Therapeutic Potential of Stem Cell-Derived Exosomes in Skin Wound Healing
Abstract
1. Introduction
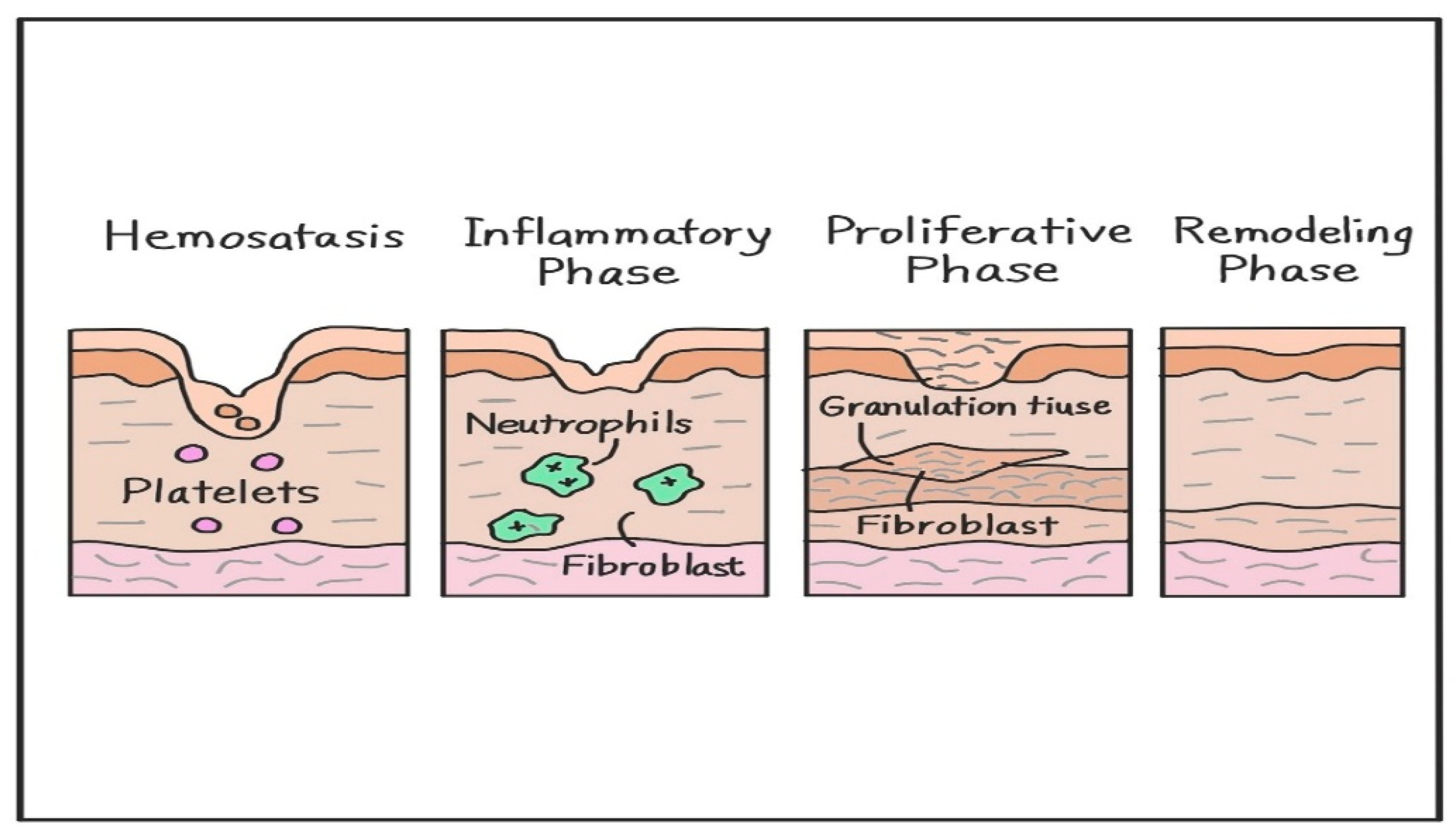
2. Molecular Mechanisms of Skin Wound Healing Process
2.1. Hemostasis
2.2. Inflammatory Phase
2.3. Proliferative Phase
2.4. Remodeling Phase
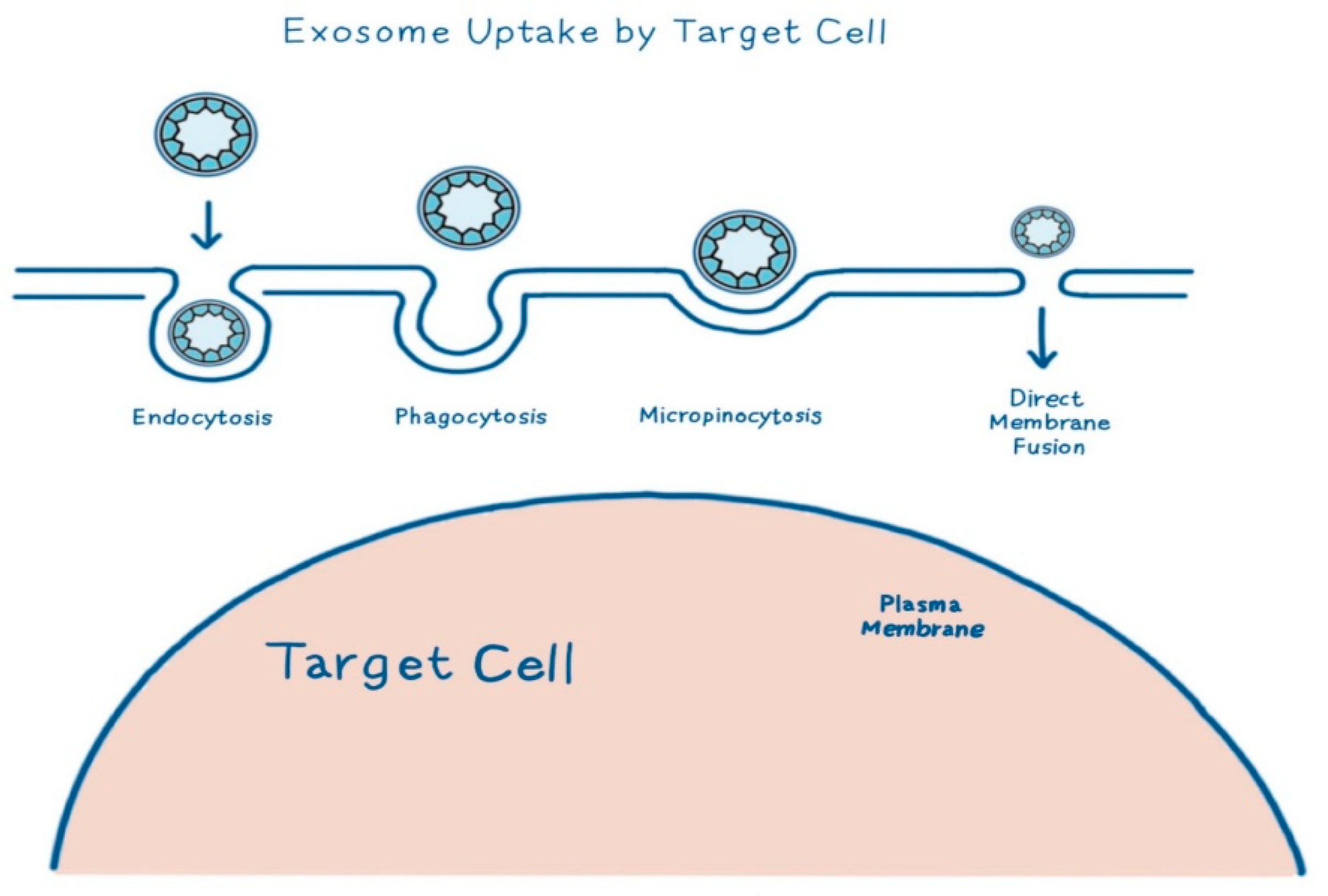
3. Exosomes
4. Stem Cell-Derived Exosome for Skin Wound Treatment
4.1. Mesenchymal Stem Cell-Derived Exosome
4.2. Adipose-Derived Stem Cell-Derived Exosome
4.3. Induced Pluripotent Stem Cell-Derived Exosome
4.4. Other Type of Stem Cell-Derived Exosome
5. Challenges and Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SC-Exos | Stem cell-derived exosomes |
| NPWT | Negative pressure wound therapy |
| MSCs | Mesenchymal stem cells |
| ECM | Extracellular matrix |
| PGDF | Platelet-derived growth factor |
| TGF-β | Transforming growth factor-β |
| VEGF | Vascular endothelial growth factor |
| FGF-2 | Fibroblast growth factor 2 |
| ADSCs | Adipose-derived stem cells |
| MMPs | Matrix metalloproteinases |
| TIMPs | Tissue inhibitors |
| MVBs | Multivesicular bodies |
| ILVs | Intraluminal vesicles |
| iPSCs | Pluripotent stem cells |
| BMMSCs | Bone marrow-derived mesenchymal stem cells |
| JMMSCs | Human jawbone marrow-derived mesenchymal stem cells |
| HUVECs | Human umbilical vein endothelial cells |
| iPSC-Exos | iPSC-derived exosomes |
| HucMSCs | Human umbilical cord MSCs |
| lncRNA | Long non-coding RNA |
| HF-MSC-Exos | Hair follicle mesenchymal stem cells |
| EV | Extracellular vesicles |
| TFF | Tangential flow filtration |
| GMP | Good Manufacturing Practice |
References
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Varela, P.; Roca, B.; López, M.; Martínez, I. Antioxidant dressing therapy vs standard wound care in chronic wounds. BMC Trials 2020, 21, 569. [Google Scholar]
- Kirsner, R.S.; Vivas, A.C. Lower-extremity ulcers: Diagnosis and management. Br. J. Dermatol. 2015, 173, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.J.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D.; Fife, C.E. Chronic wound prevalence and the associated cost of treatment in Medicare beneficiaries: Changes between 2014 and 2019. J. Med. Econ. 2023, 26, 894–901. [Google Scholar] [CrossRef]
- Popescu, V.; Cauni, V.; Petrutescu, M.S.; Rustin, M.M.; Bocai, R.; Turculet, C.R.; Doran, H.; Patrascu, T.; Lazar, A.M.; Cretoiu, D.; et al. Chronic Wound Management: From Gauze to Homologous Cellular Matrix. Biomedicines 2023, 11, 2457. [Google Scholar] [CrossRef] [PubMed]
- Kapp, S.; Santamaria, N. The financial and quality-of-life cost to patients living with a chronic wound in the community. Int. Wound J. 2017, 14, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.B.; Desai, U.; Cummings, A.K.G.; Birnbaum, H.G.; Skornicki, M.; Parsons, N. Clinical and economic burden of diabetic foot ulcers. Int. Wound J. 2021, 18, 336–348. [Google Scholar] [CrossRef]
- Li, K.; Zhu, Z.; Zhai, Y.; Chen, S. Recent Advances in Electrospun Nanofiber-Based Strategies for Diabetic Wound Healing Application. Pharmaceutics 2023, 15, 2285. [Google Scholar] [CrossRef]
- Peinemann, F.; Sauerland, S. Negative-pressure wound therapy: Systematic review of randomized controlled trials. Dtsch. Arztebl. Int. 2011, 108, 381–389. [Google Scholar]
- Trujillo-Martín, M.; García-Pérez, L.; Serrano-Aguilar, P. Effectiveness, safety and cost-effectiveness of the negative pressure wound therapy on the treatment of chronic wounds: A systematic review. Med. Clin. 2011, 137, 321–328. [Google Scholar] [CrossRef]
- Hasan, M.Y.; Teo, R.; Nather, A. Negative-pressure wound therapy for management of diabetic foot wounds: A review of the mechanism of action, clinical applications, and recent developments. Diabet. Foot Ankle 2015, 6, 27618. [Google Scholar] [CrossRef] [PubMed]
- Vuerstaek, J.D.; Vainas, T.; Wuite, J.; Nelemans, P.; Neumann, M.H.; Veraart, J.C. State-of-the-art treatment of chronic leg ulcers: A randomized controlled trial comparing vacuum-assisted closure (V.A.C.) with modern wound dressings. J. Vasc. Surg. 2006, 44, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound healing and treating wounds: Chronic wound care and management. J. Am. Acad. Dermatol. 2016, 74, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Loesche, M.; Gardner, S.E.; Kalan, L.; Horwinski, J.; Zheng, Q.; Hodkinson, B.P.; Tyldsley, A.S.; Franciscus, C.L.; Hillis, S.L.; Mehta, S.; et al. Temporal Stability in Chronic Wound Microbiota Is Associated with Poor Healing. J. Investig. Dermatol. 2017, 137, 237–244. [Google Scholar] [CrossRef]
