Titanium Implants Functionalized with Zoledronic Acid Associated with Ruterpy Accelerate Peri-Implant Repair in Healthy and Osteoporotic Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Model Design
2.3. Implants Used in the Research
2.4. Surface Treatment of Implants
2.5. In Vivo Procedures in the Osteoporotic Model
2.6. Estrous Cycle Monitoring
2.7. Ovariectomy Procedure
2.8. Implant Placement Surgery in the Tibia
2.9. Application of Fluorochromes
2.10. Euthanasia
2.11. Processing of Calcified and Decalcified Tissue Samples
2.12. Micro-CT Three-Dimensional Analysis
2.13. Epifluorescence
2.14. Qualitative Histological Evaluation
2.15. Statistical Analysis
3. Results
3.1. Confirmation of the Efficacy of Bilateral Ovariectomy Surgery
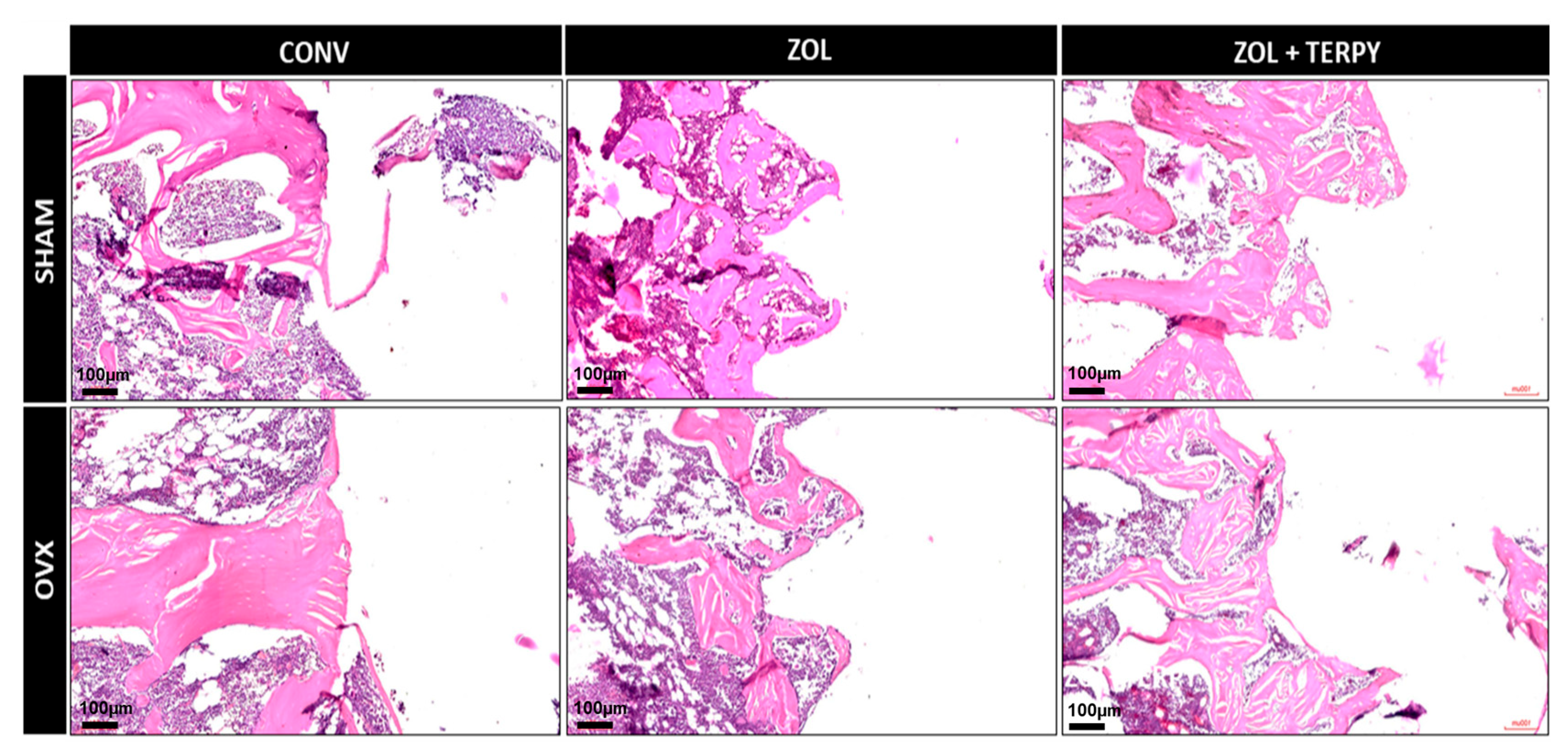
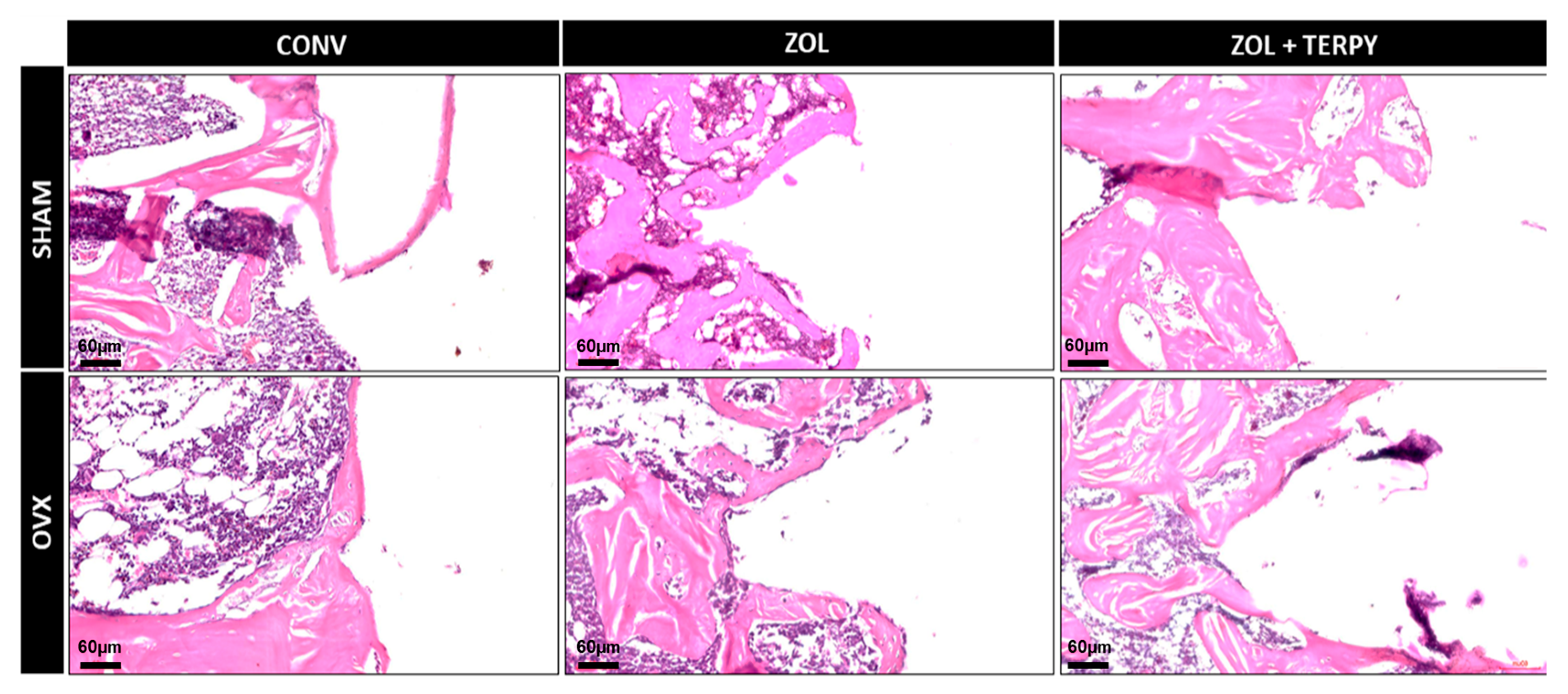
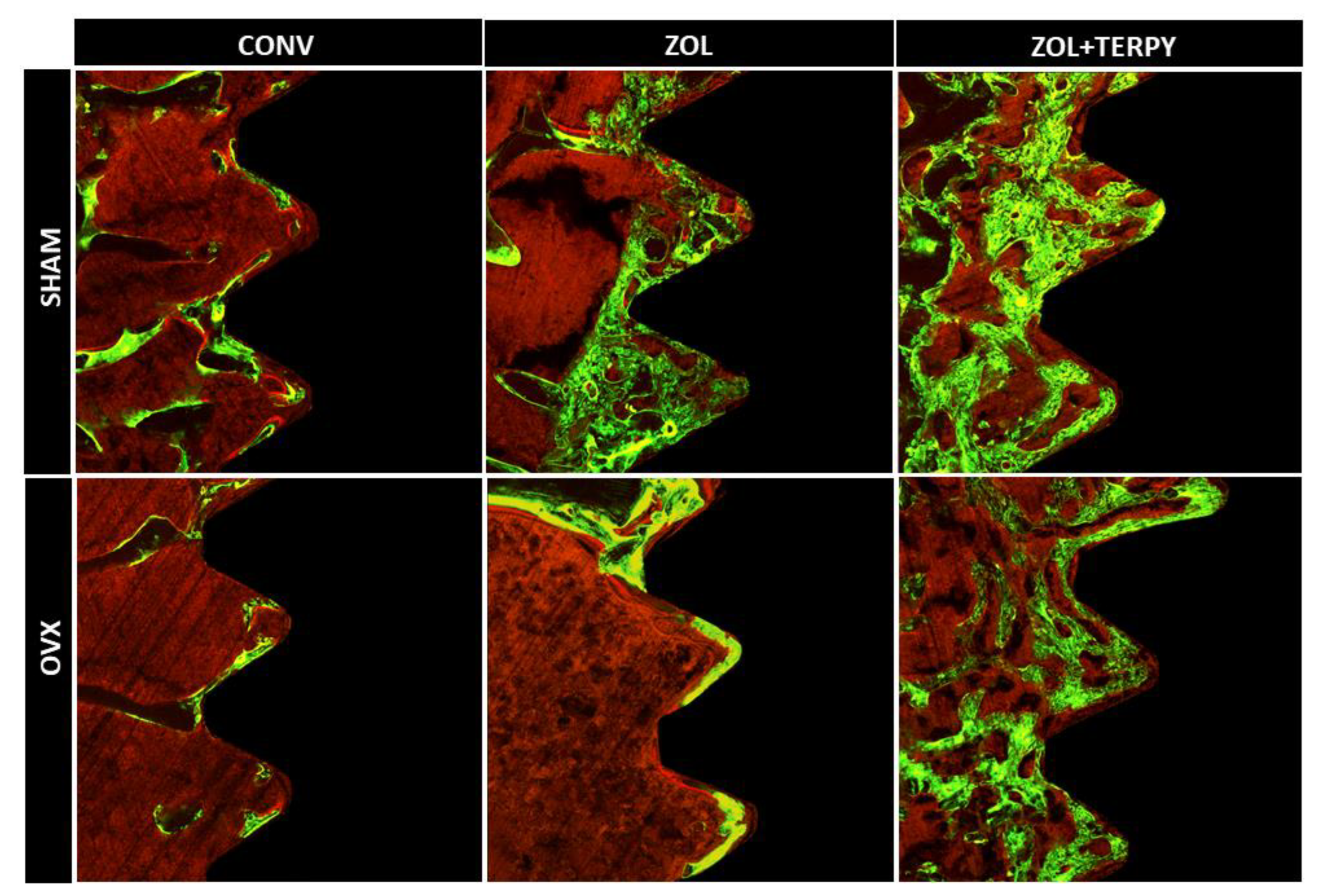
3.2. Qualitative Histological Results
3.2.1. SHAM CONV and OVX CONV Groups
3.2.2. SHAM ZOL and OVX ZOL Groups
3.2.3. SHAM ZOL + TERPY and OVX ZOL + TERPY Groups
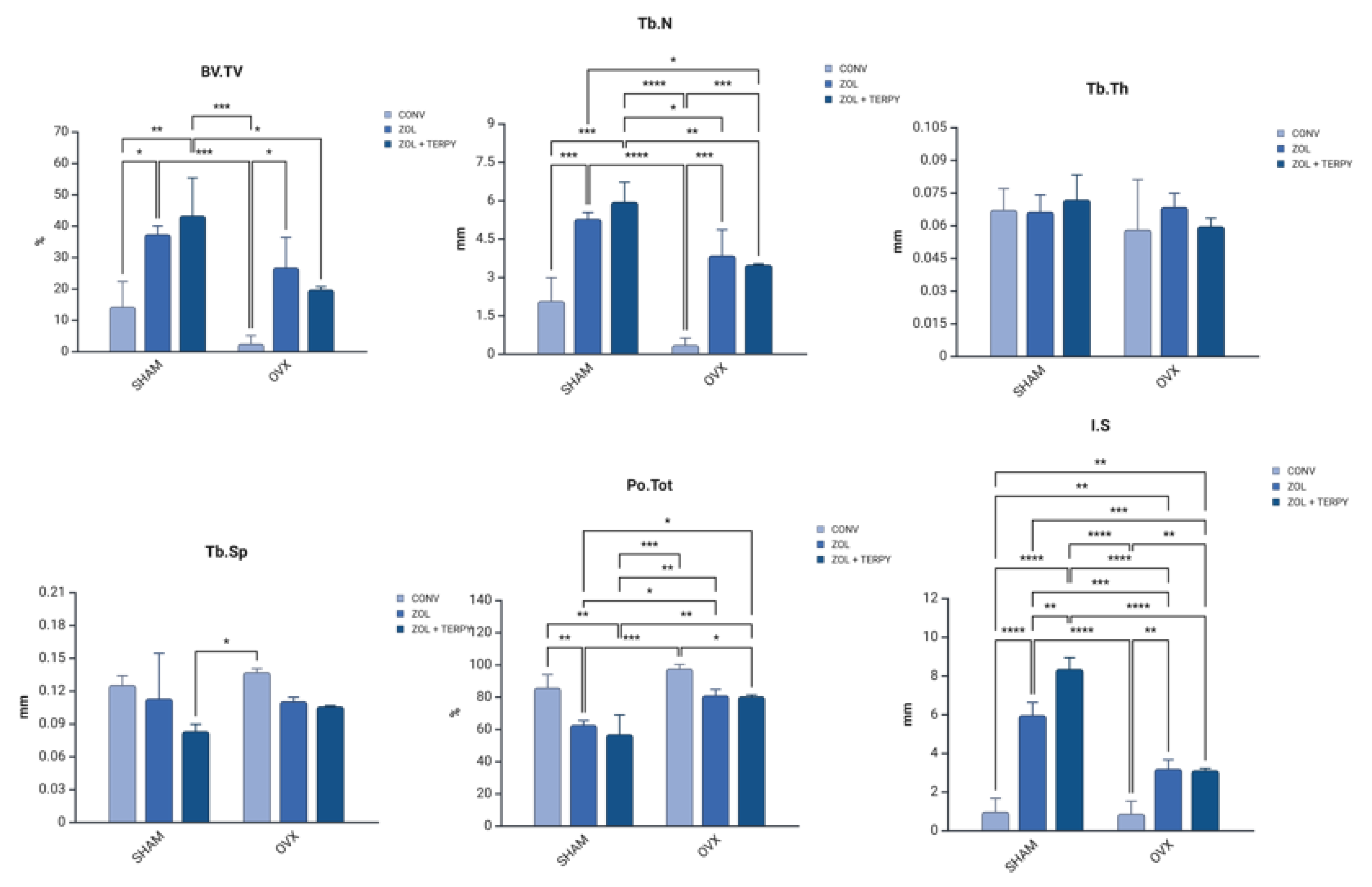
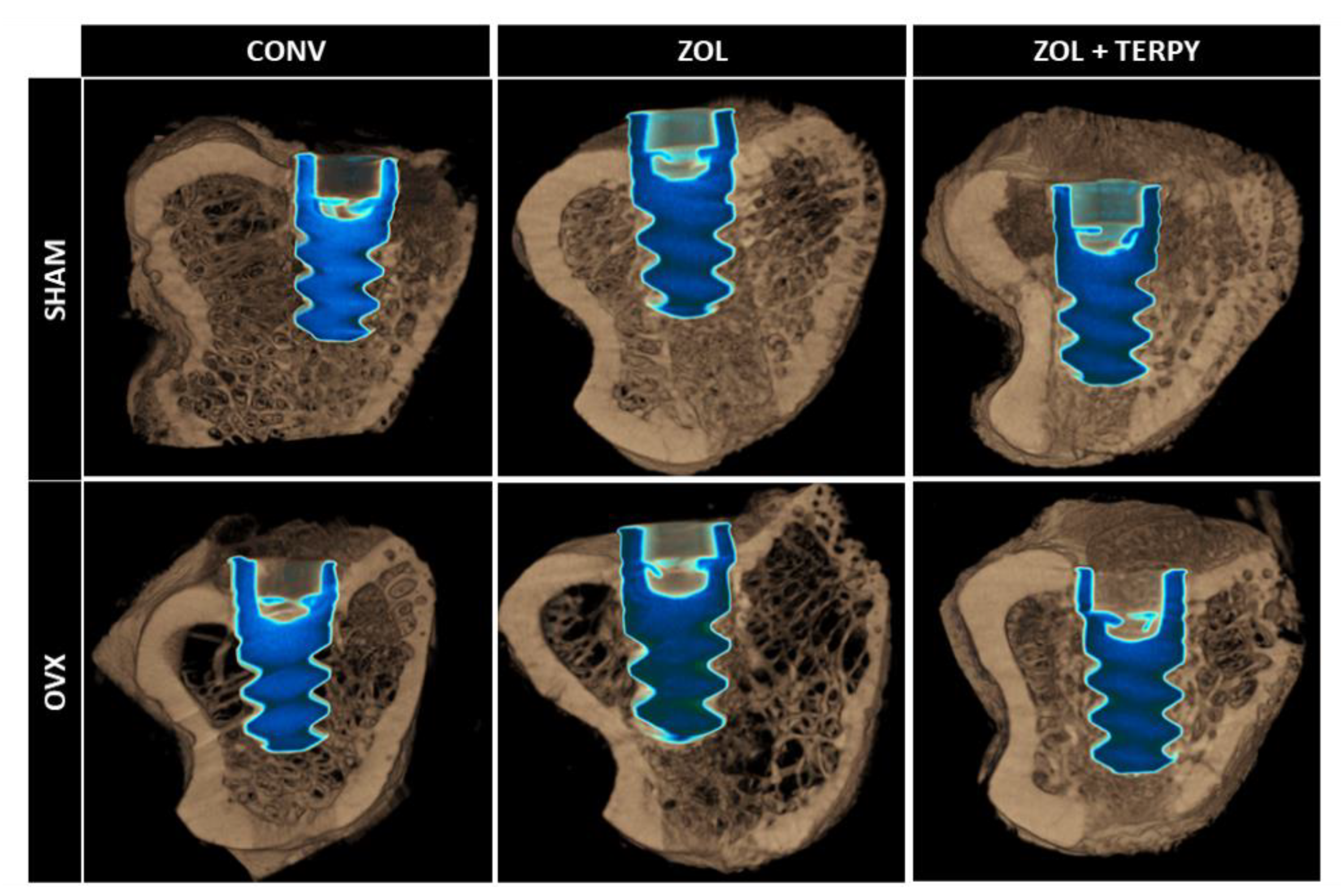
3.3. Three-Dimensional Microtomographic (Micro-CT) Results
3.4. Three-Dimensional Microtomographic Reconstructions (CTvox® Software)
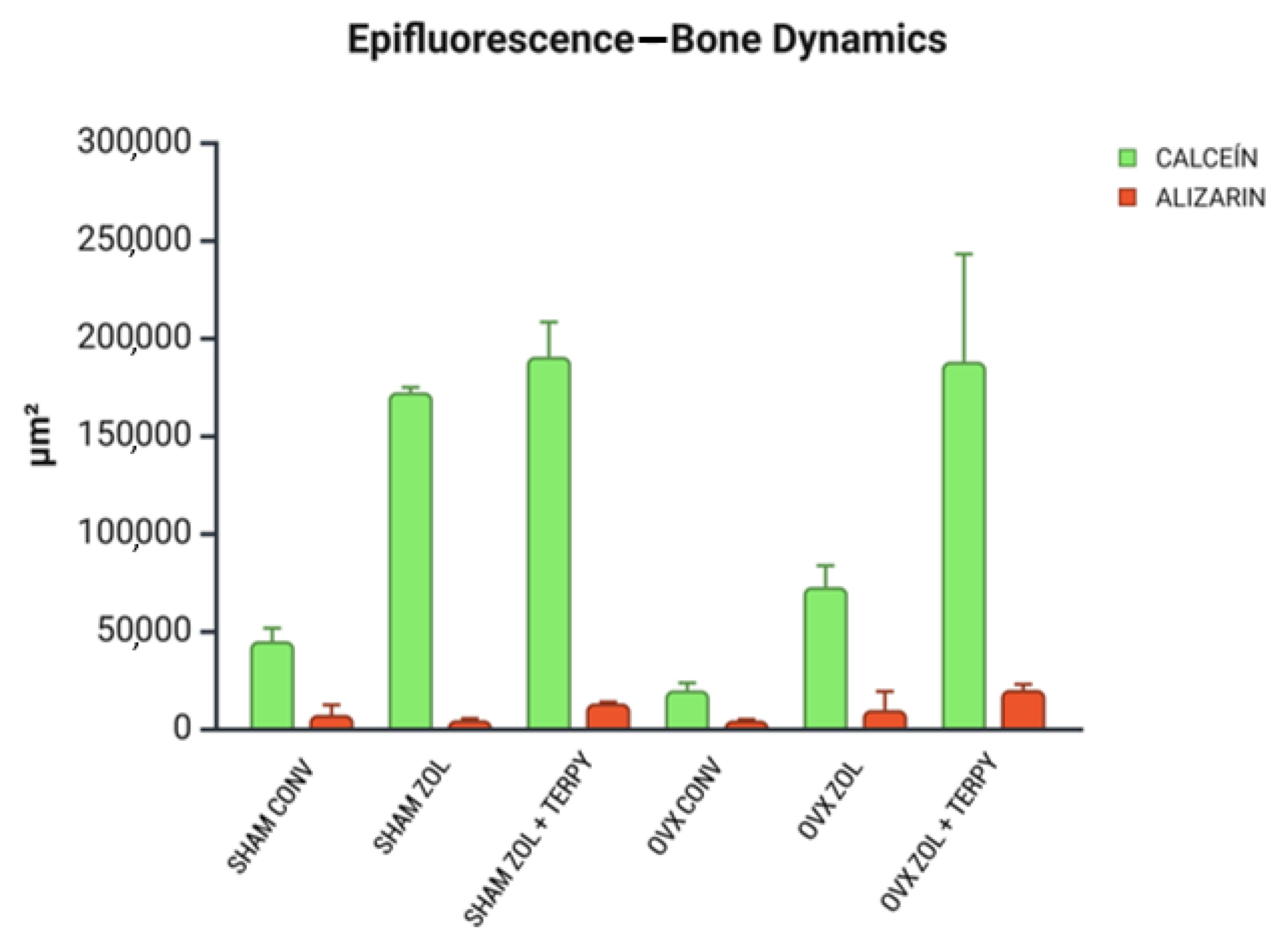
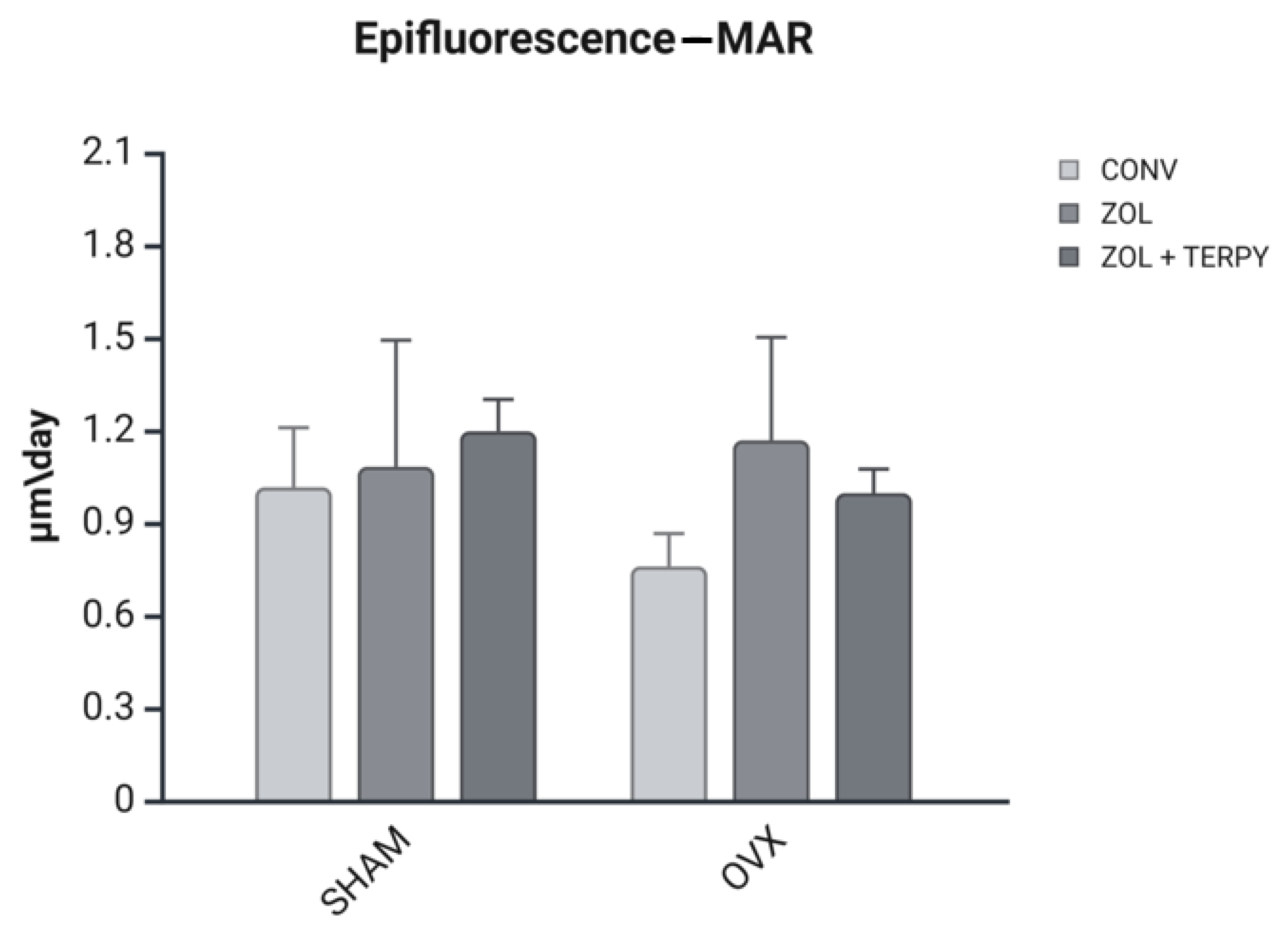
3.5. Epifluorescence Results
3.5.1. Bone Dynamics
3.5.2. Daily Mineral Apposition Rate (MAR)
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD US Health Disparities Collaborators. Life expectancy by county, race, and ethnicity in the USA, 2000–2019: A systematic analysis of health disparities. Lancet 2022, 400, 25–38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Väänänen, H.K.; Härkönen, P.L. Estrogen and bone metabolism. Maturitas 1996, 23, S65–S69. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Boelaert, K. The endocrinology of ageing: A mini-review. Gerontology 2015, 61, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Brånemark, P.I. Vital microscopy of bone marrow in rabbit. Scand. J. Clin. Lab. Investig. 1959, 11 (Suppl. 38), 1–82. [Google Scholar]
