fieremans, E.; Jespersen, S.N.; Kiselev, V.G. Quantifying Brain Microstructure with Diffusion MRI: Theory and Parameter Estimation. NMR Biomed. 2019, 32, e3998. [Google Scholar] [CrossRef] [PubMed]
- Cherstvy, A.G.; Safdari, H.; Metzler, R. Anomalous Diffusion, Nonergodicity, and Ageing for Exponentially and Logarithmically Time-Dependent Diffusivity: Striking Differences for Massive versus Massless Particles. J. Phys. D Appl. Phys. 2021, 54, 195401. [Google Scholar] [CrossRef]
- Gaurav, K.; Roy, B.; Bharti, J. A Hybrid Deep Learning Model for Human Activity Recognition Using Wearable Sensors. In Machine Intelligence and Smart Systems; Springer: Singapore, 2022; pp. 207–222. [Google Scholar]
- Ahmadkhan, K.; Ahmadirad, Z.; Karaminezhad, K.; SeyedKhamoushi, F.; Karimi, K.; Khakpash, F. A novel block-chain-based approach for enhanced food supply chain traceability and waste mitigation. Br. Food J. 2025, 1–41. [Google Scholar] [CrossRef]
- Sheykhivand, S.; Rezaii, T.Y.; Saatlo, A.N.; Romooz, N. Comparison between different methods of feature extraction in BCI systems based on SSVEP. Int. J. Ind. Math. 2017, 9, 341–347. [Google Scholar]
- Basirat, S.; Raoufi, S.; Bazmandeh, D.; Khamoushi, S.; Entezami, M. Ranking of AI-Based Criteria in Health Tourism Using Fuzzy SWARA Method. Comput. Decis. Mak. Int. J. 2025, 2, 530–545. [Google Scholar] [CrossRef]
- Sabahi, K.; Sheykhivand, S.; Mousavi, Z.; Rajabioun, M. Recognition COVID-19 cases using deep type-2 fuzzy neural networks based on chest X-ray image. Comput. Intell. Electr. Eng. 2023, 14, 75–92. [Google Scholar] [CrossRef]
- Phan, H.; Andreotti, F.; Cooray, N.; Chén, O.Y.; De Vos, M. Joint Classification and Prediction CNN Framework for Automatic Sleep Stage Classification. IEEE Trans. Biomed. Eng. 2018, 66, 1285–1296. [Google Scholar] [CrossRef]
- Sheykhivand, S.; Yousefi Rezaii, T.; Mousavi, Z.; Meshini, S. Automatic stage scoring of single-channel sleep EEG using CEEMD of genetic algorithm and neural network. Comput. Intell. Electr. Eng. 2018, 9, 15–28. [Google Scholar]