- Chen, V.; Burgess, J.L.; Verpile, R.; Tomic-Canic, M.; Pastar, I. Novel Diagnostic Technologies and Therapeutic Approaches Targeting Chronic Wound Biofilms and Microbiota. Curr. Dermatol. Rep. 2022, 11, 60–72. [Google Scholar] [CrossRef]
- Dayya, D.; O’Neill, O.J.; Huedo-Medina, T.B.; Habib, N.; Moore, J.; Iyer, K. Debridement of Diabetic Foot Ulcers. Adv. Wound Care 2022, 11, 666–686. [Google Scholar] [CrossRef]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef]
- Preda, M.B.; Neculachi, C.A.; Fenyo, I.M.; Vacaru, A.M.; Publik, M.A.; Simionescu, M.; Burlacu, A. Short lifespan of syngeneic transplanted MSC is a consequence of In Vivo apoptosis and immune cell recruitment in mice. Cell Death Dis. 2021, 12, 566. [Google Scholar] [CrossRef]
- Tutuianu, R.; Rosca, A.M.; Iacomi, D.M.; Simionescu, M.; Titorencu, I. Human mesenchymal stromal cell-derived exosomes promote In Vitro wound healing by modulating the biological properties of skin keratinocytes and fibroblasts and stimulating angiogenesis. Int. J. Mol. Sci. 2021, 22, 6239. [Google Scholar] [CrossRef]
- Boriachek, K.; Islam, M.N.; Möller, A.; Salomon, C.; Nguyen, N.T.; Hossain, M.S.A.; Yamauchi, Y.; Shiddiky, M.J.A. Biological Functions and Current Advances in Isolation and Detection Strategies for Exosome Nanovesicles. Small 2018, 14, 1702153. [Google Scholar] [CrossRef]
- Toh, W.S.; Lai, R.C.; Zhang, B.; Lim, S.K. MSC exosome works through a protein-based mechanism of action. Biochem. Soc. Trans. 2018, 46, 843–853. [Google Scholar] [CrossRef]
- Jang, H.J.; Shim, K.S.; Lee, J.; Park, J.H.; Kang, S.J.; Shin, Y.M.; Lee, J.B.; Baek, W.; Yoon, J.K. Engineering of Cell Derived-Nanovesicle as an Alternative to Exosome Therapy. Tissue Eng. Regen. Med. 2024, 21, 1–19. [Google Scholar] [CrossRef]
- Hu, L.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Exosomes from adipose-derived stem cells accelerate cutaneous wound healing by optimizing the characteristics of fibroblasts. Sci. Rep. 2016, 6, 32993. [Google Scholar]
- Li, X.; Xie, X.; Lian, W.; Shi, R.; Han, S.; Zhang, H.; Lu, L.; Li, M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Hunt, M.; Torres, M.; Bachar-Wikstrom, E.; Wikstrom, J.D. Cellular and molecular roles of reactive oxygen species in wound healing. Commun. Biol. 2024, 7, 1534. [Google Scholar] [CrossRef]
- DiPietro, L.A.; Burdick, M.; Low, Q.E.; Kunkel, S.L.; Strieter, R.M. MIP-1alpha as a critical macrophage chemoattractant in murine wound repair. J. Clin. Investig. 1998, 101, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
- van Zanten, H.G.; Saelman, E.U.; Schut-Hese, K.M.; Wu, Y.P.; Slootweg, P.J.; Nieuwenhuis, H.K.; de Groot, P.G.; Sixma, J.J. Platelet adhesion to collagen type IV under flow conditions. Blood 1996, 88, 3862–3871. [Google Scholar] [CrossRef]
- Crane, M.J.; Daley, J.M.; van Houtte, O.; Brancato, S.K.; Henry, W.L., Jr.; Albina, J.E. The Monocyte to Macrophage Transition in the Murine Sterile Wound. PLoS ONE 2014, 9, e86660. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mehta, N.D.; Zhao, Y.; DiPietro, L.A. Absence of CD4 or CD8 lymphocytes changes infiltration of inflammatory cells and profiles of cytokine expression in skin wounds, but does not impair healing. Exp. Dermatol. 2014, 23, 189–194. [Google Scholar] [CrossRef]
- Fernández-Guarino, M.; Hernández-Bule, M.L.; Bacci, S. Expression of tissue remodeling- and inflammation-related factors during the wound-healing process in humans. J. Pers. Med. 2023, 15, 14. [Google Scholar]
- Pierce, G.F.; Mustoe, T.A.; Lingelbach, J.; Masakowski, V.R.; Griffin, G.L.; Senior, R.M.; Deuel, T.F. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J. Cell Biol. 1989, 109, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, Y.; Li, L. MiR146a-loaded engineered exosomes released from silk fibroin patch promote diabetic wound healing. Nat. Commun. 2023, 14, 5678. [Google Scholar]
- Neudecker, V.; Haneklaus, M.; Jensen, O.; Khailova, L.; Masterson, J.C.; Tye, H.; Biette, K.; Jedlicka, P.; Brodsky, K.S.; Gerich, M.E.; et al. Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. J Exp Med. 2017, 214, 1737–1752. [Google Scholar] [CrossRef]
- Arora, P.D.; McCulloch, C.A. The deletion of transforming growth factor-β-induced myofibroblasts depends on growth conditions and actin organization. Am. J. Pathol. 1999, 155, 2055–2066. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Patsouris, D.; Stanojcic, M.; Abdullahi, A.; Rehou, S.; Pinto, R.; Chen, P.; Amini-Nik, S. Pathophysiologic Response to Burns in the Elderly. EBioMedicine 2015, 2, 1536–1548. [Google Scholar] [CrossRef]