- Pandey, C.; Rokaya, D.; Bhattarai, B.P. Contemporary Concepts in Osseointegration of Dental Implants: A Review. BioMed Res. Int. 2022, 2022, 6170452. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kupka, J.R.; König, J.; Al-Nawas, B.; Sagheb, K.; Schiegnitz, E. How far can we go? A 20-year meta-analysis of dental implant survival rates. Clin. Oral Investig. 2024, 28, 541. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brånemark, P.I. Osseointegration and its experimental background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Giro, G.; Chambrone, L.; Goldstein, A.; Rodrigues, J.A.; Zenóbio, E.; Feres, M.; Figueiredo, L.C.; Cassoni, A.; Shibli, J.A. Impact of osteoporosis in dental implants: A systematic review. World J. Orthop. 2015, 6, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Shibli, J.A.; Naddeo, V.; Cotrim, K.C.; Kalil, E.C.; de Avila, E.D.; Faot, F.; Faverani, L.P.; Souza, J.G.S.; Fernandes, J.C.H.; Fernandes, G.V.O. Osteoporosis’ effects on dental implants osseointegration and survival rate: A systematic review of clinical studies. Quintessence Int. 2025, 56, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Grisa, A.; Veitz-Keenan, A. Is osteoporosis a risk factor for implant survival orfailure? Evid.-Based Dent. 2018, 19, 51–52. [Google Scholar] [CrossRef] [PubMed]
- Lemos, C.A.A.; de Oliveira, A.S.; Faé, D.S.; Oliveira, H.F.F.E.; Del Rei Daltro Rosa, C.D.; Bento, V.A.A.; Verri, F.R.; Pellizzer, E.P. Do dental implants placed in patients with osteoporosis have higher risks of failure and marginal bone loss compared to those in healthy patients? A systematic review with meta-analysis. Clin. Oral Investig. 2023, 27, 2483–2493. [Google Scholar] [CrossRef] [PubMed]
