Cellulose-Based Nanofibers in Wound Dressing
Abstract
1. Introduction
2. Chemical Structure of Cellulose
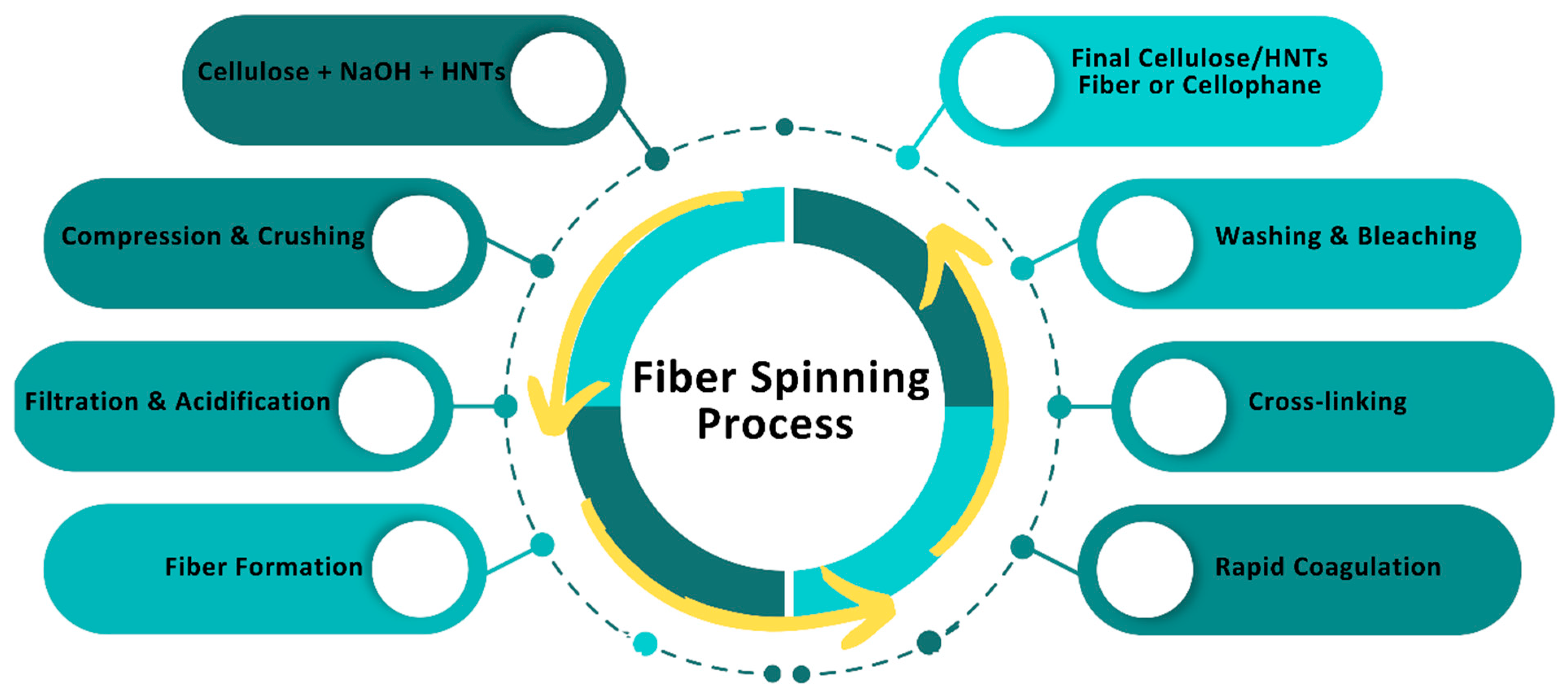
3. Preparation of Cellulose
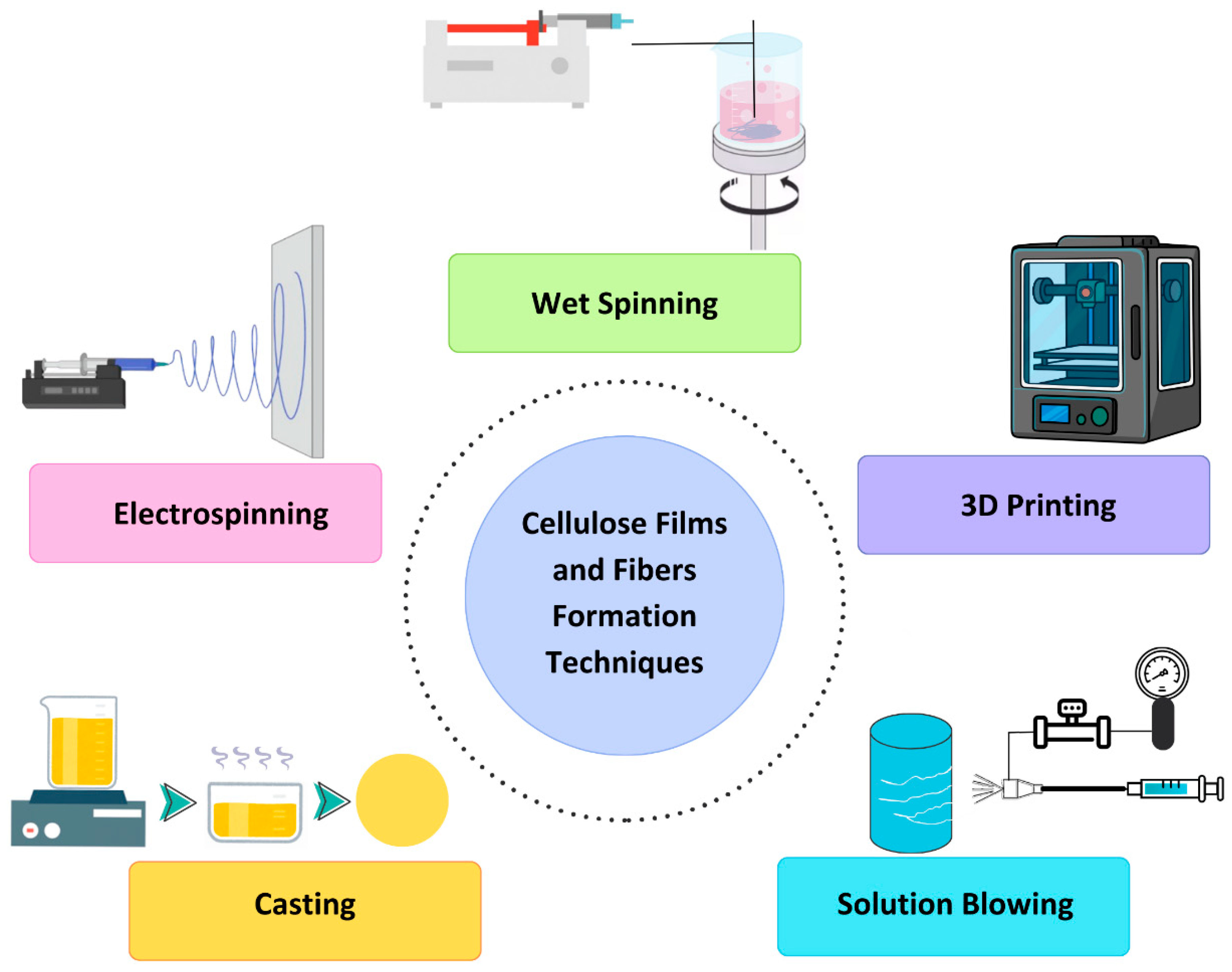
4. Formation of Films and Fibers from Cellulose
- Electrospinning: Electrospinning involves applying a high voltage to a polymer solution or melt, resulting in the formation of ultrafine fibers through the electrostatic repulsion of charged droplets. This method enables precise control over fiber morphology, including diameter and porosity, making it suitable for applications such as tissue engineering scaffolds, filtration membranes, and drug-delivery systems. However, solvent toxicity and limited scalability hinder its widespread adoption for large-scale production [28,29].
- Casting: Casting, also known as solution casting, is a widely used technique for producing cellulose films by casting solutions onto substrates and allowing them to dry. This method offers simplicity, scalability, and control over film thickness and properties. Nevertheless, challenges related to solvent recycling, film uniformity, and limited morphological control persist, influencing its applicability in various fields such as packaging, biomedical implants, and barrier coatings [30,31].
