Hyaluronic-Acid-Coated Sterosome for Dasatinib Delivery in Hepatocellular Carcinoma: Preparation, Physicochemical Characterization, and In Vitro Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
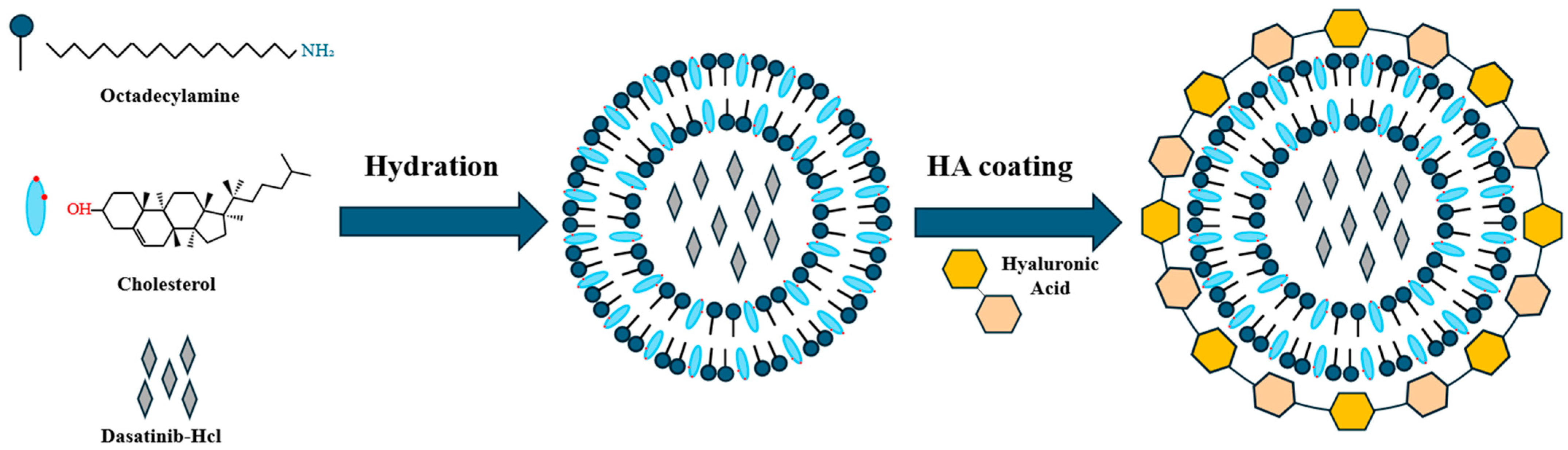
2.2. Preparation of HA-Coated Das-Loaded Sterosomes (HA-St-Das)
2.3. Characterization of St-Das and HA-St-Das
2.4. Lyophilization of Sterosome
2.5. Stability of Sterosome
2.6. In Vitro Drug Release
2.7. Cell Culture and Incubation Conditions
2.8. Cell Cytotoxicity Assay
2.9. Cellular Uptake
2.10. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Sterosomes
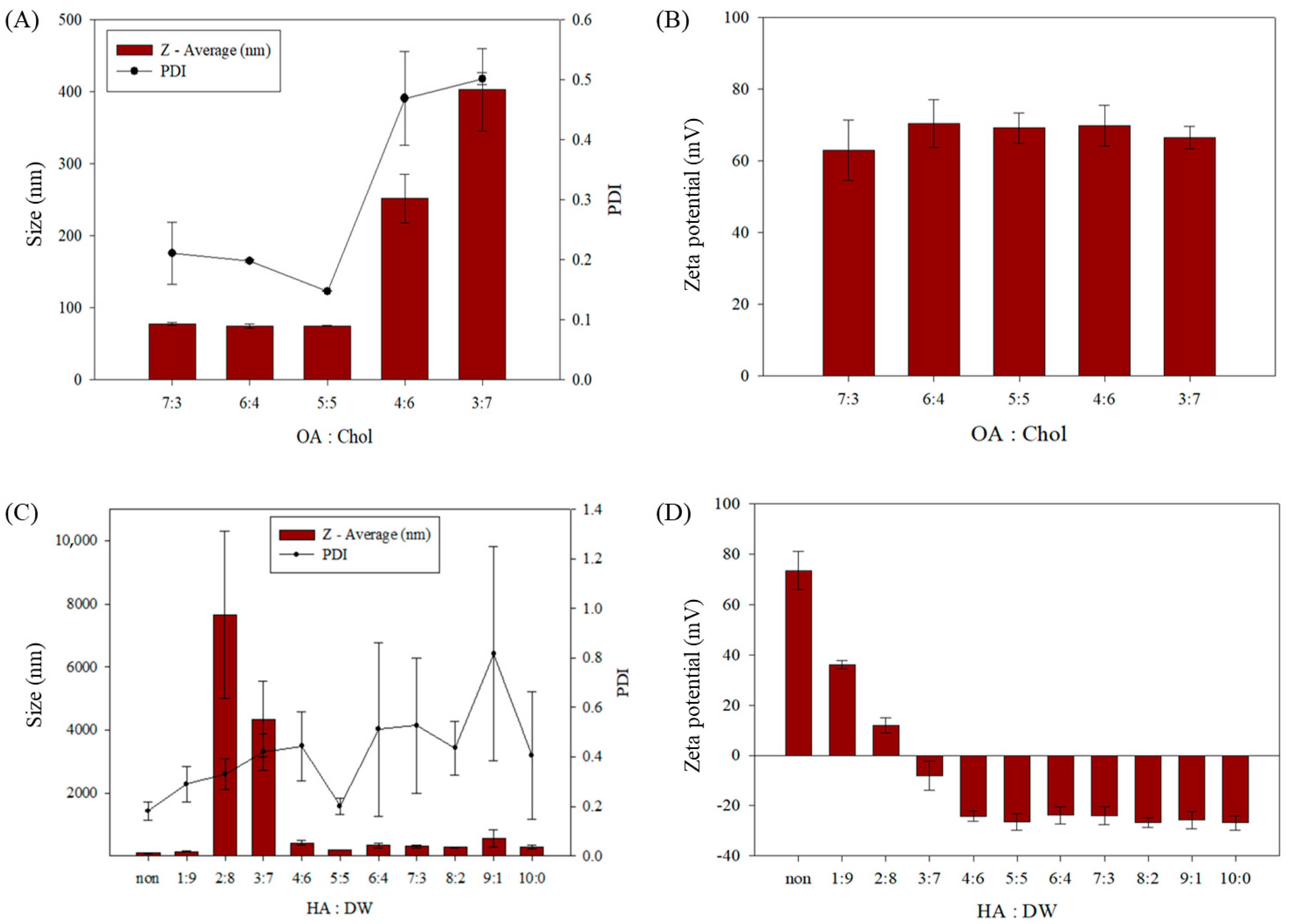
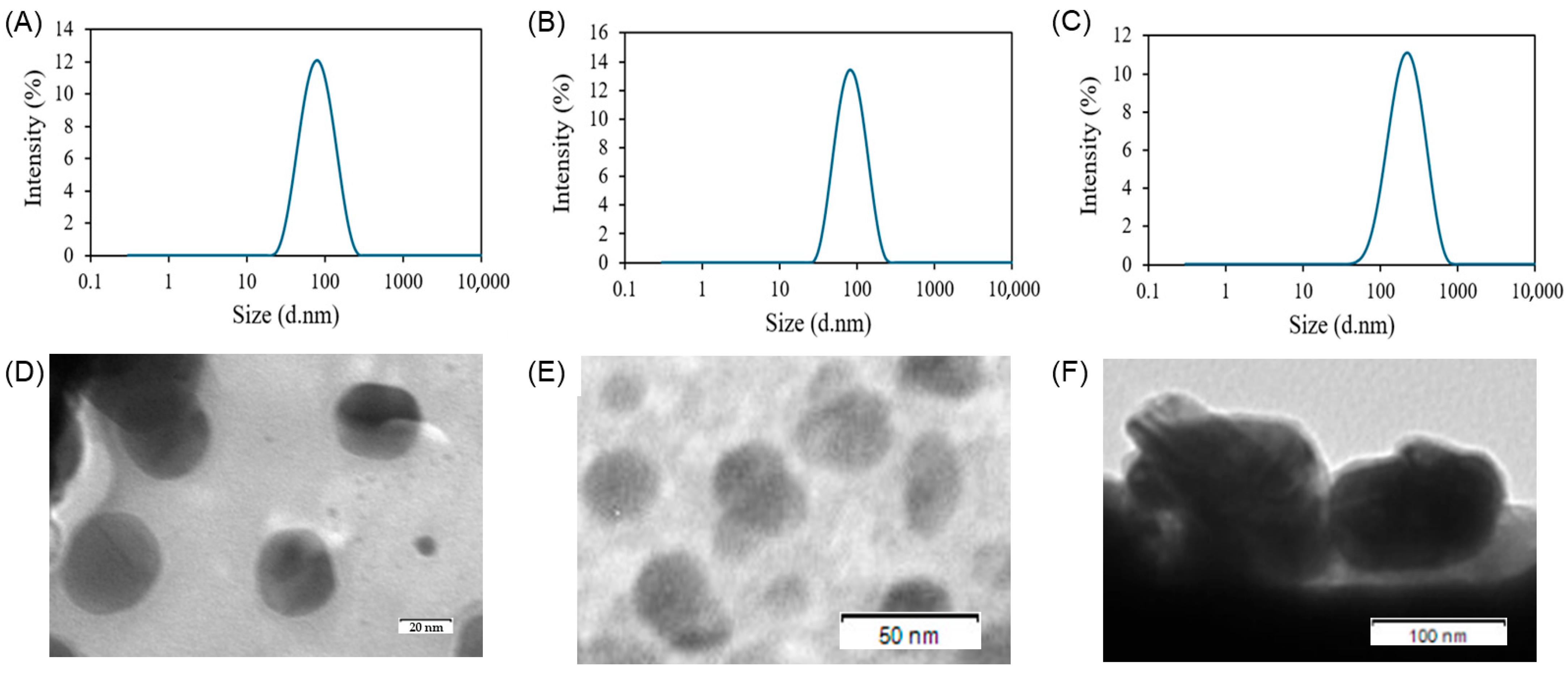
3.2. Characterization of the Sterosome
3.3. In Vitro Drug Release
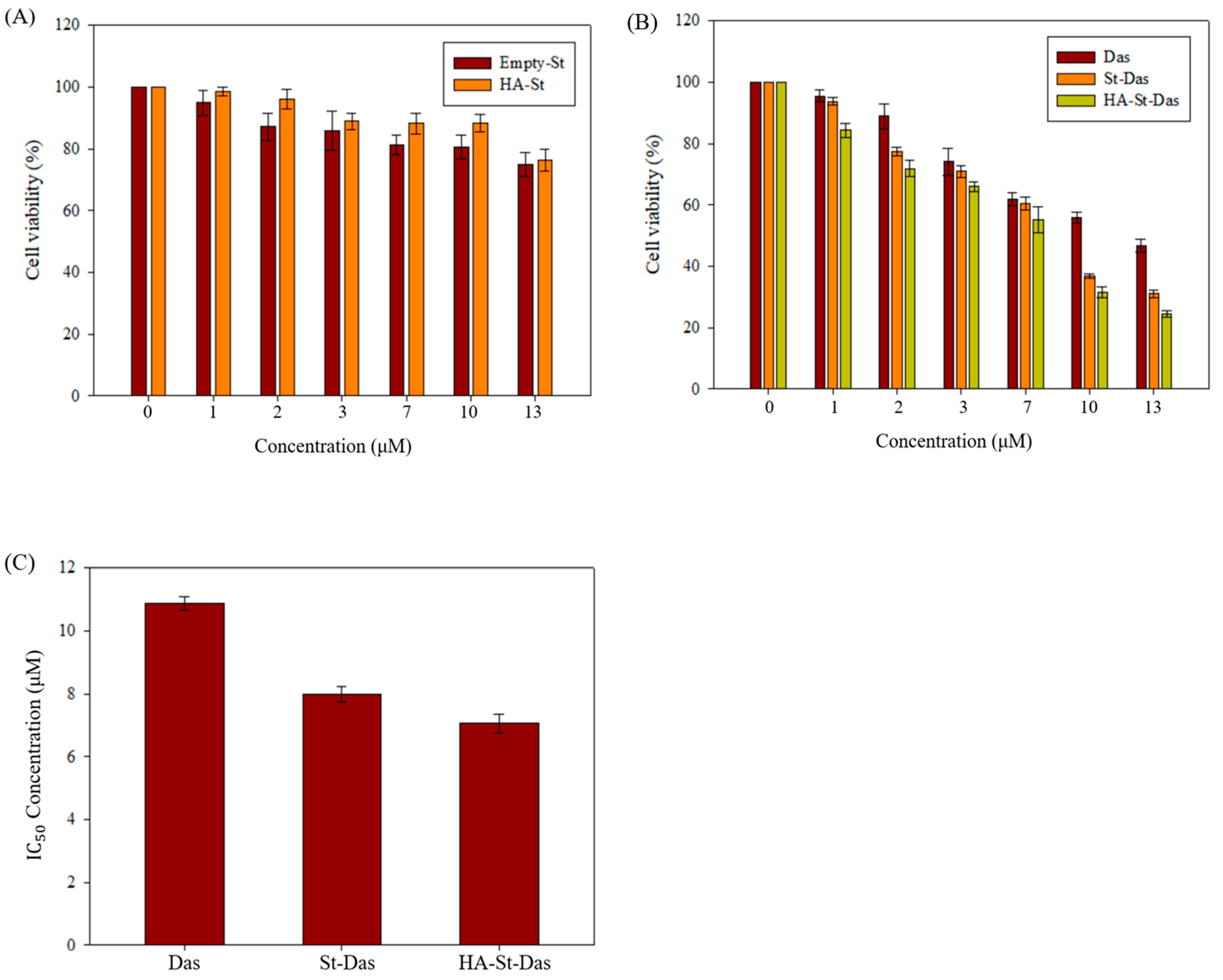
3.4. In Vitro Cytotoxicity of Sterosome
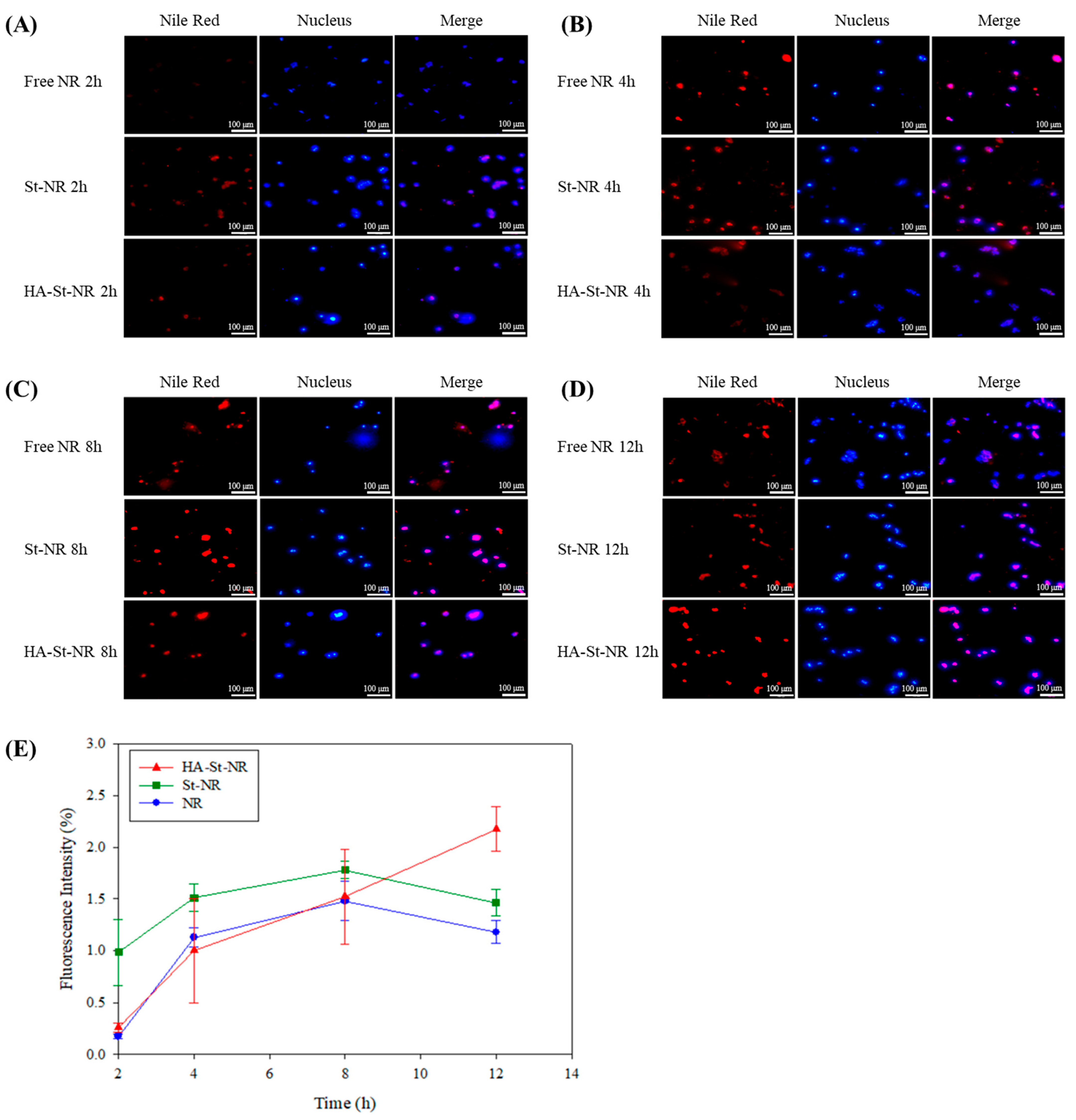
3.5. Cellular Uptake of Sterosome
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Amini, M.; Looha, M.A.; Zarean, E.; Pourhoseingholi, M.A. Global pattern of trends in incidence, mortality, and mortality-to-incidence ratio rates related to liver cancer, 1990–2019: A longitudinal analysis based on the global burden of disease study. BMC Public Health 2022, 22, 604. [Google Scholar] [CrossRef]
- Ye, S.; Pan, X.; Zou, L.; Ni, S.; Zhang, L.; Hong, Y.; Hu, K. HepG2 exosomes coated luteolin nanoparticles remodeling hepatic stellate cells and combination with sorafenib for the treatment of hepatocellular carcinoma. Cancer Nanotechnol. 2024, 15, 15. [Google Scholar] [CrossRef]
- Wu, Z.; Tang, H.; Wang, L.; Jin, X.; Lei, Z.; Yang, P.; Zhou, J. Postoperative survival analysis of hepatocellular carcinoma patients with liver cirrhosis based on propensity score matching. BMC Surg. 2022, 22, 103. [Google Scholar] [CrossRef]
