Surface Assessment of a Novel Acid-Etching Solution on CAD/CAM Dental Ceramics
Abstract
1. Introduction
2. Materials and Methods
3. Results
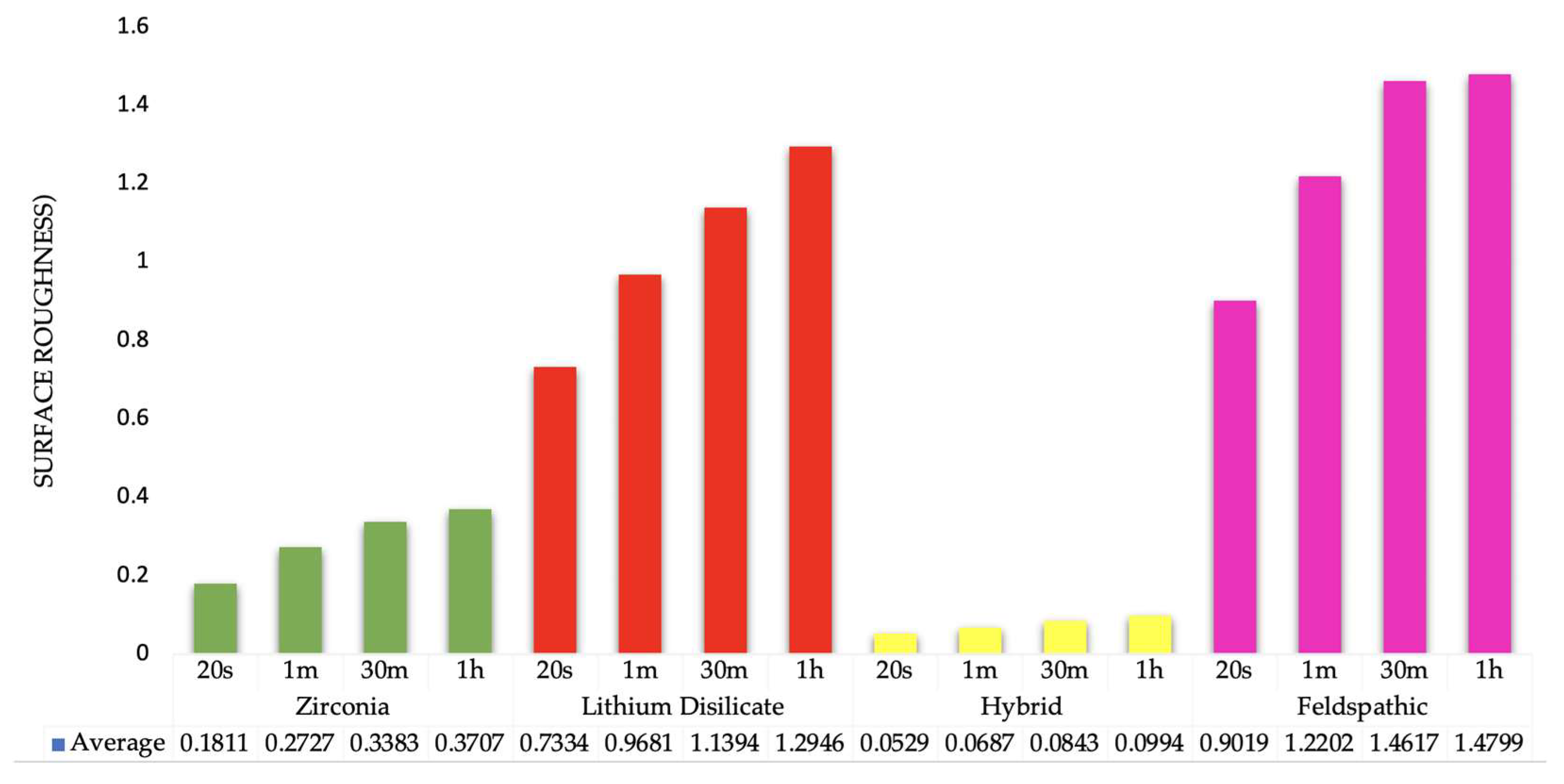
3.1. Surface Roughness Measurements
3.2. Profilometric Imaging
3.3. Scanning Electron Microscopy (SEM)
4. Discussion
4.1. Surface Chemistry and Phase Composition
4.2. Surface Roughness and Topography
4.3. SEM Analysis
4.4. Influence of Ceramic Composition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Freitas, J.S.; Souza, L.F.B.; Pereira, G.K.R.; May, L.G. Surface properties and flexural fatigue strength of an advanced lithium disilicate. J. Mech. Behav. Biomed. Mater. 2023, 147, 106154. [Google Scholar] [CrossRef]
- Fu, L.; Engqvist, H.; Xia, W. Glass–ceramics in dentistry: A review. Materials 2020, 13, 1049. [Google Scholar] [CrossRef]
- Streit, G.; Sykes, L.M. Overview of lithium disilicate as a restorative material in dentistry. S. Afr. Dent. J. 2022, 77, 495–499. [Google Scholar] [CrossRef]
- Garfias, C.S.; de Goes, M.F. Acid dissolution of ultrathin glass-ceramic and its correlation with flexural strength. J. Prosthet. Dent. 2022, 128, 1084.e1–1084.e8. [Google Scholar] [CrossRef]
- Denry, I.; Kelly, J.R. State of the art of zirconia for dental applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Denry, I. Stabilized zirconia as a structural ceramic: An overview. Dent. Mater. 2008, 24, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Schwenter, J.; Schmidli, F.; Weiger, R.; Fischer, J. Adhesive bonding to polymer infiltrated ceramic. Dent. Mater. J. 2016, 35, 796–802. [Google Scholar] [CrossRef]
- Conejo, J.; Ozer, F.; Mante, F.; Atria, P.J.; Blatz, M.B. Effect of surface treatment and cleaning on the bond strength to polymer-infiltrated ceramic network CAD-CAM material. J. Prosthet. Dent. 2021, 126, 698–702. [Google Scholar] [CrossRef]
- Praisuwanna, N.; Chiaraputt, S.; Kittikundecha, N.; Sriamporn, T. The Effect of Different Surface Treatments on the Shear Bond Strength of Resin Composite Attached to Polymer-Infiltrated Ceramic-Network Material. In Proceedings of the RSU International Research Conference 2022 on Science and Technology, Hybrid (online and on-site), 29 April 2022; pp. 283–292. [Google Scholar] [CrossRef]
- Mahrous, A.I.; Salama, A.A.; Shabaan, A.A.; Abdou, A.; Radwan, M.M. Color stability of two different resin matrix ceramics: Randomized clinical trial. BMC Oral Health 2023, 23, 665. [Google Scholar] [CrossRef]