- Lin, M.H.; Sassani, M.; Golchin, N.; Jabbari, Y.; Boymatova, Z.; Rustambekovich, J.U.; Ugli, Y.J.E.; Atajanova, S.; Turdiyeva, Y. Optimal Planning and Operation of the Smart Electrical Distribution Network Considering Stochastic Optimization Modeling and Energy Storage Systems. Oper. Res. Forum 2025, 6, 123. [Google Scholar] [CrossRef]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Beke, A.; Kumbasar, T. Learning with Type-2 Fuzzy Activation Functions To Improve the Performance of Deep Neural Networks. Eng. Appl. Artif. Intell. 2019, 85, 372–384. [Google Scholar] [CrossRef]
- Shen, T.; Wang, J.; Gou, C.; Wang, F.-Y. Hierarchical fused model with deep learning and type-2 fuzzy learning for breast cancer diagnosis. IEEE Trans. Fuzzy Syst. 2020, 28, 3204–3218. [Google Scholar] [CrossRef]
- Afsharfard, A.; Jafari, A.; Rad, Y.A.; Tehrani, H.; Kim, K.C. Modifying Vibratory Behavior of the Car Seat to Decrease the Neck Injury. J. Vib. Eng. Technol. 2023, 11, 1115–1126. [Google Scholar] [CrossRef]
- Khatami, S.S.; Shoeibi, M.; Salehi, R.; Kaveh, M. Energy-Efficient and Secure Double RIS-Aided Wireless Sensor Networks: A QoS-Aware Fuzzy Deep Reinforcement Learning Approach. J. Sens. Actuator Netw. 2025, 14, 18. [Google Scholar] [CrossRef]
- Mohammadabadi, S.M.S.; Zawad, S.; Yan, F.; Yang, L. Speed Up Federated Learning in Heterogeneous Environments: A Dynamic Tiering Approach. IEEE Internet Things J. 2024, 12, 5026–5035. [Google Scholar] [CrossRef]
- Sadeghi, S.; Niu, C. Augmenting Human Decision-Making in K-12 Education: The Role of Artificial Intelligence in Assisting the Recruitment and Retention of Teachers of Color for Enhanced Diversity and Inclusivity. Leadersh. Policy Sch. 2024, 1–21. [Google Scholar] [CrossRef]
- Mahdavimanshadi, M.; Anaraki, M.G.; Mowlai, M.; Ahmadirad, Z. A Multistage Stochastic Optimization Model for Resilient Pharmaceutical Supply Chain in COVID-19 Pandemic Based on Patient Group Priority. In Proceedings of the 2024 Systems and Information Engineering Design Symposium (SIEDS), Charlottesville, VA, USA, 3 May 2024; pp. 382–387. [Google Scholar]