- Niu, M.; Yi, M.; Li, N.; Wu, K.; Wu, K. Advances of targeted therapy for hepatocellular carcinoma. Front. Oncol. 2021, 11, 719896. [Google Scholar] [CrossRef]
- Tanaka, S.; Arii, S. Molecularly targeted therapy for hepatocellular carcinoma. Cancer Sci. 2009, 100, 1–8. [Google Scholar] [CrossRef]
- Chidambaram, M.; Manavalan, R.; Kathiresan, K. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J. Pharm. Pharm. Sci. 2011, 14, 67–77. [Google Scholar] [CrossRef]
- Sheoran, S.; Arora, S.; Samsonraj, R.; Govindaiah, P. Lipid-based nanoparticles for treatment of cancer. Heliyon 2022, 8, e09403. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, X.; Lu, X.; Tian, J. Nanoparticle drug delivery systems for synergistic delivery of tumor therapy. Front. Pharmacol. 2023, 14, 1111991. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Kashanian, S.; Bahrami, Y.; Cruz, L.J.; Motiei, M. Redox-sensitive and hyaluronic acid-functionalized nanoparticles for improving breast cancer treatment by cytoplasmic 17α-methyltestosterone delivery. Molecules 2020, 25, 1181. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; DeGiovanni, P.-J.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 44. [Google Scholar] [CrossRef]
- Gubernator, J. Active methods of drug loading into liposomes: Recent strategies for stable drug entrapment and increased in vivo activity. Expert Opin. Drug Deliv. 2011, 8, 565–580. [Google Scholar] [CrossRef]
- Cui, Z.-K.; Lafleur, M. Lamellar self-assemblies of single-chain amphiphiles and sterols and their derived liposomes: Distinct compositions and distinct properties. Colloids Surf. B Biointerfaces 2014, 114, 177–185. [Google Scholar] [CrossRef]