- Solution Blowing: Solution blowing entails extruding a cellulose solution through a spinneret while simultaneously subjecting it to a high-velocity gas stream, leading to fiber formation. This technique offers high production rates and control over fiber morphology, making it suitable for applications requiring tailored properties, such as filtration membranes and tissue engineering scaffolds [32,33].
- Phase Separation: Phase separation techniques use controlled phase separation of cellulose solutions to induce film or fiber formation. By manipulating parameters such as solvent composition and temperature, precise control over film morphology and porosity can be achieved. This method finds applications in membrane filtration, tissue engineering, and controlled release systems [34].
- 3D Printing: 3D printing involves layer-by-layer deposition of cellulose-based inks to create complex three-dimensional structures [35]. This additive manufacturing technique offers customizable designs and precise control over architecture and composition, making it ideal for fabricating custom-designed scaffolds for tissue engineering and regenerative medicine applications [36].
- Wet Spinning: Wet spinning utilizes coagulation baths to solidify cellulose solutions or dispersions extruded through spinnerets, forming fiber. This technique offers scalability and control over fiber properties such as diameter, mechanical strength, and surface chemistry, making it suitable for applications in textiles, composites, and biomedical devices [2].
5. Surface Functionalization of Cellulose Membranes
- Chemical Modification
- II.