- Cuzic, C.; Rominu, M.; Pricop, A.; Urechescu, H.; Pricop, M.O.; Rotar, R.; Cuzic, O.S.; Sinescu, C.; Jivanescu, A. Clinician’s Guide to Material Selection for All-Ceramics in Modern Digital Dentistry. Materials 2025, 18, 2235. [Google Scholar] [CrossRef]
- D’Alessandro, C.; Josic, U.; Mazzitelli, C.; Maravic, T.; Graham, L.; Barausse, C.; Mazzoni, A.; Breschi, L.; Blatz, M.B. Is zirconia surface etching a viable alternative to airborne particle abrasion? A systematic review and meta-analysis of in vitro studies. J. Dent. 2024, 151, 105394. [Google Scholar] [CrossRef]
- Murillo-Gómez, F.; Hernández-Víquez, J.R.; Sauma-Montes de Oca, J.R.; Vargas-Vargas, C.; González-Vargas, N.; Vega-Baudrit, J.R.; Chavarría-Bolaños, D. Mechanical, Adhesive and Surface Properties of a Zirconia-Reinforced Lithium Silicate CAD/CAM Ceramic Exposed to Different Etching Protocols. Materials 2024, 17, 5039. [Google Scholar] [CrossRef] [PubMed]
- Yahyazadehfar, N.; Azimi Zavaree, M.; Shayegh, S.S.; Yahyazadehfar, M.; Hooshmand, T.; Hakimaneh, S.M.R. Effect of different surface treatments on surface roughness, phase transformation, and biaxial flexural strength of dental zirconia. J. Dent. Res. Dent. Clin. Dent. Prospect. 2021, 15, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.M.; Kumaraswamy, M.; Thomas, D.; Boraiah, S.; Singh Rana, K. Zirconia in Restorative Dentistry. In Zirconia—New Advances, Structure, Fabrication and Applications, 1st ed.; Al-Naib, U.M.B., Ed.; IntechOpen: London, UK, 2023; pp. 1–17. [Google Scholar]
- Conner, C.; Andretti, F.; Hernandez, A.; Rojas-Rueda, S.; Azpiazu-Flores, F.X.; Morrow, B.M.; Garcia-Godoy, F.; Jurado, C.A.; Alshabib, A. Surface Evaluation of a Novel Acid-Etching Solution for Zirconia and Lithium Disilicate. Materials 2025, 18, 2912. [Google Scholar] [CrossRef] [PubMed]
- Sundfeld Neto, D.; Naves, L.Z.; Costa, A.R.; Correr, A.B.; Consani, S.; Borges, G.A.; Correr-Sobrinho, L. The effect of hydrofluoric acid concentration on the bond strength and morphology of the surface and interface of glass ceramics to a resin cement. Oper. Dent. 2015, 40, 470–479. [Google Scholar] [CrossRef]
- Hallmann, L.; Ulmer, P.; Wille, S.; Polonskyi, O.; Köbel, S.; Trottenberg, T.; Bornholdt, S.; Haase, F.; Kersten, H.; Kern, M. Effect of surface treatments on the properties and morphological change of dental zirconia. J. Prosthet. Dent. 2016, 115, 341–349. [Google Scholar] [CrossRef]
- Bebsh, M.; Haimeur, A.; França, R. The Effect of Different Surface Treatments on the Micromorphology and the Roughness of Four Dental CAD/CAM Lithium Silicate-Based Glass-Ceramics. Ceramics 2021, 4, 467–475. [Google Scholar] [CrossRef]