- de Medeiros, F.C.F.L.; Kudo, G.A.H.; Leme, B.G.; Saraiva, P.P.; Verri, F.R.; Honório, H.M.; Pellizzer, E.P.; Santiago Junior, J.F. Dental implants in patients with osteoporosis: A systematic review with meta-analysis. Int. J. Oral Maxillofac. Surg. 2018, 47, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Holahan, C.M.; Koka, S.; Kennel, K.A.; Weaver, A.L.; Assad, D.A.; Regennitter, F.J.; Kademani, D. Efeito do estado osteoporótico na sobrevivência de implantes dentários de titânio. Int. J. Oral Maxillofac. Implant. 2008, 23, 905–910. [Google Scholar]
- Amin, U.; McPartland, A.; O’Sullivan, M.; Silke, C. An overview of the management of osteoporosis in the aging female population. Women’s Health 2023, 19, 17455057231176655. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gehrke, B.; Alves Coelho, M.C.; Brasil d’Alva, C.; Madeira, M. Long-term consequences of osteoporosis therapy with bisphosphonates. Arch. Endocrinol. Metab. 2023, 68, e220334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smit, D.L.; Zillikens, M.C.; de Jongh, R.T. Zoledroninezuur zinvol bij osteopenie? [Zoledronic acid; useful in osteopenia?]. Ned. Tijdschr. Geneeskd. 2019, 163, D3626. (In Dutch) [Google Scholar] [PubMed]
- He, B.; Zhao, J.Q.; Zhang, M.Z.; Quan, Z.X. Zoledronic acid and fracture risk: A meta-analysis of 12 randomized controlled trials. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Peter, B.; Pioletti, D.P.; Laïb, S.; Bujoli, B.; Pilet, P.; Janvier, P.; Guicheux, J.; Zambelli, P.Y.; Bouler, J.M.; Gauthier, O. Calcium phosphate drug delivery system: Influence of local zoledronate release on bone implant osteointegration. Bone 2005, 36, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Luo, E.; Hu, J.; Xue, J.; Zhu, S.; Li, J. Effect of combined local treatment with ovariectomized rats. Bone 2009, 44, 225–232. [Google Scholar] [CrossRef]
