Micro-CT and Histomorphometric Analysis of Degradability and New Bone Formation of Anodized Mg-Ca System
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Clinical Evaluation
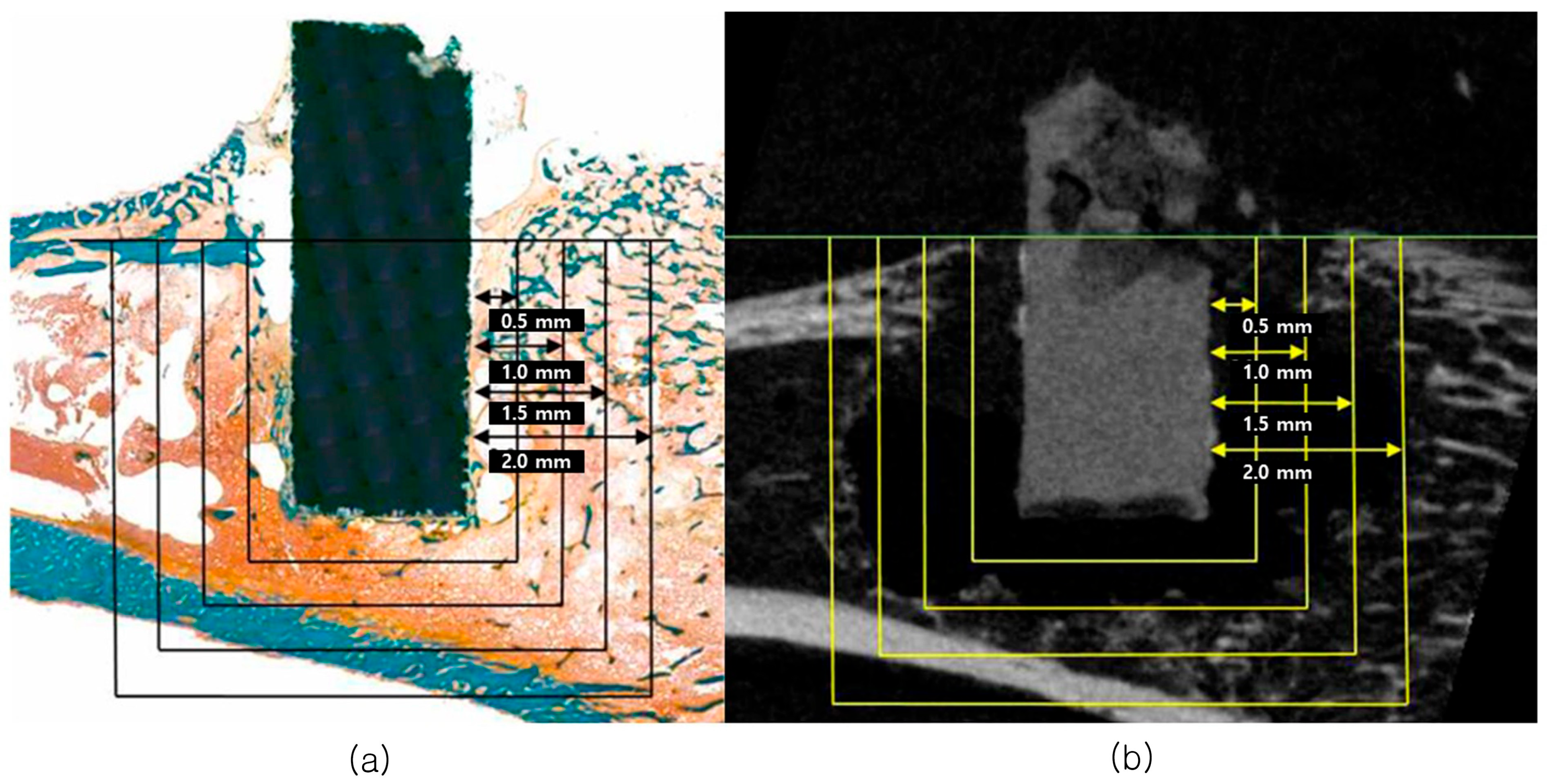
3.2. Micro-CT Analysis
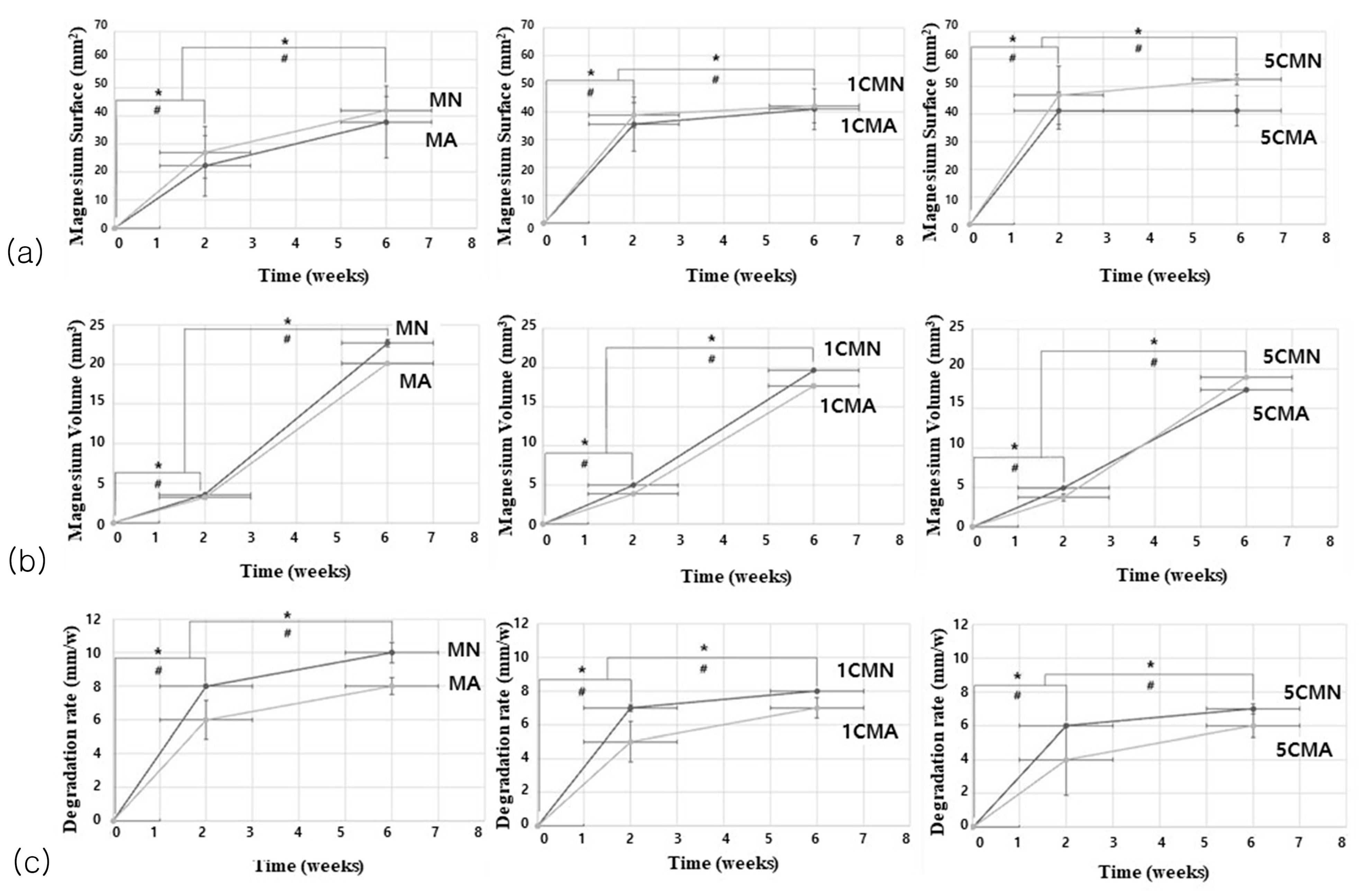
3.2.1. Mg Implant Analysis
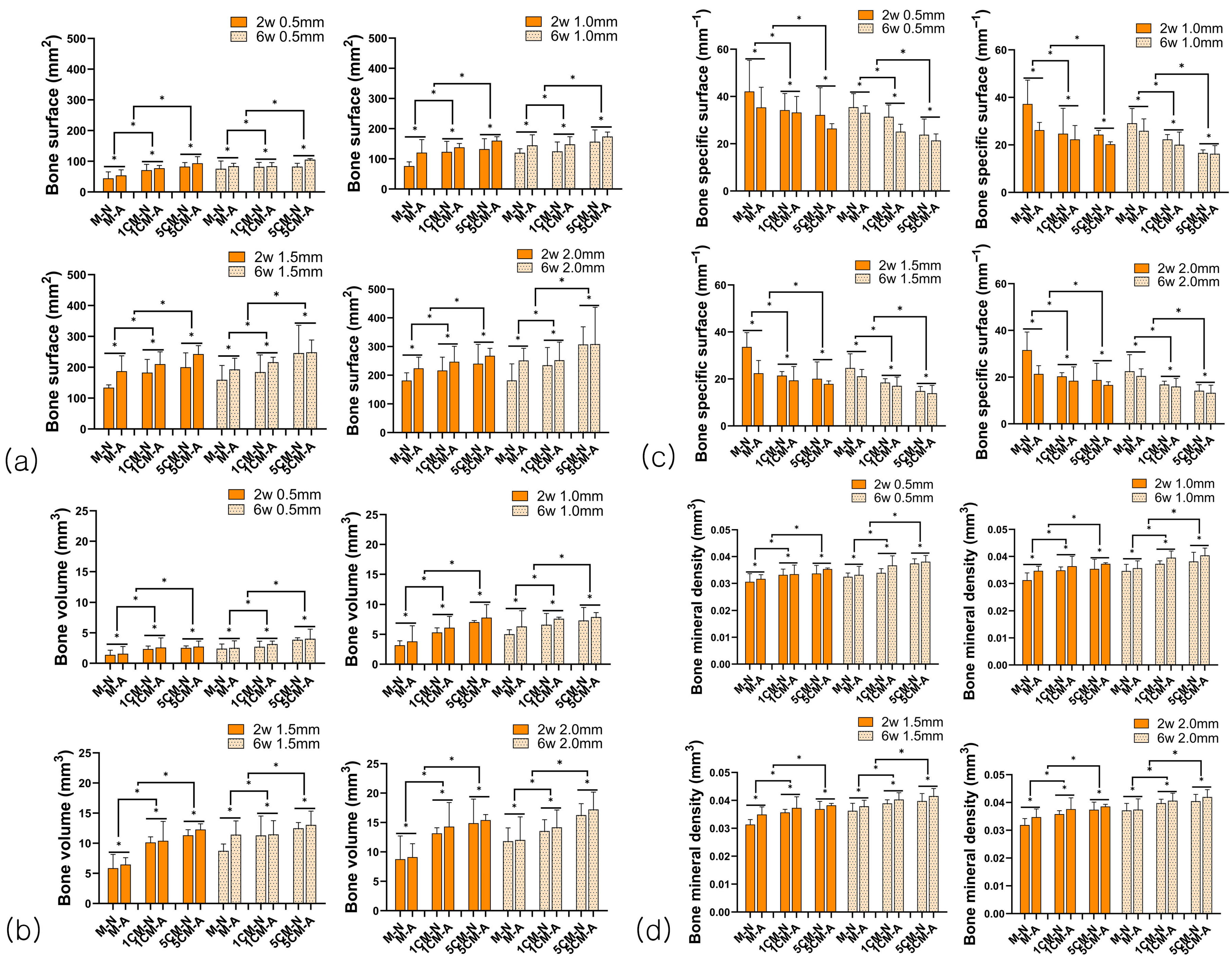
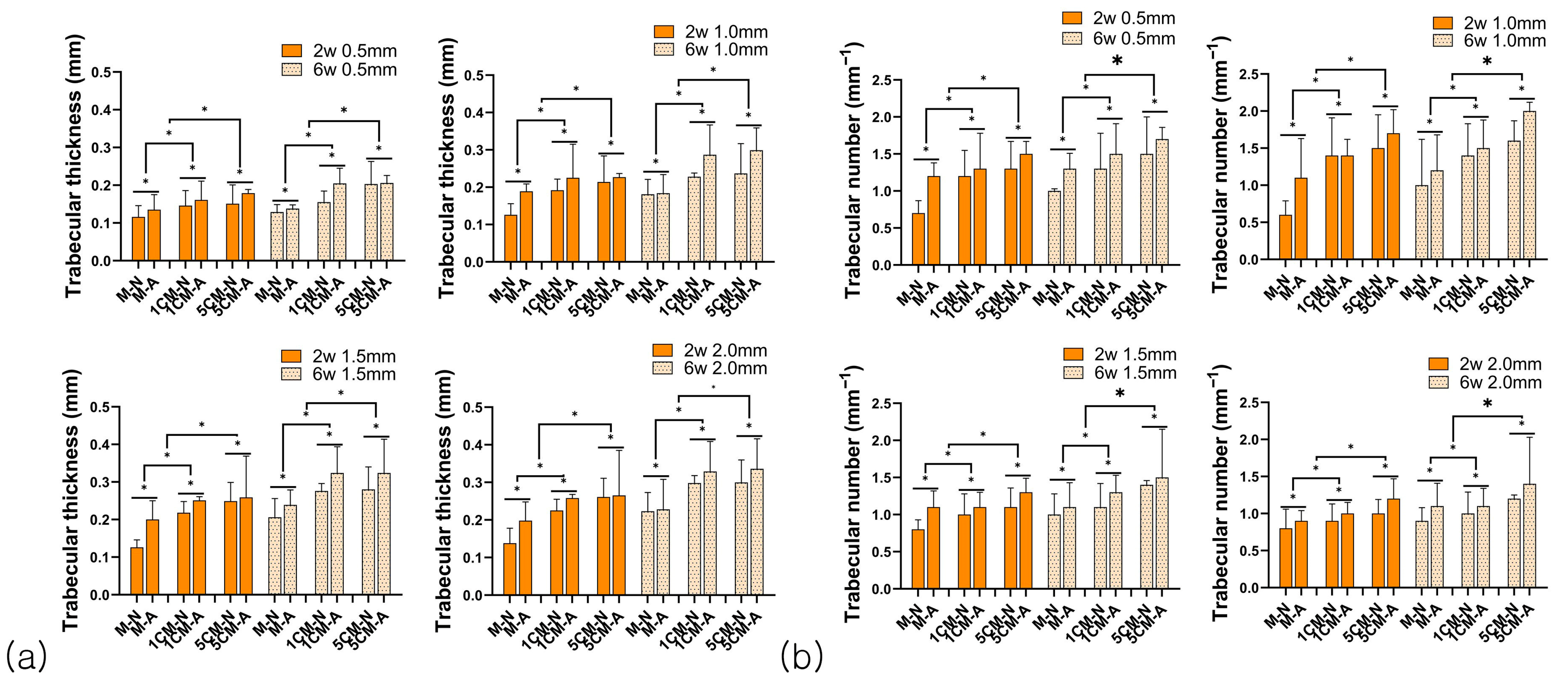
3.2.2. Bone Formation Analysis
3.3. Histomorphometric Analysis
3.3.1. Goldner Trichrome Stain
3.3.2. The Amount of Absorbed Cylindrical Mg Implant, Bone-Implant Contact Rate (BIC), and Bone Amount (BA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Mg-Ca | Magnesium–calcium |
| ROI | Region of interest |
| Mg | Magnesium |
| H2 | Hydrogen gas |
| Cl− | Chlorine ions |
| Ca | Calcium |
| Ca2+ | Calcium ions |
| MS | Magnesium surface |
| MV | Magnesium volume |
| DR | Degradation rate |
| BS | Bone surface |
| BV | Bone volume |
| BS/BV | Bone surface ratio |
| BMD | Bone mineral density |
| Tb.Th | Trabecular thickness |
| Tb.N | Trabecular number |
| HU | Hounsfield unit |
| BIC | Bone-to-implant contact ratio |
| BA | Bone amount |
| MAO | Micro-arc oxidation |
| Mg(OH)2 | Magnesium hydroxide |
References
- Song, Y.; Zhang, S.; Li, J.; Zhao, C.; Zhang, X. Electrodeposition of Ca–P coatings on biodegradable Mg alloy: In vitro biomineralization behavior. Acta Biomater. 2010, 6, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Okutan, B.; Schwarze, U.Y.; Berger, L.; Martinez, D.C.; Herber, V.; Suljevic, O.; Plocinski, T.; Swieszkowski, W.; Santos, S.G.; Schindl, R. The combined effect of zinc and calcium on the biodegradation of ultrahigh-purity magnesium implants. Biomater. Adv. 2023, 146, 213287. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, X.; Wang, Y.; Tao, T.; Wang, Z.; Yuan, L.; Han, B. The biomimetics of Mg2+-concentration-resolved microenvironment for bone and cartilage repairing materials design. Biomimetics 2022, 7, 227. [Google Scholar] [CrossRef]
