Design and Development of a Real-Time Pressure-Driven Monitoring System for In Vitro Microvasculature Formation
Abstract
1. Introduction
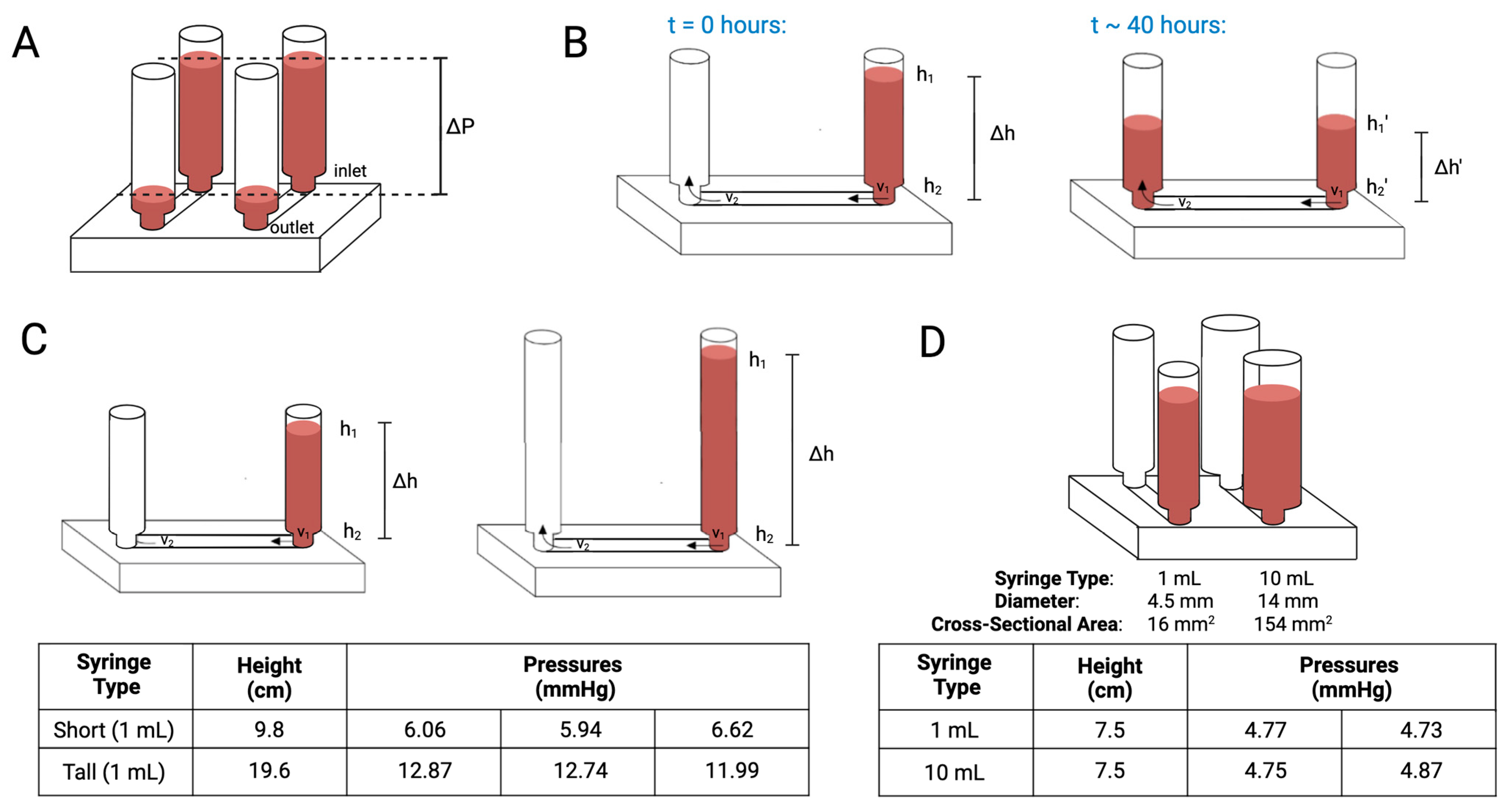
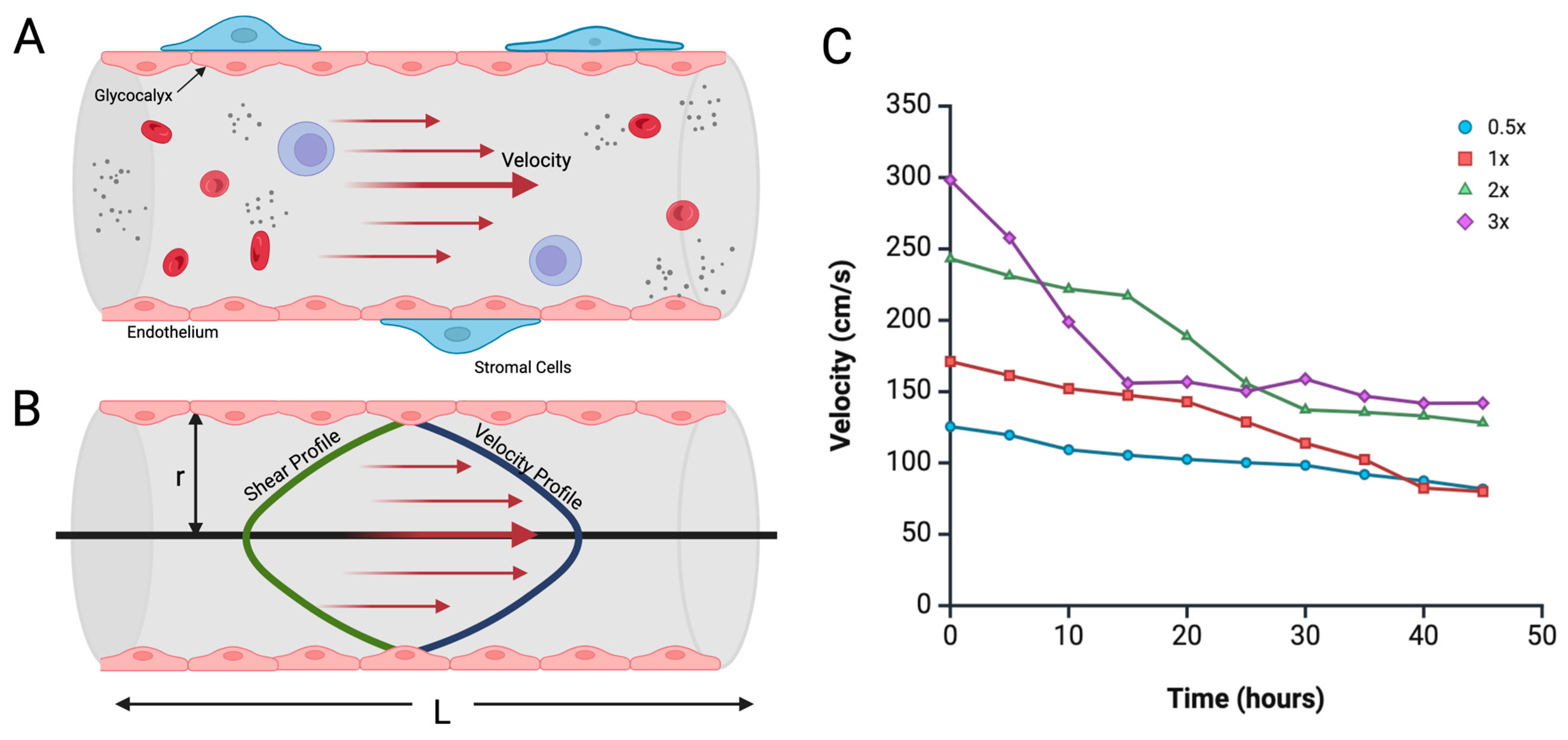
2. Theory and Design
3. Materials and Methods
3.1. Biological Setup
3.1.1. Cell Culture
3.1.2. Cell Seeding Within 3D Vessel Formation Assay
3.1.3. Immunofluorescent Staining
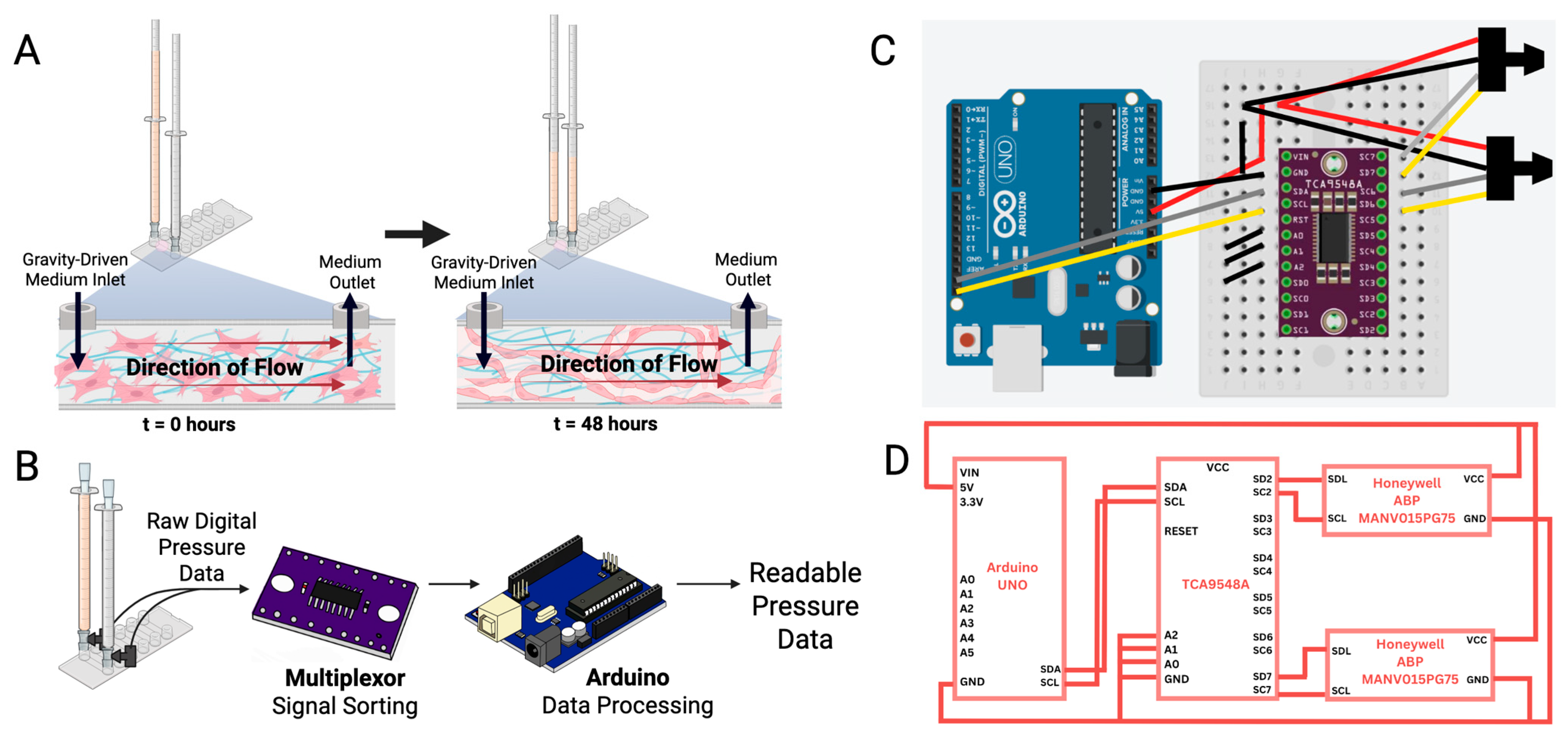
3.2. Data Acquisition and Analysis
3.2.1. Pressure Sensor Selection
3.2.2. Pressure Sensor Calibration and Signal Conditioning
3.2.3. Pressure Signal Acquisition
3.2.4. Incubator Camera Setup
3.2.5. Programming Code
4. Results
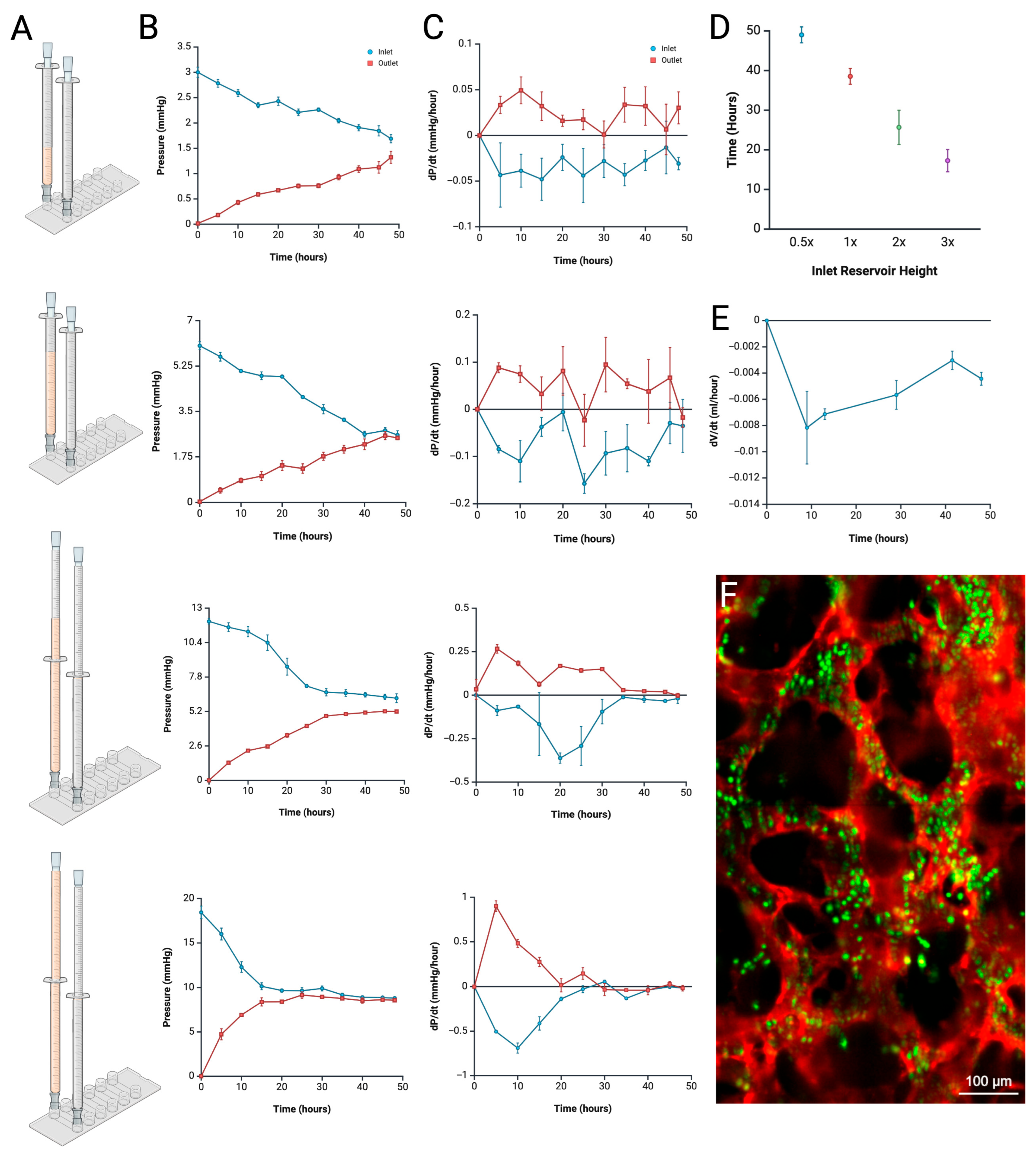
4.1. Pressure Measurement and Profile Analysis
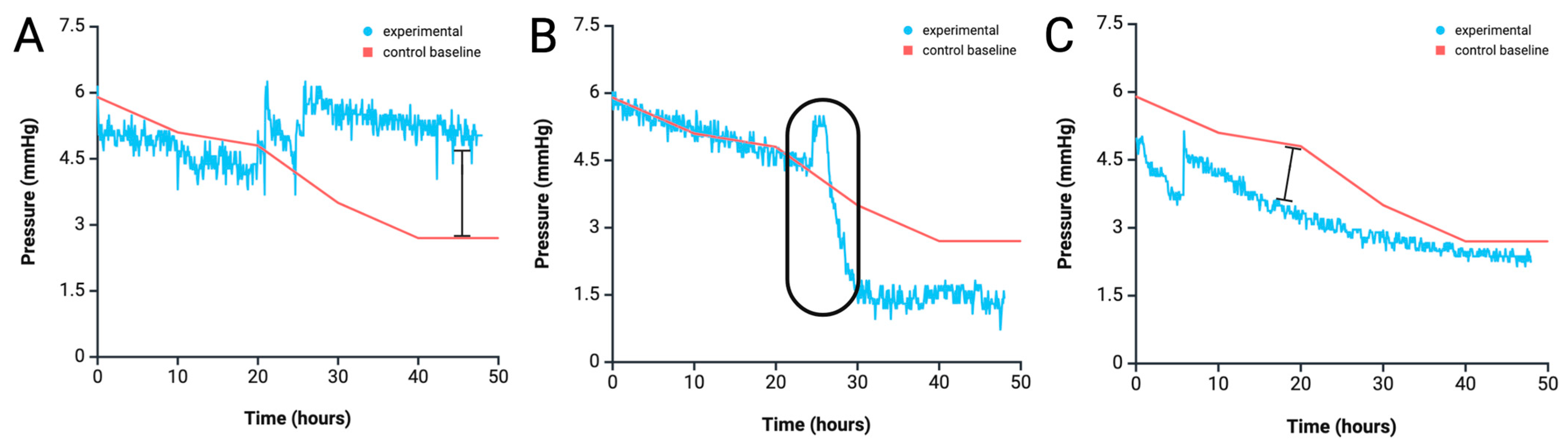
4.2. Anomaly Detection from Pressure Profile Analysis
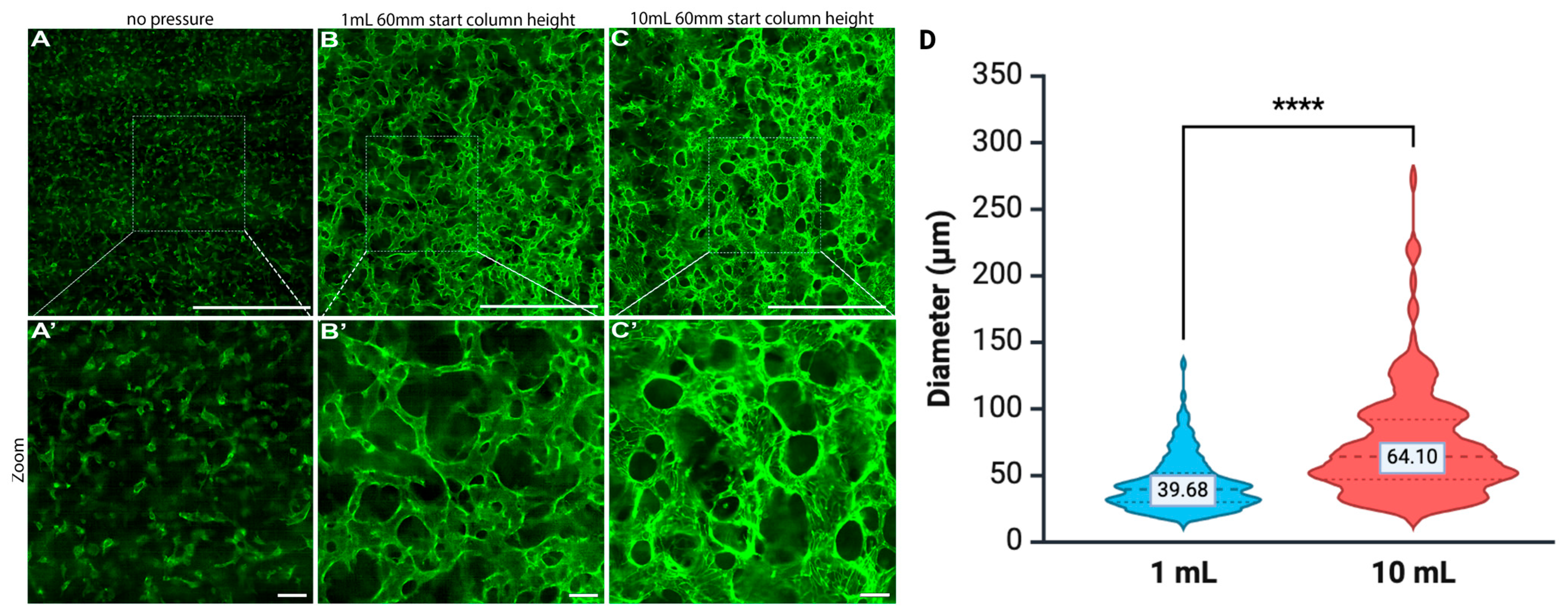
4.3. Duration of Pressure Exposure Influencing Vessel Dilation
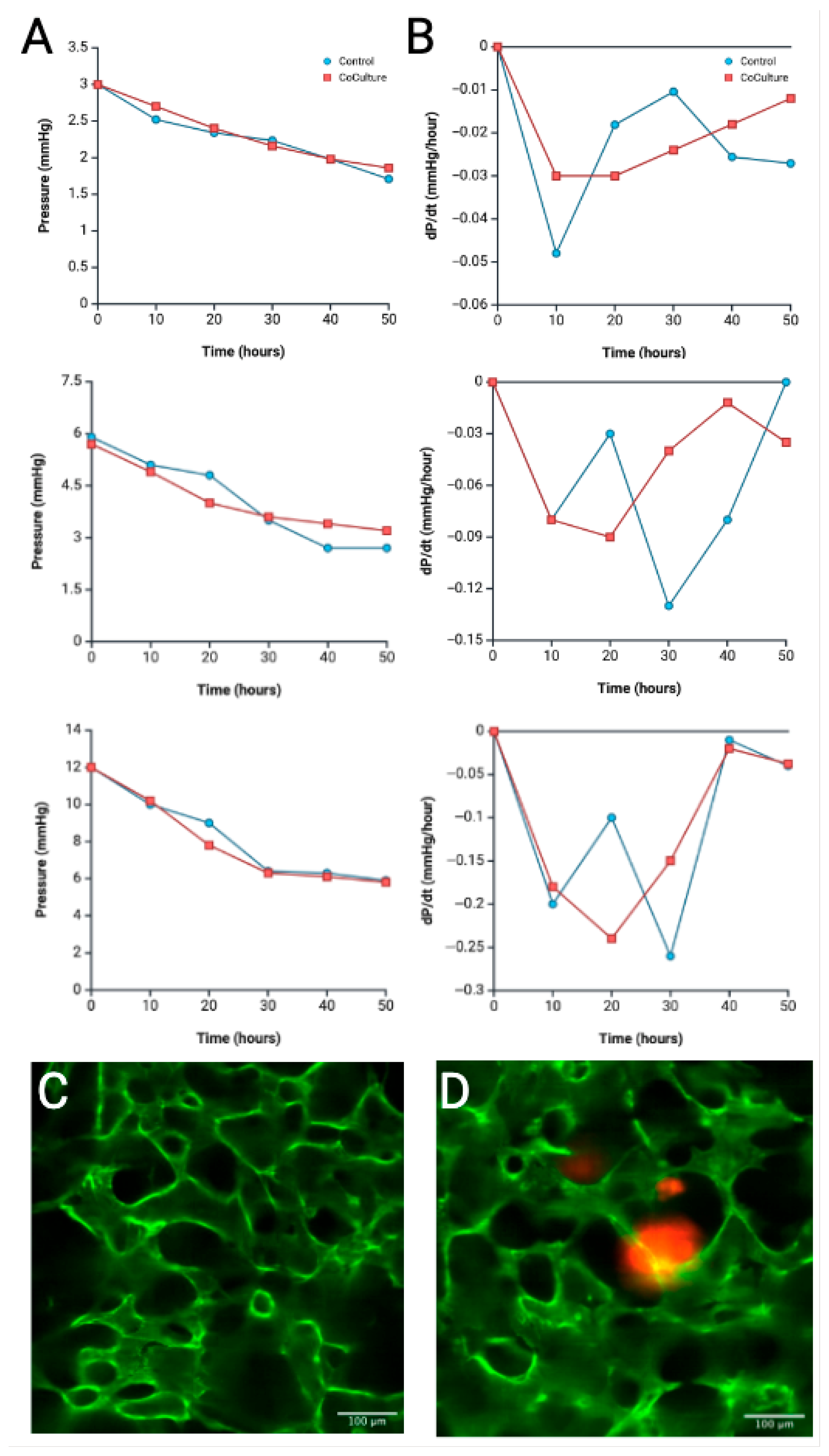
4.4. Tumoroid Co-Culture Pressure Profile Analysis
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HUVECs | Human Umbilical Vein Endothelial Cells |
| R-VECs | “Reset” Vascular Endothelial Cells |
| ECs | Endothelial Cells |
References
- Smith, Q.; Gerecht, S. Going with the flow: Microfluidic platforms in vascular tissue engineering. Curr. Opin. Chem. Eng. 2014, 3, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Naderi-Meshkin, H.; Cornelius, V.A.; Eleftheriadou, M.; Potel, K.N.; Setyaningsih, W.A.W.; Margariti, A. Vascular organoids: Unveiling advantages, applications, challenges, and disease modelling strategies. Stem Cell Res. Ther. 2023, 14, 292. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Li, F.; Lv, J.; He, Y.; Lu, D.; Yamada, T.; Ono, N. Microfluidic analysis of pressure drop and flow behavior in hypertensive micro vessels. Biomed. Microdevices 2015, 17, 60. [Google Scholar] [CrossRef] [PubMed]
- Lake, J.R.; Heyde, K.C.; Ruder, W.C. Low-cost feedback-controlled syringe pressure pumps for microfluidics applications. PLoS ONE 2017, 12, e0175089. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Ingber, D. Microfluidic organs-on-chip. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Hofer, M.; Lutolf, M.P. Engineering organoids. Nat. Rev. Mater. 2021, 6, 402–420. [Google Scholar] [CrossRef]
- Lee, S.; Park, C.; Han, J.W.; Kim, J.Y.; Cho, K.; Kim, E.J.; Kim, S.; Lee, S.J.; Oh, S.Y.; Tanaka, Y.; et al. Direct Reprogramming of Human Dermal Fibroblasts Into Endothelial Cells Using ER71/ETV2. Circ. Res. 2017, 120, 848–861. [Google Scholar] [CrossRef]
- Kim, T.M.; Lee, R.H.; Kim, M.S.; Lewis, C.A.; Park, C. ETV2/ER71, the key factor leading the paths to vascular regeneration and angiogenic reprogramming. Stem Cell Res. Ther. 2023, 14, 41. [Google Scholar] [CrossRef]
- Palikuqi, B.; Nguyen, D.T.; Li, G.; Schreiner, R.; Pellegata, A.F.; Liu, Y.; Redmond, D.; Geng, F.; Lin, Y.; Gomez-Salinero, J.M.; et al. Adaptable haemodynamic endothelial cells for organogenesis and tumorigenesis. Nature 2020, 585, 426–432. [Google Scholar] [CrossRef]
- Esch, E.W.; Bahinski, A.; Huh, D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 2015, 14, 248–260. [Google Scholar] [CrossRef]