- Azadmanesh, M.; Roshanian, J.; Georgiev, K.; Todrov, M.; Hassanalian, M. Synchronization of Angular Velocities of Chaotic Leader-Follower Satellites Using a Novel Integral Terminal Sliding Mode Controller. Aerosp. Sci. Technol. 2024, 150, 109211. [Google Scholar] [CrossRef]
- Ahmadirad, Z. Evaluating the Influence of AI on Market Values in Finance: Distinguishing Between Authentic Growth and Speculative Hype. Int. J. Adv. Res. Humanit. Law 2024, 1, 50–57. [Google Scholar] [CrossRef]
- Sadeghi, S.; Marjani, T.; Hassani, A.; Moreno, J. Development of Optimal Stock Portfolio Selection Model in the Tehran Stock Exchange by Employing Markowitz Mean-Semivariance Model. J. Financ. Issues 2022, 20, 47–71. [Google Scholar] [CrossRef]
- Ahmadirad, Z. The Beneficial Role of Silicon Valley’s Technological Innovations and Venture Capital in Strengthening Global Financial Markets. Int. J. Mod. Achiev. Sci. Eng. Technol. 2024, 1, 9–17. [Google Scholar] [CrossRef]
- Dokhanian, S.; Sodagartojgi, A.; Tehranian, K.; Ahmadirad, Z.; Moghaddam, P.K.; Mohsenibeigzadeh, M. Exploring the Impact of Supply Chain Integration and Agility on Commodity Supply Chain Performance. World J. Adv. Res. Rev. 2024, 22, 441–450. [Google Scholar] [CrossRef]
- Ahmadirad, Z. The Effects of Bitcoin ETFs on Traditional Markets: A Focus on Liquidity, Volatility, and Investor Behavior. Curr. Opin. 2024, 4, 697–706. [Google Scholar] [CrossRef]
- Ahmadirad, Z. The Banking and Investment in the Future: Unveiling Opportunities and Research Necessities for Long-Term Growth. Int. J. Appl. Res. Manag. Econ. Account. 2024, 1, 34–41. [Google Scholar] [CrossRef]
- Gudarzi Farahani, Y.; Mirarab Baygi, S.A.; Abbasi Nahoji, M.; Roshdieh, N. Presenting the Early Warning Model of Financial Systemic Risk in Iran’s Financial Market Using the LSTM Model. Int. J. Financ. Manag. Account. 2026, 11, 29–38. [Google Scholar]