- Manescu, V.; Antoniac, I.; Antoniac, A.; Laptoiu, D.; Paltanea, G.; Ciocoiu, R.; Nemoianu, I.V.; Gruionu, L.G.; Dura, H. Bone regeneration induced by patient-adapted Mg alloy-based scaffolds for bone defects: Present and future perspectives. Biomimetics 2023, 8, 618. [Google Scholar]
- Wang, Y.; Wei, M.; Gao, J.; Hu, J.; Zhang, Y. Corrosion process of pure magnesium in simulated body fluid. Mater. Lett. 2008, 62, 2181–2184. [Google Scholar] [CrossRef]
- Kannan, M.B.; Raman, R.S. In vitro degradation and mechanical integrity of calcium-containing magnesium alloys in modified-simulated body fluid. Biomaterials 2008, 29, 2306–2314. [Google Scholar] [CrossRef]
- Wolf, F.I.; Cittadini, A. Chemistry and biochemistry of magnesium. Mol. Asp. Med. 2003, 24, 3–9. [Google Scholar] [CrossRef]
- Krause, A.; Von der Höh, N.; Bormann, D.; Krause, C.; Bach, F.-W.; Windhagen, H.; Meyer-Lindenberg, A. Degradation behaviour and mechanical properties of magnesium implants in rabbit tibiae. J. Mater. Sci. 2010, 45, 624–632. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Yamamoto, A.; Watanabe, A.; Sugahara, K.; Tsubakino, H.; Fukumoto, S. Improvement of corrosion resistance of magnesium alloys by vapor deposition. Scr. Mater. 2001, 44, 1039–1042. [Google Scholar] [CrossRef]
- Wang, H.; Shi, Z.M.; Yang, K. Magnesium and magnesium alloys as degradable metallic biomaterials. Adv. Mater Res. 2008, 32, 207–210. [Google Scholar] [CrossRef]
- Reifenrath, J.; Krause, A.; Bormann, D.; Von Rechenberg, B.; Windhagen, H.; Meyer-Lindenberg, A. Profound differences in the in-vivo-degradation and biocompatibility of two very similar rare-earth containing Mg-alloys in a rabbit model. Mater. Und Werkst. 2010, 41, 1054–1061. [Google Scholar] [CrossRef]
- Zhang, B.; Geng, L.; Huang, L.; Zhang, X.; Dong, C. Enhanced mechanical properties in fine-grained Mg–1.0 Zn–0.5 Ca alloys prepared by extrusion at different temperatures. Scr. Mater. 2010, 63, 1024–1027. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Geng, L. Research on Mg-Zn-Ca Alloy as Degradable Biomaterial, Biomater—Physics and Chemistry; InTech: London, UK, 2011. [Google Scholar]