- Ingber, D.E. Cellular mechanotransduction: Putting all the pieces together again. FASEB J. 2006, 20, 811–827. [Google Scholar] [CrossRef]
- Wong, A.K.; Llanos, P.; Boroda, N.; Rosenberg, S.R.; Rabbany, S.Y. A Parallel-Plate Flow Chamber for Mechanical Characterization of Endothelial Cells Exposed to Laminar Shear Stress. Cell. Mol. Bioeng. 2016, 9, 127–138. [Google Scholar] [CrossRef]
- Bogorad, M.I.; DeStefano, J.; Karlsson, J.; Wong, A.D.; Gerecht, S.; Searson, P.C. Review: In vitro microvessel models. Lab Chip 2015, 15, 4242–4255. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Pessell, A.F.; Zhu, N.; Searson, P.C. Tissue-Engineered Microvessels: A Review of Current Engineering Strategies and Applications. Adv. Healthc. Mater. 2024, 13, e2303419. [Google Scholar] [CrossRef]
- Guyton, A.C.; Hall, J.E. The Microcirculation and the Lymphatic System: Capillary Fluid Exchange, Interstitial Flow, and Lymph Flow. In Guyton and Hall Textbook of Medical Physiology; Harcourt College Publishers: San Diego, CA, USA, 1990. [Google Scholar]
- Paradiso, A.; Volpi, M.; Martinez, D.C.; Jaroszewicz, J.; Costantini, M.; Swieszkowski, W. Engineering Biomimetic Microvascular Capillary Networks in Hydrogel Fibrous Scaffolds via Microfluidics-Assisted Co-Axial Wet-Spinning. ACS Appl. Mater. Interfaces 2024, 16, 65927–65941. [Google Scholar] [CrossRef]
- Li, H.; Shang, Y.; Zeng, J.; Matsusaki, M. Technology for the formation of engineered microvascular network models and their biomedical applications. Nano Converg. 2024, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.Y.; Yu, A.X.; Qi, B.; Zhou, M.; Li, Z.H.; Wang, W.Y. Analysis of blood flow and local expression of angiogenesis-associated growth factors in infected wounds treated with negative pressure wound therapy. Mol. Med. Rep. 2014, 9, 1749–1754. [Google Scholar] [CrossRef]