- Hu, J.C.; Zheng, C.X.; Sui, B.D.; Liu, W.J.; Jin, Y. Mesenchymal stem cell-derived exosomes: A novel and potential remedy for cutaneous wound healing and regeneration. World J. Stem Cells 2022, 14, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, D.; Wang, Z. Exosomal miR-17–92 derived from human mesenchymal stem cells promotes wound healing. Stem Cell Res. Ther. 2023, 14, 123. [Google Scholar]
- Yuan, R.; Dai, X.; Li, Y.; Li, C.; Liu, L. Exosomes from miR-29a-modified adipose-derived mesenchymal stem cells reduce excessive scar formation by inhibiting TGF-β2/Smad3 signaling. Mol. Med. Rep. 2021, 24, 758. [Google Scholar] [CrossRef]
- Ladwig, G.P.; Robson, M.C.; Liu, R.; Kuhn, M.A.; Muir, D.F.; Schultz, G.S. Ratios of activated matrix metalloproteinase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair. Regen. 2002, 10, 26–37. [Google Scholar] [CrossRef]
- Tamai, K.; Tanaka, N.; Nakano, T.; Kakazu, E.; Kondo, Y.; Inoue, J.; Shiina, M.; Fukushima, K.; Hoshino, T.; Sano, K.; et al. Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem. Biophys. Res. Commun. 2010, 399, 384–390. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Liu, D. Exosomal microRNAs from Mesenchymal Stem Cells: Novel Therapeutic Effect in Wound Healing. Tissue Eng. Regen. Med. 2023, 20, 647–660. [Google Scholar] [CrossRef]
- Haraszti, R.; Didiot, M.; Sapp, E.; Leszyk, J.; Shaffer, S.; Rockwell, H.; Gao, F.; Narain, N.; DiFiglia, M.; Kiebish, M.; et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J. Extracell. Vesicles 2016, 5, 32570. [Google Scholar] [CrossRef]
- Hu, Y.; Rao, S.S.; Wang, Z.X.; Cao, J.; Tan, Y.J.; Luo, J.; Li, H.M.; Zhang, W.S.; Chen, C.Y.; Xie, H. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 2018, 8, 169–184. [Google Scholar] [CrossRef]
- Yasuda, T.; Watanabe, H.; Hirosawa, K.M.; Suzuki, K.G.N.; Suga, K.; Hanashima, S. Fluorescence Spectroscopic Analysis of Lateral and Transbilayer Fluidity of Exosome Membranes. Langmuir 2022, 38, 14695–14703. [Google Scholar] [CrossRef]
- Charoenviriyakul, C.; Takahashi, Y.; Nishikawa, M.; Takakura, Y. Preservation of exosomes at room temperature using lyophilization. Int. J. Pharm. 2018, 553, 1–7. [Google Scholar] [CrossRef]
- Arishe, O.O.; Priviero, F.; Wilczynski, S.A.; Webb, R.C. Exosomes as Intercellular Messengers in Hypertension. Int. J. Mol. Sci. 2021, 22, 11685. [Google Scholar] [CrossRef]
- Li, D.; Zhang, P.; Yao, X.; Li, H.; Shen, H.; Li, X.; Wu, J.; Lu, X. Exosomes Derived from miR-133b-Modified Mesenchymal Stem Cells Promote Recovery After Spinal Cord Injury. Front. Neurosci. 2018, 12, 845. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ao, Q.; Wang, X.; Cao, Y.; Liu, Y.; Zheng, S.G.; Tian, X. Mesenchymal Stem Cell-Derived Exosomes: A Promising Biological Tool in Nanomedicine. Front. Pharmacol. 2021, 11, 590470. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, J.; Niu, X.; Hu, G.; Guo, S.; Li, Q.; Xie, Z.; Zhang, C.; Wang, Y. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J. Transl. Med. 2015, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Van Badiavas, E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis In Vitro. Stem Cells Dev. 2015, 24, 1635–1647. [Google Scholar] [CrossRef]
- He, M.; Halima, M.; Xie, Y.; Schaaf, M.J.M.; Meijer, A.H.; Wang, M. Ginsenoside Rg1 Acts as a Selective Glucocorticoid Receptor Agonist with Anti-Inflammatory Action without Affecting Tissue Regeneration in Zebrafish Larvae. Cells 2020, 9, 1107. [Google Scholar] [CrossRef]
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B. MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cells Int. 2019, 2019, 7132708. [Google Scholar] [CrossRef]
- Liu, W.; Yu, M.; Xie, D.; Wang, L.; Ye, C.; Zhu, Q.; Liu, F.; Yang, L. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res. Ther. 2020, 11, 259. [Google Scholar] [CrossRef]
- Liu, Z.; Gan, L.; Zhang, T.; Ren, Q.; Sun, C. Melatonin alleviates adipose inflammation through elevating α-ketoglutarate and diverting adipose-derived exosomes to macrophages in mice. J. Pineal Res. 2018, 64, e12455. [Google Scholar] [CrossRef]
- Shabbir, A.; Zisa, D.; Leiker, M.; Johnston, C.; Lin, H.; Lee, T. Muscular dystrophy therapy by nonautologous mesenchymal stem cells: Muscle regeneration without immunosuppression and inflammation. Transplantation 2009, 87, 1275–1282. [Google Scholar] [CrossRef]
- Shabbir, A.; Zisa, D.; Lin, H.; Mastri, M.; Roloff, G.; Suzuki, G.; Lee, T. Activation of host tissue trophic factors through JAK-STAT3 signaling: A mechanism of mesenchymal stem cell-mediated cardiac repair. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1428–H1438. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, A.; Zisa, D.; Suzuki, G.; Lee, T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: A noninvasive therapeutic regimen. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1888–H1897. [Google Scholar] [CrossRef] [PubMed]