- Bakhsheshi-Rad, H.; Idris, M.; Abdul-Kadir, M.; Ourdjini, A.; Medraj, M.; Daroonparvar, M.; Hamzah, E. Mechanical and bio-corrosion properties of quaternary Mg–Ca–Mn–Zn alloys compared with binary Mg–Ca alloys. Mater. Des. 2014, 53, 283–292. [Google Scholar] [CrossRef]
- Ai, Y.; Luo, C.; Liu, J. Twinning of CaMgSi phase in a cast Mg–1.0 Ca–0.5 Si–0.3 Zr alloy. Acta Mater. 2007, 55, 531–538. [Google Scholar] [CrossRef]
- Son, W.w.; Zhu, X.; Shin, H.i.; Ong, J.L.; Kim, K.h. In vivo histological response to anodized and anodized/hydrothermally treated titanium implants. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 66, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rath, B.; Tingart, M.; Eschweiler, J. Role of implants surface modification in osseointegration: A systematic review. J. Biomed. Mater. Res. Part A 2020, 108, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Makkar, P.; Sarkar, S.K.; Padalhin, A.R.; Moon, B.-G.; Lee, Y.S.; Lee, B.T. In vitro and in vivo assessment of biomedical Mg–Ca alloys for bone implant applications. J. Appl. Biomater. Funct. Mater. 2018, 16, 126–136. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Lee, K.; Park, S.; Lim, H.; Park, C.; Bae, J.; Yun, K. Biological evaluation of anodized biodegradable magnesium-calcium alloys. Acta Phys. Pol. A 2016, 129, 728–735. [Google Scholar] [CrossRef]
- Li, H.; Lu, S.; Qin, W.; Han, L.; Wu, X. Improving the wear properties of AZ31 magnesium alloy under vacuum low-temperature condition by plasma electrolytic oxidation coating. Acta Astronaut. 2015, 116, 126–131. [Google Scholar] [CrossRef]
- Sul, Y.-T.; Johansson, C.B.; Albrektsson, T. Oxidized titanium screws coated with calcium ions and their performance in rabbit bone. Int. J. Oral Maxillofac. Implant 2002, 17, 625–634. [Google Scholar]