- Jufri, N.F.; Mohamedali, A.; Avolio, A.; Baker, M.S. Mechanical stretch: Physiological and pathological implications for human vascular endothelial cells. Vasc. Cell 2015, 7, 8. [Google Scholar] [CrossRef]
- Retailleau, K.; Duprat, F.; Arhatte, M.; Ranade, S.S.; Peyronnet, R.; Martins, J.R.; Jodar, M.; Moro, C.; Offermanns, S.; Feng, Y.; et al. Piezo1 in Smooth Muscle Cells Is Involved in Hypertension-Dependent Arterial Remodeling. Cell Rep. 2015, 13, 1161–1171. [Google Scholar] [CrossRef]
- Lu, D.; Kassab, G.S. Role of shear stress and stretch in vascular mechanobiology. J. R. Soc. Interface 2011, 8, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, N.; Plotnikova, L.; Singh, P.; Giniatullin, R.; Hamalainen, R.H. Functional Characterization of Mechanosensitive Piezo1 Channels in Trigeminal and Somatic Nerves in a Neuron-on-Chip Model. Int. J. Mol. Sci. 2022, 23, 1370. [Google Scholar] [CrossRef]
- Ranade, S.S.; Qiu, Z.; Woo, S.H.; Hur, S.S.; Murthy, S.E.; Cahalan, S.M.; Xu, J.; Mathur, J.; Bandell, M.; Coste, B.; et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl. Acad. Sci. USA 2014, 111, 10347–10352. [Google Scholar] [CrossRef]
- Fang, J.S.; Coon, B.G.; Gillis, N.; Chen, Z.; Qiu, J.; Chittenden, T.W.; Burt, J.M.; Schwartz, M.A.; Hirschi, K.K. Shear-induced Notch-Cx37-p27 axis arrests endothelial cell cycle to enable arterial specification. Nat. Commun. 2017, 8, 2149. [Google Scholar] [CrossRef]
