Comparative Evaluation of Color Stability in Bioactive and Conventional Resin Cements Under Thermal Stress Conditions
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | ACTIVA BioACTIVE cement |
| ANOVA | One-way analysis of variance |
| Bis-GMA | Bisphenol A diglycidyl methacrylate |
| BPA | Bisphenol A |
| CIE | Commission International de L’Eclairage |
| GLM | General linear model |
| HEMA | Hydroxyethyl methacrylate |
| ISO | International Organization for Standardization |
| LED | Light-emitting diode |
| MDP | Methacryloyloxydecyl dihydrogen phosphate |
| OMB | 2-Hydroxy-4-methoxybenzophenone |
| PR | Predicta Bioactive Cement |
| RBCs | Resin-based cements |
| SEM | Scanning electron microscopy |
| TEGDMA | Triethyleneglycol dimethacrylate |
| UDMA | Urethane dimethacrylate |
| UV | Ultraviolet |
| WAT | Whiteness acceptability threshold |
| WPT | Whiteness perceptibility threshold |
Appendix A
| N | Mean | SD | SE | 95% Confidence Interval for Mean | Minimum | Maximum | Coefficient of Variation (CV) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||||
| ΔE00-1 (baseline–5000 cycles) | PN | 10 | 3.72 | 0.69 | 0.22 | 3.23 | 4.21 | 2.90 | 4.83 | 18.43% |
| PR | 10 | 3.61 | 1.26 | 0.40 | 2.71 | 4.51 | 2.06 | 5.73 | 34.78% | |
| AC | 10 | 5.81 | 0.90 | 0.28 | 5.16 | 6.45 | 3.63 | 6.83 | 15.50% | |
| ΔE00-2 (baseline–10,000 cycles) | PN | 10 | 4.31 | 0.50 | 0.16 | 3.95 | 4.67 | 3.35 | 4.97 | 11.58% |
| PR | 10 | 3.83 | 0.97 | 0.31 | 3.14 | 4.53 | 2.63 | 5.50 | 25.32% | |
| AC | 10 | 7.31 | 1.05 | 0.33 | 6.55 | 8.06 | 5.22 | 8.70 | 14.43% | |
| ΔE00-3 (baseline–15,000 cycles) | PN | 10 | 4.47 | 0.51 | 0.16 | 4.10 | 4.84 | 3.80 | 5.35 | 11.52% |
| PR | 10 | 3.77 | 0.52 | 0.16 | 3.40 | 4.14 | 3.07 | 4.36 | 13.78% | |
| AC | 10 | 9.41 | 0.92 | 0.29 | 8.75 | 10.06 | 7.69 | 10.74 | 9.75% | |
| ΔWID-1 (baseline–5000 cycles) | PN | 10 | −6.13 | 1.13 | 0.36 | −6.94 | −5.32 | −7.91 | −4.11 | 18.39% |
| PR | 10 | −3.45 | 1.75 | 0.55 | −4.71 | −2.20 | −7.48 | −1.43 | 50.66% | |
| AC | 10 | −6.41 | 1.40 | 0.44 | −7.41 | −5.41 | −8.06 | −3.93 | 21.83% | |
| ΔWID-2 (baseline–10,000 cycles) | PN | 10 | −8.92 | 1.24 | 0.39 | −9.80 | −8.03 | −11.70 | −7.58 | 13.87% |
| PR | 10 | −6.17 | 2.33 | 0.74 | −7.84 | −4.50 | −9.88 | −2.32 | 37.81% | |
| AC | 10 | −9.06 | 1.99 | 0.63 | −10.49 | −7.64 | −12.55 | −5.59 | 21.94% | |
