Adhesive Performance of Zirconia and Lithium Disilicate Maryland Cantilever Restorations on Prepared and Non-Prepared Abutment Teeth: An In Vitro Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Group Allocation
- Group 1: Zirconia restorations on non-prepared teeth.
- Group 2: Zirconia restorations on prepared teeth.
- Group 3: Lithium disilicate restorations on non-prepared teeth.
- Group 4: Lithium disilicate restorations on prepared teeth.
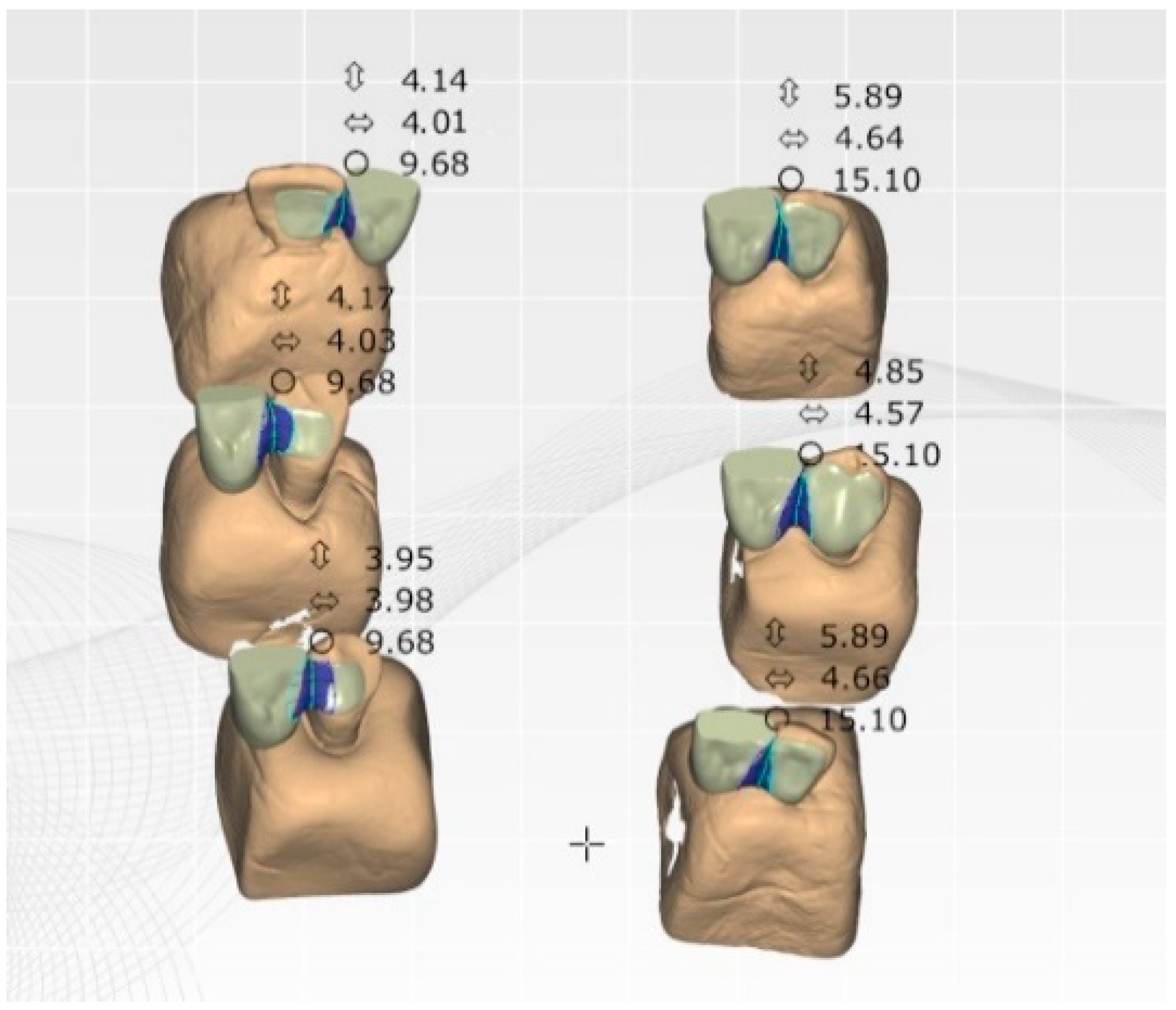
2.2. Restoration Design and Fabrication
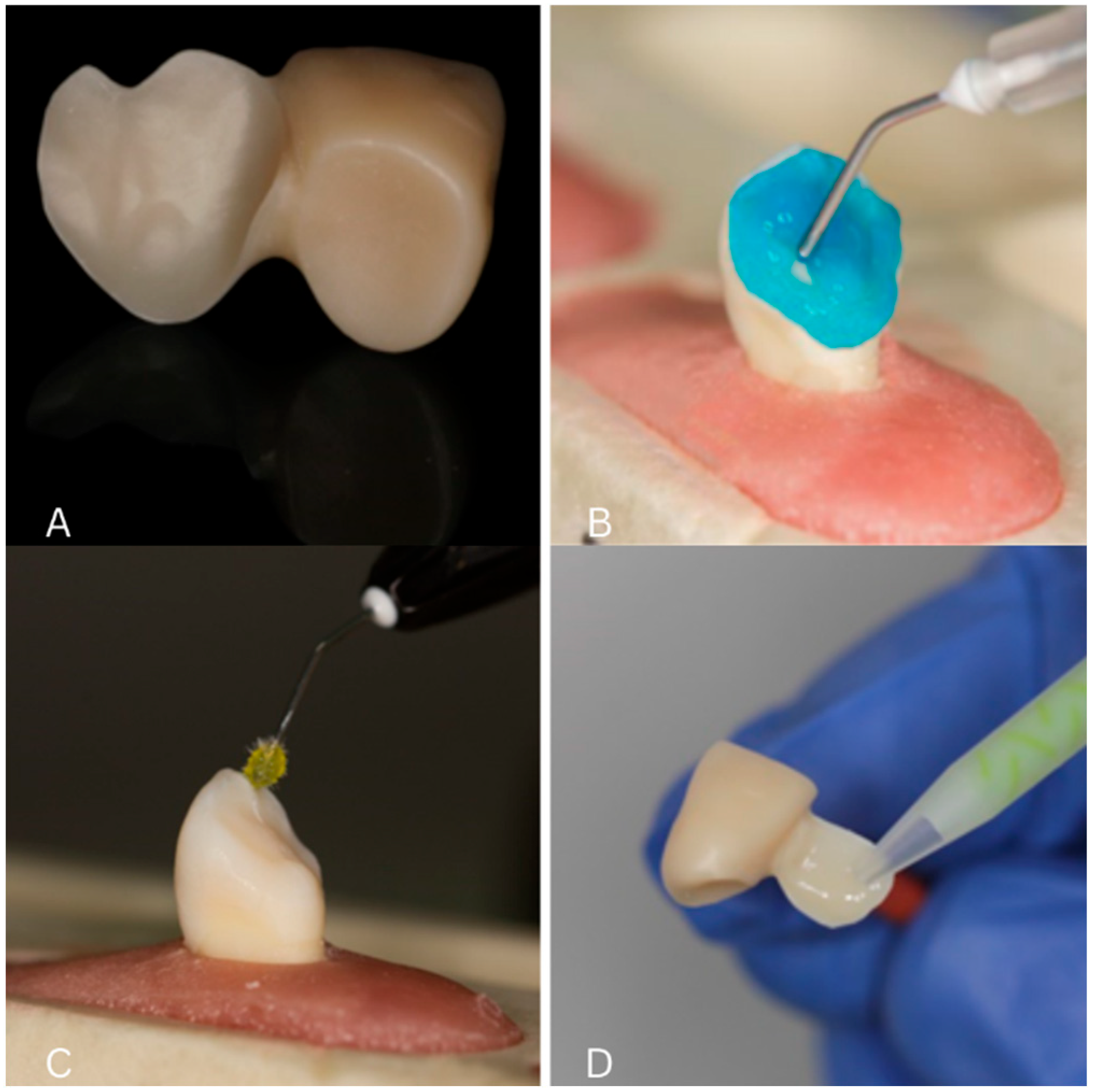
2.3. Surface Conditioning Protocols
2.4. Cementation Procedure
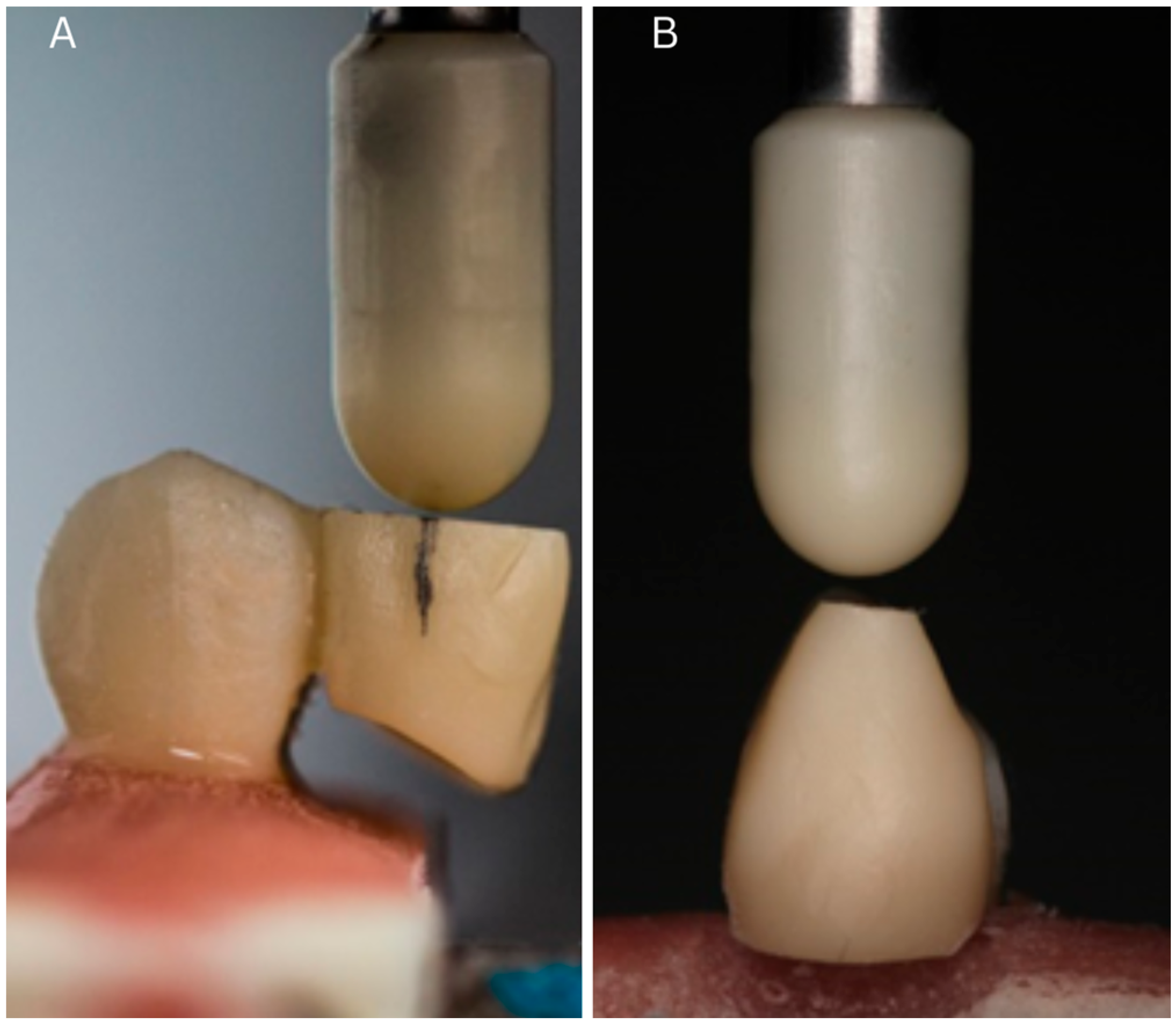
2.5. Mechanical Testing
2.6. Statistical Analysis
3. Results
3.1. Group Comparisons
3.2. Failure Mode
