Titanium–Aluminum–Vanadium Surfaces Generated Using Sequential Nanosecond and Femtosecond Laser Etching Provide Osteogenic Nanotopography on Additively Manufactured Implants
Abstract
1. Introduction
2. Materials and Methods
2.1. Disk Production
2.2. Laser Treatment
2.3. Surface Laser Treatment Verification
2.4. Surface Characterization
2.4.1. Scanning Electron Microscopy
2.4.2. Contact Angle Analysis
2.4.3. Roughness Analysis
2.4.4. Surface Chemistry
2.5. Cell Culture
2.6. Cellular Response
2.7. Statistical Analysis
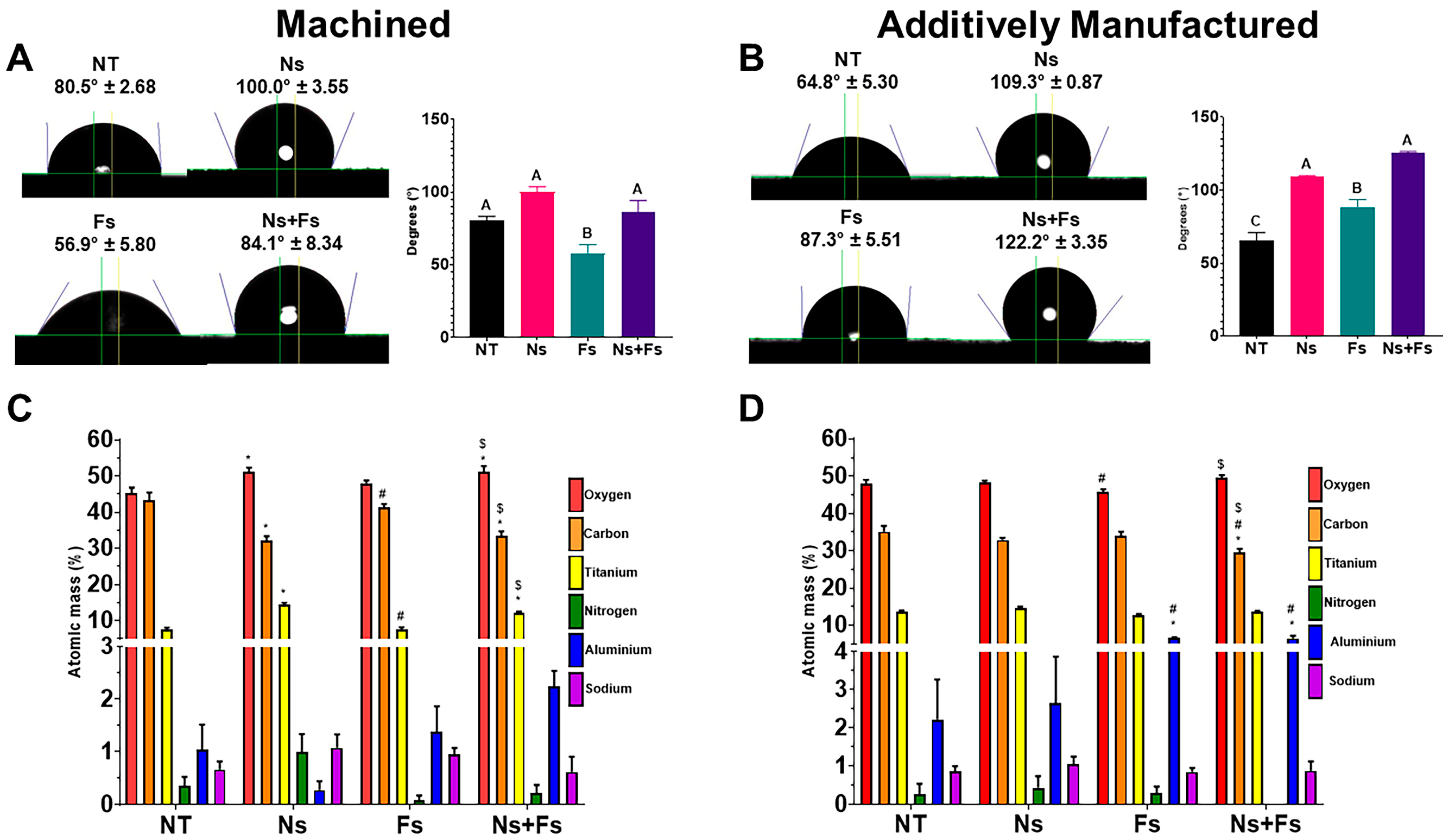
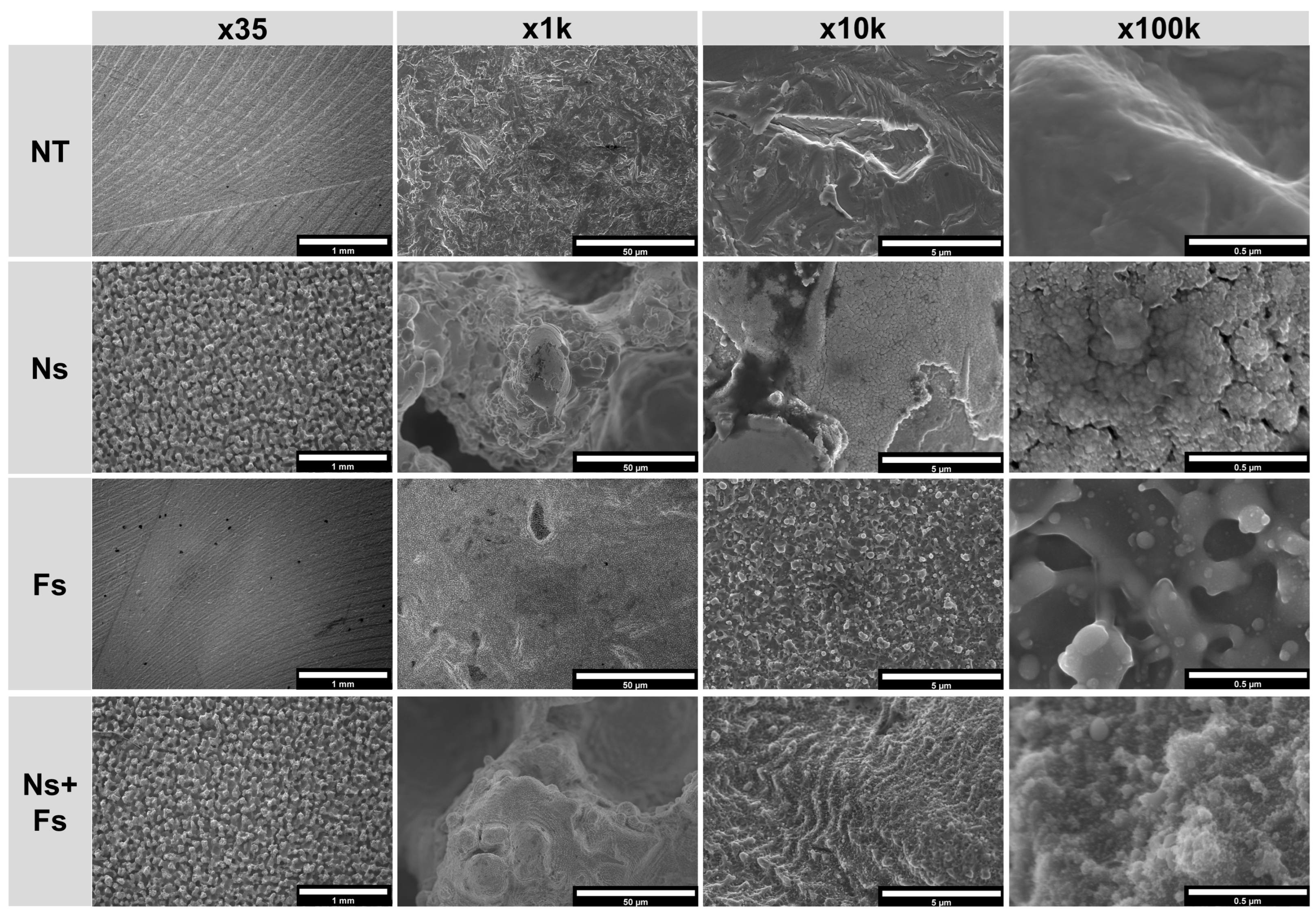
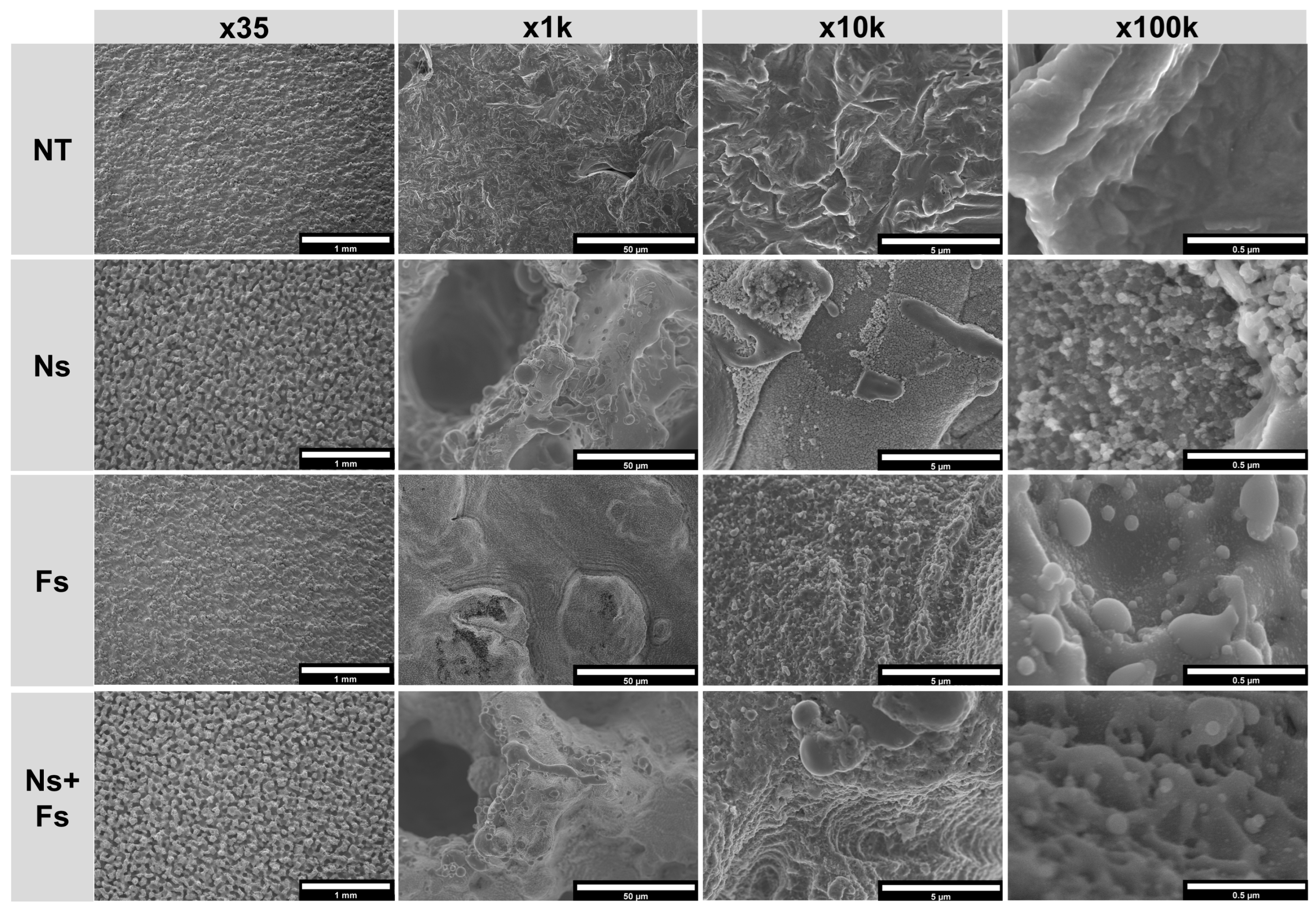
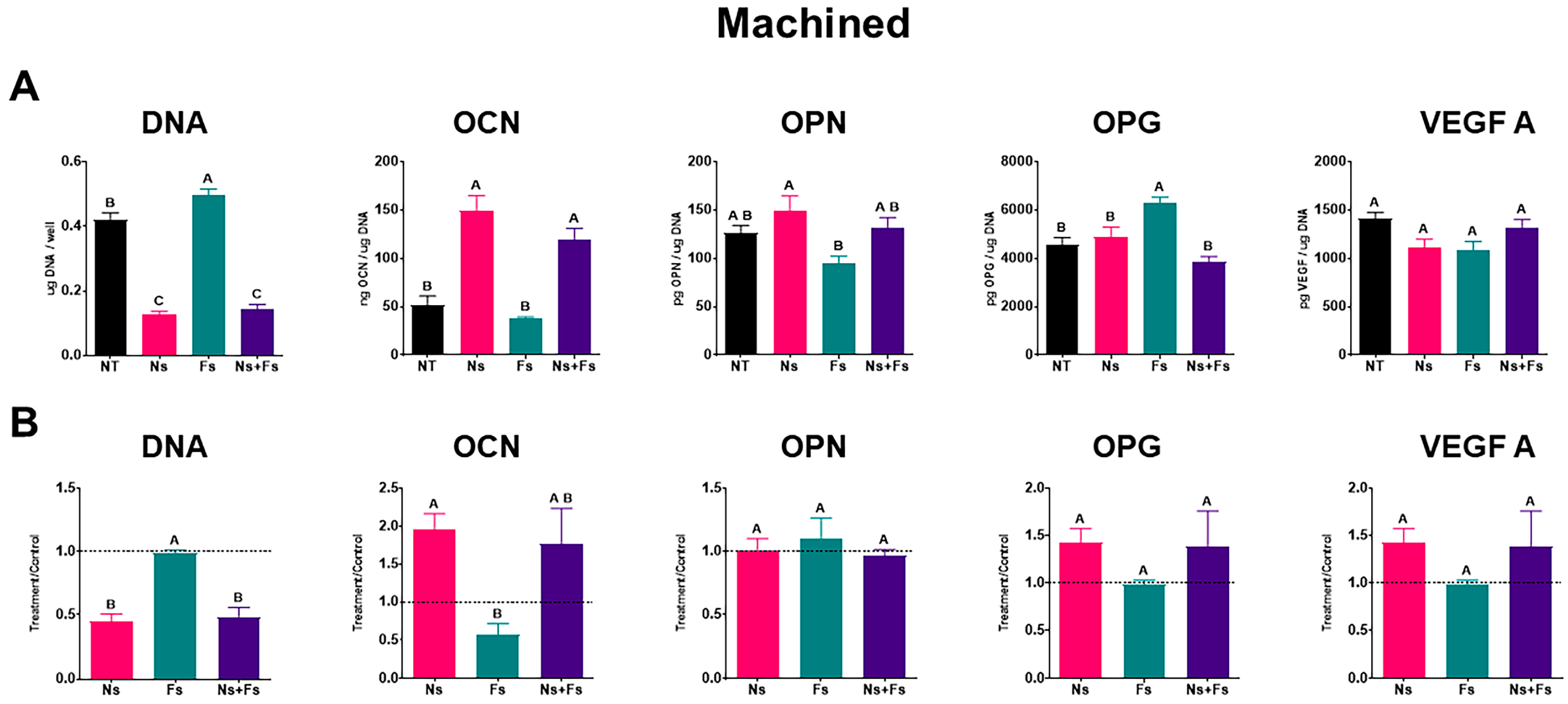
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ti6Al4V | Titanium–aluminum–vanadium |
| MSCs | Bone marrow stromal cells |
| M | Machined |
| AM | Additively manufactured |
| NT | No laser treatment |
| Ns | Nanosecond laser |
| Fs | Femtosecond laser |
| Ns+Fs | Nanosecond followed by femtosecond laser |
| XPS | X-ray photoelectron spectroscopy |
| LSM | laser scanning microscopy |
| SEM | scanning electron microscopy |
| TCPS | tissue culture polystyrene |
| OCN | Osteocalcin |
| OPN | Osteopontin |
| OPG | Osteoprotegerin |
| VEGF | vascular endothelial growth factor 165 |
| IL-1β | interleukin-1 beta |
| IL-10 | interleukin-10 |
| LIPSS | laser-induced periodic surface structures |
| GM | growth media |
| OM | osteogenic media |
| DMLS | direct metal laser sintering |
| HIP | hot isostatic pressing |
| T | laser-treated |
| L | Lighting |
| ΔE | change in color variable |
| Sa | arithmetic mean deviation |
| Sz | maximum peak to valley distance |
| ELISA | enzyme-linked immunosorbent assay |
| ANOVA | one-way analysis of variance |
| T/C | treatment/control ratios |
References
- Cohen, D.J.; Cheng, A.; Kahn, A.; Aviram, M.; Whitehead, A.J.; Hyzy, S.L.; Clohessy, R.M.; Boyan, B.D.; Schwartz, Z. Novel Osteogenic Ti-6Al-4V Device for Restoration of Dental Function in Patients with Large Bone Deficiencies: Design, Development and Implementation. Sci. Rep. 2016, 6, 20493. [Google Scholar] [CrossRef]