- Khorsandi, H.; Mohsenibeigzadeh, M.; Tashakkori, A.; Kazemi, B.; Khorashadi Moghaddam, P.; Ahmadirad, Z. Driving Innovation in Education: The Role of Transformational Leadership and Knowledge Sharing Strategies. Curr. Opin. 2024, 4, 505–515. [Google Scholar] [CrossRef]
- Nezhad, K.K.; Ahmadirad, Z.; Mohammadi, A.T. The Dynamics of Modern Business: Integrating Research Findings into Practical Management; Nobel Sciences: Palm Coast, FL, USA, 2024. [Google Scholar]
- Nawaser, K.; Jafarkhani, F.; Khamoushi, S.; Yazdi, A.; Mohsenifard, H.; Gharleghi, B. The Dark Side of Digitalization: A Visual Journey of Research through Digital Game Addiction and Mental Health. IEEE Eng. Manag. Rev. 2024, 1, 1–27. [Google Scholar] [CrossRef]
- Motta de Castro, E.; Bozorgmehrian, F.; Carrola, M.; Koerner, H.; Samouei, H.; Asadi, A. Sulfur-Driven Reactive Processing of Multiscale Graphene/Carbon Fiber-Polyether Ether Ketone (PEEK) Composites with Tailored Crystallinity and Enhanced Mechanical Performance. Compos. Part B Eng. 2025, 295, 112180. [Google Scholar] [CrossRef]
- Golkarieh, A.; Rezvani Boroujeni, S.; Kiashemshaki, K.; Deldadehasl, M.; Aghayarzadeh, H.; Ramezani, A. Breakthroughs in Brain Tumor Detection: Leveraging Deep Learning and Transfer Learning for MRI-Based Classification. Comput. Decis. Mak. Int. J. 2025, 2, 708–722. [Google Scholar] [CrossRef]
- Rezaeianjam, M.; Khabazian, A.; Khabazian, T.; Ghorbani, F.; Abbasi, T.; Asghari, S.; Heidari, F.; Shiri, A.; Naderi, M. Efficacy of Ozone Therapy in Dentistry with Approach of Healing, Pain Management, and Therapeutic Outcomes: A Systematic Review of Clinical Trials. BMC Oral Health 2025, 25, 433. [Google Scholar] [CrossRef]
- Sajjadi Mohammadabadi, S.M.; Seyedkhamoushi, F.; Mostafavi, M.; Borhani Peikani, M. Examination of AI’s Role in Diagnosis, Treatment, and Patient Care. In Transforming Gender-Based Healthcare with AI and Machine Learning; Gupta, M., Kumar, R., Lu, Z., Eds.; CRC Press: Boca Raton, FL, USA, 2024; pp. 221–238. [Google Scholar] [CrossRef]
- Golkarieh, A.; Kiashemshaki, K.; Rezvani Boroujeni, S.; Sadi Isakan, N. Advanced U-Net Architectures with CNN Backbones for Automated Lung Cancer Detection and Segmentation in Chest CT Images. arXiv 2025. [Google Scholar] [CrossRef]