3.3. Summary of Fracture Resistance Values (Table 4)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, R.; Laverty, D.P. The use of all-ceramic resin-bonded bridges in the anterior aesthetic zone. Dent. Update 2017, 44, 230–232, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Sailer, I.; Ioannidis, A.; Zwahlen, M.; Makarov, N.; Pjetursson, B.E. A systematic review of the survival and complication rates of resin-bonded fixed dental prostheses after a mean observation period of at least 5 years. Clin. Oral Implant. Res. 2017, 28, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Yue, L.; Liao, Y.; Liu, W.; Zhang, H.; Li, X.; Wang, H.; Shen, J. The effect of various sandblasting conditions on surface changes of dental zirconia and shear bond strength between zirconia core and indirect composite resin. J. Adv. Prosthodont. 2015, 7, 214–223. [Google Scholar] [CrossRef]
- Valente, F.; Talou, G.; Mazzitelli, C. Effect of 10-MDP-based primer on shear bond strength between zirconia and new experimental resin cement. Materials 2020, 13, 235. [Google Scholar] [CrossRef] [PubMed]
- Scaminaci Russo, D.; Cinelli, F.; Sarti, C.; Giachetti, L. Adhesion to zirconia: A systematic review of current conditioning methods and bonding materials. Dent. J. 2019, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Quigley, N.P.; Loo, D.S.S.; Choy, C.; Ha, W.N. Clinical efficacy of methods for bonding to zirconia: A systematic review. J. Prosthet. Dent. 2021, 125, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Piascik, J.R.; Swift, E.J.; Braswell, K.; Stoner, B.R. Surface fluorination of zirconia: Adhesive bond strength comparison to commercial primers. Dent. Mater. 2012, 28, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Gabbert, O.; Ohlmann, B.; Schmitter, M.; Gilde, H.; Ruef, T.; Rammelsberg, P. Fracture behaviour of zirconia ceramic cantilever fixed dental prostheses in vitro. Acta Odontol. Scand. 2008, 66, 200–206. [Google Scholar] [CrossRef]
- Kern, M.; Passia, N.; Sasse, M.; Yazigi, C. Ten-year outcome of zirconia ceramic cantilever resin-bonded fixed dental prostheses and the influence of the reasons for missing incisors. J. Dent. 2017, 65, 51–55. [Google Scholar] [CrossRef]
- Alshahrani, F.A.; Yilmaz, B.; Seidt, J.D.; McGlumphy, E.A.; Brantley, W.A. A load-to-fracture and strain analysis of monolithic zirconia cantilevered frameworks. J. Prosthet. Dent. 2017, 118, 752–758. [Google Scholar] [CrossRef]
- Dental Direkt GmbH. DD Bio ZWISO [Internet]. Available online: https://www.dentaldirekt.de/en/products/materials/zirconium-dioxide/white-zirconium-dioxide/dd-bio-zwiso (accessed on 3 February 2024).