- Cui, Z.-K.; Kim, S.; Baljon, J.J.; Doroudgar, M.; Lafleur, M.; Wu, B.M.; Aghaloo, T.; Lee, M. Design and characterization of a therapeutic non-phospholipid liposomal nanocarrier with osteoinductive characteristics to promote bone formation. ACS Nano 2017, 11, 8055–8063. [Google Scholar] [CrossRef]
- Lee, C.-S.; Kim, S.; Fan, J.; Hwang, H.S.; Aghaloo, T.; Lee, M. Smoothened agonist sterosome immobilized hybrid scaffold for bone regeneration. Sci. Adv. 2020, 6, eaaz7822. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhang, X.; Kang, M.; Lee, C.-S.; Kim, L.; Hadaya, D.; Aghaloo, T.L.; Lee, M. Complementary modulation of BMP signaling improves bone healing efficiency. Biomaterials 2023, 302, 122335. [Google Scholar] [CrossRef]
- Cieślak, A.; Wauthoz, N.; Orellana, A.N.; Lautram, N.; Béjaud, J.; Hureaux, J.; Lafleur, M.; Benoit, J.-P.; Salomon, C.J.; Bastiat, G. Stealth nanocarriers based sterosomes using PEG post-insertion process. Eur. J. Pharm. Biopharm. 2017, 115, 31–38. [Google Scholar] [CrossRef]
- Lee, C.-S.; Hsu, G.C.-Y.; Sono, T.; Lee, M.; James, A.W. Development of a biomaterial scaffold integrated with osteoinductive oxysterol liposomes to enhance hedgehog signaling and bone repair. Mol. Pharm. 2021, 18, 1677–1689. [Google Scholar] [CrossRef]
- Ye, S.; Wen, J.; Ye, W.-H.; Li, Z.; Huang, X.; Chen, S.; Ma, J.-C.; Wu, Y.; Chen, R.; Cui, Z.-K. A facile and smart strategy to enhance bone regeneration with efficient vitamin D3 delivery through sterosome technology. J. Control. Release 2024, 370, 140–151. [Google Scholar] [CrossRef] [PubMed]
- AbouSamra, M.M.; Afifi, S.M.; Galal, A.F.; Kamel, R. Rutin-loaded Phyto-Sterosomes as a potential approach for the treatment of hepatocellular carcinoma: In-vitro and in-vivo studies. J. Drug Deliv. Sci. Technol. 2023, 79, 104015. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, Y.; Xu, X.; Chen, Z.; Ma, L.; Wang, Y.; Guo, X.; Li, J.; Wang, X. Construction of pH-sensitive targeted micelle system co-delivery with curcumin and dasatinib and evaluation of anti-liver cancer. Drug Deliv. 2022, 29, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Tian, Y.; Yuan, X.; Wu, H.; Liu, Q.; Pestell, R.G.; Wu, K. The role of CD44 in epithelial–mesenchymal transition and cancer development. OncoTargets Ther. 2015, 8, 3783–3792. [Google Scholar] [CrossRef]
- Brave, M.; Goodman, V.; Kaminskas, E.; Farrell, A.; Timmer, W.; Pope, S.; Harapanhalli, R.; Saber, H.; Morse, D.; Bullock, J. Sprycel for chronic myeloid leukemia and Philadelphia chromosome–positive acute lymphoblastic leukemia resistant to or intolerant of imatinib mesylate. Clin. Cancer Res. 2008, 14, 352–359. [Google Scholar] [CrossRef]
- Talpaz, M.; Shah, N.P.; Kantarjian, H.; Donato, N.; Nicoll, J.; Paquette, R.; Cortes, J.; O’Brien, S.; Nicaise, C.; Bleickardt, E. Dasatinib in imatinib-resistant Philadelphia chromosome–positive leukemias. N. Engl. J. Med. 2006, 354, 2531–2541. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, X.; Wang, H.; Fan, R.; Hu, Y.; Hu, X.; Li, J. Dasatinib self-assembled nanoparticles decorated with hyaluronic acid for targeted treatment of tumors to overcome multidrug resistance. Drug Deliv. 2021, 28, 670–679. [Google Scholar] [CrossRef]
- Chang, A.Y.; Wang, M. Molecular mechanisms of action and potential biomarkers of growth inhibition of dasatinib (BMS-354825) on hepatocellular carcinoma cells. BMC Cancer 2013, 13, 267. [Google Scholar] [CrossRef]
- Carvalho, M.P.; Costa, E.C.; Miguel, S.P.; Correia, I.J. Tumor spheroid assembly on hyaluronic acid-based structures: A review. Carbohydr. Polym. 2016, 150, 139–148. [Google Scholar] [CrossRef]