- Al-Nuaimi, D.A.; Rutsche, D.; Abukar, A.; Hiebert, P.; Zanetti, D.; Cesarovic, N.; Falk, V.; Werner, S.; Mazza, E.; Giampietro, C. Hydrostatic pressure drives sprouting angiogenesis via adherens junction remodelling and YAP signalling. Commun. Biol. 2024, 7, 940. [Google Scholar] [CrossRef] [PubMed]
- Barrasa-Ramos, S.; Dessalles, C.A.; Hautefeuille, M.; Barakat, A.I. Mechanical regulation of the early stages of angiogenesis. J. R. Soc. Interface 2022, 19, 20220360. [Google Scholar] [CrossRef]
- Salwen, S.A.; Szarowski, D.H.; Turner, J.N.; Bizios, R. Three-dimensional changes of the cytoskeleton of vascular endothelial cells exposed to sustained hydrostatic pressure. Med. Biol. Eng. Comput. 1998, 36, 520–527. [Google Scholar] [CrossRef]
- Yoshino, D.; Sato, K.; Sato, M. Endothelial Cell Response Under Hydrostatic Pressure Condition Mimicking Pressure Therapy. Cell. Mol. Bioeng. 2015, 8, 296–303. [Google Scholar] [CrossRef]
- Prystopiuk, V.; Fels, B.; Simon, C.S.; Liashkovich, I.; Pasrednik, D.; Kronlage, C.; Wedlich-Soldner, R.; Oberleithner, H.; Fels, J. A two-phase response of endothelial cells to hydrostatic pressure. J. Cell Sci. 2018, 131, jcs206920. [Google Scholar] [CrossRef]
- Shin, H.Y.; Underwood, R.M.; Fannon, M.W. Fluid pressure is a magnitude-dependent modulator of early endothelial tubulogenic activity: Implications related to a potential tissue-engineering control parameter. Tissue Eng. Part A 2012, 18, 2590–2600. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, D.; Funamoto, K.; Sato, K.; Kenry; Sato, M.; Lim, C.T. Hydrostatic Pressure Promotes Endothelial Tube Formation through Aquaporin 1 and Ras-ERK Signaling. Commun. Biol. 2020, 3, 152. [Google Scholar] [CrossRef]