- Entezami, Z.; Davis, C.H.; Entezami, M. An AI-Assisted Topic Model of the Media Literacy Research Literature. Media Lit. Acad. Res. 2025, 8, 5–28. [Google Scholar] [CrossRef]
- Mohaghegh, A.; Huang, C. Feature-Guided Sampling Strategy for Adaptive Model Order Reduction of Convection-Dominated Problems. arXiv 2025. [Google Scholar] [CrossRef]
- Ahmadirad, Z. The Role of AI and Machine Learning in Supply Chain Optimization. Int. J. Mod. Achiev. Sci. Eng. Technol. 2025, 2, 1–8. [Google Scholar] [CrossRef]
- Dehghanpour Abyaneh, M.; Narimani, P.; Javadi, M.S.; Golabchi, M.; Attarsharghi, S.; Hadad, M. Predicting Surface Roughness and Grinding Forces in UNS S34700 Steel Grinding: A Machine Learning and Genetic Algorithm Approach to Coolant Effects. Physchem 2024, 4, 495–523. [Google Scholar] [CrossRef]
- Mohammadabadi, S.M.S.; Yang, L.; Yan, F.; Zhang, J. Communication-Efficient Training Workload Balancing for Decentralized Multi-Agent Learning. In Proceedings of the 2024 IEEE 44th International Conference on Distributed Computing Systems (ICDCS), Jersey City, NJ, USA, 23–26 July 2024; pp. 680–691. [Google Scholar] [CrossRef]
- Sajjadi Mohammadabadi, S.M. From Generative AI to Innovative AI: An Evolutionary Roadmap. arXiv 2025. [Google Scholar] [CrossRef]
- Barati-Nia, A.; Parrott, A.E.; Sorenson, K.; Moug, D.M.; Khosravifar, A. Comparing Cyclic Direct Simple Shear Behavior of Fine-Grained Soil Prepared with SHANSEP or Recompression Approaches. In Geotechnical Frontiers; ASCE Library: Reston, VA, USA, 2025; pp. 419–429. [Google Scholar] [CrossRef]
- Asadi, M.; Taheri, R. Enhancing Peer Assessment and Engagement in Online IELTS Writing Course through a Teacher’s Multifaceted Approach and AI Integration. Technol. Assist. Lang. Educ. 2024, 2, 94–117. [Google Scholar] [CrossRef]
- Abbasi, E.; Dwyer, E. The Efficacy of Commercial Computer Games as Vocabulary Learning Tools for EFL Students: An Empirical Investigation. Sunshine State TESOL J. 2024, 16, 24–35. [Google Scholar] [CrossRef]