- Biomolecule Immobilization
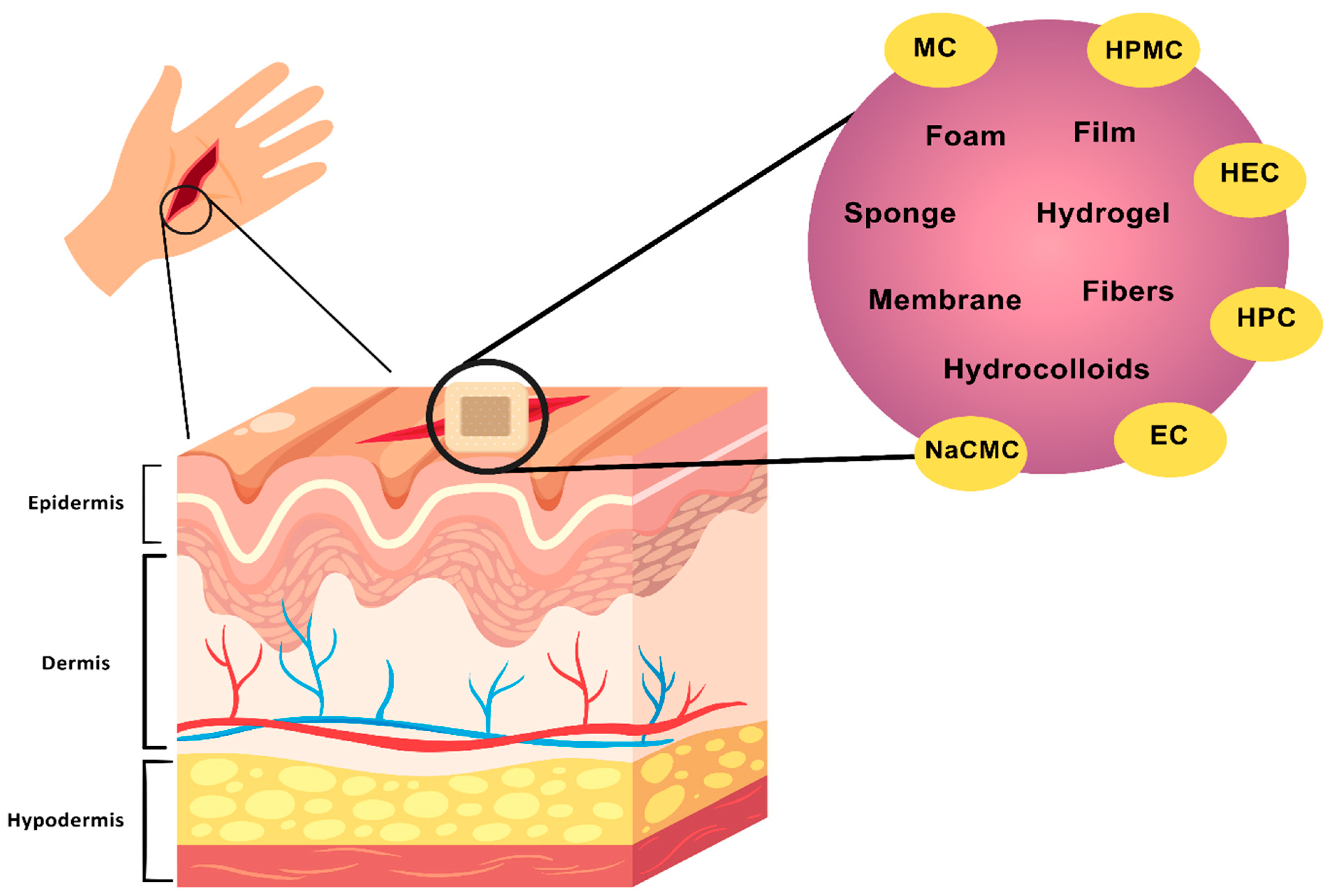
6. Cellulose Membranes in Wound Healing
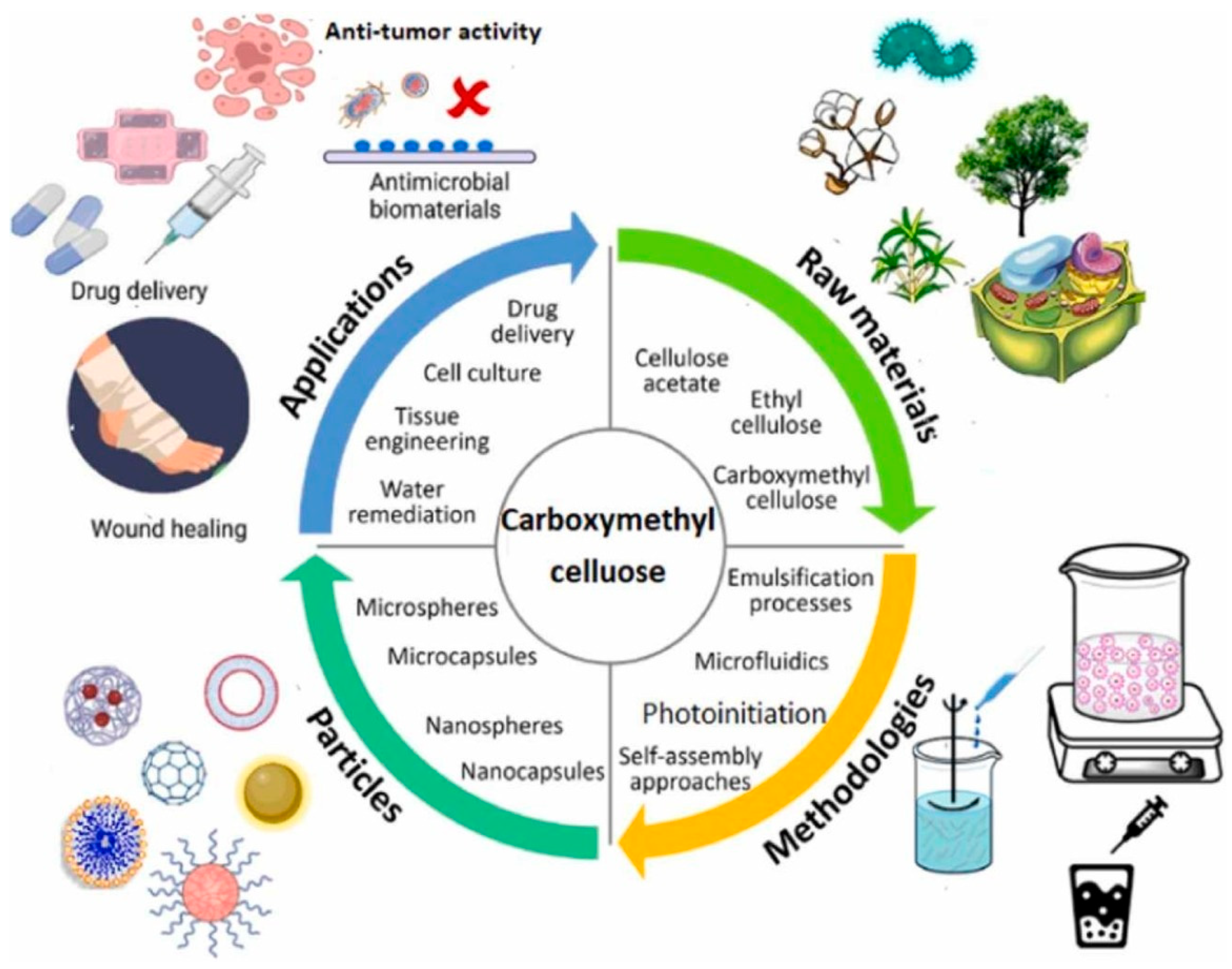
7. Cellulose Derivatives
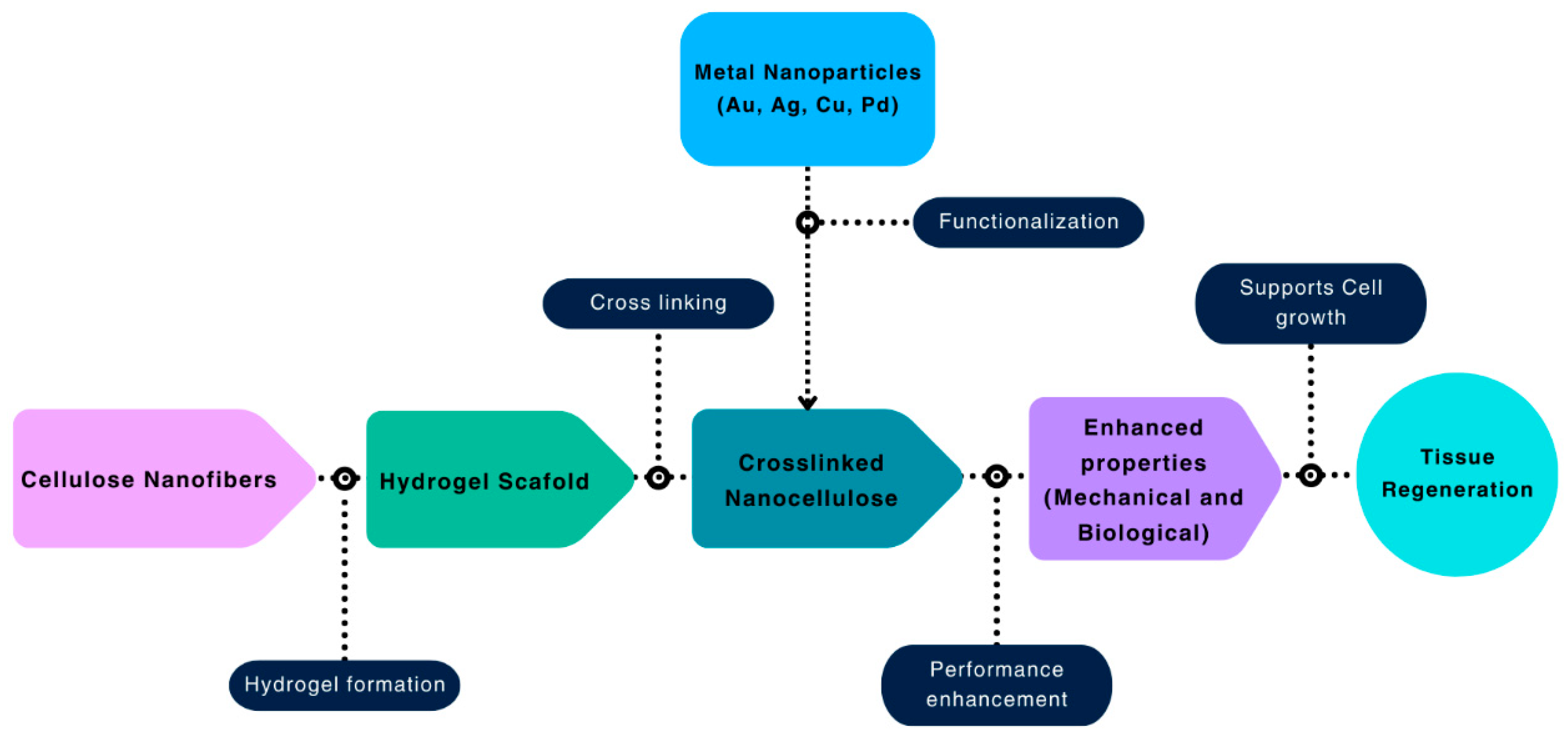
8. Nanoparticle Additions for Added Functionalities
9. Membranes Based on Cellulose Derivatives
10. Applications in Wound Healing
11. Applications in Drug Delivery
12. Applications in Tissue Engineering
13. Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elisseeff, J.; Puleo, C.; Yang, F.; Sharma, B. Advances in skeletal tissue engineering with hydrogels. Orthod. Craniofac. Res. 2005, 8, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, S.; Isogai, A.; Iwata, T. Structure and Mechanical Properties of Wet-Spun Fibers Made from Natural Cellulose Nanofibers. Biomacromolecules 2011, 12, 831–836. [Google Scholar] [CrossRef]
- Dutta, S.D.; Patel, D.K.; Lim, K.T. Functional cellulose-based hydrogels as extracellular matrices for tissue engineering. J. Biol. Eng. 2019, 13, 55. [Google Scholar] [CrossRef]
- Nasri-Nasrabadi, B.; Mehrasa, M.; Rafienia, M.; Bonakdar, S.; Behzad, T.; Gavanji, S. Porous starch/cellulose nanofibers composite prepared by salt leaching technique for tissue engineering. Carbohydr. Polym. 2014, 108, 232–238. [Google Scholar] [CrossRef]
- Dodda, J.M.; Azar, M.G.; Bělský, P.; Šlouf, M.; Brož, A.; Bačáková, L.; Remiš, T. Biocompatible hydrogels based on chitosan, cellulose/starch, PVA and PEDOT:PSS with high flexibility and high mechanical strength. Cellulose 2022, 29, 6697–6717. [Google Scholar] [CrossRef]