- Lee, C.-S.; Na, K. Photochemically triggered cytosolic drug delivery using pH-responsive hyaluronic acid nanoparticles for light-induced cancer therapy. Biomacromolecules 2014, 15, 4228–4238. [Google Scholar] [CrossRef]
- Gao, C.; Guo, H.; Downey, L.; Marroquin, C.; Wei, J.; Kuo, P.C. Osteopontin-dependent CD44v6 expression and cell adhesion in HepG2 cells. Carcinogenesis 2003, 24, 1871–1878. [Google Scholar] [CrossRef]
- Pedrosa, S.S.; Pereira, P.; Correia, A.; Gama, F. Targetability of hyaluronic acid nanogel to cancer cells: In vitro and in vivo studies. Eur. J. Pharm. Sci. 2017, 104, 102–113. [Google Scholar] [CrossRef]
- Hwang, H.S.; Lee, C.-S. Recent progress in hyaluronic-acid-based hydrogels for bone tissue engineering. Gels 2023, 9, 588. [Google Scholar] [CrossRef]
- Eliaz, R.E.; Szoka, F.C., Jr. Liposome-encapsulated doxorubicin targeted to CD44: A strategy to kill CD44-overexpressing tumor cells. Cancer Res. 2001, 61, 2592–2601. [Google Scholar]
- Wang, T.; Hou, J.; Su, C.; Zhao, L.; Shi, Y. Hyaluronic acid-coated chitosan nanoparticles induce ROS-mediated tumor cell apoptosis and enhance antitumor efficiency by targeted drug delivery via CD44. J. Nanobiotechnol. 2017, 15, 7. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, J.; Lee, C.S.; Kim, S.; Chen, C.; Aghaloo, T.; Lee, M. Apatite-binding nanoparticulate agonist of hedgehog signaling for bone repair. Adv. Funct. Mater. 2020, 30, 1909218. [Google Scholar] [CrossRef]
- Ravisankar, P.; Anusha, S.; Babu, P.S. Development and validation of UV-spectrophotometric method for determination of dasatinib in bulk and pharmaceutical dosage form and its degradation behaviour under various stress conditions. Int. J. Pharm. Sci. Rev. Res 2020, 53, 45–50. [Google Scholar]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Almalik, A.; Donno, R.; Cadman, C.J.; Cellesi, F.; Day, P.J.; Tirelli, N. Hyaluronic