- Pazouki, S.; Jamshidi, M.B.; Jalali, M.; Tafreshi, A. Transformative Impact of AI and Digital Technologies on the FinTech Industry: A Comprehensive Review. Int. J. Adv. Res. Humanit. Law 2025, 2, 1–27. [Google Scholar] [CrossRef]
- Pazouki, S.; Jamshidi, M.B.; Jalali, M.; Tafreshi, A. Artificial Intelligence and Digital Technologies in Finance: A Comprehensive Review. J. Econ. Financ. Account. Stud. 2025, 7, 54–69. [Google Scholar] [CrossRef]
- Karkehabadi, A.; Sadeghmalakabadi, S. Evaluating Deep Learning Models for Architectural Image Classification: A Case Study on the UC Davis Campus. In Proceedings of the 2024 IEEE 8th International Conference on Information and Communication Technology (CICT), Prayagraj, India, 6–8 December 2024; pp. 1–6. [Google Scholar] [CrossRef]
- Naseri, S. AI in Architecture and Urban Design and Planning: Case Studies on Three AI Applications. GSC Adv. Res. Rev. 2024, 21, 565–577. [Google Scholar] [CrossRef]
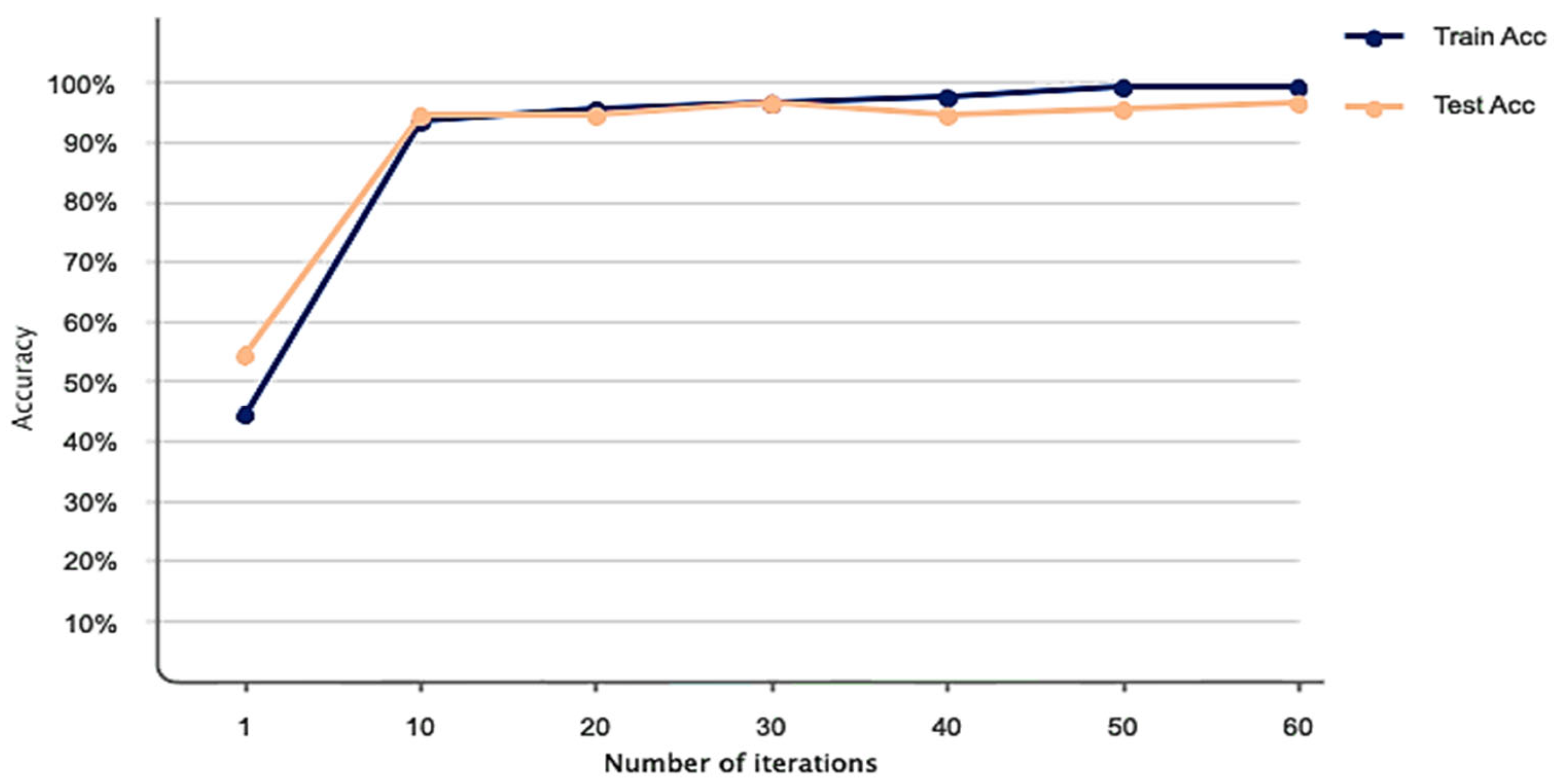
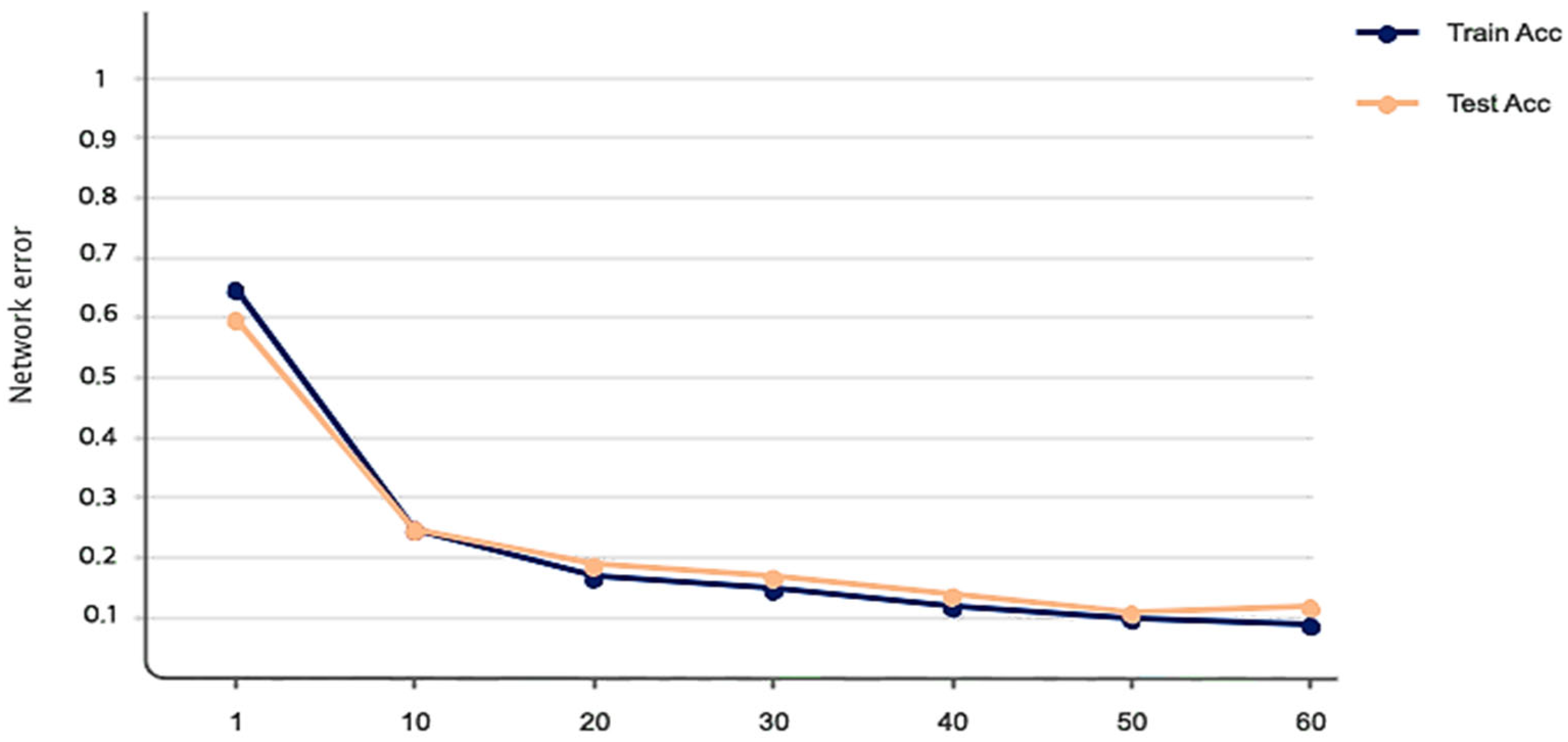

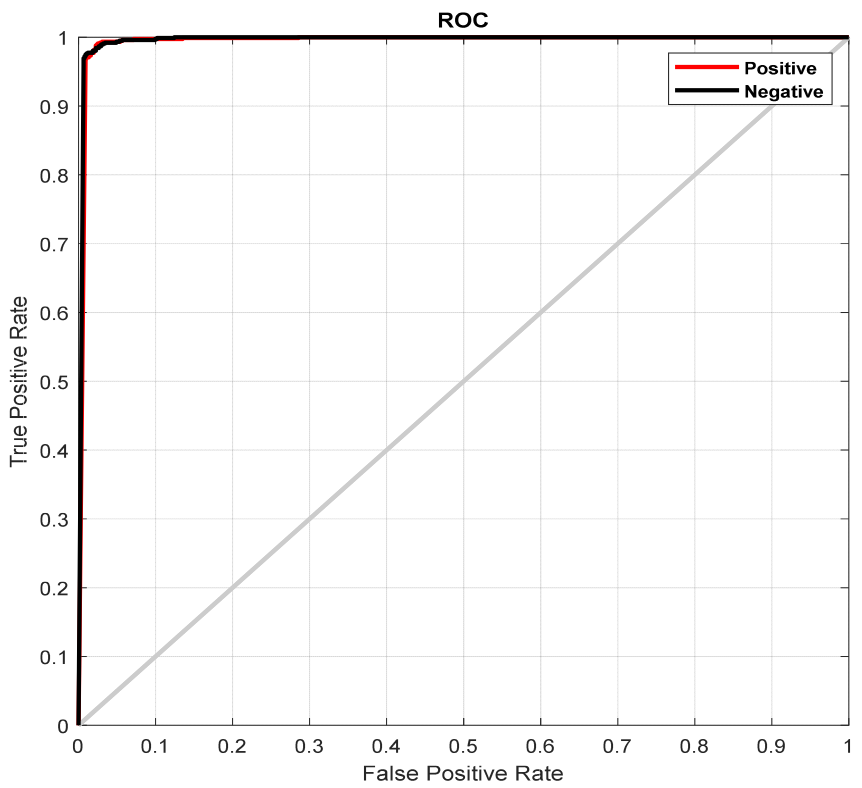

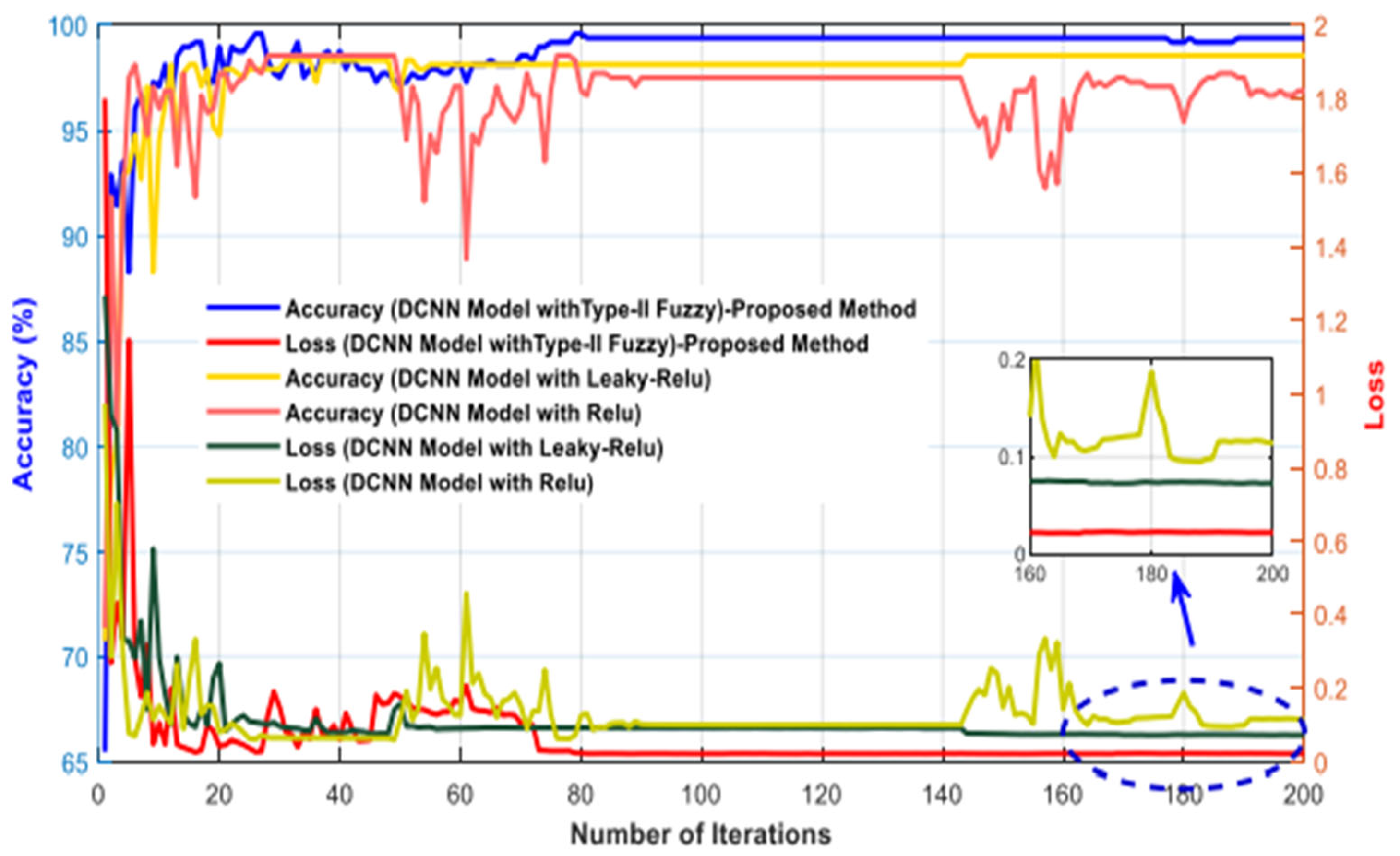

| Studies | Method |
|---|---|
| Chiari et al. [13] | Dynamic indices of postural control |
| Norris et al. [14] | Posturographic indices |
| Li et al. [15] | Postural control |
| Kiwanara et al. [16] | Postural control based on dissociated sensory systems |
| Richmond et al. [17] | CSP microstructural integrity |
| Da Oliveira et al. [18] | Core stability and sensorimotor training |
| Plereti et al. [19] | Machine learning |
| Tuncer et al. [20] | Postural control |
| Xu et al. [21] | Posture utilizing |
| Cherstvy et al. [22] | Diffusion processes with exponential and logarithmic time-dependent diffusion coefficients. |
| Novikov et al. [23] | MRS in cancer diagnosis and management |
| Layer Number | Layer Type | Size and Filter Steps | Number of Filters | Output Value | Padding | Activation Function |
|---|---|---|---|---|---|---|
| 1 | Convolution1 | 12 × 1/8 × 1 | 16 | 1034 × 16 | yes | Type II Fuzzy |