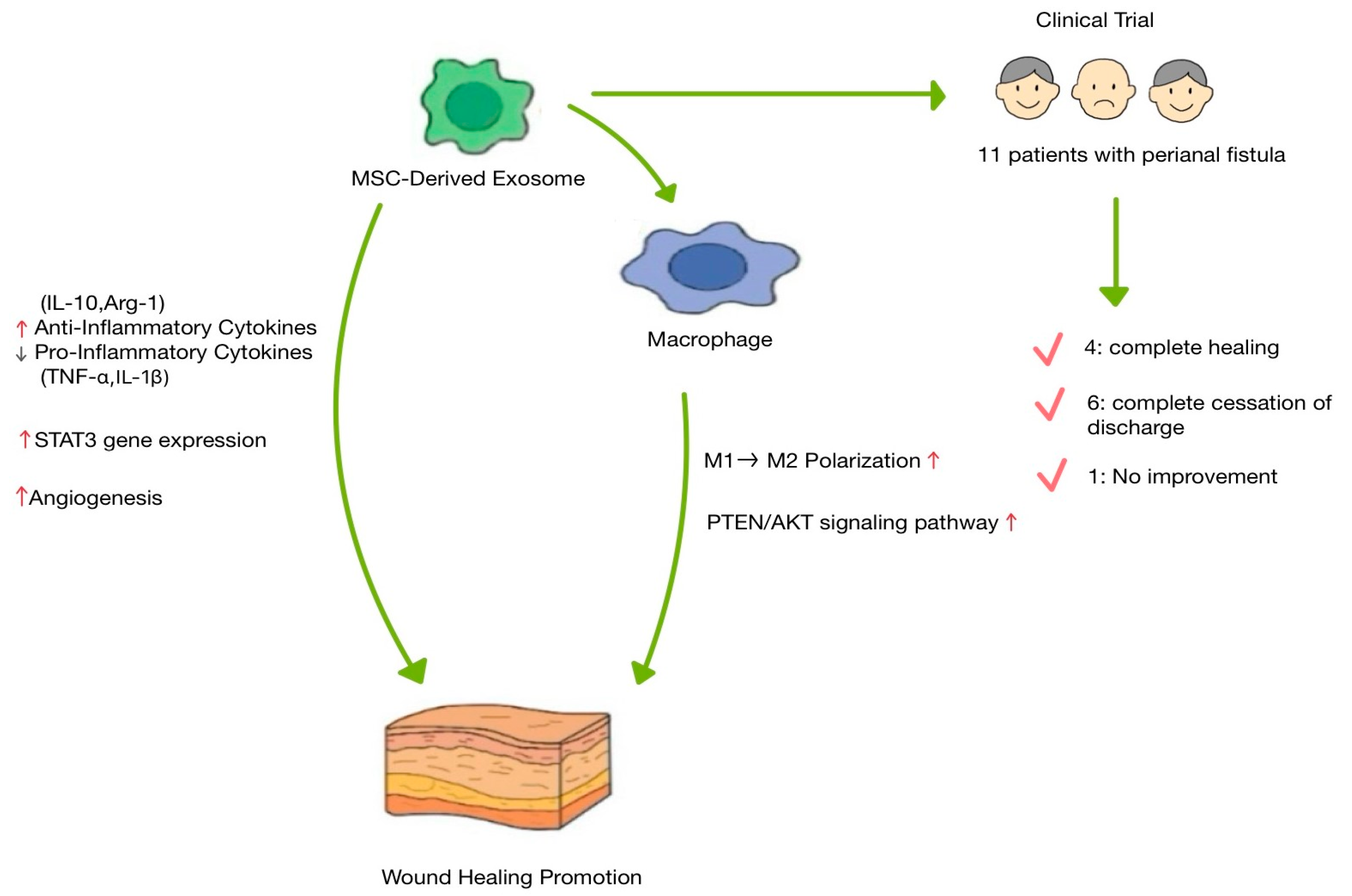
- Pak, H.; Hadizadeh, A.; Heirani-Tabasi, A.; Soleimani, M.; Asbagh, R.A.; Fazeli, M.S.; Kazemeini, A.; Keshvari, A.; Keramati, M.R.; Salahshour, F.; et al. Safety and efficacy of injection of human placenta mesenchymal stem cells derived exosomes for treatment of complex perianal fistula in non-Crohn’s cases: Clinical trial phase I. Gastroenterol. Hepatol. 2023, 38, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Maciel Gutiérrez, V.M.; Gutiérrez Guillen, S.G.; Centeno Flores, M.W.; Valenzuela Pérez, J.A.; Abarca Rendón, F.M.; Hernández García, F.S.; De la Cerda Trujillo, L.F.; Gómez Torres, G.Á. Safety of allogeneic adipose tissue-derived mesenchymal stem cells for the treatment of complex perianal fistulas not associated with Crohn’s disease: A phase I clinical trial. Dis. Colon Rectum 2021, 64, 328–334. [Google Scholar] [CrossRef]
- Nazari, H.; Naei, V.Y.; Tabasi, A.H.; Badripour, A.; Akbari Asbagh, R.; Keramati, M.R.; Sharifi, A.; Behboudi, B.; Kazemeini, A.; Abbasi, M.; et al. Advanced regenerative medicine strategies for treatment of perianal fistula in Crohn’s disease. Inflamm. Bowel Dis. 2022, 28, 133–142. [Google Scholar] [CrossRef]
- Garcia-Arranz, M.; Garcia-Olmo, D.; Herreros, M.D.; Gracia-Solana, J.; Guadalajara, H.; de la Portilla, F.; Baixauli, J.; Garcia-Garcia, J.; Ramirez, J.M.; Sanchez-Guijo, F.; et al. Autologous adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistula: A randomized clinical trial with long-term follow-up. Stem Cells Transl. Med. 2020, 9, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, M.Y.; Tai, H.C.; Cheng, N.C. Cell sheet composed of adipose-derived stem cells demonstrates enhanced skin wound healing with reduced scar formation. Acta Biomater. 2018, 77, 191–200. [Google Scholar] [CrossRef]
- Liu, S.C.; Bamodu, O.A.; Kuo, K.T.; Fong, I.H.; Lin, C.C.; Yeh, C.T.; Chen, S.G. Adipose-derived stem cell induced-tissue repair or wound healing is mediated by the concomitant upregulation of miR-21 and miR-29b expression and activation of the AKT signaling pathway. Arch. Biochem. Biophys. 2021, 705, 108895. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Shan, X.; Xu, L.; Yu, W.; Zhang, M.; Lei, C.; Xu, N.; Lin, J.; Wang, B. Adipose-derived stem cells combined with platelet-rich plasma enhance wound healing in a rat model of full-thickness skin defects. Stem Cell Res. Ther. 2021, 12, 226. [Google Scholar] [CrossRef]
- Cai, Y.; Li, J.; Jia, C.; He, Y.; Deng, C. Therapeutic applications of adipose cell-free derivatives: A review. Stem Cell Res. Ther. 2020, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bai, X.; Shen, K.; Luo, L.; Zhao, M.; Xu, C.; Jia, Y.; Xiao, D.; Li, Y.; Gao, X.; et al. Exosomes derived from adipose mesenchymal stem cells promote diabetic chronic wound healing through SIRT3/SOD2. Cells 2022, 11, 2568. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, H.; Liu, J.; Chen, Y.; He, R.R.; Liu, B. Mitochondrial Sirtuin 3: New emerging biological function and therapeutic target. Theranostics 2020, 10, 8315–8342. [Google Scholar] [CrossRef]
- Yang, S.; Xu, M.; Meng, G.; Lu, Y. SIRT3 deficiency delays diabetic skin wound healing via oxidative stress and necroptosis enhancement. J. Cell Mol. Med. 2020, 24, 4415–4427. [Google Scholar] [CrossRef]
- Karppinen, S.M.; Heljasvaara, R.; Gullberg, D.; Tasanen, K.; Pihlajaniemi, T. Toward understanding scarless skin wound healing and pathological scarring. F1000Research 2019, 8, 787. [Google Scholar] [CrossRef]
- van den Broek, L.J.; Limandjaja, G.C.; Niessen, F.B.; Gibbs, S. Human hypertrophic and keloid scar models: Principles, limitations and future challenges from a tissue engineering perspective. Exp. Dermatol. 2014, 23, 382–386. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.; Liang, P.; Ren, L.; Zeng, J.; Zhang, M.; Zhang, P.; Huang, X. Protective role of microRNA-29a in denatured dermis and skin fibroblast cells after thermal injury. Biol. Open 2016, 5, 211–219. [Google Scholar] [CrossRef]
- Guo, L.; Huang, X.; Liang, P.; Zhang, P.; Zhang, M.; Ren, L.; Zeng, J.; Cui, X.; Huang, X. Role of XIST/miR-29a/LIN28A pathway in denatured dermis and human skin fibroblasts (HSFs) after thermal injury. J. Cell Biochem. 2018, 119, 1463–1474. [Google Scholar] [CrossRef]