- Stadelmann, V.A.; Gauthier, O.; Terrier, A.; Bouler, J.M.; Pioletti, D.P. Implants delivering bisphosphonate locally increase periprosthetic bone density in an osteoporotic sheep model. A pilot study. Eur. Cell Mater. 2008, 16, 10–16. [Google Scholar] [CrossRef]
- Bobyn, J.D.; McKenzie, K.; Karabasz, D.; Krygier, J.J.; Tanzer, M. Locally delivered bisphosphonate for enhancement of bone formation and implant fixation. J. Bone Jt. Surg. Am. 2009, 91 (Suppl. 6), 23–31. [Google Scholar] [CrossRef]
- Li, Y.F.; Li, X.D.; Bao, C.Y.; Chen, Q.M.; Zhang, H.; Hu, J. Promotion of peri-implant bone healing by systemically administered parathyroid hormone (1-34) and zoledronic acid adsorbed onto the implant surface. Osteoporos. Int. 2013, 24, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pathak, J.L.; Hu, X.; Jin, Y.; Wu, Z.; Al-Baadani, M.A.; Wu, S.; Zhang, H.; Farkasdi, S.; Liu, Y.; et al. Sustained release of zoledronic acid from mesoporous TiO2-layered implant enhances implant osseointegration in osteoporotic condition. J. Biomed. Nanotechnol. 2018, 14, 1965–1978. [Google Scholar] [CrossRef]
- Korn, P.; Kramer, I.; Schlottig, F.; Tödtman, N.; Eckelt, U.; Bürki, A.; Ferguson, S.J.; Kautz, A.; Schnabelrauch, M.; Range, U.; et al. Systemic sclerostin antibody treatment increases osseointegration and biomechanical competence of zoledronic- acid-coated dental implants in a rat osteoporosis model. Eur. Cell Mater. 2019, 37, 333–346. [Google Scholar] [CrossRef]
- Paludetto, L.V.; Monteiro, N.G.; Breseghello, I.; de Souza Batista, F.R.; Antoniali, C.; Lisboa-Filho, P.N.; Okamoto, R. Smart Delivery of Biomolecules Interfering with Peri-Implant Repair in Osteoporotic Rats. Int. J. Mol. Sci. 2024, 25, 8963. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Inoue, B.K.N.; Paludetto, L.V.; Monteiro, N.G.; Batista, F.R.S.; Kitagawa, I.L.; da Silva, R.S.; Antoniali, C.; Lisboa Filho, P.N.; Okamoto, R. Synergic Action of Systemic Risedronate and Local Rutherpy in Peri-implantar Repair of Ovariectomized Rats: Biomechanical and Molecular Analysis. Int. J. Mol. Sci. 2023, 24, 16153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Neto, F.C.; Breseghello, I.; Paludetto, L.V.; Frasnelli, S.C.; de Souza Batista, F.R.; Blay, A.; Boni, A.; Júnior, F.C.; Okamoto, R. Effect of Er:YAG laser bone bed milling, with or without photobiomodulation, on the bone repair process of additive manufacturing implants in rats. J. Photochem. Photobiol. 2025, 26–27, 100260. [Google Scholar] [CrossRef]
- Gomes-Ferreira, P.H.S.; de Oliveira, D.; Frigério, P.B.; de Souza Batista, F.R.; Grandfield, K.; Okamoto, R. Teriparatide improves microarchitectural characteristics of peri-implant bone in orchiectomized rats. Osteoporos. Int. 2020, 31, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Yogui, F.C. Caracterização e Avaliação Randomizada de Superfícies de Liga de Titânio Modificada por Hidroxiapatita e Hidroxia- 635 Patita Incorporada com Estrôncio em Ratas Saudáveis e Osteoporóticas. Master’s Thesis, Universidade Estadual Paulista ‘’Julio de Mesquita Filho’’, Araçatuba, Brazil, 2020. [Google Scholar]
- Tanaka, M.; Ejiri, S.; Toyooka, E.; Kohno, S.; Ozawa, H. Effects of ovariectomy on trabecular structures of rat alveolar bone. J. Periodontal Res. 2002, 37, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Long, J.A.; Evans, H.M. The Oestrus Cycle in the Rat and Its Related Phenomena; University of California Press: Berkeley, CA, USA, 1922; Volume 6, pp. 1–148. [Google Scholar]
- Sayed, A.A.; Soliman, A.M.; Fahmy, S.R.; Marzouk, M. Antiosteoporotic effect of Coelatura aegyptiaca shell powder on ovariectomized rats. Afr. J. Pharm. Pharmacol. 2013, 7, 2406–2416. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro- computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Green, J.R. Preclinical pharmacology of zoledronic acid. Semin. Oncol. 2002, 29 (Suppl. 21), 3–11. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.H.M.; Pelegrino, M.T.; Pieretti, J.C.; Seabra, A.B. How can nitric oxide help osteogenesis? AIMS Mol. Sci. 2020, 7, 29–48. [Google Scholar] [CrossRef]