| ΔWID-3 (baseline–15,000 cycles) | PN | 10 | −9.56 | 0.67 | 0.21 | −10.04 | −9.08 | −10.23 | −8.21 | 7.03% |
| PR | 10 | −7.45 | 1.52 | 0.48 | −8.54 | −6.36 | −10.05 | −4.14 | 20.47% | |
| AC | 10 | −10.67 | 1.95 | 0.62 | −12.07 | −9.27 | −13.93 | −7.34 | 18.30% | |
References
- Lepora, N.F.; Verschure, P.; Prescott, T.J. The state of the art in biomimetics. Bioinspir. Biomim. 2013, 8, 013001. [Google Scholar] [CrossRef]
- Zafar, M.S.; Amin, F.; Fareed, M.A.; Ghabbani, H.; Riaz, S.; Khurshid, Z.; Kumar, N. Biomimetic Aspects of Restorative Dentistry Biomaterials. Biomimetics 2020, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Dionysopoulos, D.; Gerasimidou, O. Biomimetic Dentistry: Basic Principles and Protocols. ARC J. Dent. Sci. 2020, 5, 1–3. [Google Scholar]
- Attik, N.; Richert, R.; Garoushi, S. Biomechanics, Bioactive and Biomimetic philosophy in restorative dentistry—Quo vadis? J. Dent. 2024, 148, 105036. [Google Scholar] [CrossRef] [PubMed]
- Mazzitelli, C.; Paolone, G.; Sabbagh, J.; Scotti, N.; Vichi, A. Color Stability of Resin Cements after Water Aging. Polymers 2023, 15, 655. [Google Scholar] [CrossRef]
- Singer, L.; Fouda, A.; Bourauel, C. Biomimetic approaches and materials in restorative and regenerative dentistry: Review article. BMC Oral Health 2023, 23, 105. [Google Scholar] [CrossRef]
- Heboyan, A.; Vardanyan, A.; Karobari, M.I.; Marya, A.; Avagyan, T.; Tebyaniyan, H.; Mustafa, M.; Rokaya, D.; Avetisyan, A. Dental Luting Cements: An Updated Comprehensive Review. Molecules 2023, 28, 1619. [Google Scholar] [CrossRef]
- Al-Saleh, S.; Aboghosh, T.W.; Hazazi, M.S.; Binsaeed, K.A.; Almuhaisen, A.M.; Tulbah, H.I.; Al-Qahtani, A.S.; Shabib, S.; Binhasan, M.; Vohra, F.; et al. Polymer-Based Bioactive Luting Agents for Cementation of All-Ceramic Crowns: An SEM, EDX, Microleakage, Fracture Strength, and Color Stability Study. Polymers 2021, 13, 4227. [Google Scholar] [CrossRef]
- Falacho, R.; Marques, J.; Palma, P.; Roseiro, L.; Caramelo, F.; Ramos, J.; Guerra, F.; Blatz, M. Luting indirect restorations with resin cements versus composite resins: Effects of preheating and ultrasound energy on film thickness. J. Esthet. Restor. Dent. 2021, 34, 641–649. [Google Scholar] [CrossRef]
- Maravić, T.; Mazzitelli, C.; Mancuso, E.; Del Bianco, F.; Josić, U.; Cadenaro, M.; Breschi, L.; Mazzoni, A. Resin composite cements: Current status and a novel classification proposal. J. Esthet. Restor. Dent. 2023, 35, 1085–1097. [Google Scholar] [CrossRef]