| 2 | Pooling1 | 2 × 1/2 × 1 | 16 | 517 × 16 | no | |
| 3 | Convolution2 | 3 × 1/1 × 1 | 32 | 517 × 32 | yes | Type II Fuzzy |
| 4 | Pooling2 | 2 × 1/2 × 1 | 32 | 258 × 32 | no | |
| 5 | Convolution3 | 3 × 1/1 × 1 | 64 | 258 × 64 | yes | Type II Fuzzy |
| 6 | Pooling3 | 2 × 1/2 × 1 | 64 | 129 × 64 | no | |
| 7 | Convolution4 | 3 × 1/1 × 1 | 80 | 129 × 80 | yes | Type II Fuzzy |
| 8 | Pooling4 | 2 × 1/2 × 1 | 80 | 64 × 80 | no | |
| 9 | Convolution5 | 3 × 1/1 × 1 | 80 | 64 × 80 | yes | Type II Fuzzy |
| 10 | Pooling5 | 2 × 1/2 × 1 | 80 | 32 × 80 | no | |
| 11 | Convolution6 | 3 × 1/1 × 1 | 80 | 32 × 80 | yes | Type II Fuzzy |
| 12 | Pooling6 | 2 × 1/2 × 1 | 80 | 16 × 80 | no | |
| 13 | Convolution7 | 3 × 1/1 × 1 | 80 | 16 × 80 | yes | Type II Fuzzy |
| 14 | Pooling7 | 2 × 1/2 × 1 | 80 | 8 × 80 | no | |
| 15 | Convolution8 | 3 × 1/1 × 1 | 80 | 8 × 80 | Yes | Type II Fuzzy |
| 16 | Pooling8 | 2 × 1/2 × 1 | 80 | 4 × 80 | no | |
| 17 | Convolution9 | 3 × 1/1 × 1 | 80 | 8 × 80 | yes | Type II Fuzzy |
| 18 | Pooling9 | 2 × 1/2 × 1 | 80 | 2 × 80 | no | |
| 19 | Convolution10 | 3 × 1/1 × 1 | 80 | 2 × 80 | yes | Type II Fuzzy |
| 20 | Pooling10 | 2 × 1/2 × 1 | 80 | 1 × 80 | no | |
| 21 | Fully-connected | - | 100 | 100 | - | |
| 22 | Softmax | - | 1 | 2 | - |
| Parameters | Search Space | Optimal Value |
|---|---|---|
| Optimizer | RMSProp, Adam, Sgd, Adamax, Adadelta | Adam |
| Cost function | MSE, Cross-entropy | Cross-Entropy |
| Number of convolution layers | 3, 5, 6, 10, 15 | 10 |
| Filters in the first convolution layer | 16, 32, 64, 128 | 16 |
| Filters in the second convolution layer | 16, 32, 64, 128 | 32 |
| Filters in another convolution layer | 16, 32, 64, 128 | 32 |
| Size of filter in the first convolution layer | 3, 16, 32, 64, 128 | 128 |
| Size of filter in another convolution layers | 3, 16, 32, 64, 128 | 16 |
| Size of filter before the first convolution layer | 0.2, 0.3, 0.4, 0.5 | 0.4 |
| Dropout rate after the first convolution layer | 0.2, 0.3, 0.4, 0.5 | 0.4 |