- De La Garza-Ramos, M.A.; Estupiñan-Lopez, F.H.; Gaona-Tiburcio, C.; Beltrán-Novelo, L.G.; Zambrano-Robledo, P.; Cabral-Miramontes, J.; Almeraya-Calderón, F. Materials Electrochemical Behavior of Ti6Al4V Alloy Used in Dental Implants Immersed in Streptococcus gordonii and Fusobacterium nucleatum Solutions. Materials 2020, 13, 4185. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. BioMed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef]
- Boyan, B.D.; Hummert, T.W.; Dean, D.D.; Schwartz, Z. Role of Material Surfaces in Regulating Bone and Cartilage Cell Response. Biomaterials 1996, 17, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Kolarovszki, B.; Ficsor, S.; Frank, D.; Katona, K.; Soos, B.; Turzo, K. Unlocking the Potential: Laser Surface Modifications for Titanium Dental Implants. Lasers Med. Sci. 2024, 39, 162. [Google Scholar] [CrossRef]
- Khoo, L.K.; Sakdajeyont, W.; Khanijou, M.; Seriwatanachai, D.; Kiattavorncharoen, S.; Pairuchvej, V.; Wongsirichat, N. Titanium Fixture Implants Treated by Laser in Dentistry: Review Article. J. Oral. Maxillofac. Surg. Med. Pathol. 2019, 31, 381–385. [Google Scholar] [CrossRef]
- Guo, W.; He, X.; Song, J.; Cao, Z.; Hu, W.; Tan, Y.; Dong, S.; Ma, Y.; Wang, K. Multiscale Hierarchical Micro- and Nano-Surface Induced by High-Repetition-Rate Femtosecond Laser Promote Peri-Implant Osseointegration. ACS Appl. Bio. Mater. 2025, 8, 1621–1634. [Google Scholar] [CrossRef]
- Schweitzer, L.; Schoon, J.; Bläß, N.; Huesker, K.; Neufend, J.V.; Siemens, N.; Bekeschus, S.; Schlüter, R.; Schneider, P.; Uhlmann, E.; et al. Ultraviolet Laser Induced Periodic Surface Structures Positively Influence Osteogenic Activity on Titanium Alloys. Front. Bioeng. Biotechnol. 2024, 12, 1462232. [Google Scholar] [CrossRef]
- Łukaszewska-Kuska, M.; Krawczyk, P.; Buchwald, T.; Martyła, A.; Zinchenko, V.; Majchrowski, R.; Przekop, R.E.; Dorocka-Bobkowska, B. Electrodeposited Hydroxyapatite Coating on Titanium After Ultrashort-Pulsed Laser Processing for a Novel Surface of Endosseous Implants. Dent. Med. Probl. 2024, 61, 909–918. [Google Scholar] [CrossRef]
- Nevins, M.; Kim, D.M.; Jun, S.-H.; Guze, K.; Schupbach, P.; Nevins, M.L. Histologic Evidence of a Connective Tissue Attachment to Laser Microgrooved Abutments: A Canine Study. Int. J. Periodontics Restor. Dent. 2010, 30, 245–255. [Google Scholar]