- Bacchi, A.; Cesar, P.F. Advances in ceramics for dental applications. In Dental Biomaterials; Ferracane, J.L., Pfeifer, C.S., Bertassoni, L.E., Eds.; Elsevier: Philadelphia, PA, USA, 2022; Volume 66, pp. 591–602. [Google Scholar]
- Malallah, A.D.; Hasan, N.H. Classification and Generations of Dental Zirconia. In Zirconia—New Advances, Structure, Fabrication and Applications; Basheer, M., Al-Naib, U., Eds.; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Nejat, A.H. Overview of Current Dental Ceramics. In Updates in Prosthodontics; Jahangiri, L., Ed.; Elsevier: Philadelphia, PA, USA, 2025; Volume 69, pp. 155–171. [Google Scholar]
- Awada, A.; Nathanson, D. Mechanical properties of resin-ceramic CAD/CAM restorative materials. J. Prosthet. Dent. 2015, 114, 587–593. [Google Scholar] [CrossRef]
- Phark, J.H.; Duarte, S., Jr. Microstructural considerations for novel lithium disilicate glass ceramics: A review. J. Esthet. Restor. Dent. 2022, 34, 92–103. [Google Scholar] [CrossRef]
- Benalcázar-Jalkh, E.B.; Bergamo, E.T.; Campos, T.M.; Coelho, P.G.; Sailer, I.; Yamaguchi, S.; Alves, L.M.M.; Witek, L.; Tebcherani, S.M.; Bonfante, E.A. A narrative review on polycrystalline ceramics for dental applications and proposed update of a classification system. Materials 2023, 16, 7541. [Google Scholar] [CrossRef]
- Martins, J.D.; Moura, D.; Lima, C.M.; de Carvalho, R.; Leite, F.; Souza, R. Surface Treatment and Cementation of Lithium Silicate Ceramics Containing ZrO2. Oper. Dent. 2022, 47, 202–213. [Google Scholar] [CrossRef]
- Kim, S.-H.; Oh, K.C.; Moon, H.-S. Effects of Surface-Etching Systems on the Shear Bond Strength of Dual-Polymerized Resin Cement and Zirconia. Materials 2024, 17, 3096. [Google Scholar] [CrossRef]
- Flamant, Q.; García Marro, F.; Roa Rovira, J.J.; Anglada, M. Hydrofluoric acid etching of dental zirconia. Part 1: Etching mechanism and surface characterization. J. Eur. Ceram. Soc. 2016, 36, 121–134. [Google Scholar] [CrossRef]
- Zogheib, L.V.; Bona, A.D.; Kimpara, E.T.; McCabe, J.F. Effect of hydrofluoric acid etching duration on the roughness and flexural strength of a lithium disilicate-based glass ceramic. Braz. Dent. J. 2011, 22, 45–50. [Google Scholar] [CrossRef]
- Blatz, M.B.; Alvarez, M.; Sawyer, K.; Brindis, M. How to Bond Zirconia: The APC Concept. Compend. Contin. Educ. Dent. 2016, 37, 611–618. [Google Scholar]