- Padma, S.; Umesh, S.; Asokan, S.; Srinivas, T. Bite force measurement based on fiber Bragg grating sensor. J. Biomed. Opt. 2017, 22, 107002. [Google Scholar] [CrossRef] [PubMed]
- Takaki, P.; Vieira, M.; Bommarito, S. Maximum bite force analysis in different age groups. Int. Arch. Otorhinolaryngol. 2014, 18, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Yazigi, C.; Kern, M. Clinical evaluation of zirconia cantilevered single-retainer resin-bonded fixed dental prostheses replacing missing canines and posterior teeth. J. Dent. 2022, 116, 103907. [Google Scholar] [CrossRef] [PubMed]
- Romînu, M.; Bratu, D.; Hurst, J. Aluminablasting of human tooth enamel. J. Mater. Sci. Mater. Med. 1997, 8, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Habibzadeh, S.; Khamisi, F.; Mosaddad, S.A.; Fernandes, G.V.O.; Heboyan, A. Full-ceramic resin-bonded fixed dental prostheses: A systematic review. J. Appl. Biomater. Funct. Mater. 2024, 22, 22808000241250118. [Google Scholar] [CrossRef]
- Bömicke, W.; Rathmann, F.; Pilz, M.; Bermejo, J.L.; Waldecker, M.; Ohlmann, B.; Rammelsberg, P.; Zenthöfer, A. Clinical performance of posterior inlay-retained and wing-retained monolithic zirconia resin-bonded fixed partial dentures: Stage one results of a randomized controlled trial. J. Prosthodont. 2021, 30, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Bömicke, W.; Karl, J.; Rammelsberg, P. Minimally invasive prosthetic restoration of posterior tooth loss with resin-bonded, wing-retained, and inlay-retained fixed dental prostheses fabricated from monolithic zirconia: A clinical report of two patients. J. Prosthet. Dent. 2017, 117, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.A.; de Lima, E.A.; Mendonça, L.S.; de Oliveira, J.E.; Rizuto, A.V.; de Araújo Silva Tavares, Á.F.; da Silva, R.B. Can universal adhesive systems bond to zirconia? J. Esthet. Restor. Dent. 2019, 31, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chae, S.Y.; Lee, Y.; Han, G.J.; Cho, B.H. Effects of multipurpose, universal adhesives on resin bonding to zirconia ceramic. Oper. Dent. 2015, 40, 55–62. [Google Scholar] [CrossRef]
- Bonilla, E.; Zhao, Z.; Maslucan, R.; Frimpong, C.; Al Khalifah, S. Influence of core build-up designs on preventing early failure of composite resin core in molars under traction forces: A finite element analysis study. J. Calif. Dent. Assoc. 2024, 52, 2426250. [Google Scholar] [CrossRef]
- Hatch, J.P.; Shinkai, R.S.; Sakai, S.; Rugh, J.; Paunovich, E. Determinants of masticatory performance in dentate adults. Arch. Oral Biol. 2001, 46, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Nakamura, T.; Matsumura, H.; Ban, S.; Kobayashi, T. Current status of zirconia restoration. J. Prosthodont. Res. 2013, 57, 236–261. [Google Scholar] [CrossRef]