- Kuhlmann, J.; Bartsch, I.; Willbold, E.; Schuchardt, S.; Holz, O.; Hort, N.; Höche, D.; Heineman, W.R.; Witte, F. Fast escape of hydrogen from gas cavities around corroding magnesium implants. Acta Biomater. 2013, 9, 8714–8721. [Google Scholar] [CrossRef]
- Huang, J.; Song, G.-L.; Atrens, A.; Dargusch, M. What activates the Mg surface—A comparison of Mg dissolution mechanisms. J. Mater. Sci. Technol. 2020, 57, 204–220. [Google Scholar] [CrossRef]
- Arnett, T.R. Acid–base regulation of bone metabolism. Int. Congr. Ser. 2007, 1297, 255–267. [Google Scholar] [CrossRef]
- Janning, C.; Willbold, E.; Vogt, C.; Nellesen, J.; Meyer-Lindenberg, A.; Windhagen, H.; Thorey, F.; Witte, F. Magnesium hydroxide temporarily enhancing osteoblast activity and decreasing the osteoclast number in peri-implant bone remodelling. Acta Biomater. 2010, 6, 1861–1868. [Google Scholar] [CrossRef]
- Xue, D.; Yun, Y.; Tan, Z.; Dong, Z.; Schulz, M.J. In vivo and in vitro degradation behavior of magnesium alloys as biomaterials. J. Mater. Sci. Technol. 2012, 28, 261–267. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Berglund, I.S.; Brar, H.S.; Dolgova, N.; Acharya, A.P.; Keselowsky, B.G.; Sarntinoranont, M.; Manuel, M.V. Synthesis and characterization of Mg-Ca-Sr alloys for biodegradable orthopedic implant applications. J. Biomed. Mater. Res. Part B 2012, 100, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guan, S.; Li, W.; Wang, H.; Chen, J.; Wang, Y.; Wang, H. In vivo degradation and bone response of a composite coating on Mg–Zn–Ca alloy prepared by microarc oxidation and electrochemical deposition. J. Biomed. Mater. Res. Part B 2012, 100, 533–543. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, M.; Wu, K. Microarc oxidation coating formed on SiCw/AZ91 magnesium matrix composite and its corrosion resistance. Mater. Lett. 2005, 59, 1727–1731. [Google Scholar] [CrossRef]