- Nichols Scott, P.; Storm, W.L.; Koh, A.; Schoenfisch, M.H. Local Delivery of Nitric Oxide: Targeted Delivery of Therapeutics to Bone and Connective Tissues. Adv. Drug Deliv. Rev. 2012, 64, 1177–1188. [Google Scholar] [CrossRef]
- Liu, W.; Meng, Z.; Wang, G. The Efficacy of Nitrates for Bone Health: A Systematic Review and Meta-Analysis of Observational and Randomized Controlled Studies. Front. Endocrinol. 2022, 13, 833932. [Google Scholar] [CrossRef]
- Russell, R.G.G.; Watts, N.B.; Ebetino, F.H.; Rogers, M.J. Mechanisms of action of bisphosphonates: Similarities and differences and their potential influence on clinical efficacy. Osteoporos. Int. 2008, 19, 733–759. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Nitric oxide and bone. Ann. N. Y. Acad. Sci. 2010, 1192, 391–403. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, D.S.; Park, S.Y.; Baek, S.W.; Jung, J.W.; Kim, T.H.; Han, D.K. Nitric Oxide-Releasing Bioinspired Scaffold for Exquisite Regeneration of Osteoporotic Bone via Regulation of Homeostasis. Adv. Sci. 2023, 10, e2205336. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.W.; Chow, J.W. Nitric oxide synthase expression in bone cells. Bone 1998, 23, 1–6. [Google Scholar] [CrossRef] [PubMed]


| Group Name | N | Description of the Group |
|---|---|---|
| SHAM CONV | 11 | Implants without surface functionalization installed in healthy rats |
| SHAM ZOL | 11 | Implants functionalized with zoledronic acid installed in healthy rats |
| SHAM ZOL + TERPY | 11 | Implants functionalized with zoledronic acid + ruterpy installed in healthy rats |
| OVX CONV | 11 | Implants without surface functionalization installed in osteoporotic rats |
| OVX ZOL | 11 | Implants functionalized with zoledronic acid installed in osteoporotic rats |