- Juste-Lanas, Y.; Hervas-Raluy, S.; Garcia-Aznar, J.M.; Gonzalez-Loyola, A. Fluid flow to mimic organ function in 3D in vitro models. APL Bioeng. 2023, 7, 031501. [Google Scholar] [CrossRef]
- Schwartz, E.A.; Bizios, R.; Medow, M.S.; Gerritsen, M.E. Exposure of human vascular endothelial cells to sustained hydrostatic pressure stimulates proliferation. Involvement of the αV integrins. Circ. Res. 1999, 84, 315–322. [Google Scholar] [CrossRef]
- Nakajima, H.; Mochizuki, N. Flow pattern-dependent endothelial cell responses through transcriptional regulation. Cell Cycle 2017, 16, 1893–1901. [Google Scholar] [CrossRef]
- Ehlers, H.; Olivier, T.; Trietsch, S.J.; Vulto, P.; Burton, T.P.; van den Broek, L.J. Microfluidic artery-on-a-chip model with unidirectional gravity-driven flow for high-throughput applications. Lab Chip 2025, 25, 2376–2389. [Google Scholar] [CrossRef]
- Sudo, R.; Chung, S.; Zervantonakis, I.K.; Vickerman, V.; Toshimitsu, Y.; Griffith, L.G.; Kamm, R.D. Transport-mediated angiogenesis in 3D epithelial coculture. FASEB J. 2009, 23, 2155–2164. [Google Scholar] [CrossRef]
- Rapp, J.; Ness, J.; Wolf, J.; Hospach, A.; Liang, P.; Hug, M.J.; Agostini, H.; Schlunck, G.; Lange, C.; Bucher, F. 2D and 3D in vitro angiogenesis assays highlight different aspects of angiogenesis. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167028. [Google Scholar] [CrossRef]