- Song, G.-L.; Atrens, A. Recently deepened insights regarding Mg corrosion and advanced engineering applications of Mg alloys. J. Magnes. Alloy 2023, 11, 3948–3991. [Google Scholar] [CrossRef]
- Gu, X.; Li, N.; Zhou, W.; Zheng, Y.; Zhao, X.; Cai, Q.; Ruan, L. Corrosion resistance and surface biocompatibility of a microarc oxidation coating on a Mg–Ca alloy. Acta Biomater. 2011, 7, 1880–1889. [Google Scholar] [CrossRef]
- Fischerauer, S.; Kraus, T.; Wu, X.; Tangl, S.; Sorantin, E.; Hänzi, A.; Löffler, J.F.; Uggowitzer, P.J.; Weinberg, A. In vivo degradation performance of micro-arc-oxidized magnesium implants: A micro-CT study in rats. Acta Biomater. 2013, 9, 5411–5420. [Google Scholar] [CrossRef]
- Song, G.-L.; Shi, Z. Anodization and corrosion of magnesium (Mg) alloys. In Corrosion Prevention of Magnesium Alloys; Elsevier: Amsterdam, The Netherlands, 2013; pp. 232–281. [Google Scholar]
- Moreno, J.; Merlo, J.L.; Renno, A.C.; Canizo, J.; Buchelly, F.J.; Pastore, J.I.; Katunar, M.R.; Cere, S. In vitro characterization of anodized magnesium alloy as a potential biodegradable material for biomedical applications. Electrochim. Acta 2023, 437, 141463. [Google Scholar] [CrossRef]
- Brar, H.S.; Ball, J.P.; Berglund, I.S.; Allen, J.B.; Manuel, M.V. A study of a biodegradable Mg–3Sc–3Y alloy and the effect of self-passivation on the in vitro degradation. Acta Biomater. 2013, 9, 5331–5340. [Google Scholar] [CrossRef]
- Witte, F.; Ulrich, H.; Palm, C.; Willbold, E. Biodegradable magnesium scaffolds: Part II: Peri-implant bone remodeling. J. Biomed. Mater. Res. Part A 2007, 81, 757–765. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
- Stadlinger, B.; Lode, A.T.; Eckelt, U.; Range, U.; Schlottig, F.; Hefti, T.; Mai, R. Surface-conditioned dental implants: An animal study on bone formation. J. Clin. Periodontol. 2009, 36, 882–891. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Scotchford, C.A.; Gilmore, C.P.; Cooper, E.; Leggett, G.J.; Downes, S. Protein adsorption and human osteoblast-like cell attachment and growth on alkylthiol on gold self-assembled monolayers. J. Biomed. Mater. Res. 2002, 59, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloys 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Martinez, D.C.; Borkam-Schuster, A.; Helmholz, H.; Dobkowska, A.; Luthringer-Feyerabend, B.; Płociński, T.; Willumeit-Römer, R.; Święszkowski, W. Bone cells influence the degradation interface of pure Mg and WE43 materials: Insights from multimodal in vitro analysis. Acta Biomater. 2024, 187, 471–490. [Google Scholar] [CrossRef]
- Prakash, T. Review on nanostructured semiconductors for dye sensitized solar cells. Electron. Mater. Lett. 2012, 8, 231–243. [Google Scholar] [CrossRef]
- Martinez, H.; Davarpanah, M.; Missika, P.; Celletti, R.; Lazzara, R. Optimal implant stabilization in low density bone. Clin. Oral Implant. Res. 2001, 12, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Berglund, I.S.; Jacobs, B.Y.; Allen, K.D.; Kim, S.E.; Pozzi, A.; Allen, J.B.; Manuel, M.V. Peri-implant tissue response and biodegradation performance of a Mg–1.0 Ca–0.5 Sr alloy in rat tibia. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Cui, Y.; Sun, S.; Zhang, Y.; Ge, J.; Yin, W.; Li, P.; Wang, Y. Versatile application of magnesium-related bone implants in the treatment of bone defects. Mater. Today Bio 2025, 31, 101635. [Google Scholar] [CrossRef]
- Hung, C.-C.; Chaya, A.; Liu, K.; Verdelis, K.; Sfeir, C. The role of magnesium ions in bone regeneration involves the canonical Wnt signaling pathway. Acta Biomater. 2019, 98, 246–255. [Google Scholar] [CrossRef]
- Ali, A.; Ikram, F.; Iqbal, F.; Fatima, H.; Mehmood, A.; Kolawole, M.Y.; Chaudhry, A.A.; Siddiqi, S.A.; Rehman, I.U. Improving the in vitro degradation, mechanical and biological properties of AZ91-3Ca Mg alloy via hydrothermal calcium phosphate coatings. Front. Mater. 2021, 8, 715104. [Google Scholar] [CrossRef]