- Hernandes, D.K.; Arrais, C.A.; Lima, E.; Cesar, P.F.; Rodrigues, J.A. Influence of resin cement shade on the color and translucency of ceramic veneers. J. Appl. Oral Sci. 2016, 24, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, P.; Pietruska, M.J.; Ladewig, J.; Fischer, C.; Sader, R.; Weigl, P. Effect of cement type, luting protocol, and ceramic abutment material on the shade of cemented titanium-based lithium disilicate crowns and surrounding peri-implant soft tissue: A spectrophotometric analysis. J. Adv. Prosthodont. 2024, 16, 231–243. [Google Scholar] [CrossRef]
- Bresser, R.A.; Gerdolle, D.; van den Heijkant, I.A.; Sluiter-Pouwels, L.M.A.; Cune, M.S.; Gresnigt, M.M.M. Up to 12 years clinical evaluation of 197 partial indirect restorations with deep margin elevation in the posterior region. J. Dent. 2019, 91, 103227. [Google Scholar] [CrossRef]
- Alshabib, A.; AlDosary, K.; Algamaiah, H. A Comprehensive Review of Resin Cements: Bonding Mechanisms and Polymerization Reactions. Saudi Dent. J. 2023, 36, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Santi, M.R.; Lins, R.B.E.; Sahadi, B.O.; Denucci, G.C.; Soffner, G.; Martins, L.R.M. Influence of inorganic composition and filler particle morphology on the mechanical properties of self-adhesive resin cements. Restor. Dent. Endod. 2022, 47, e32. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.; Martins, F.; Reis, J.A.; Maurício, P.D.; Ramírez-Fernández, M.P. Color Assessment of Feldspathic Ceramic with Two Different Thicknesses, Using Multiple Polymeric Cements. Polymers 2023, 15, 397. [Google Scholar] [CrossRef]
- Mancuso, E.; Mazzitelli, C.; Maravic, T.; Pitta, J.; Mengozzi, A.; Comba, A.; Baldi, A.; Scotti, N.; Mazzoni, A.; Fehmer, V.; et al. The influence of finishing lines and margin location on enamel and dentin removal for indirect partial restorations: A micro-CT quantitative evaluation. J. Dent. 2022, 127, 104334. [Google Scholar] [CrossRef]
- Hasanain, F.A. Effect of Ageing, Staining and Polishing on the Colour Stability of a Single, a Group Shade and Nano Fill Dental Composite: An In-vitro Study. J. Clin. Diagn. Res. 2022, 16, ZC26–ZC30. [Google Scholar] [CrossRef]