- Zhang, Y.; Lawn, B.R. Novel zirconia materials in dentistry. J. Dent. Res. 2018, 97, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Hajaj, T.; Lile, I.E.; Veja, I.; Titihazan, F.; Rominu, M.; Negruțiu, M.L.; Sinescu, C.; Novac, A.C.; Talpos Niculescu, S.; Zaharia, C. Influence of Pontic Length on the Structural Integrity of Zirconia Fixed Partial Dentures (FPDs). J. Funct. Biomater. 2025, 16, 116. [Google Scholar] [CrossRef]
- Torabzadeh, H.; Ghasemi, A.; Dabestani, A.; Razmavar, S. Fracture resistance of teeth restored with direct and indirect composite restorations. J. Dent. 2013, 10, 417–425. [Google Scholar] [PubMed] [PubMed Central]
- Silva, N.R.D.; Araujo, G.M.; Vila-Nova, T.E.L.; Bezerra, M.G.P.G.; Calderon, P.D.S.; Özcan, M.; Souza, R.O.A.E. Which Zirconia Surface-cleaning Strategy Improves Adhesion of Resin Composite Cement after Saliva Contamination? A Systematic Review and Meta-Analysis. J. Adhes. Dent. 2022, 24, 175–186. [Google Scholar] [CrossRef]
- Koutayas, S.O.; Kern, M.; Ferraresso, F.; Strub, J.R. Influence of framework design on fracture strength of mandibular anterior all-ceramic resin-bonded fixed partial dentures. Int. J. Prosthodont. 2002, 15, 223–229. [Google Scholar]
- Malgaj, T.; Papšík, R.; Abram, A.; Kocjan, A.; Jevnikar, P. Bonding Performance of Surface-Treated Zirconia Cantilevered Resin-Bonded Fixed Dental Prostheses: In Vitro Evaluation and Finite Element Analysis. Materials 2023, 16, 2646. [Google Scholar] [CrossRef]
- Bourgi, R.; Kharouf, N.; Cuevas-Suárez, C.E.; Lukomska-Szymanska, M.; Haikel, Y.; Hardan, L. A Literature Review of Adhesive Systems in Dentistry: Key Components and Their Clinical Applications. Appl. Sci. 2024, 14, 8111. [Google Scholar] [CrossRef]
- Zoidis, P.; Papathanasiou, I.; Polyzois, G. Zirconia in adhesive dentistry: A review of bonding techniques and clinical protocols. Eur. J. Esthet. Dent. 2019, 14, 110–126. [Google Scholar]
- Creugers, N.H.; Snoek, P.A.; Van ‘t Hof, M.A.; Käyser, A.F. Clinical performance of resin-bonded bridges: A 5-year prospective study. Part III: Failure characteristics and survival after rebonding. J. Oral Rehabil. 1990, 17, 179–186. [Google Scholar] [CrossRef]
- Panyasuksri, N.; Angkasith, P.; Yavirach, A.; Chaijareenont, P.; Saokaew, S.; Kanchanasurakit, S. Clinical Efficacy of Anterior Ceramic Materials in Resin-Bonded Fixed Dental Prostheses with Different Bridge Designs—A Systematic Review and Meta-Analysis. Prosthesis 2025, 7, 41. [Google Scholar] [CrossRef]