- Mohamed, A.; El-Aziz, A.M.; Breitinger, H.-G. Study of the degradation behavior and the biocompatibility of Mg–0.8 Ca alloy for orthopedic implant applications. J. Magnes. Alloys 2019, 7, 249–257. [Google Scholar] [CrossRef]
- Paul, W.; Sharma, C.P. Nanoceramic matrices: Biomedical applications. Am. J. Biochem. Biotechnol. 2006, 2, 41–48. [Google Scholar] [CrossRef]
- Vormann, J. Magnesium: Nutrition and metabolism. Mol. Asp. Med. 2003, 24, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Zreiqat, H.; Howlett, C.; Zannettino, A.; Evans, P.; Schulze-Tanzil, G.; Knabe, C.; Shakibaei, M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J. Biomed. Mater. Res. 2002, 62, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, D.; Van Rietbergen, B.; Laib, A.; Ruegsegger, P. The ability of three-dimensional structural indices to reflect mechanical aspects of trabecular bone. Bone 1999, 25, 55–60. [Google Scholar] [CrossRef]
- Parkinson, I.H.; Forbes, D.; Sutton-Smith, P.; Fazzalari, N.L. Model-Independent 3D Descriptors of Vertebral Cancellous Bone Architecture. J. Osteoporos. 2010, 2010, 641578. [Google Scholar] [CrossRef] [PubMed]
- Blawert, C.; Dietzel, W.; Ghali, E.; Song, G. Anodizing treatments for magnesium alloys and their effect on corrosion resistance in various environments. Adv. Eng. Mater. 2006, 8, 511–533. [Google Scholar] [CrossRef]
- Rahman, M.; Dutta, N.K.; Roy Choudhury, N. Magnesium alloys with tunable interfaces as bone implant materials. Front. Bioeng. Biotechnol. 2020, 8, 564. [Google Scholar] [CrossRef]
- Shalabi, M.; Gortemaker, A.; Hof, M.V.t.; Jansen, J.; Creugers, N. Implant surface roughness and bone healing: A systematic review. J. Dent. Res. 2006, 85, 496–500. [Google Scholar] [CrossRef]
- Uppal, G.; Thakur, A.; Chauhan, A.; Bala, S. Magnesium based implants for functional bone tissue regeneration—A review. J. Magnes. Alloy 2014, 10, 356–386. [Google Scholar] [CrossRef]
- Davies, J. Mechanisms of endosseous integration. Int. J. Prosthodont. 1998, 11, 391–401. [Google Scholar]



| Surface Treatment | Group | Material | Composition (wt%) | |