- Song, Y.; You, Y.; Xu, X.; Lu, J.; Huang, X.; Zhang, J.; Zhu, L.; Hu, J.; Wu, X.; Xu, X.; et al. Adipose-derived mesenchymal stem cell-derived exosomes biopotentiated extracellular matrix hydrogels accelerate diabetic wound healing and skin regeneration. Adv. Sci. 2023, 10, e2304023. [Google Scholar] [CrossRef]
- Nosrati, H.; Aramideh Khouy, R.; Nosrati, A.; Khodaei, M.; Banitalebi-Dehkordi, M.; Ashrafi-Dehkordi, K.; Sanami, S.; Alizadeh, Z. Nanocomposite scaffolds for accelerating chronic wound healing by enhancing angiogenesis. J. Nanobiotechnol. 2021, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; He, J.; Guo, B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef] [PubMed]
- Amengual-Tugores, A.M.; Ráez-Meseguer, C.; Forteza-Genestra, M.A.; Monjo, M.; Ramis, J.M. Extracellular vesicle-based hydrogels for wound healing applications. Int. J. Mol. Sci. 2023, 24, 4104. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Huang, J.; Heng, S.; Zhang, W.; Liu, Y.; Xia, T.; Ji, C.; Zhang, L.J. Dermal extracellular matrix molecules in skin development, homeostasis, wound regeneration and diseases. Semin. Cell Dev. Biol. 2022, 128, 137–144. [Google Scholar] [CrossRef]
- Ju, Y.; Hu, Y.; Yang, P.; Xie, X.; Fang, B. Extracellular vesicle-loaded hydrogels for tissue repair and regeneration. Mater. Today Bio 2022, 18, 100522. [Google Scholar] [CrossRef]
- Beachley, V.; Ma, G.; Papadimitriou, C.; Gibson, M.; Corvelli, M.; Elisseeff, J. Extracellular matrix particle-glycosaminoglycan composite hydrogels for regenerative medicine applications. J. Biomed. Mater. Res. A 2018, 106, 147–159. [Google Scholar] [CrossRef]
- Chen, X.; Li, K.; Chen, J.; Tan, S. Breakthrough in large-scale production of iPSCs-derived exosomes to promote clinical applications. Front. Bioeng. Biotechnol. 2023, 11, 1257186. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ebisawa, K.; Kambe, M.; Kasai, T.; Suga, H.; Nakamura, K.; Narita, Y.; Ogata, A.; Kamei, Y. <Editors’ Choice> Effects of exosomes derived from the induced pluripotent stem cells on skin wound healing. Nagoya J. Med. Sci. 2018, 80, 141–153. [Google Scholar]
- Galiano, R.D.; Michaels, J., 5th; Dobryansky, M.; Levine, J.P.; Gurtner, G.C. Quantitative and reproducible murine model of excisional wound healing. Wound Repair. Regen. 2004, 12, 485–492. [Google Scholar] [CrossRef]
- Geerlings, S.E.; Hoepelman, A.I. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol. Med. Microbiol. 1999, 26, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Hayes, N.V.; Blackburn, E.; Boyle, M.M.; Russell, G.A.; Frost, T.M.; Morgan, B.J.; Gullick, W.J. Expression of neuregulin 4 splice variants in normal human tissues and prostate cancer and their effects on cell motility. Endocr. Relat. Cancer 2010, 18, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Lee, J.; Kim, Y.J.; Rhee, W.J.; Park, J.H. Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. Int. J. Mol. Sci. 2018, 19, 1715. [Google Scholar] [CrossRef] [PubMed]
- Pandel, R.; Poljšak, B.; Godic, A.; Dahmane, R. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013, 2013, 930164. [Google Scholar] [CrossRef]
- Tigges, J.; Krutmann, J.; Fritsche, E.; Haendeler, J.; Schaal, H.; Fischer, J.W.; Kalfalah, F.; Reinke, H.; Reifenberger, G.; Stühler, K.; et al. The hallmarks of fibroblast ageing. Mech. Ageing Dev. 2014, 138, 26–44. [Google Scholar] [CrossRef]
- Boukamp, P. Skin aging: A role for telomerase and telomere dynamics? Curr. Mol. Med. 2005, 5, 171–177. [Google Scholar] [CrossRef]
- Oh, M.; Kim, Y.J.; Son, Y.J.; Yoo, H.S.; Park, J.H. Promotive effects of human induced pluripotent stem cell-conditioned medium on the proliferation and migration of dermal fibroblasts. Biotechnol. Bioprocess. Eng. 2017, 22, 561–568. [Google Scholar] [CrossRef]
- Panepucci, R.A.; Siufi, J.L.; Silva, W.A., Jr.; Proto-Siquiera, R.; Neder, L.; Orellana, M.; Rocha, V.; Covas, D.T.; Zago, M.A. Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells 2004, 22, 1263–1278. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, J.; Manuchehrabadi, N.; Weir, M.D.; Zhu, Z.; Xu, H.H. Umbilical cord and bone marrow mesenchymal stem cell seeding on macroporous calcium phosphate for bone regeneration in rat cranial defects. Biomaterials 2013, 34, 9917–9925. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Karahuseyinoglu, S. Concise review: Human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells 2007, 25, 2886–2895. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, H.; Xu, W.; Wang, B.; Wu, H.; Tao, Y.; Zhang, B.; Wang, M.; Mao, F.; Yan, Y.; et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis In Vivo and In Vitro. Stem Cell Res. Ther. 2013, 4, 34. [Google Scholar] [CrossRef]