- Alakija, F. Silicon Nitride and Metalized Halloysite Nanotube for Enhanced Bacteriostatic Activity, Wound Healing, and Tissue Regeneration. Ph.D. Dissertation, Louisiana Tech University, Ruston, LA, USA, 2023. [Google Scholar]
- Peng, W.; Yan, Y.; Zhang, D.; Zhou, Y.; Na, D.; Xiao, C.; Zhang, J. Preparation of thermal stable supported metal (Cu, Au, Pd) nanoparticles via cross-linking cellulose gel confinement strategy. Colloids Surf. A Coll. Surf. A 2021, 624, 126809. [Google Scholar] [CrossRef]
- Ma, N.; Cheung, D.Y.; Butcher, J.T. Incorporating nanocrystalline cellulose into a multifunctional hydrogel for heart valve tissue engineering applications. J. Biomed. Mater. Res. A 2022, 110, 76–91. [Google Scholar] [CrossRef]
- Maharjan, B.; Park, J.; Kaliannagounder, V.K.; Awasthi, G.P.; Joshi, M.K.; Park, C.H.; Kim, C.S. Regenerated cellulose nanofiber reinforced chitosan hydrogel scaffolds for bone tissue engineering. Carbohydr. Polym. 2021, 251, 117023. [Google Scholar] [CrossRef]
- Jing, W.; Chunxi, Y.; Yizao, W.; Honglin, L.; Fang, H.; Kerong, D.; Yuan, H. Laser Patterning of Bacterial Cellulose Hydrogel and its Modification With Gelatin and Hydroxyapatite for Bone Tissue Engineering. Soft Mater. 2013, 11, 173–180. [Google Scholar] [CrossRef]
- Burger, D.; Beaumont, M.; Rosenau, T.; Tamada, Y. Porous silk fibroin/cellulose hydrogels for bone tissue engineering via a novel combined process based on sequential regeneration and porogen leaching. Molecules 2020, 25, 5097. [Google Scholar] [CrossRef]