- Iorio-Siciliano, V.; Matarasso, R.; Guarnieri, R.; Nicolò, M.; Farronato, D.; Matarasso, S. Soft Tissue Conditions and Marginal Bone Levels of Implants with a Laser-Microtextured Collar: A 5-Year, Retrospective, Controlled Study. Clin. Oral. Implant. Res. 2015, 26, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, R.; Grande, M.; Ippoliti, S.; Iorio-Siciliano, V.; Riccitiello, F.; Farronato, D. Influence of a Laser-Lok Surface on Immediate Functional Loading of Implants in Single-Tooth Replacement: Three-Year Results of a Prospective Randomized Clinical Study on Soft Tissue Response and Esthetics. Int. J. Periodontics Restor. Dent. 2015, 35, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Farronato, D.; Mangano, F.; Briguglio, F.; Iorio-Siciliano, V.; Riccitiello, F.; Guarnieri, R. Influence of Laser-Lok Surface on Immediate Functional Loading of Implants in Single-Tooth Replacement: A 2-Year Prospective Clinical Study. Int. J. Periodontics Restor. Dent. 2014, 34, 79–89. [Google Scholar] [CrossRef]
- Webster, T.J.; Ejiofor, J.U. Increased Osteoblast Adhesion on Nanophase Metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials 2004, 25, 4731–4739. [Google Scholar] [CrossRef]
- Matos, G.R.M. Surface Roughness of Dental Implant and Osseointegration. J. Maxillofac. Oral. Surg. 2021, 20, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Schwartz, Z.; Lohmann, C.H.; Sylvia, V.L.; Cochran, D.L.; Dean, D.D.; Puzas, J.E. Pretreatment of Bone with Osteoclasts Affects Phenotypic Expression of Osteoblast-like Cells. J. Orthop. Res. 2003, 21, 638–647. [Google Scholar] [CrossRef]
- Gittens, R.A.; Olivares-Navarrete, R.; Schwartz, Z.; Boyan, B.D. Implant Osseointegration and the Role of Microroughness and Nanostructures: Lessons for Spine Implants. Acta Biomater. 2014, 10, 3363–3371. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Hyzy, S.L.; Slosar, P.J.; Schneider, J.M.; Schwartz, Z.; Boyan, B.D. Implant Materials Generate Different Peri-Implant Inflammatory Factors. Spine 2015, 40, 399–404. [Google Scholar] [CrossRef]