- Li, T.; Yan, Y.; Wang, B.; Qian, H.; Zhang, X.; Shen, L.; Wang, M.; Zhou, Y.; Zhu, W.; Li, W.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013, 22, 845–854. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells 2015, 33, 2158–2168. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Du, Z.; Wu, T.; Yang, C. Hair follicle mesenchymal stem cell exosomal lncRNA H19 inhibited NLRP3 pyroptosis to promote diabetic mouse skin wound healing. Aging 2023, 15, 791–809. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lin, L.; Yu, S.; Xia, R.; Zheng, L. Long non-coding RNA H19 acts as a microRNA-194 sponge to inhibit the apoptosis and promote the proliferation of hypertrophic scar fibroblasts. Can. J. Physiol. Pharmacol. 2021, 99, 1288–1297. [Google Scholar] [CrossRef]
- Anand, S.; Majeti, B.K.; Acevedo, L.M.; Murphy, E.A.; Mukthavaram, R.; Scheppke, L.; Huang, M.; Shields, D.J.; Lindquist, J.N.; Lapinski, P.E.; et al. MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat. Med. 2010, 16, 909–914. [Google Scholar] [CrossRef]
- Lai, J.J.; Chau, Z.L.; Chen, S.Y.; Hill, J.J.; Korpany, K.V.; Liang, N.W.; Lin, L.H.; Lin, Y.H.; Liu, J.K.; Liu, Y.C.; et al. Exosome processing and characterization approaches for research and technology development. Adv. Sci. 2022, 9, e2103222. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation: Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [PubMed]
- Visan, K.S.; Lobb, R.J.; Ham, S.; Lima, L.G.; Palma, C.; Edna, C.P.Z.; Wu, L.Y.; Gowda, H.; Datta, K.K.; Hartel, G.; et al. Comparative analysis of tangential flow filtration and ultracentrifugation, both combined with subsequent size exclusion chromatography, for the isolation of small extracellular vesicles. J. Extracell. Vesicles 2022, 11, e12266. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Welch, J.L.; Madison, M.N.; Margolick, J.B.; Galvin, S.; Gupta, P.; Martínez-Maza, O.; Dash, C.; Okeoma, C.M. Effect of prolonged freezing of semen on exosome recovery and biologic activity. Sci. Rep. 2017, 7, 45034. [Google Scholar] [CrossRef]
- Gelibter, S.; Marostica, G.; Mandelli, A.; Siciliani, S.; Podini, P.; Finardi, A.; Furlan, R. The impact of storage on extracellular vesicles: A systematic study. J. Extracell. Vesicles 2022, 11, e12162. [Google Scholar] [CrossRef]
- Budgude, P.; Kale, V.; Vaidya, A. Cryopreservation of mesenchymal stromal cell-derived extracellular vesicles using trehalose maintains their ability to expand hematopoietic stem cells In Vitro. Cryobiology 2021, 98, 152–163. [Google Scholar] [CrossRef]
- Bosch, S.; de Beaurepaire, L.; Allard, M.; Mosser, M.; Heichette, C.; Chrétien, D.; Jegou, D.; Bach, J.M. Trehalose prevents aggregation of exosomes and cryodamage. Sci. Rep. 2016, 6, 36162. [Google Scholar] [CrossRef]
- Susa, F.; Limongi, T.; Borgione, F.; Peiretti, S.; Vallino, M.; Cauda, V.; Pisano, R. Comparative studies of different preservation methods and relative freeze-drying formulations for extracellular vesicle pharmaceutical applications. ACS Biomater. Sci. Eng. 2023, 9, 5871–5885. [Google Scholar] [CrossRef]
- Trenkenschuh, E.; Richter, M.; Heinrich, E.; Koch, M.; Fuhrmann, G.; Friess, W. Enhancing the stabilization potential of lyophilization for extracellular vesicles. Adv. Healthc. Mater. 2022, 11, e2100538. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yuan, W.; Zheng, Y.; Zhu, X.; Jin, B.; Yang, T.; Yan, Y.; Xu, W.; Chen, H.; Gao, J.; et al. Delivery of engineered extracellular vesicles with miR-29b editing system for muscle atrophy therapy. J. Nanobiotechnol. 2022, 20, 304. [Google Scholar] [CrossRef]
- Jung, J.W.; Kwon, M.; Choi, J.C.; Shin, J.W.; Park, I.W.; Choi, B.W.; Kim, J.Y. Familial occurrence of pulmonary embolism after intravenous, adipose tissue-derived stem cell therapy. Yonsei Med. J. 2013, 54, 1293–1296. [Google Scholar] [CrossRef]
- Heo, J.S.; Choi, Y.; Kim, H.O. Adipose-derived mesenchymal stem cells promote M2 macrophage phenotype through exosomes. Stem Cells Int. 2019, 2019, 7921760. [Google Scholar] [CrossRef] [PubMed]
- Rebmann, V.; König, L.; Nardi, F.d.S.; Wagner, B.; Manvailer, L.F.; Horn, P.A. The potential of HLA-G-bearing extracellular vesicles as a future element in HLA-G immune biology. Front. Immunol. 2016, 7, 173. [Google Scholar] [CrossRef]