- Pang, M.; Huang, Y.; Meng, F.; Zhuang, Y.; Liu, H.; Du, M.; Cai, Y. Application of bacterial cellulose in skin and bone tissue engineering. Eur. Polym. J. 2020, 122, 109365. [Google Scholar] [CrossRef]
- Phatchayawat, P.P.; Khamkeaw, A.; Yodmuang, S.; Phisalaphong, M. 3D bacterial cellulose-chitosan-alginate-gelatin hydrogel scaffold for cartilage tissue engineering. Biochem. Eng. J. 2022, 184, 108476. [Google Scholar] [CrossRef]
- Yu, X.; Li, X.; Kan, L.; Pan, P.; Wang, X.; Liu, W.; Zhang, J. Double network microcrystalline cellulose hydrogels with high mechanical strength and biocompatibility for cartilage tissue engineering scaffold. Int. J. Biol. Macromol. 2023, 238, 124113. [Google Scholar] [CrossRef]
- Boyer, C.; Figueiredo, L.; Pace, R.; Lesoeur, J.; Rouillon, T.; Le Visage, C.; Rethore, G. Laponite nanoparticle-associated silated hydroxypropylmethyl cellulose as an injectable reinforced interpenetrating network hydrogel for cartilage tissue engineering. Acta Biomater. 2018, 65, 112–122. [Google Scholar] [CrossRef]
- Doench, I.; Ahn Tran, T.; David, L.; Montembault, A.; Viguier, E.; Gorzelanny, C.; Osorio-Madrazo, A. Cellulose Nanofiber-Reinforced Chitosan Hydrogel Composites for Intervertebral Disc Tissue Repair. Biomimetics 2019, 4, 19. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Malinen, M.M.; Lauren, P.; Lou, Y.R.; Kuisma, S.W.; Kanninen, L.; Yliperttula, M. Nanofibrillar cellulose hydrogel promotes three-dimensional liver cell culture. J. Control. Release 2012, 164, 291–298. [Google Scholar] [CrossRef]
- Bacterial Cellulose—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/bacterial-cellulose (accessed on 20 April 2024).