- Cohen, D.J.; Van Duyn, C.M.; Deng, J.; Lodi, M.K.; Gallagher, M.B.; Sugar, J.T.; Rawlinson, J.J.; Ghosh, P.; Boyan, B.D.; Schwartz, Z. Bone Marrow Stromal Cells Generate a Pro-Healing Inflammasome When Cultured on Titanium-Aluminum-Vanadium Surfaces with Microscale/Nanoscale Structural Features. Biomimetics 2025, 10, 66. [Google Scholar] [CrossRef]
- Epperlein, N.; Menzel, F.; Schwibbert, K.; Koter, R.; Bonse, J.; Sameith, J.; Krüger, J.; Toepel, J. Influence of Femtosecond Laser Produced Nanostructures on Biofilm Growth on Steel. Appl. Surf. Sci. 2017, 418, 420–424. [Google Scholar] [CrossRef]
- Mao, B.; Siddaiah, A.; Liao, Y.; Menezes, P.L. Laser Surface Texturing and Related Techniques for Enhancing Tribological Performance of Engineering Materials: A Review. J. Manuf. Process 2020, 53, 153–173. [Google Scholar] [CrossRef]
- Lackington, W.A.; Schweizer, P.; Khokhlova, M.; Cancellieri, C.; Guimond, S.; Chopard-Lallier, A.; Hofstetter, J.; Schmutz, P.; Maeder, X.; Rottmar, M. Femtosecond Laser-Texturing the Surface of Ti-Based Implants to Improve Their Osseointegration Capacity. Adv. Mater. Interfaces 2022, 9, 2201164. [Google Scholar] [CrossRef]
- Khan, M.R.; Mordan, N.; Parkar, M.; Salih, V.; Donos, N.; Brett, P.M. Atypical Mesenchymal Stromal Cell Responses to Topographic Modifications of Titanium Biomaterials Indicate Cytoskeletal- and Genetic Plasticity-Based Heterogeneity of Cells. Stem Cells Int. 2019, 2019, 5214501. [Google Scholar] [CrossRef]
- Šugár, P.; Antala, R.; Šugárová, J.; Kováčik, J.; Pata, V. Study on Surface Roughness, Morphology, and Wettability of Laser-Modified Powder Metallurgy-Processed Ti-Graphite Composite Intended for Dental Application. Bioengineering 2023, 10, 1406. [Google Scholar] [CrossRef]
- Dumas, V.; Guignandon, A.; Vico, L.; Mauclair, C.; Zapata, X.; Linossier, M.T.; Bouleftour, W.; Granier, J.; Peyroche, S.; Dumas, J.C.; et al. Femtosecond Laser Nano/Micro Patterning of Titanium Influences Mesenchymal Stem Cell Adhesion and Commitment. Biomed. Mater. 2015, 10, 055002. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C. Femtosecond Laser Structuring of Titanium Implants. Appl. Surf. Sci. 2007, 253, 7272–7280. [Google Scholar] [CrossRef]
- Choudhury, S.; Joshi, A.; Agrawal, A.; Nain, A.; Bagde, A.; Patel, A.; Syed, Z.Q.; Asthana, S.; Chatterjee, K. NIR-Responsive Deployable and Self-Fitting 4D-Printed Bone Tissue Scaffold. ACS Appl. Mater. Interfaces 2024, 16, 49135–49147. [Google Scholar] [CrossRef]