- Chen, Y.F.; Luh, F.; Ho, Y.S.; Yen, Y. Exosomes: A review of biologic function, diagnostic and targeted therapy applications, and clinical trials. J. Biomed. Sci. 2024, 31, 67. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pachler, K.; Lener, T.; Streif, D.; Dunai, Z.A.; Desgeorges, A.; Feichtner, M.; Öller, M.; Schallmoser, K.; Rohde, E.; Gimona, M. A good manufacturing practice-grade standard protocol for exclusively human mesenchymal stromal cell-derived extracellular vesicles. Cytotherapy 2017, 19, 458–472. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Q.; Cheng, L.; Wang, Y.; Li, M.; Yang, Q.; Hu, L.; Lou, D.; Li, J.; Dong, X.; et al. Exosome detection via the ultrafast-isolation system: EXODUS. Nat. Methods 2021, 18, 212–218. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Miller, R.; Stoppato, M.; Sere, Y.Y.; Coles, A.; Didiot, M.C.; Wollacott, R.; Sapp, E.; Dubuke, M.L.; Li, X.; et al. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol. Ther. 2018, 26, 2838–2847. [Google Scholar] [CrossRef]
- Kim, J.Y.; Rhim, W.K.; Yoo, Y.I.; Kim, D.S.; Ko, K.W.; Heo, Y.; Park, C.G.; Han, D.K. Defined MSC exosome with high yield and purity to improve regenerative activity. J. Tissue Eng. 2021, 12, 20417314211008626. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, T.; Kong, J.; Chen, H. ChatExosome: An artificial intelligence (AI) agent based on deep learning of exosomes spectroscopy for hepatocellular carcinoma (HCC) diagnosis. Anal. Chem. 2025, 97, 4643–4652. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, B.; Zhai, D.; Wu, C. Three-dimensional printing of bioceramic-induced macrophage exosomes: Immunomodulation and osteogenesis/angiogenesis. NPG Asia Mater. 2021, 13, 72. [Google Scholar] [CrossRef]
- Lotfy, A.; AboQuella, N.M.; Wang, H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res. Ther. 2023, 14, 66. [Google Scholar] [CrossRef]
- Stojadinovic, O.; Brem, H.; Vouthounis, C.; Lee, B.; Fallon, J.; Stallcup, M.; Merchant, A.; Galiano, R.D.; Tomic-Canic, M. Molecular pathogenesis of chronic wounds: The role of beta-catenin and c-myc in the inhibition of epithelialization and wound healing. Am. J. Pathol. 2005, 167, 59–69. [Google Scholar] [CrossRef]
- Yager, D.R.; Zhang, L.Y.; Liang, H.X.; Diegelmann, R.F.; Cohen, I.K. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J. Investig. Dermatol. 1996, 107, 743–748. [Google Scholar] [CrossRef]
- Qin, Q.; Haba, D.; Takizawa, C.; Tomida, S.; Horinouchi, A.; Katagiri, M.; Nomura, S.; Nakagami, G. Candidate biomarkers for hard-to-heal wounds revealed by single-cell RNA sequencing of wound fluid in murine wound models. Wound Repair Regen. 2025, 33, e70038. [Google Scholar] [CrossRef] [PubMed]
- Beneat, A.; Rueda, V.; Patel, H.; Brune, Z.; Sherry, B.; Shih, A.; Kaplan, S.; Rao, A.; Lee, A.; Varghese, A.; et al. Elevation of plasma IL-15 and RANTES as potential biomarkers of healing in chronic venous ulcerations: A pilot study. Biomolecules 2025, 15, 395. [Google Scholar] [CrossRef]
- Ojeda-Hernández, D.D.; Hernández-Sapiéns, M.A.; Reza-Zaldívar, E.E.; Canales-Aguirre, A.; Matías-Guiu, J.A.; Matías-Guiu, J.; Mateos-Díaz, J.C.; Gómez-Pinedo, U.; Sancho-Bielsa, F. Exosomes and Biomaterials: In Search of a New Therapeutic Strategy for Multiple Sclerosis. Life 2022, 12, 1417. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Yang, L. Hydrogel Encapsulation: Taking the Therapy of Mesenchymal Stem Cells and Their Derived Secretome to the Next Level. Front. Bioeng. Biotechnol. 2022, 10, 859927. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, H.; Wan, R.; Cai, Y.; Liu, Y.; Wu, Y.; Yang, Y.; Chen, J.; Zhang, D.; Luo, Z.; et al. Biomaterials-Based Technologies in Skeletal Muscle Tissue Engineering. Adv. Healthc. Mater. 2024, 13, e2304196. [Google Scholar] [CrossRef]
- Lee, J.H.; Won, Y.J.; Kim, H.; Choi, M.; Lee, E.; Ryoou, B.; Lee, S.G.; Cho, B.S. Adipose tissue-derived mesenchymal stem cell-derived exosomes promote wound healing and tissue regeneration. Int. J. Mol. Sci. 2023, 24, 10434. [Google Scholar] [CrossRef] [PubMed]
- Ghauri, Z.H.; Islam, A.; Qadir, M.A.; Gull, N.; Haider, B.; Khan, R.U.; Riaz, T. Development and evaluation of pH-sensitive biodegradable ternary blended hydrogel films (chitosan/guar gum/PVP) for drug delivery application. Sci. Rep. 2021, 11, 21255. [Google Scholar] [CrossRef] [PubMed]
- Tawanwongsri, W.; Vachiramon, V. Skin necrosis after intradermal injection of lyophilized exosome: A case report and a review of the literature. J. Cosmet. Dermatol. 2024, 23, 1597–1603. [Google Scholar] [CrossRef]
- Ha, D.H.; Kim, S.D.; Lee, J.; Kwon, H.H.; Park, G.H.; Yang, S.H.; Jung, J.Y.; Lee, J.H.; Park, S.R.; Youn, J.; et al. Toxicological evaluation of exosomes derived from human adipose tissue-derived mesenchymal stem/stromal cells. Regul. Toxicol. Pharmacol. 2020, 115, 104686. [Google Scholar] [CrossRef]