- Brown, A.J. XLIII.—On an acetic ferment which forms cellulose. J. Chem. Soc. Trans. 1886, 49, 432–439. [Google Scholar] [CrossRef]
- Dugan, J.M.; Gough, J.E.; Eichhorn, S.J. Bacterial cellulose scaffolds and cellulose nanowhiskers for tissue engineering. Nanomedicine 2013, 8, 287–298. [Google Scholar] [CrossRef]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.L.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26, 419–431. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl. Mater. Today 2018, 11, 34–49. [Google Scholar] [CrossRef]
- Keshipour, S.; Maleki, A. Modification of Cellulose. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–54. [Google Scholar]
- Cumpstey, I. Chemical Modification of Polysaccharides. Int. Sch. Res. Not. 2013, 1, 417672. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef]
- Kulichikhin, V.; Makarov, I.; Mironova, M.; Golova, L.; Vinogradov, M.; Shandryuk, G.; Arkharova, N. A role of coagulant in structure formation of fibers and films spun from cellulose solutions. Materials 2020, 13, 3495. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Kramar, A.; Rodríguez, O.I.; González-Gaitano, G.; González-Benito, J. Solution casting of cellulose acetate films: Influence of surface substrate and humidity on wettability, morphology and optical properties. Cellulose 2023, 30, 2037–2052. [Google Scholar] [CrossRef]
- Khare, V.P.; Greenberg, A.R.; Kelley, S.S.; Pilath, H.; Juhn Roh, I.; Tyber, J. Synthesis and characterization of dense and porous cellulose films. J. Appl. Polym. Sci. 2007, 105, 1228–1236. [Google Scholar] [CrossRef]
- Sun, G.; Wang, Y.; Zhang, Y.; Han, W.; Shang, S. Formation Mechanism of Fibrous Web in the Solution Blowing Process. ACS Omega 2022, 7, 20584–20595. [Google Scholar] [CrossRef]
- Medeiros, E.S.; Glenn, G.M.; Klamczynski, A.P.; Orts, W.J.; Mattoso, L.H.C. Solution blow spinning: A new method to produce micro-and nanofibers from polymer solutions. J. Appl. Polym. Sci. 2009, 113, 2322–2330. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Makarova, V.V.; Anokhina, T.S.; Ignatenko, V.Y.; Brantseva, T.V.; Volkov, A.V.; Antonov, S.V. Diffusion and phase separation at the morphology formation of cellulose membranes by regeneration from N-methylmorpholine N-oxide solutions. Cellulose 2018, 25, 2515–2530. [Google Scholar] [CrossRef]
- Firmanda, A.; Syamsu, K.; Sari, Y.W.; Cabral, J.; Pletzer, D.; Mahadik, B.; Fahma, F. 3D printed cellulose based product applications. Mater. Chem. Front. 2022, 6, 254–279. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Yao, Q.; Ji, C.; Liu, J.; Zhu, Q. 3D printing with cellulose materials. Cellulose 2018, 25, 4275–4301. [Google Scholar] [CrossRef]
- Han, C.D.; Segal, L. A study of fiber extrusion in wet spinning. II. Effects of spinning conditions on fiber formation. J. Appl. Polym. Sci. 1970, 14, 2999–3019. [Google Scholar] [CrossRef]
- Bognitzki, M.; Czado, W.; Frese, T.; Schaper, A.; Hellwig, M.; Steinhart, M.; Wendorff, J.H. Nanostructured fibers via electrospinning. Adv. Mater. 2001, 13, 70–72. [Google Scholar] [CrossRef]
- Frenot, A.; Henriksson, M.W.; Walkenström, P. Electrospinning of cellulose-based nanofibers. J. Appl. Polym. Sci. 2007, 103, 1473–1482. [Google Scholar] [CrossRef]
- Huang, C.; Thomas, N.L. Fabrication of porous fibers via electrospinning: Strategies and applications. Polym. Rev. 2020, 60, 595–647. [Google Scholar] [CrossRef]
- Shao, W.; Wu, J.; Liu, H.; Ye, S.; Jiang, L.; Liu, X. Novel bioactive surface functionalization of bacterial cellulose membrane. Carbohydr. Polym. 2017, 178, 270–276. [Google Scholar] [CrossRef]
- Orlando, I.; Basnett, P.; Nigmatullin, R.; Wang, W.; Knowles, J.C.; Roy, I. Chemical modification of bacterial cellulose for the development of an antibacterial wound dressing. Front. Bioeng. Biotechnol. 2020, 8, 557885. [Google Scholar] [CrossRef]
- Bora, U.; Kannan, K.; Nahar, P. A simple method for functionalization of cellulose membrane for covalent immobilization of biomolecules. J. Memb. Sci. 2005, 250, 215–222. [Google Scholar] [CrossRef]
- Bai, L.; Liu, Y.; Ding, A.; Ren, N.; Li, G.; Liang, H. Surface coating of UF membranes to improve antifouling properties: A comparison study between cellulose nanocrystals (CNCs) and cellulose nanofibrils (CNFs). Chemosphere 2019, 217, 76–84. [Google Scholar] [CrossRef]