- da Silva, E.M.; Miragaya, L.; Sabrosa, C.E.; Maia, L.C. Stability of the bond between two resin cements and an yttria-stabilized zirconia ceramic after six months of aging in water. J. Prosthet. Dent. 2014, 112, 568–575. [Google Scholar] [CrossRef]
- Inokoshi, M.; De Munck, J.; Minakuchi, S.; Van Meerbeek, B. Meta-analysis of bonding effectiveness to zirconia ceramics. J. Dent. Res. 2014, 93, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Olariu, I.; Marian, D.; Veja (Ilyes), I.; Flueras, R.; Popovici, R.A.; Pitic (Cot), D.E.; Stana, H.A.; Vaida, L.L.; Lile, I.E. Exploring Dentists’ Preferences in Selecting Adhesive Systems: A Survey Analysis. Appl. Sci. 2024, 14, 10119. [Google Scholar] [CrossRef]
- Hajaj, T.; Marian, D.; Zaharia, C.; Niculescu, S.T.; Negru, R.M.; Titihazan, F.; Rominu, M.; Sinescu, C.; Novac, A.C.; Dobrota, G.; et al. Fracture Resistance of CAD/CAM-Fabricated Zirconia and Lithium Disilicate Crowns with Different Margin Designs: Implications for Digital Dentistry. J. Funct. Biomater. 2025, 16, 205. [Google Scholar] [CrossRef]
- Gavrilovici, A.M.; Anitas, E.M.; Chirigiu, L.; Bica, I.; Negrutiu, M.L. Magnetodielectric Effects in Magnetorheological Elastomers Based on Polymer Fabric, Silicone Rubber, and Magnetorheological Suspension. Adv. Polym. Technol. 2019, 2019, 1983547. [Google Scholar] [CrossRef]
- Sinescu, C.; Bradu, A.; Duma, V.-F.; Topala, F.; Negrutiu, M.; Podoleanu, A.G. Effects of Temperature Variations during Sintering of Metal Ceramic Tooth Prostheses Investigated Non-Destructively with Optical Coherence Tomography. Appl. Sci. 2017, 7, 552. [Google Scholar] [CrossRef]
- Zaharia, C.; Duma, V.-F.; Sinescu, C.; Socoliuc, V.; Craciunescu, I.; Turcu, R.P.; Marin, C.N.; Tudor, A.; Rominu, M.; Negrutiu, M.-L. Dental Adhesive Interfaces Reinforced with Magnetic Nanoparticles: Evaluation and Modeling with Micro-CT versus Optical Microscopy. Materials 2020, 13, 3908. [Google Scholar] [CrossRef]

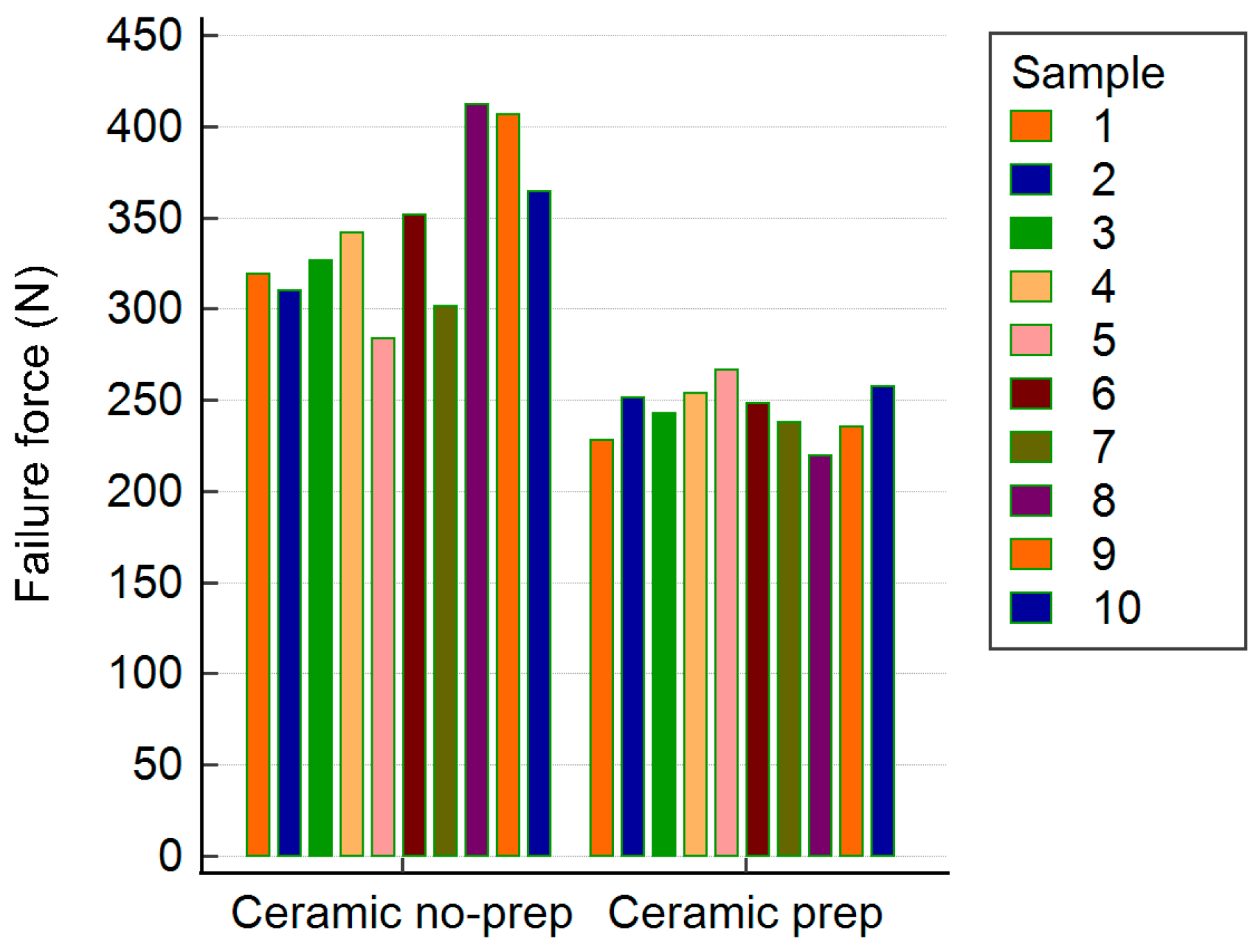

| Zirconia Samples | Max Fracture Resistance (N) Non-Prepared Teeth | Max Fracture Resistance (N) Prepared Teeth |
|---|---|---|
| 1 | 404.86 | 260.81 |
| 2 | 294.71 | 227.01 |
| 3 | 344.3 | 190.72 |
| 4 | 378.03 | 230.03 |
| 5 | 327.84 | 248.58 |
| 6 | 243.37 | 220.33 |
| 7 | 418.42 | 243.3 |
| 8 | 447 | 207.45 |
| 9 | 255.17 | 237.84 |
| 10 | 298.16 | 195.81 |
| Mean Values | 341.19 | 226.19 |
| Median | 336.07 | 228.52 |
| SD | 69.71 | 22.88 |
| Lithium Disilicate Restorations | Max Fracture Resistance (N) Non-Prepared Teeth | Max Fracture Resistance (N) Prepared Teeth |