- BomBač, D.; Brojan, M.; FajFar, P.; Kosel, F.; Turk, R. Review of Materials in Medical Applications Pregled Materialov v Medicinskih Aplikacijah. RMZ Mater. Geoenviron. 2007, 54, 471–499. [Google Scholar]
- Zhao, X.; Chen, J.; Lin, X.; Huang, W. Study on Microstructure and Mechanical Properties of Laser Rapid Forming Inconel 718. Mater. Sci. Eng. A 2008, 478, 119–124. [Google Scholar] [CrossRef]
- Bidaux, J.-E.; Closuit, C.; Rodriguez-Arbaizar, M.; Zufferey, D.; Carreño-Morelli, E. Metal Injection Moulding of Low Modulus Ti–Nb Alloys for Biomedical Applications. Powder Metall. 2013, 56, 263–266. [Google Scholar] [CrossRef]
- Tardelli, J.D.C.; Otani, L.B.; Batalha, R.L.; Alves, F.; Pereira-da-Siva, M.A.; Bagnato, V.S.; Gargarella, P.; Bolfarini, C.; dos Reis, A.C. Atomic Interaction S. Aureus/Machined and Additive Manufacturing Ti-6Al-4V and Ti-35Nb-7Zr-5Ta Disks for Dental Implants. J. Biomed. Mater. Res. B Appl. Biomater. 2024, 112, e35508. [Google Scholar] [CrossRef]
- Yu, M.; Wan, Y.; Ren, B.; Wang, H.; Zhang, X.; Qiu, C.; Liu, A.; Liu, Z. 3D Printed Ti−6Al−4V Implant with a Micro/Nanostructured Surface and Its Cellular Responses. ACS Omega 2020, 5, 31738–31743. [Google Scholar] [CrossRef]
- Jacobs, T.W.; Dillon, J.T.; Cohen, D.J.; Boyan, B.D.; Schwartz, Z. Different Methods to Modify the Hydrophilicity of Titanium Implants with Biomimetic Surface Topography to Induce Variable Responses in Bone Marrow Stromal Cells. Biomimetics 2024, 9, 227. [Google Scholar] [CrossRef]
- Lian, Z.; Xu, J.; Yu, Z.; Yu, P.; Ren, W.; Wang, Z.; Yu, H. Bioinspired Reversible Switch Between Underwater Superoleophobicity/Superaerophobicity and Oleophilicity/ Aerophilicity and Improved Antireflective Property on the Nanosecond Laser-Ablated Superhydrophobic Titanium Surfaces. ACS Appl. Mater. Interfaces 2019, 12, 6573–6580. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.H.; Ngo, C.V.; Chun, D.M. Controlling the Wetting Properties of Superhydrophobic Titanium Surface Fabricated by UV Nanosecond-Pulsed Laser and Heat Treatment. Nanomaterials 2018, 8, 766. [Google Scholar] [CrossRef]