- Bhanthumnavin, W.; Wanichapichart, P.; Taweepreeda, W.; Sirijarukula, S.; Paosawatyanyong, B. Surface modification of bacterial cellulose membrane by oxygen plasma treatment. Surf. Coat. Technol. 2016, 306, 272–278. [Google Scholar] [CrossRef]
- Teixeira, S.R.Z.; Reis, E.M.D.; Apati, G.P.; Meier, M.M.; Nogueira, A.L.; Garcia, M.C.F.; Porto, L.M. Biosynthesis and functionalization of bacterial cellulose membranes with cerium nitrate and silver nanoparticles. Mater. Res. 2019, 22, e20190054. [Google Scholar] [CrossRef]
- Hon, D.N.S. Chemical modification of cellulose. In Chemical Modification of Lignocellulosic Materials; Routledge: London, UK, 2017; pp. 97–127. [Google Scholar]
- Teixeira, M.A.; Paiva, M.C.; Amorim, M.T.P.; Felgueiras, H.P. Electrospun nanocomposites containing cellulose and its derivatives modified with specialized biomolecules for an enhanced wound healing. Nanomaterials 2020, 10, 557. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ul-Islam, M.; Ikram, M.; Islam, S.U.; Ullah, M.W.; Israr, M.; Jang, J.H.; Yoon, S.; Park, J.K. Preparation and structural characterization of surface modified microporous bacterial cellulose scaffolds: A potential material for skin regeneration applications in vitro and in vivo. Int. J. Biol. Macromol. 2018, 117, 1200–1210. [Google Scholar] [CrossRef]
- Junior, S.; Sarubbo, M.; Costa, S.; Sarubbo, L.A. Bacterial cellulose as a versatile biomaterial for wound dressing application. Molecules 2022, 27, 5580. [Google Scholar] [CrossRef]
- Andriani, D.; Apriyana, A.Y.; Karina, M. The optimization of bacterial cellulose production and its applications: A review. Cellulose 2020, 27, 6747–6766. [Google Scholar] [CrossRef]
- Swingler, S.; Gupta, A.; Gibson, H.; Kowalczuk, M.; Heaselgrave, W.; Radecka, I. Recent advances and applications of bacterial cellulose in biomedicine. Polymers 2021, 13, 412. [Google Scholar] [CrossRef]
- Farooq, U.; Ullah, M.W.; Yang, Q.; Aziz, A.; Xu, J.; Zhou, L.; Wang, S. High-density phage particles immobilization in surface-modified bacterial cellulose for ultra-sensitive and selective electrochemical detection of Staphylococcus aureus. Biosens. Bioelectron 2020, 157, 112163. [Google Scholar] [CrossRef]
- Khalid, A.; Ullah, H.; UI Islam, M.; Wahid, F. Bacterial cellulose–TiO2 nanocomposites promote healing and tissue regeneration in burn mice model. RSC Adv. 2017, 7, 47662–47668. [Google Scholar] [CrossRef]
- Kamalipooya, S.; Fahimirad, S.; Abtahi, H.; Golmohammadi, M.; Satari, M.; Dadashpour, M.; Nasrabadi, D. Diabetic wound healing function of PCL/cellulose acetate nanofiber engineered with chitosan/cerium oxide nanoparticles. Int. J. Pharm. 2024, 653, 123880. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, H.; Yu, F.; Pei, Y.; Luo, X. Superclear, Porous Cellulose Membranes with Chitosan-Coated Nanofibers for Visualized Cutaneous Wound Healing Dressing. ACS Appl. Mater. Interfaces 2020, 12, 24370–24379. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, S.; Huang, J. Natural Cellulose Substance Based Energy Materials. Chem. Asian J. 2021, 16, 378–396. [Google Scholar] [CrossRef]
- Livazovic, S.; Li, Z.; Behzad, A.R.; Peinemann, K.V.; Nunes, S.P. Cellulose multilayer membranes manufacture with ionic liquid. J. Membr. Sci. 2015, 490, 282–293. [Google Scholar] [CrossRef]
- Snell, F.D. Laboratory Production of Viscose. Ind. Eng. Chem. 1925, 17, 197–198. [Google Scholar] [CrossRef]
- Greis, K.; Bethke, K.; Stückrath, J.B.; Ingber, T.T.K.; Valiyaveettil, S.; Rademann, K. One-Pot Synthesis of Xanthate-Functionalized Cellulose for the Detection of Micromolar Copper(II) and Nickel(II) Ions. CLEAN Soil Air Water 2019, 47, 1900179. [Google Scholar] [CrossRef]
- Franz, H. Process for Production of Cuprammonium Cellulose Articles. Available online: https://patents.google.com/patent/US3110546A/en (accessed on 4 March 2024).
- Johnson, D.L. Compounds Dissolved in Cyclic Amine Oxides. Available online: https://patents.google.com/patent/US3447939A/en (accessed on 4 March 2024).
- Fink, H.P.; Weigel, P.; Purz, H.J.; Ganster, J. Structure formation of regenerated cellulose materials from NMMO-solutions. Prog. Polym. Sci. 2001, 26, 1473–1524. [Google Scholar] [CrossRef]
- Kuo, Y.N.; Hong, J. A new method for cellulose membrane fabrication and the determination of its characteristics. J. Colloid Interface Sci. 2005, 285, 232–238. [Google Scholar] [CrossRef]
- AgriKnowledgeシステム. Available online: https://agriknowledge.affrc.go.jp/RN/2030910649 (accessed on 7 March 2024).