|---|---|---|---|---|
| Ca | Mg | |||
| No Treatment (N) | MN | Pure Mg | - | 100 |
| 1CMN | Mg-1Ca | 1 | 99 | |
| 5CMN | Mg-5Ca | 5 | 95 | |
| Anodic Oxidation Treatment (A) | MA | Pure Mg | - | 100 |
| 1CMA | Mg-1Ca | 1 | 99 | |
| 5CMA | Mg-5Ca | 5 | 95 | |
| Final voltage | 120 V |
| Coating time | 15 min |
| Room temperature | 20–25 °C |
| pH | 7–8 |
| Electrolyte solution | Calcium gluconate (4 g/L) |
| Sodium hexametaphosphate (3 g/L) | |
| Sodium hydroxide (6 g/L) |
| Parameters | Pre-Set Values |
|---|---|
| Filter | A1 0.5 mm |
| Voltage | 50 kV |
| Current | 201 mA |
| Frame averaging | 5 |
| Rotation step | 0.7° |
| Parameter Name | Symbol | Unit |
|---|---|---|
| Bone mineral density | BMD | g/cm2 |
| Bone volume | BV | mm3 |
| Bone surface | BS | mm2 |
| Bone specific surface | BS/BV | mm−1 |
| Trabecular thickness | Tb.Th | mm |
| Trabecular number | Tb.N | mm−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Jung, Y.; Lee, Y.-S.; Choi, S.-W.; Hwang, G.; Yun, K. Micro-CT and Histomorphometric Analysis of Degradability and New Bone Formation of Anodized Mg-Ca System. Biomimetics 2025, 10, 583. https://doi.org/10.3390/biomimetics10090583
Kim J, Jung Y, Lee Y-S, Choi S-W, Hwang G, Yun K. Micro-CT and Histomorphometric Analysis of Degradability and New Bone Formation of Anodized Mg-Ca System. Biomimetics. 2025; 10(9):583. https://doi.org/10.3390/biomimetics10090583
Chicago/Turabian StyleKim, Jihyun, Yoona Jung, Yong-Seok Lee, Seong-Won Choi, Geelsu Hwang, and Kwidug Yun. 2025. "Micro-CT and Histomorphometric Analysis of Degradability and New Bone Formation of Anodized Mg-Ca System" Biomimetics 10, no. 9: 583. https://doi.org/10.3390/biomimetics10090583
APA StyleKim, J., Jung, Y., Lee, Y.-S., Choi, S.-W., Hwang, G., & Yun, K. (2025). Micro-CT and Histomorphometric Analysis of Degradability and New Bone Formation of Anodized Mg-Ca System. Biomimetics, 10(9), 583. https://doi.org/10.3390/biomimetics10090583