- Lopes, G.C.; Perdigão, J.; Baptista, D.; Ballarin, A. Does a self-etching ceramic primer improve bonding to lithium disilicate ceramics? Bond Strengths and FESEM Analyses. Oper. Dent. 2019, 44, 210–218. [Google Scholar] [CrossRef]
- Ansari, S.; Jahedmanesh, N.; Cascione, D.; Zafarnia, P.; Shah, K.C.; Wu, B.M.; Moshaverinia, A. Effects of an etching solution on the adhesive properties and surface microhardness of zirconia dental ceramics. J. Prosthet. Dent. 2018, 120, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chiang, C.-C.; Craciun, V.; Deane, G.M.; Ren, F.; Esquivel-Upshaw, J.F. Yttrium ion release and phase transformation in yttria-stabilized zirconia under acidic conditions: Implications for dental implant durability. Materials 2025, 18, 3311. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, J.; Gremillard, L.; Virkar, A.V.; Clarke, D.R. The tetragonal-monoclinic transformation in zirconia: Lessons learned and future trends. J. Am. Ceram. Soc. 2009, 92, 1901–1920. [Google Scholar] [CrossRef]
- Veríssimo, A.H.; Moura, D.M.D.; Tribst, J.P.M.; Araújo, A.M.M.; Leite, F.P.P.; Souza, R.O.A. Effect of hydrofluoric acid concentration and etching time on resin-bond strength to different glass ceramics. Braz. Oral Res. 2019, 33, e041. [Google Scholar] [CrossRef] [PubMed]
- Poulon-Quintin, A.; Ogden, E.; Large, A.; Vaudescal, M.; Labrugère, C.; Bartala, M.; Bertrand, C. Chemical surface modification of lithium disilicate needles of a silica-based ceramic after HF-etching and ultrasonic bath cleaning: Impact on the chemical bonding with silane. Dent. Mater. 2021, 37, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.W.; Matinlinna, J.P. Insights on Ceramics as Dental Materials. Part II: Chemical Surface Treatments. Silicon 2011, 3, 117–123. [Google Scholar] [CrossRef]
- Ramakrishnaiah, R.; Alkheraif, A.A.; Divakar, D.D.; Matinlinna, J.P.; Vallittu, P.K. The effect of hydrofluoric acid etching duration on the surface micromorphology, roughness, and wettability of dental ceramics. Int. J. Mol. Sci. 2016, 17, 822. [Google Scholar] [CrossRef] [PubMed]
- You, G.E.; Lim, M.J.; Min, K.S.; Yu, M.K.; Lee, K.W. Surface property changes observed in zirconia during etching with high-concentration hydrofluoric acid over various immersion times. Dent. Mater. J. 2024, 43, 52–57. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, S.J.; Shim, J.S.; Lee, K.W. Effect of zirconia surface treatment using nitric acid-hydrofluoric acid on the shear bond strengths of resin cements. J. Adv. Prosthodont. 2017, 9, 77–84. [Google Scholar] [CrossRef]