- Sokolowski, G.; Szczesio, A.; Bociong, K.; Kaluzinska, K.; Lapinska, B.; Sokolowski, J.; Domarecka, M.; Lukomska-Szymanska, M. Dental Resin Cements-The Influence of Water Sorption on Contraction Stress Changes and Hydroscopic Expansion. Material 2018, 11, 973. [Google Scholar] [CrossRef]
- do Nascimento Santos, J.V.; Magalhães, G.d.A.P.; Costa Leite, J.V.; Pacheco, R.R.; Puppin-Rontani, R.M.; Ferracane, J.L.; Lima, R.B.W. From names to concepts: Unraveling bioactivity in restorative dental materials. J. Am. Dent. Assoc. 2025, 156, 355–373.e2. [Google Scholar] [CrossRef]
- Schmalz, G.; Hickel, R.; Price, R.B.; Platt, J.A. Bioactivity of Dental Restorative Materials: FDI Policy Statement. Int. Dent. J. 2023, 73, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Giannini, M.; Sauro, S. “Bioactivity” in Restorative Dentistry: Standing for the Use of Innovative Materials to Improve the Longevity of Restorations in Routine Dental Practice. J. Adhes. Dent. 2021, 23, 176–178. [Google Scholar] [PubMed]
- Kunert, M.; Piwonski, I.; Hardan, L.; Bourgi, R.; Sauro, S.; Inchingolo, F.; Lukomska-Szymanska, M. Dentine Remineralisation Induced by “Bioactive” Materials through Mineral Deposition: An In Vitro Study. Nanomaterials 2024, 14, 274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, N.; Weir, M.D.; Reynolds, M.A.; Bai, Y.; Xu, H.H.K. Bioactive Dental Composites and Bonding Agents Having Remineralizing and Antibacterial Characteristics. Dent. Clin. N. Am. 2017, 61, 669–687. [Google Scholar] [CrossRef]
- Vohra, F.; Altwaim, M.; Alshuwaier, A.S.; Alomayri, A.; Al Deeb, M.; AlFawaz, Y.F.; Alrabiah, M.; Al Ahdal, K.; Al Deeb, L.; Abduljabbar, T. Bond integrity and microleakage of dentin-bonded crowns cemented with bioactive cement in comparison to resin cements: In vitro study. J. Appl. Biomater. Funct. Mater. 2020, 18, 2280800020905768. [Google Scholar] [CrossRef]
- Parkell. Advertorial: At Last, an Outstanding Universal Cement That Closes the Gap Against Microleakage! Dent. Prod. Rep. 2022, 56, 63. [Google Scholar]
- Chinadet, W.; Pengpue, P.; Chaijareenont, P. Investigating the impact of surface treatments on tensile bond strength between pediatric prefabricated zirconia crowns and primary maxillary incisors with various types of luting cement: An in vitro study. Eur. Arch. Paediatr. Dent. 2024, 25, 677–684. [Google Scholar] [CrossRef]