acid-coated chitosan nanoparticles: Molecular weight-dependent effects on morphology and hyaluronic acid presentation. J. Control. Release 2013, 172, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Pasarin, D.; Ghizdareanu, A.-I.; Enascuta, C.E.; Matei, C.B.; Bilbie, C.; Paraschiv-Palada, L.; Veres, P.-A. Coating materials to increase the stability of liposomes. Polymers 2023, 15, 782. [Google Scholar] [CrossRef] [PubMed]
- Amorim, S.; Pashkuleva, I.; Reis, C.A.; Reis, R.L.; Pires, R.A. Tunable layer-by-layer films containing hyaluronic acid and their interactions with CD44. J. Mater. Chem. B 2020, 8, 3880–3885. [Google Scholar] [CrossRef]
- Bayer, I.S. Hyaluronic acid and controlled release: A review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef]
- Hanieh, P.N.; Forte, J.; Di Meo, C.; Ammendolia, M.G.; Del Favero, E.; Cantù, L.; Rinaldi, F.; Marianecci, C.; Carafa, M. Hyaluronic acid derivative effect on niosomal coating and interaction with cellular mimetic membranes. Molecules 2021, 26, 3434. [Google Scholar] [CrossRef]
- Lee, C.-S.; Park, W.; Park, S.-j.; Na, K. Endolysosomal environment-responsive photodynamic nanocarrier to enhance cytosolic drug delivery via photosensitizer-mediated membrane disruption. Biomaterials 2013, 34, 9227–9236. [Google Scholar] [CrossRef]
- Niko, Y.; Klymchenko, A.S. Emerging solvatochromic push–pull dyes for monitoring the lipid order of biomembranes in live cells. J. Biochem. 2021, 170, 163–174. [Google Scholar] [CrossRef]
- Treuel, L.; Brandholt, S.; Maffre, P.; Wiegele, S.; Shang, L.; Nienhaus, G.U. Impact of protein modification on the protein corona on nanoparticles and nanoparticle–cell interactions. ACS Nano 2014, 8, 503–513. [Google Scholar] [CrossRef]