- Sokolowski, G.; Szczesio-Wlodarczyk, A.; Szynkowska-Józ’wik, M.I.; Stopa, W.; Sokolowski, J.; Kopacz, K.; Bociong, K. The Shear Bond Strength of Resin-Based Luting Cement to Zirconia Ceramics after Different Surface Treatments. Materials 2023, 16, 5433. [Google Scholar] [CrossRef]
- Botelho, M.G.; Dangay, S.; Shih, K.; Lam, W.Y.H. The effect of surface treatments on dental zirconia: An analysis of biaxial flexural strength, surface roughness, and phase transformation. J. Dent. 2018, 75, 65–73. [Google Scholar] [CrossRef]
- Lee, H.; Woo, C.; Paek, J.; Geis-Gerstorfer, J. Effects of ultrasonic acid treatment on resin-zirconia bond strength. In Proceedings of the 40th European Prosthodontic Association (EPA) 65th German Society for Prosthetic Dentistry and Biomaterials (DGPro) Joint Scientific Meeting, Halle, Germany, 29 September–1 October 2016. [Google Scholar]
- Ruyter, E.I.; Vajeeston, N.; Knarvang, T.; Kvam, K. A novel etching technique for surface treatment of zirconia ceramics to improve adhesion of resin-based luting cements. Acta Biomater. Odontol. Scand. 2017, 3, 36–46. [Google Scholar] [CrossRef]
- Almirabi, R.S.; Alzahrani, K.M. Effect of the intaglio surface treatment and thickness of different types of yttria-stabilized tetragonal zirconia polycrystalline materials on the flexural strength: In-vitro study. Materials 2024, 17, 5256. [Google Scholar] [CrossRef]
- Niizuma, Y.; Kobayashi, M.; Toyama, T.; Manabe, A. Effect of etching with low-concentration hydrofluoric acid on the bond strength of CAD/CAM resin block. Dent. Mater. J. 2020, 39, 1000–1008. [Google Scholar] [CrossRef]
- Turker, I.; Yuzbasioglu, E. Optical Properties of CAD/CAM Interpenetrating Phase Composites—An Overview. Glass Ceram. 2024, 81, 217–224. [Google Scholar] [CrossRef]
- Oliveira, L.T.; de Castro, E.F.; Azevedo, V.L.; de Andrade, O.S.; Faraoni, J.J.; Palma-Dibb, R.G.; Dias, C.T.S.; Giannini, M. Effect of ceramic conditioners on surface morphology, roughness, contact angle, adhesion, microstructure, and composition of CAD/CAM ceramics. Oper. Dent. 2023, 48, 277–293. [Google Scholar] [CrossRef]
- Porto, T.S.; da Silva, I.G.M.; de Freitas Vallerini, B.; de Goes, M.F. Different surface treatment strategies on etchable CAD-CAM materials: Part 1—Effect on the surface morphology. J. Prosthet. Dent. 2023, 130, 761–769. [Google Scholar] [CrossRef]


| Type of Ceramic | HF Application Time |
|---|---|
| Zirconia (ZirCAD, Ivoclar) | Not recommended |
| Lithium Disilicate (Amber Mill, Hassbio) | 20 s |
| Hybrid Ceramic (CeraSmart, GC) | 60 s |