| OVX ZOL + TERPY | 11 | Implants functionalized with zoledronic acid + ruterpy installed in osteoporotic rats |
| SHAM CONV | SHAM ZOL | SHAM ZOL + TERPY | OVX CONV | OVX ZOL | OVX ZOL + TERPY | Statistical Difference | |
|---|---|---|---|---|---|---|---|
| BV.TV (%) | 14.24 ± 7.98 | 37.32 ± 2.60 | 43.26 ± 12.00 | 2.435 ± 2.54 | 26.72 ± 9.58 | 19.71 ± 0.84 | SHAM CONV × SHAM ZOL = 0.01 SHAM CONV × SHAM ZOL + TERPY = 0.0037 OVX CONV × SHAM ZOL = 0.0007819 OVX CONV × OVX ZOL = 0.01400 OVX CONV × SHAM ZOL + TERPY = 0.0001822 SHAM ZOL + TERPY × OVX ZOL + TERPY = 0.01731 |
| Tb.Th (mm) | 0.067 ± 0.009 | 0.066 ± 0.007 | 0.071 ± 0.011 | 0.058 ± 0.022 | 0.068 ± 0.006 | 0.059 ± 0.003 | no |
| Tb.N (per/mm) | 2.067 ± 0.905 | 5.278 ± 0.247 | 5.941 ± 0.765 | 0.351 ± 0.260 | 3.842 ± 1.007 | 3.483 ± 0.029 | SHAM CONV × SHAM ZOL = 0.0006514 SHAM CONV × SHAM ZOL + TERPY = 0.0001 OVX CONV × SHAM ZOL ≤.0001 OVX CONV × OVX ZOL = 0.0003016 OVX CONV × SHAM ZOL + TERPY ≤0.0001 OVX CONV × OVX ZOL + TERPY = 0.0008137 SHAM ZOL × OVX ZOL + TERPY = 0.04907 OVX ZOL × SHAM ZOL + TERPY = 0.01870 SHAM ZOL + TERPY × OVX ZOL + TERPY = 0.006051 |
| Tb.Sp (mm) | 0.125 ± 0.008 | 0.112 ± 0.041 | 0.083 ± 0.006 | 0.136 ± 0.003 | 0.110 ± 0.003 | 0.105 ± 0.000 | OVX CONV × SHAM ZOL + TERPY = 0.02496 |
| Po.Tot (%) | 85.75 ± 7.989 | 62.68 ± 2.607 | 56.74 ± 12.000 | 97.57 ± 2.543 | 80.91 ± 3.660 | 80.29 ± 0.849 | SHAM CONV × SHAM ZOL = 0.007166 SHAM CONV × SHAM ZOL + TERPY = 0.0011 OVX CONV × SHAM ZOL = 0.0002067 OVX CONV × SHAM ZOL + TERPY ≤0.0001 OVX CONV × OVX ZOL + TERPY = 0.04829 SHAM ZOL × OVX ZOL = 0.03534 SHAM ZOL × OVX ZOL + TERPY = 0.04333 OVX ZOL × SHAM ZOL + TERPY = 0.005048 SHAM ZOL + TERPY × OVX ZOL + TERPY = 0.0061510 |