|---|---|---|
| 1 | 319.41 | 227.94 |
| 2 | 309.74 | 251.08 |
| 3 | 326.32 | 242.59 |
| 4 | 342.02 | 253.62 |
| 5 | 283.41 | 266.44 |
| 6 | 351.57 | 248.35 |
| 7 | 301.55 | 237.69 |
| 8 | 412.28 | 219.45 |
| 9 | 406.55 | 235.14 |
| 10 | 364.29 | 257.11 |
| Mean Values | 341.71 | 243.94 |
| Median | 334.17 | 245.47 |
| SD | 42.89 | 14.21 |
| Zirconia Restorations | Lithium Disilicate Restorations | |
|---|---|---|
| Mean | 283.69 | 292.83 |
| SD | 77.65 | 59.02 |
| Minimum | 190.72 | 219.45 |
| Maximum | 447 | 412.28 |
| 95% CI | 247.34–320.03 | 265.21–320.45 |
| Group | Material | Preparation | Mean ± SD (N) | Min–Max (N) |
|---|---|---|---|---|
| Group 1—Zirconia, Non-prepared | Zirconia | No | 341.19 ± 69.71 | 243.37–447.00 |
| Group 2—Zirconia, Prepared | Zirconia | Yes | 226.19 ± 22.88 | 190.72–260.81 |
| Group 3—Lithium Disilicate, Non-prep | Lithium Disilicate | No | 341.71 ± 42.89 | 283.41–412.28 |
| Group 4—Lithium Disilicate, Prepared | Lithium Disilicate | Yes | 243.94 ± 14.21 | 219.45–266.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajaj, T.; Lile, I.E.; Negru, R.M.; Niculescu, S.T.; Stuparu, S.; Rominu, M.; Sinescu, C.; Albu, P.; Titihazan, F.; Veja, I. Adhesive Performance of Zirconia and Lithium Disilicate Maryland Cantilever Restorations on Prepared and Non-Prepared Abutment Teeth: An In Vitro Comparative Study. Biomimetics 2025, 10, 413. https://doi.org/10.3390/biomimetics10070413
Hajaj T, Lile IE, Negru RM, Niculescu ST, Stuparu S, Rominu M, Sinescu C, Albu P, Titihazan F, Veja I. Adhesive Performance of Zirconia and Lithium Disilicate Maryland Cantilever Restorations on Prepared and Non-Prepared Abutment Teeth: An In Vitro Comparative Study. Biomimetics. 2025; 10(7):413. https://doi.org/10.3390/biomimetics10070413
Chicago/Turabian StyleHajaj, Tareq, Ioana Elena Lile, Radu Marcel Negru, Serban Talpos Niculescu, Sami Stuparu, Mihai Rominu, Cosmin Sinescu, Paul Albu, Florina Titihazan, and Ioana Veja. 2025. "Adhesive Performance of Zirconia and Lithium Disilicate Maryland Cantilever Restorations on Prepared and Non-Prepared Abutment Teeth: An In Vitro Comparative Study" Biomimetics 10, no. 7: 413. https://doi.org/10.3390/biomimetics10070413
APA StyleHajaj, T., Lile, I. E., Negru, R. M., Niculescu, S. T., Stuparu, S., Rominu, M., Sinescu, C., Albu, P., Titihazan, F., & Veja, I. (2025). Adhesive Performance of Zirconia and Lithium Disilicate Maryland Cantilever Restorations on Prepared and Non-Prepared Abutment Teeth: An In Vitro Comparative Study. Biomimetics, 10(7), 413. https://doi.org/10.3390/biomimetics10070413