- Liu, M.C.; Shih, H.C.; Wu, J.G.; Weng, T.W.; Wu, C.Y.; Lu, J.C.; Tung, Y.C. Electrofluidic pressure sensor embedded microfluidic device: A study of endothelial cells under hydrostatic pressure and shear stress combinations. Lab Chip 2013, 13, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Cheri, M.S.; Shahraki, H.; Sadeghi, J.; Moghaddam, M.S.; Latifi, H. Measurement and control of pressure driven flows in microfluidic devices using an optofluidic flow sensor. Biomicrofluidics 2014, 8, 054123. [Google Scholar] [CrossRef]
- Li, Y.S.; Haga, J.H.; Chien, S. Molecular basis of the effects of shear stress on vascular endothelial cells. J. Biomech. 2005, 38, 1949–1971. [Google Scholar] [CrossRef] [PubMed]
- Baudin, B.; Bruneel, A.; Bosselut, N.; Vaubourdolle, M. A protocol for isolation and culture of human umbilical vein endothelial cells. Nat. Protoc. 2007, 2, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Puca, L.; Bareja, R.; Prandi, D.; Shaw, R.; Benelli, M.; Karthaus, W.R.; Hess, J.; Sigouros, M.; Donoghue, A.; Kossai, M.; et al. Patient derived organoids to model rare prostate cancer phenotypes. Nat. Commun. 2018, 9, 2404. [Google Scholar] [CrossRef]
- Honeywell. Basic Board Mount Pressure Sensors ABP Series—High Accuracy, Compensated/Amplified; Honeywell: Moines, IA, USA, 2021. [Google Scholar]
- Zhou, R.; Brislinger, D.; Fuchs, J.; Lyons, A.; Langthaler, S.; Hauser, C.A.E.; Baumgartner, C. Vascularised organoids: Recent advances and applications in cancer research. Clin. Transl. Med. 2025, 15, e70258. [Google Scholar] [CrossRef]
- Long, Y.; Dan, Y.; Jiang, Y.; Ma, J.; Zhou, T.; Fang, L.; Wang, Z. Colorectal Cancer Cell-Derived Extracellular Vesicles Promote Angiogenesis Through JAK/STAT3/VEGFA Signaling. Biology 2024, 13, 873. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Lee, S.S.; Bae, Y.K. Colorectal cancer cells differentially impact migration and microRNA expression in endothelial cells. Oncol. Lett. 2019, 18, 6361–6370. [Google Scholar] [CrossRef] [PubMed]