| Batch size | 4, 8, 10, 16, 32, 64 | 16 |
| Learning rate | 0.01, 0.001, 0.0001 | 0.001 |
| Performance Metric | Stroke (%) | Normal (%) |
|---|---|---|
| Sensitivity | 97.22 | 97.4 |
| Accuracy | 97.81 | 96.3 |
| Specificity | 96.66 | 97.7 |
| Precision | 96.65 | 97.7 |
| Function | Stroke | Normal |
|---|---|---|
| ReLU | 10,100 | 333 |
| Leaky ReLU | 11,542 | 421 |
| Fuzzy sets | 12,101 | 900 |
| Model | Architecture/Type | Key Parameters | Input Features |
|---|---|---|---|
| SVM | RBF kernel | C = 1.0, gamma = ‘scale’ | 29 handcrafted |
| MLP | 1 hidden layer | 100 neurons, ReLU, Adam, LR = 0.001 | 29 handcrafted |
| CNN | 2 Conv + Dense | Filters = 32/64, ReLU, 100 dense | Time series raw |
| VGG19 | Pre-trained (fine-tuned) | LR = 1 × 10−4, top layers modified | Resized image format |
| ResNet50 | Pre-trained (fine-tuned) | 10 trainable layers, Adam | Resized image format |
| Xception | Pre-trained (fine-tuned) | Dropout = 0.5, Adam, batch = 32 | Resized image format |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najipour, A.; Khorramymehr, S.; Razeghi, M.; Hassani, K. Deep Learning-Based Evaluation of Postural Control Impairments Caused by Stroke Under Altered Sensory Conditions. Biomimetics 2025, 10, 586. https://doi.org/10.3390/biomimetics10090586
Najipour A, Khorramymehr S, Razeghi M, Hassani K. Deep Learning-Based Evaluation of Postural Control Impairments Caused by Stroke Under Altered Sensory Conditions. Biomimetics. 2025; 10(9):586. https://doi.org/10.3390/biomimetics10090586
Chicago/Turabian StyleNajipour, Armin, Siamak Khorramymehr, Mehdi Razeghi, and Kamran Hassani. 2025. "Deep Learning-Based Evaluation of Postural Control Impairments Caused by Stroke Under Altered Sensory Conditions" Biomimetics 10, no. 9: 586. https://doi.org/10.3390/biomimetics10090586
APA StyleNajipour, A., Khorramymehr, S., Razeghi, M., & Hassani, K. (2025). Deep Learning-Based Evaluation of Postural Control Impairments Caused by Stroke Under Altered Sensory Conditions. Biomimetics, 10(9), 586. https://doi.org/10.3390/biomimetics10090586