| Feldspathic Porcelain (VitaBLOCS, Vita) | 60–90 s |
| Material | Commercial Brand | Crystalline Microstructure | Mechanical Properties CTE | Clinical Indication |
|---|---|---|---|---|
| Zirconia [1] | e.max ZirCAD® (Ivoclar) Block size: 12 × 14 × 18 mm | Homogeneous fine Y2O3: 4.0–6.0 ZrO2: 87.0–95.0% HfO2: 1.0–5.0, Al2O3: 0.0–1.0 Other oxides: <0.2 | σ: ≥900 MPa Ra: 0.22 µm KIc: 5.14 ± 0.07 MPa·m1/2 E: 70 ± 2 GPa CTE: 10.6 ± 0.1 × 10−6 K−1 (100–400 °C) | Full-contour crowns, 3-unit bridges, and 4- and multi-unit bridges with max. 2 pontics, Crown copings, 3 unit- and multi-unit bridge frameworks with max. 2 pontics |
| Glass-Ceramic Lithium disilicate [1] | Amber Mill® (Hassbio) Block size: 12 × 14 × 18 mm | Needle–like crystals (approx. 70 vol%); Composition: Li2Si2O5; Size: 3–6 µm (length) | σ: 350–450 MPa Ra: 0.21 µm KIc: 0.8–1.5 MPa·m1/2 E: ~70 GPa CTE: 10.2 ± 0.4 × 10−6 K−1 (100–400 °C), 10.6 ± 0.35 × 10−6 K−1 (100–500 °C) | Crowns, Veneers Occlusal veneers (table tops) ≥ 1.0 mm, Inlays, Onlays, Partial crowns, 3-unit bridges in the anterior and posterior region (2nd premolar as the terminal abutment) Hybrid abutments in the anterior and posterior region as a single-tooth restoration, Hybrid abutment crowns in the anterior and posterior region |
| Hybrid Ceramic | CeraSmart (GC America) Block size: 12 × 14.0 × 18 mm | ~71–80% ceramic nanoparticles (silica + zirconia) in a resin matrix (UDMA, Bis-MEPP, Bis-GMA) Highly dispersed nano-sized fillers bonded in a resilient resinous matrix | σ: ~220 MPa KIc: ~1.2–1.6 MPa·m0.5 E: ~10–15 GPa Sa ≈ 0.05–0.15 µm (post-polishing) CTE: ~30–50 × 10−6/°C (resin-like behavior) ~1.0 GPa (Vickers) |
|
| Feldspathic Ceramic | VitaBLOCS, (Vita) Block size: 10 × 12 × 15 mm | Feldspathic ceramic Glass matrix with fine feldspar crystals (~60–70 vol% crystalline phase) Homogeneous crystalline-glass microstructure CAD/CAM blocks, sintered glass-ceramic | σ: ~130–160 MPa KIc: ~1.1–1.5 MPa·m0.5 E: ~45–65 GPa Sa ≈ 0.15–0.3 µm (depends on finish) CTE: ~9–10 × 10−6/°C ~5.5 GPa (Vickers) |
|
| Zirconia (Z) | Lithium Disilicate (L) | Hybrid (Hy) | Feldspathic (F) | |
|---|---|---|---|---|
| Etching time | ||||
| 20 s | Group 1 (Z20) | Group 5 (L20) | Group 9 (Hy20) | Group 13 (F20) |
| 1 min | Group 2 (Z1m) | Group 6 (L1m) | Group 10 (Hy1m) | Group 14 (F1m) |
| 30 min | Group 3 (Z30) | Group 7 (L30) | Group 11 (Hy30) | Group 15 (F30) |
| 1 h | Group 4 (Z1h) | Group 8 (L1h) | Group 12 (Hy1h) | Group 16 (F1h) |
| Application Time | Mean Surface Roughness in Microns (µm) (Standard Deviation) 1 | |||