- Gulati, K.; Ding, C.; Guo, T.; Guo, H.; Yu, H.; Liu, Y. Craniofacial Therapy: Advanced Local Therapies from Nano-Engineered Titanium Implants to Treat Craniofacial Conditions. Int. J. Oral Sci. 2023, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Brånemark, R.; Emanuelsson, L.; Palmquist, A.; Thomsen, P. Bone Response to Laser-Induced Micro- and Nano-Size Titanium Surface Features. Nanomedicine 2011, 7, 220–227. [Google Scholar] [CrossRef]
- Wang, G.; Li, J.; Lv, K.; Zhang, W.; Ding, X.; Yang, G.; Liu, X.; Jiang, X. Surface Thermal Oxidation on Titanium Implants to Enhance Osteogenic Activity and in Vivo Osseointegration. Sci. Rep. 2016, 6, 31769. [Google Scholar] [CrossRef]
- Shu, T.; Wang, X.; Li, M.; Ma, S.; Cao, J.; Sun, G.; Lai, T.; Liu, S.; Li, A.; Qu, Z.; et al. Nanoscaled Titanium Oxide Layer Provokes Quick Osseointegration on 3D-Printed Dental Implants: A Domino Effect Induced by Hydrophilic Surface. ACS Nano 2024, 18, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced Bone Apposition to a Chemically Modified SLA Titanium Surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef]
- Yan, S.; Li, M.; Komasa, S.; Agariguchi, A.; Yang, Y.; Zeng, Y.; Takao, S.; Zhang, H.; Tashiro, Y.; Kusumoto, T.; et al. Decontamination of Titanium Surface Using Different Methods: An In Vitro Study. Materials 2020, 13, 2287. [Google Scholar] [CrossRef]
- Aita, H.; Hori, N.; Takeuchi, M.; Suzuki, T.; Yamada, M.; Anpo, M.; Ogawa, T. The Effect of Ultraviolet Functionalization of Titanium on Integration with Bone. Biomaterials 2009, 30, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jeon, H.J.; Jung, A.; Kim, J.; Kim, J.Y.; Lee, S.H.; Kim, H.; Yeom, M.S.; Choe, W.; Gweon, B.; et al. Improvement of Osseointegration Efficacy of Titanium Implant Through Plasma Surface Treatment. Biomed. Eng. Lett. 2022, 12, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Berger, M.B.; Nelson, F.R.; Donahue, H.J.; Schwartz, Z. The Biological Basis for Surface-Dependent Regulation of Osteogenesis and Implant Osseointegration. J. Am. Acad. Orthop. Surg. 2022, 30, E894–E898. [Google Scholar] [CrossRef]