- Åberg, C. Kinetics of nanoparticle uptake into and distribution in human cells. Nanoscale Adv. 2021, 3, 2196–2212. [Google Scholar] [CrossRef] [PubMed]
- Lindauer, M.; Hochhaus, A. Dasatinib. In Small Molecules in Oncology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 27–65. [Google Scholar]
- Arpicco, S.; Lerda, C.; Dalla Pozza, E.; Costanzo, C.; Tsapis, N.; Stella, B.; Donadelli, M.; Dando, I.; Fattal, E.; Cattel, L. Hyaluronic acid-coated liposomes for active targeting of gemcitabine. Eur. J. Pharm. Biopharm. 2013, 85, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Cho, H.-J.; Yoon, H.Y.; Yoon, I.-S.; Ko, S.-H.; Shim, J.-S.; Cho, J.-H.; Park, J.H.; Kim, K.; Kwon, I.C. Hyaluronic acid derivative-coated nanohybrid liposomes for cancer imaging and drug delivery. J. Control. Release 2014, 174, 98–108. [Google Scholar] [CrossRef] [PubMed]


| OA:Cholesterol | Size (nm) | Zeta Potential (mV) | PDI |
|---|---|---|---|
| 7:3 | 1.9 | 8.4 | 0.05 |
| 6:4 | 3.0 | 6.6 | 0.01 |
| 5:5 | 75.1 ± 0.7 | 4.2 | 0.01 |
| 4:6 | 34.2 | 5.7 | 0.08 |
| 3:7 | 57.7 | 3.1 | 0.01 |
| HA:DW (v/v) | Size (nm) | Zeta Potential (mV) | PDI |
|---|---|---|---|
| non | 89.1 ± 6.2 | 73.6 ± 7.6 | 0.18 ± 0.04 |
| 1:9 | 137.5 ± 15.4 | 36.0 ± 1.6 | 0.29 ± 0.07 |
| 2:8 | 7660.3 ± 2642.3 | 11.9 ± 3.0 | 0.33 ± 0.06 |
| 3:7 | 4346.7 ± 1197.3 | −8.0 ± 5.7 | 0.42 ± 0.07 |
| 4:6 | 409.9 ± 72.6 | −24.2 ± 2.1 | 0.44 ± 0.14 |
| 5:5 | 203.4 ± 1.2 | −26.4 ± 3.2 | 0.20 ± 0.03 |
| 6:4 | 333.7 ± 71.8 | −23.8 ± 3.4 | 0.51 ± 0.35 |
| 7:3 | 299.9 ± 41.4 | −24.0 ± 3.5 | 0.53 ± 0.27 |
| 8:2 | 276.3 ± 9.4 | −26.7 ± 1.9 | 0.44 ± 0.11 |
| 9:1 | 563.4 ± 274.1 | −25.8 ± 3.4 | 0.82 ± 0.43 |
| 10:0 | 284.7 ± 69.6 | −26.8 ± 2.9 | 0.41 ± 0.26 |
| Size (nm) | Zeta Potential (mV) | PDI | EE (%) | DLC (%) | |
|---|---|---|---|---|---|