|---|---|---|---|---|
| Zirconia | Lithium Disilicate | Hybrid | Feldspathic | |
| 20 s | Group 1 0.1811 (0.043) | Group 5 0.7334 (0.082) | Group 9 0.0528 (0.008) | Group 13 0.9019 (0.102) |
| 1 min | Group 2 0.2727 (0.041) | Group 6 0.9680 (0.066) | Group 10 0.0686 (0.007) | Group 14 1.2202 (0.166) |
| 30 min | Group 3 0.3382 (0.037) | Group 7 1.1394 (0.098) | Group 11 0.0843 (0.006) | Group 15 1.4617 (0.100) |
| 1 h | Group 4 0.3706 (0.074) | Group 8 1.2946 (0.123) | Group 12 0.0994 (0.016) | Group 16 1.4799 (0.096) |
| Sum of Squares | df | Mean Square | F | Sig. | |
|---|---|---|---|---|---|
| Between Groups | 65.250 | 15 | 4.350 | 664.323 | <0.001 |
| Within Groups | 1.467 | 224 | 0.007 | ||
| Total | 66.717 | 239 |
| Etching Time | Group Number (Standard Deviation) | |||
|---|---|---|---|---|
| 20 s | (Group 1) 0.181 (0.043) b | (Group 5) 0.733 (0.082) d | (Group 9) 0.053 (0.008) a | (Group 13) 0.902 (0.102) d |
| 1 min | (Group 2) 0.273 (0.041) b | (Group 6) 0.968 (0.066) d | (Group 10) 0.069 (0.007) a | (Group 14) 1.220 (0.166) d |
| 30 min | (Group 3) 0.338 (0.037) b | (Group 7) 1.139 (0.098) d | (Group 11) 0.084 (0.006) a | (Group 15) 1.461 (0.100) d |
| 1 h | (Group 4) 0.371 (0.074) c | (Group 8) 1.295 (0.123) d | (Group 12) 0.099 (0.016) a | (Group 16) 1.479 (0.096) d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andretti, F.; Jurado, C.A.; Antal, M.; Hernandez, A.I.; Rojas-Rueda, S.; Garcia-Godoy, F.; Morrow, B.R.; Nurrohman, H. Surface Assessment of a Novel Acid-Etching Solution on CAD/CAM Dental Ceramics. Biomimetics 2025, 10, 508. https://doi.org/10.3390/biomimetics10080508
Andretti F, Jurado CA, Antal M, Hernandez AI, Rojas-Rueda S, Garcia-Godoy F, Morrow BR, Nurrohman H. Surface Assessment of a Novel Acid-Etching Solution on CAD/CAM Dental Ceramics. Biomimetics. 2025; 10(8):508. https://doi.org/10.3390/biomimetics10080508
Chicago/Turabian StyleAndretti, Fabio, Carlos A. Jurado, Mark Antal, Alfredo I. Hernandez, Silvia Rojas-Rueda, Franklin Garcia-Godoy, Brian R. Morrow, and Hamid Nurrohman. 2025. "Surface Assessment of a Novel Acid-Etching Solution on CAD/CAM Dental Ceramics" Biomimetics 10, no. 8: 508. https://doi.org/10.3390/biomimetics10080508
APA StyleAndretti, F., Jurado, C. A., Antal, M., Hernandez, A. I., Rojas-Rueda, S., Garcia-Godoy, F., Morrow, B. R., & Nurrohman, H. (2025). Surface Assessment of a Novel Acid-Etching Solution on CAD/CAM Dental Ceramics. Biomimetics, 10(8), 508. https://doi.org/10.3390/biomimetics10080508