- Checchi, V.; Forabosco, E.; Della Casa, G.; Kaleci, S.; Giannetti, L.; Generali, L.; Bellini, P. Color Stability Assessment of Single- and Multi-Shade Composites Following Immersion in Staining Food Substances. Dent. J. 2024, 12, 285. [Google Scholar] [CrossRef]
- Rohym, S.; Tawfeek, H.E.M.; Kamh, R. Effect of coffee on color stability and surface roughness of newly introduced single shade resin composite materials. BMC Oral Health 2023, 23, 236. [Google Scholar] [CrossRef]
- Gomez-Polo, C.; Portillo Munoz, M.; Lorenzo Luengo, M.C.; Vicente, P.; Galindo, P.; Martin Casado, A.M. Comparison of the CIELab and CIEDE2000 color difference formulas. J. Prosthet. Dent. 2016, 115, 65–70. [Google Scholar] [CrossRef]
- Pérez Gómez, M.d.M.; Ghinea, R.I.; Rivas, M.; Yebra, A.; Ionescu, A.; Paravina, R.; Herrera, L. Development of a customized whiteness index for dentistry based on CIELAB color space. Dent. Mater. 2016, 32, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhu, J.; Yu, M.; Jin, C.; Huang, C. Effect of aging and bleaching on the color stability and surface roughness of a recently introduced single-shade composite resin. J. Dent. 2024, 143, 104917. [Google Scholar] [CrossRef] [PubMed]
- ISO 7491:2000; Dental Materials e Determination of Color Stability. ISO: Geneva, Switzerland, 2017; Published (Edition 2, 2000) Reviewed and Confirmed in 2023.
- Pérez, M.M.; Herrera, L.J.; Carrillo, F.; Pecho, O.E.; Dudea, D.; Gasparik, C.; Ghinea, R.; Bona, A.D. Whiteness difference thresholds in dentistry. Dent. Mater. 2019, 35, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Paravina, R.D.; Perez, M.M.; Ghinea, R. Acceptability and perceptibility thresholds in dentistry: A comprehensive review of clinical and research applications. J. Esthet. Restor. Dent. 2019, 31, 103–112. [Google Scholar] [CrossRef]
- Morresi, A.L.; D’Amario, M.; Capogreco, M.; Gatto, R.; Marzo, G.; D’Arcangelo, C.; Monaco, A. Thermal cycling for restorative materials: Does a standardized protocol exist in laboratory testing? A literature review. J. Mech. Behav. Biomed. Mater. 2014, 29, 295–308. [Google Scholar] [CrossRef]
- Müller, J.A.; Rohr, N.; Fischer, J. Evaluation of ISO 4049: Water sorption and water solubility of resin cements. Eur. J. Oral Sci. 2017, 125, 141–150. [Google Scholar] [CrossRef]
- Benkeser, S.M.; Karlin, S.; Rohr, N. Effect of curing mode of resin composite cements on water sorption, color stability, and biaxial flexural strength. Dent. Mater. 2024, 40, 897–906. [Google Scholar] [CrossRef]