- Komeya, M.; Hayashi, K.; Nakamura, H.; Yamanaka, H.; Sanjo, H.; Kojima, K.; Sato, T.; Yao, M.; Kimura, H.; Fujii, T.; et al. Pumpless microfluidic system driven by hydrostatic pressure induces and maintains mouse spermatogenesis in vitro. Sci. Rep. 2017, 7, 15459. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suresh, G.; Pearson, B.E.; Schreiner, R.; Lin, Y.; Rafii, S.; Rabbany, S.Y. Design and Development of a Real-Time Pressure-Driven Monitoring System for In Vitro Microvasculature Formation. Biomimetics 2025, 10, 501. https://doi.org/10.3390/biomimetics10080501
Suresh G, Pearson BE, Schreiner R, Lin Y, Rafii S, Rabbany SY. Design and Development of a Real-Time Pressure-Driven Monitoring System for In Vitro Microvasculature Formation. Biomimetics. 2025; 10(8):501. https://doi.org/10.3390/biomimetics10080501
Chicago/Turabian StyleSuresh, Gayathri, Bradley E. Pearson, Ryan Schreiner, Yang Lin, Shahin Rafii, and Sina Y. Rabbany. 2025. "Design and Development of a Real-Time Pressure-Driven Monitoring System for In Vitro Microvasculature Formation" Biomimetics 10, no. 8: 501. https://doi.org/10.3390/biomimetics10080501
APA StyleSuresh, G., Pearson, B. E., Schreiner, R., Lin, Y., Rafii, S., & Rabbany, S. Y. (2025). Design and Development of a Real-Time Pressure-Driven Monitoring System for In Vitro Microvasculature Formation. Biomimetics, 10(8), 501. https://doi.org/10.3390/biomimetics10080501