| IS (mm) | 0.963 ± 0.693 | 5.959 ± 0.657 | 8.347 ± 0.579 | 0.861 ± 0.650 | 3.184 ± 0.465 | 3.108 ± 0.093 | SHAM CONV × SHAM ZOL = <0.0001 SHAM CONV × OVX ZOL = 0.004228 SHAM CONV × SHAM ZOL + TERPY ≤0.000 SHAM CONV × OVX ZOL + TERPY = 0.005554 OVX CONV × SHAM ZOL ≤0.0001 OVX CONV × OVX ZOL = 0.002953 OVX CONV × SHAM ZOL + TERPY ≤0.0001 OVX CONV × OVX ZOL + TERPY = 0.003866 SHAM ZOL × OVX ZOL = 0.0006402 SHAM ZOL × SHAM ZOL + TERPY = 0.002356 SHAM ZOL × OVX ZOL + TERPY = 0.0005000 OVX ZOL × SHAM ZOL + TERPY ≤0.0001 SHAM ZOL + TERPY × OVX ZOL + TERPY ≤0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paludetto, L.V.; Breseghello, I.; Frasnelli, S.C.T.; Batista, F.R.d.S.; Botacin, P.R.; Antoniali, C.; Lisboa-Filho, P.N.; Okamoto, R. Titanium Implants Functionalized with Zoledronic Acid Associated with Ruterpy Accelerate Peri-Implant Repair in Healthy and Osteoporotic Rats. Biomimetics 2025, 10, 547. https://doi.org/10.3390/biomimetics10080547
Paludetto LV, Breseghello I, Frasnelli SCT, Batista FRdS, Botacin PR, Antoniali C, Lisboa-Filho PN, Okamoto R. Titanium Implants Functionalized with Zoledronic Acid Associated with Ruterpy Accelerate Peri-Implant Repair in Healthy and Osteoporotic Rats. Biomimetics. 2025; 10(8):547. https://doi.org/10.3390/biomimetics10080547
Chicago/Turabian StylePaludetto, Laura Vidoto, Isadora Breseghello, Sabrina Cruz Tfaile Frasnelli, Fábio Roberto de Souza Batista, Paulo Roberto Botacin, Cristina Antoniali, Paulo Noronha Lisboa-Filho, and Roberta Okamoto. 2025. "Titanium Implants Functionalized with Zoledronic Acid Associated with Ruterpy Accelerate Peri-Implant Repair in Healthy and Osteoporotic Rats" Biomimetics 10, no. 8: 547. https://doi.org/10.3390/biomimetics10080547
APA StylePaludetto, L. V., Breseghello, I., Frasnelli, S. C. T., Batista, F. R. d. S., Botacin, P. R., Antoniali, C., Lisboa-Filho, P. N., & Okamoto, R. (2025). Titanium Implants Functionalized with Zoledronic Acid Associated with Ruterpy Accelerate Peri-Implant Repair in Healthy and Osteoporotic Rats. Biomimetics, 10(8), 547. https://doi.org/10.3390/biomimetics10080547