- Vichi, A.; Ferrari, M.; Davidson, C.L. Color and opacity variations in three different resin-based composite products after water aging. Dent. Mater. 2004, 20, 530–534. [Google Scholar] [CrossRef]
- Shibuya, K.; Ohara, N.; Ono, S.; Matsuzaki, K.; Yoshiyama, M. Influence of 10-MDP concentration on the adhesion and physical properties of self-adhesive resin cements. Restor. Dent. Endod. 2019, 44, e45. [Google Scholar] [CrossRef]
- Delgado, A.H.S.; Owji, N.; Ashley, P.; Young, A.M. Varying 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP) level improves polymerisation kinetics and flexural strength in self-adhesive, remineralising composites. Dent. Mater. 2021, 37, 1366–1376. [Google Scholar] [CrossRef]
- Andreucci, A.; Baroudi, K.; Freitas, M.; Amaral, M.; Aguiar, F.; Zanatta, R.; Liporoni, P. Color Stability and Degree of Conversion of Light-cured Resin Cements. Open Dent. J. 2023, 17, e187421062305150. [Google Scholar] [CrossRef]
- Pissaia, J.; Guanaes, B.; Kintopp, C.; Correr, G.; Cunha, L.; Gonzaga, C. Color stability of ceramic veneers as a function of resin cement curing mode and shade: 3-year follow-up. PLoS ONE 2019, 14, e0219183. [Google Scholar] [CrossRef] [PubMed]
- Perroni, A.P.; Amaral, C.; Kaizer, M.R.; Moraes, R.R.; Boscato, N. Shade of Resin-Based Luting Agents and Final Color of Porcelain Veneers. J. Esthet. Restor. Dent. 2016, 28, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Bal, L.; Öztürk, C. The effect of UV aging on the color stability and translucency of luting agents cemented to different CAD/CAM materials. BMC Oral Health 2025, 25, 628. [Google Scholar] [CrossRef] [PubMed]
- Radwanski, M.; Zmyslowska-Polakowska, E.; Osica, K.; Krasowski, M.; Sauro, S.; Hardan, L.; Lukomska-Szymanska, M. Mechanical properties of modern restorative “bioactive” dental materials—An in vitro study. Sci. Rep. 2025, 15, 3552. [Google Scholar] [CrossRef]
- Oei, J.D.; Mishriky, M.; Barghi, N.; Rawls, H.R.; Cardenas, H.L.; Aguirre, R.; Whang, K. Development of a low-color, color stable, dual cure dental resin. Dent. Mater. 2013, 29, 405–412. [Google Scholar] [CrossRef]
- Michelsen, V.B.; Moe, G.; Skålevik, R.; Jensen, E.; Lygre, H. Quantification of organic eluates from polymerized resin-based dental restorative materials by use of GC/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 850, 83–91. [Google Scholar] [CrossRef]