| Empty sterosome | 75.1 ± 0.7 | 69.0 ± 4.2 | 0.15 ± 0.01 | - | - |
| St-Das | 89.1 ± 6.2 | 73.6 ± 7.6 | 0.18 ± 0.01 | 77.81 ± 1.37 | 10.08 ± 0.18 |
| HA-St-Das | 203.0 ± 1.2 | −26.4 ± 3.2 | 0.20 ± 0.03 | 57.78 ± 0.21 | 7.48 ± 0.03 |
| Das | St-Das | HA-St-Das | |
|---|---|---|---|
| IC50 (μM) | 10.87 ± 0.22 | 7.99 ± 0.25 | 7.05 ± 0.29 |
| Size (nm) | Zeta Potential (mV) | PDI | EE (%) | DLC (%) | |
|---|---|---|---|---|---|
| St-NR | 96.2 ± 9.8 | 61.7 ± 6.9 | 0.17 ± 0.03 | 62.96 ± 3.65 | 8.15 ± 0.47 |
| HA-St-NR | 178.4 ± 10.1 | −34.1 ± 5.5 | 0.19 ± 0.04 | 51.21 ± 5.33 | 6.63 ± 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.Y.; Lee, J.M.; Lee, C.-S.; Hwang, H.S. Hyaluronic-Acid-Coated Sterosome for Dasatinib Delivery in Hepatocellular Carcinoma: Preparation, Physicochemical Characterization, and In Vitro Evaluation. Biomimetics 2025, 10, 552. https://doi.org/10.3390/biomimetics10080552
Lee CY, Lee JM, Lee C-S, Hwang HS. Hyaluronic-Acid-Coated Sterosome for Dasatinib Delivery in Hepatocellular Carcinoma: Preparation, Physicochemical Characterization, and In Vitro Evaluation. Biomimetics. 2025; 10(8):552. https://doi.org/10.3390/biomimetics10080552
Chicago/Turabian StyleLee, Chae Yeong, Jeong Min Lee, Chung-Sung Lee, and Hee Sook Hwang. 2025. "Hyaluronic-Acid-Coated Sterosome for Dasatinib Delivery in Hepatocellular Carcinoma: Preparation, Physicochemical Characterization, and In Vitro Evaluation" Biomimetics 10, no. 8: 552. https://doi.org/10.3390/biomimetics10080552
APA StyleLee, C. Y., Lee, J. M., Lee, C.-S., & Hwang, H. S. (2025). Hyaluronic-Acid-Coated Sterosome for Dasatinib Delivery in Hepatocellular Carcinoma: Preparation, Physicochemical Characterization, and In Vitro Evaluation. Biomimetics, 10(8), 552. https://doi.org/10.3390/biomimetics10080552