- Henriksen, K.; Bollerslev, J.; Everts, V.; Karsdal, M.A. Osteoclast Activity and Subtypes as a Function of Physiology and Pathology-Implications for Future Treatments of Osteoporosis. Endocr. Rev. 2011, 32, 31–63. [Google Scholar] [CrossRef]
- Deng, J.; Van Duyn, C.; Cohen, D.J.; Schwartz, Z.; Boyan, B.D. Strategies for Improving Impaired Osseointegration in Compromised Animal Models. J. Dent. Res. 2024, 103, 467–476. [Google Scholar] [CrossRef]
- Khan, M.R.; Donos, N.; Salih, V.; Brett, P.M. The Enhanced Modulation of Key Bone Matrix Components by Modified Titanium Implant Surfaces. Bone 2012, 50, 1–8. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Valderrama, P.; Jones, A.A.; Wilson, T.G.; Seibl, R.; Cochran, D.L. Bone Apposition around Two Different Sandblasted and Acid-Etched Titanium Implant Surfaces: A Histomorphometric Study in Canine Mandibles. Clin. Oral. Implant. Res. 2008, 19, 233–241. [Google Scholar] [CrossRef]
- Mckee, M.D.; Nanci, A. Osteopontin: An Interfacial Extracellular Matrix Protein in Mineralized Tissues. Connect. Tissue Res. 1996, 35, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, Z.; Olivares-Navarrete, R.; Wieland, M.; Cochran, D.L.; Boyan, B.D. Mechanisms Regulating Increased Production of Osteoprotegerin by Osteoblasts Cultured on Microstructured Titanium Surfaces. Biomaterials 2009, 30, 3390–3396. [Google Scholar] [CrossRef]
- Langenbach, F.; Handschel, J. Effects of Dexamethasone, Ascorbic Acid and β-Glycerophosphate on the Osteogenic Differentiation of Stem Cells in Vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef]
- Boskey, A.L.; Robey, P.G. The Regulatory Role of Matrix Proteins in Mineralization of Bone. In Osteoporos, 4th ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 235–255. [Google Scholar] [CrossRef]
- Beck, G.R.; Zerler, B.; Moran, E. Phosphate Is a Specific Signal for Induction of Osteopontin Gene Expression. Proc. Natl. Acad. Sci. USA 2000, 97, 8352–8357. [Google Scholar] [CrossRef]
- Berger, M.B.; Bosh, K.B.; Jacobs, T.W.; Joshua Cohen, D.; Schwartz, Z.; Boyan, B.D. Growth Factors Produced by Bone Marrow Stromal Cells on Nanoroughened Titanium–Aluminum–Vanadium Surfaces Program Distal MSCs into Osteoblasts via BMP2 Signaling. J. Orthop. Res. 2021, 39, 1908–1920. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Serrabona, J.; Bosch, B.M.; Díez-Tercero, L.; Gil, F.J.; Camps-Font, O.; Valmaseda-Castellón, E.; Gay-Escoda, C.; Sánchez-Garcés, M.Á. Evaluation of the Inflammatory and Osteogenic Response Induced by Titanium Particles Released During Implantoplasty of Dental Implants. Sci. Rep. 2022, 12, 15790. [Google Scholar] [CrossRef] [PubMed]
- Paul, N.E.; Skazik, C.; Harwardt, M.; Bartneck, M.; Denecke, B.; Klee, D.; Salber, J.; Zwadlo-Klarwasser, G. Topographical Control of Human Macrophages by a Regularly Microstructured Polyvinylidene Fluoride Surface. Biomaterials 2008, 29, 4056–4064. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castejon, G.; Brough, D. Understanding the Mechanism of IL-1β Secretion. Cytokine Growth Factor. Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and Therapeutic Potential of Interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dillon, J.T.; Cohen, D.J.; McLean, S.; Fan, H.; Boyan, B.D.; Schwartz, Z. Titanium–Aluminum–Vanadium Surfaces Generated Using Sequential Nanosecond and Femtosecond Laser Etching Provide Osteogenic Nanotopography on Additively Manufactured Implants. Biomimetics 2025, 10, 507. https://doi.org/10.3390/biomimetics10080507
Dillon JT, Cohen DJ, McLean S, Fan H, Boyan BD, Schwartz Z. Titanium–Aluminum–Vanadium Surfaces Generated Using Sequential Nanosecond and Femtosecond Laser Etching Provide Osteogenic Nanotopography on Additively Manufactured Implants. Biomimetics. 2025; 10(8):507. https://doi.org/10.3390/biomimetics10080507
Chicago/Turabian StyleDillon, Jonathan T., David J. Cohen, Scott McLean, Haibo Fan, Barbara D. Boyan, and Zvi Schwartz. 2025. "Titanium–Aluminum–Vanadium Surfaces Generated Using Sequential Nanosecond and Femtosecond Laser Etching Provide Osteogenic Nanotopography on Additively Manufactured Implants" Biomimetics 10, no. 8: 507. https://doi.org/10.3390/biomimetics10080507
APA StyleDillon, J. T., Cohen, D. J., McLean, S., Fan, H., Boyan, B. D., & Schwartz, Z. (2025). Titanium–Aluminum–Vanadium Surfaces Generated Using Sequential Nanosecond and Femtosecond Laser Etching Provide Osteogenic Nanotopography on Additively Manufactured Implants. Biomimetics, 10(8), 507. https://doi.org/10.3390/biomimetics10080507