- Boonen, I.; De Nys, S.; Vervliet, P.; Covaci, A.; Van Landuyt, K.L.; Duca, R.C.; Godderis, L.; Denison, M.S.; Elskens, M. Assessing the estrogenic activity of chemicals present in resin based dental composites and in leachates of commercially available composites using the ERα-CALUX bioassay. Dent. Mater. 2021, 37, 1834–1844. [Google Scholar] [CrossRef]
- Turhan Bal, B.; Bankoğlu Güngör, M.; Karakoca Nemli, S.; Aydın, C.; Kaşko Arıcı, Y. Effect of ultraviolet protective agents on maxillofacial silicone elastomer, part 1: Color stability after artificial aging. J. Prosthet. Dent. 2023, 129, 513–519. [Google Scholar] [CrossRef]
- Alzahrani, B.; Alshabib, A.; Awliya, W. The Depth of Cure, Sorption and Solubility of Dual-Cured Bulk-Fill Restorative Materials. Materials 2023, 16, 6673. [Google Scholar] [CrossRef]
- Almeida, J.R.; Schmitt, G.U.; Kaizer, M.R.; Boscato, N.; Moraes, R.R. Resin-based luting agents and color stability of bonded ceramic veneers. J. Prosthet. Dent. 2015, 114, 272–277. [Google Scholar] [CrossRef]
- Birant, S.; Gümüştaş, B. The effect of thermal aging on microhardness and SEM/EDS for characterisation bioactive filling materials. BMC Oral Health 2024, 24, 1142. [Google Scholar] [CrossRef]
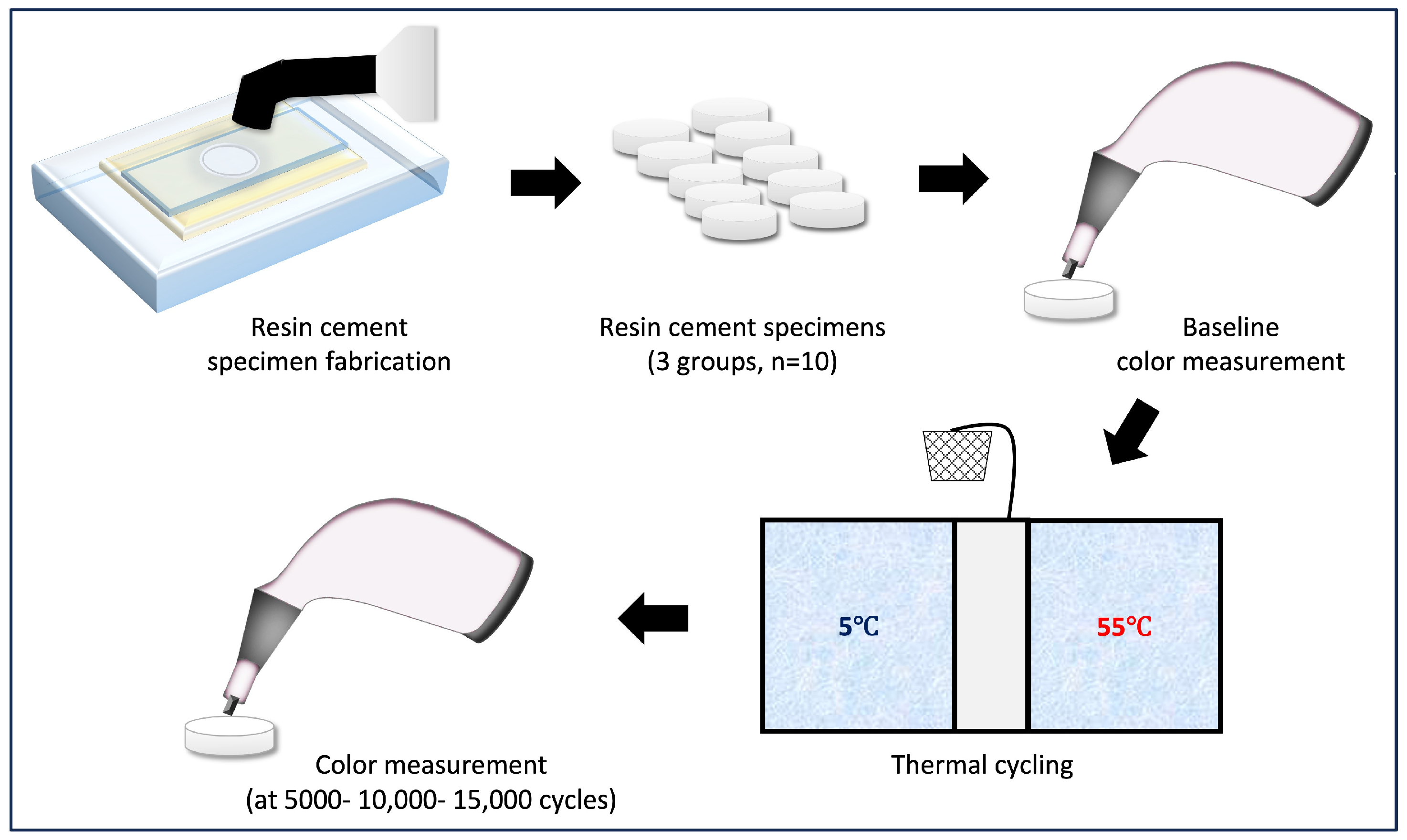

| Resin-Based Cement (Shade) | Abbreviation | Manufacturer (Lot #) | Composition | Application Instructions |
|---|---|---|---|---|
| Panavia SA Universal (A2) | PN | Kuraray Noritake Dental, Tokyo, Japan (#140200) | Paste A: MDP, Bis-GMA, TEGDMA, hydrophobic aromatic dimethacrylate, HEMA, silanated barium glass filler, silanated colloidal silica, dl-camphorquinone, peroxide, catalysts, pigments Paste B: hydrophobic aromatic dimethacrylate, silane coupling agent, silanated barium glass filler, aluminum oxide filler, surface-treated sodium fluoride (less than 1%), dl-camphorquinone, accelerators, pigments | Dispense equal amounts of paste A and B and mix for 10 s. Apply and light cure for 10 s |
| ACTIVA BioACTIVE cement (A2) | AC | Pulpdent, Watertown, MA, USA (#221118) | Diurethane and other methacrylates with modified polyacrylic acid, silica, sodium fluoride | Place cement and light cure for 20 s |