- Prasai, A.; Jay, J.W.; Jupiter, D.; Wolf, S.E.; El Ayadi, A. Role of exosomes in dermal wound healing: A systematic review. J. Investig. Dermatol. 2022, 142, 662–678.e8. [Google Scholar] [CrossRef] [PubMed]


| Stem Cell Source | Study Types | Key Mechanisms | Therapeutic Outcome | Reference |
|---|---|---|---|---|
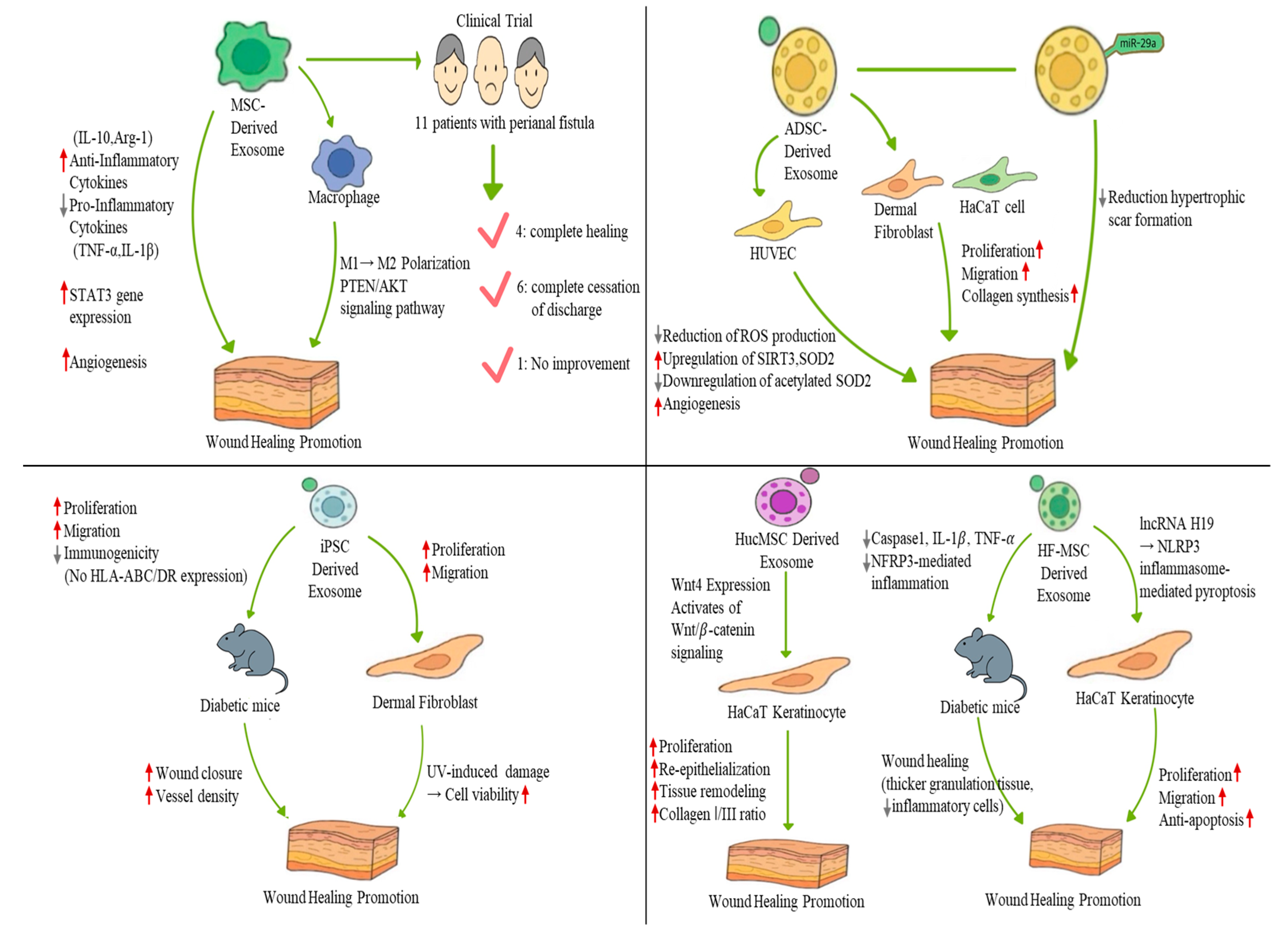
| MSC -derived exosomes | In vitro and in vivo | - Induces M2 macrophage polarization (↑ Arg-1, CD206) - Impaired exosome secretion leads to reduced M2 polarization and delayed wound healing | - Promoted wound regeneration in skin wound models - Increased M2 markers and accelerated wound healing | [53] |
| - Inhibits M1 polarization and promotes M2 polarization of macrophages - Enhances IL-10, Arg-1 secretion; reduces IL-1β, TNF-α expression - Activates the PTEN/AKT signaling pathway | - Improved M2 polarization and wound healing in vivo - Promoted angiogenesis and collagen synthesis in skin wounds | [54] | ||
| - Promotes fibroblast proliferation and migration - Enhances tube formation by endothelial cells - Increases STAT3 gene expression | - Improved cellular functions related to proliferation, migration, and angiogenesis in normal and diabetic fibroblasts | [51] | ||
| Clinical trial | - Immunomodulatory effects of MSC-exosomes utilized for chronic inflammation-related condition | - Among 11 patients with complex perianal fistula: → Four showed complete healing → Six had complete cessation of discharge → One had no improvement | [59] | |
| ADSC -derived exosomes | In vitro and in vivo | - Exosomes internalized by fibroblasts - Promotes fibroblast proliferation, migration, and collagen synthesis | - Accelerated wound healing in a mouse model - Enhanced collagen deposition - IV delivery is more effective than local injection | [23] |
| - Reduces ROS production in HUVECs - Enhances mitochondrial function - Upregulates SIRT3 and SOD2, downregulates acetylated SOD2 | - Promoted angiogenesis and wound closure in diabetic wounds - Protected endothelial cells under high-glucose conditions | [67] | ||
| - miR-29a inhibits fibroblast proliferation, migration, and collagen deposition - Activates the TGF-β2/Smad3 signaling pathway | - Reduced hypertrophic scar formation - Improved wound healing and dermis repair in thermal injury model | [38] | ||
| - Sustained exosome release (95% in 72 h) from thermosensitive ECM hydrogel - Promotes fibroblast migration, collagen synthesis, and tube formation | - 92% wound closure in diabetic ulcers - Enhanced wound regeneration in both diabetic and normal wound models | [74] | ||
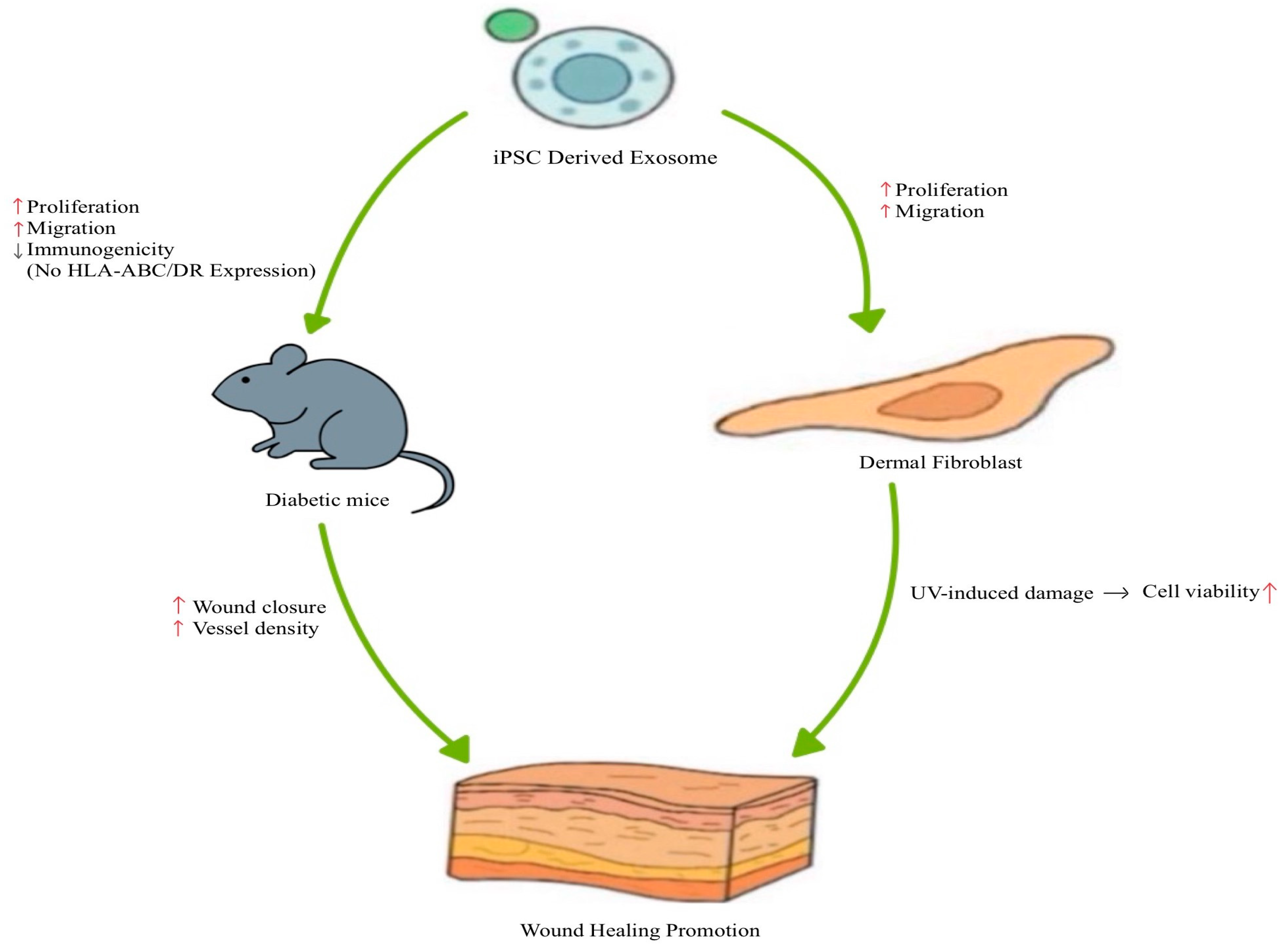
| iPSC -derived exosomes | In vitro and in vivo | - Enhances fibroblast proliferation and migration (from diabetic mice) - Exhibits low immunogenicity (no HLA-ABC/DR expression) | - Accelerated wound closure in diabetic mice - Increased vessel density by day 7 post-treatment | [83] |
| - Enhances fibroblast proliferation and migration - Proposed to mediate anti-aging effects | - Improved cell viability following UV-induced damage | [87] | ||
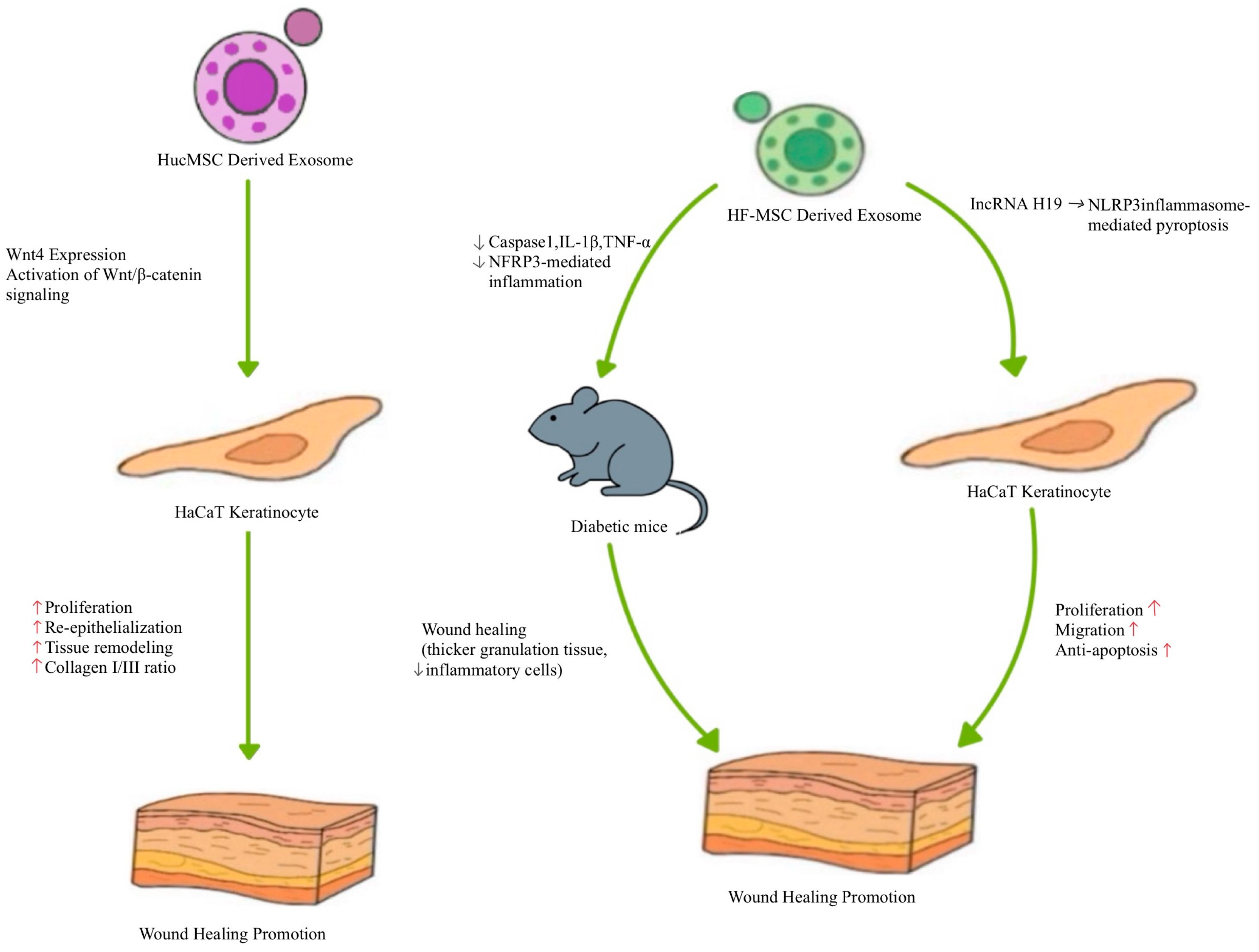
| hUCMSC, derived exosomes | In vitro and in vivo | - Wnt4 expression activates Wnt/β-catenin signaling - Protects HaCaT cells and fibroblasts from heat stress-induced apoptosis | - Enhanced re-epithelialization and tissue remodeling in second-degree burn wounds - Increased collagen I/III ratio - Improved fibroblast proliferation post-heat stress | [97] |
| HF-MSC derived exosomes | In vitro and in vivo | - lncRNA H19 modulates NLRP3 inflammasome-mediated pyroptosis - Promotes fibroblast proliferation, migration, and anti-apoptosis | - Improved wound healing in diabetic mouse skin (thicker granulation tissue, fewer inflammatory cells) - Downregulated caspase-1, IL-1β, TNF-α (reduced inflammation) | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, C.; Choi, Y.J.; Lee, T.-J. Therapeutic Potential of Stem Cell-Derived Exosomes in Skin Wound Healing. Biomimetics 2025, 10, 546. https://doi.org/10.3390/biomimetics10080546
Jo C, Choi YJ, Lee T-J. Therapeutic Potential of Stem Cell-Derived Exosomes in Skin Wound Healing. Biomimetics. 2025; 10(8):546. https://doi.org/10.3390/biomimetics10080546
Chicago/Turabian StyleJo, ChanBee, Yun Ji Choi, and Tae-Jin Lee. 2025. "Therapeutic Potential of Stem Cell-Derived Exosomes in Skin Wound Healing" Biomimetics 10, no. 8: 546. https://doi.org/10.3390/biomimetics10080546
APA StyleJo, C., Choi, Y. J., & Lee, T.-J. (2025). Therapeutic Potential of Stem Cell-Derived Exosomes in Skin Wound Healing. Biomimetics, 10(8), 546. https://doi.org/10.3390/biomimetics10080546