| Predicta Bioactive Cement (A2) | PR | Parkell, Edgewood, NY, USA (#23017) | Base component: glass oxide, Bis-GMA, UDMA, HEMA, TMPTMA, BTHQ, calcium fluoride, photoinitiators Catalyst component: 10-MDP, HEMA, UDMA, TMPTMA, cumene hydroperoxide, photoinitiators | Dispense and light cure for 30 s |
| Measurement | (Mean ± SD) | ||
|---|---|---|---|
| PN | PR | AC | |
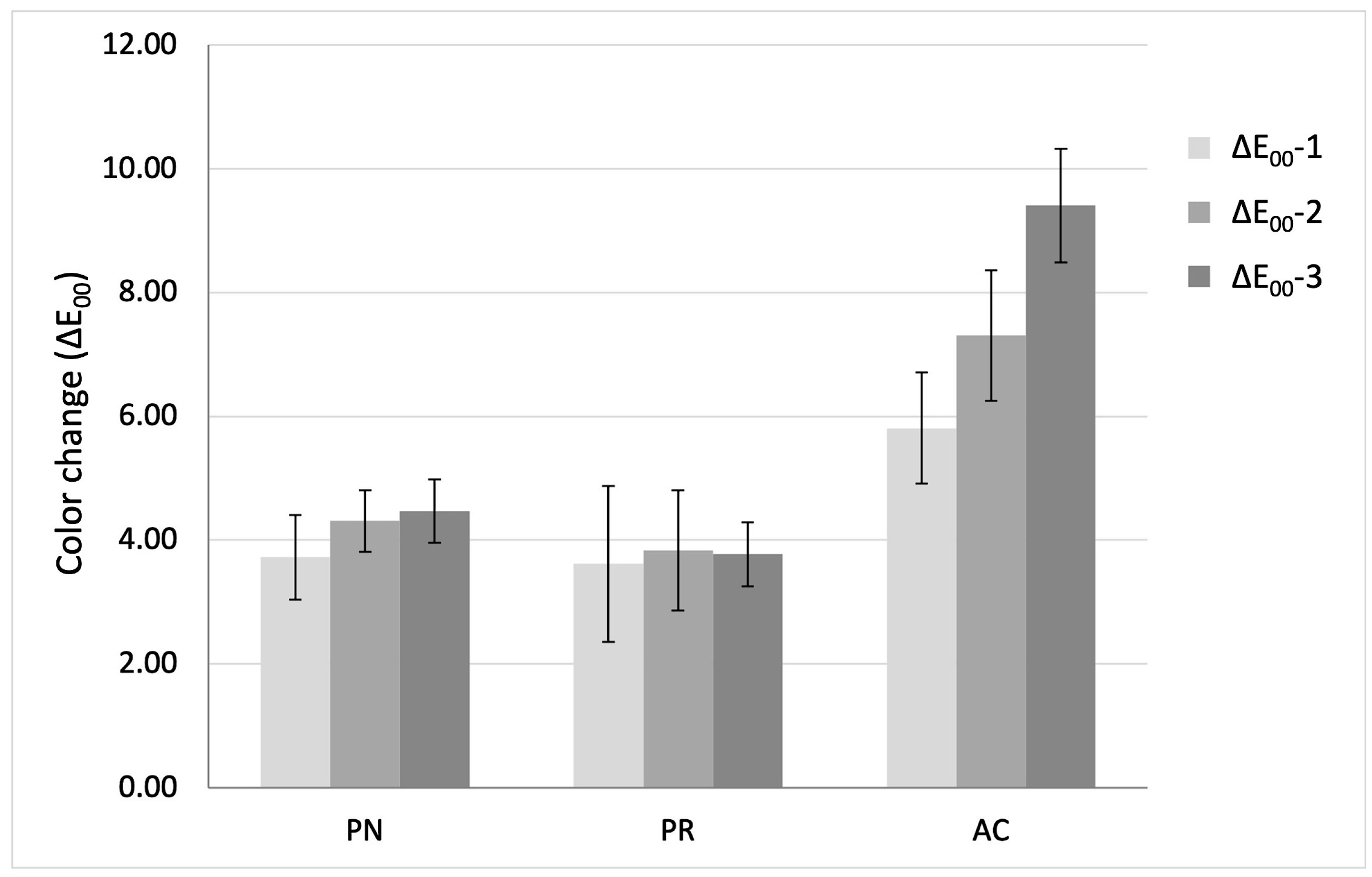
| ΔE00-1 (baseline–5000 cycles) | 3.72 ± 0.69 Bb | 3.61 ± 1.26 Ba | 5.81 ± 0.90 Ac |
| ΔE00-2 (baseline–10,000 cycles) | 4.31 ± 0.50 Ba | 3.83 ± 0.97 Ba | 7.31 ± 1.05 Ab |
| ΔE00-3 (baseline–15,000 cycles) | 4.47 ± 0.51 Ba | 3.77 ± 0.52 Ba | 9.41 ± 0.92 Aa |
| Measurement | Mean ± SD | |||
|---|---|---|---|---|
| PN | PR | AC | ||
| ΔWID-1 (baseline–5000 cycles) | 6.13 ± 0.36 Ab | 3.45 ± 0.55 Bc | 6.41 ± 0.44 Ac | |
| Color parameters | ΔL | 3.1 ± 1.23 | 3.68 ± 2.38 | 3.54 ± 1.27 |
| Δa | 0.39 ± 0.27 | 0.35 ± 0.33 | 4.83 ± 0.68 | |
| Δb | 6.19 ±0.79 | 4.11 ± 0.76 | −2.73 ± 0.59 | |
| ΔWID-2 (baseline–10,000 cycles) | 8.92 ± 0.39 Aa | 6.17 ± 0.74 Bb | 9.06 ± 0.63 Ab | |
| Color parameters | ΔL | 2.86 ± 1.01 | 2.61 ± 2.39 | 3.46 ± 1.32 |
| Δa | 0.74 ± 0.30 | 0.75 ± 0.36 | 6.27 ± 0.89 | |
| Δb | 7.87 ± 0.77 | 5.24 ± 1.04 | −3.40 ± 0.79 | |
| ΔWID-3 (baseline–15,000 cycles) | 9.56 ± 0.21 Aa | 7.45 ± 0.48 Ba | 10.67 ± 0.62 Aa | |
| Color parameters | ΔL | 2.17 ± 1.21 | 1.93 ± 1.27 | 4.04 ± 1.23 |
| Δa | 0.40 ± 0.24 | 0.87 ± 0.34 | 7.86 ± 0.81 | |
| Δb | 8.85 ± 0.74 | 5.83 ± 0.56 | −5.03 ± 0.72 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turkistani, A.; Yeslam, H.E. Comparative Evaluation of Color Stability in Bioactive and Conventional Resin Cements Under Thermal Stress Conditions. Biomimetics 2025, 10, 432. https://doi.org/10.3390/biomimetics10070432
Turkistani A, Yeslam HE. Comparative Evaluation of Color Stability in Bioactive and Conventional Resin Cements Under Thermal Stress Conditions. Biomimetics. 2025; 10(7):432. https://doi.org/10.3390/biomimetics10070432
Chicago/Turabian StyleTurkistani, Alaa, and Hanin E. Yeslam. 2025. "Comparative Evaluation of Color Stability in Bioactive and Conventional Resin Cements Under Thermal Stress Conditions" Biomimetics 10, no. 7: 432. https://doi.org/10.3390/biomimetics10070432
APA StyleTurkistani, A., & Yeslam, H. E. (2025). Comparative Evaluation of Color Stability in Bioactive and Conventional Resin Cements Under Thermal Stress Conditions. Biomimetics, 10(7), 432. https://doi.org/10.3390/biomimetics10070432