- Zhang, H.; Wu, J.; Zhang, J.; He, J. 1-Allyl-3-methylimidazolium Chloride Room Temperature Ionic Liquid: A New and Powerful Nonderivatizing Solvent for Cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Vitz, J.; Erdmenger, T.; Haensch, C.; Schubert, U.S. Extended dissolution studies of cellulose in imidazolium based ionic liquids. Green Chem. 2009, 11, 417–424. [Google Scholar] [CrossRef]
- Cooper, G.K.; Sandberg, K.R.; Hinck, J.F. Trimethylsilyl cellulose as precursor to regenerated cellulose fiber. J. Appl. Polym. Sci. 1981, 26, 3827–3836. [Google Scholar] [CrossRef]
- Ali, O. High Performance Regenerated Cellulose Membranes from Trimethylsilyl Cellulose. Ph.D. Thesis, King Abdullah University of Science and Technology, Thuwal, Saudi Arabia, 2024. [Google Scholar]
- Puspasari, T.; Pradeep, N.; Peinemann, K.V. Crosslinked cellulose thin film composite nanofiltration membranes with zero salt rejection. J. Membr. Sci. 2015, 491, 132–137. [Google Scholar] [CrossRef]
- Lin, W.C.; Lien, C.C.; Yeh, H.J.; Yu, C.M.; Hsu, S. Bacterial cellulose and bacterial cellulose–chitosan membranes for wound dressing applications. Carbohydr. Polym. 2013, 94, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Laçin, N.T. Development of biodegradable antibacterial cellulose-based hydrogel membranes for wound healing. Int. J. Biol. Macromol. 2014, 67, 22–27. [Google Scholar] [CrossRef]
- Fernandes, S.N.; Geng, Y.; Vignolini, S.; Glover, B.J.; Trindade, A.C.; Canejo, J.P.; Godinho, M.H. Structural Color and Iridescence in Transparent Sheared Cellulosic Films. Macromol. Chem. Phys. 2013, 214, 25–32. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Qiao, K.; Zheng, Y.; Guo, S.; Tan, J.; Chen, X.; Li, J.; Wang, J. Hydrophilic nanofiber of bacterial cellulose guided the changes in the micro-structure and mechanical properties of nf-BC/PVA composites hydrogels. Compos. Sci. Technol. 2015, 118, 47–54. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, Y.; Wen, X.; Lin, Q.; Chen, X.; Wu, Z. Silver nanoparticle/bacterial cellulose gel membranes for antibacterial wound dressing: Investigation in vitro and in vivo. Biomed. Mater. 2014, 9, 035005. [Google Scholar] [CrossRef]
- Li, Q.; Lu, F.; Zhou, G.; Yu, K.; Lu, B.; Xiao, Y.; Dai, F.; Wu, D.; Lan, G. Silver Inlaid with Gold Nanoparticle/Chitosan Wound Dressing Enhances Antibacterial Activity and Porosity, and Promotes Wound Healing. Biomacromolecules 2017, 18, 3766–3775. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Duan, W.; Cao, F.; Lv, Q.; Zhang, S.; She, J.; Yang, L.; He, B.; Hou, Y.; et al. Sprayable oxidized cellulose nanofiber hydrogel with rapid hemostatic ability for skin wound healing. Int. J. Biol. Macromol. 2025, 310, 143264. [Google Scholar] [CrossRef]
- Zhang, S.; Gatsi, B.; Yao, X.; Jin, Y.; Amhal, H. Cellulose nanofiber-reinforced antimicrobial and antioxidant multifunctional hydrogel with self-healing, adhesion for enhanced wound healing. Carbohydr. Polym. 2025, 352, 123189. [Google Scholar] [CrossRef]
- Santos, A.E.; Cotta, T.; Santos, J.P.; Camargos, J.S.; Carmo, A.C.; Alcântara, E.G.; Fleck, C.; Copola, A.G.; Nogueira, J.M.; Silva, G.A.; et al. Bioactive cellulose acetate nanofiber loaded with annatto support skeletal muscle cell attachment and proliferation. Front. Bioeng. Biotechnol. 2023, 11, 1116917. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Murugesan, B.; Chinnalagu, D.K.; Cai, Y.; Malliappan, S.P.; Balasekar, P.; Rengasamy, G.; Chinniah, K.; Mahalingam, S. Multifunctional silk fibroin and cellulose acetate composite nanofibers incorporated with palladium and platinum nanoparticles for enhanced wound healing: Comprehensive characterization and in vivo assessment. Colloids Surf. A Physicochem. Eng. Asp. 2024, 684, 133153. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Dey, A.; Hasan, T.; Pati, S.; Edinur, H.A.; Abdul Kari, Z.; Mohd Noor, N.H.; Ray, R.R. Bacterial Cellulose: Production, Characterization, and Application as Antimicrobial Agent. Int. J. Mol. Sci. 2021, 22, 12984. [Google Scholar] [CrossRef]
| ILs/Anion | DiMIM | C2MIM | C3MIM | C4MIM | Ally1MIM |
|---|---|---|---|---|---|
| F− | 2 wt% [66] | ||||
| Cl− | 10–14 wt% [66] | No solution [66] | 20 wt% [64] | 15 wt% [65] | |
| Br− | 1–2 wt% [66] | 1–2 wt% [66] | 2–3 wt% [66] | No solution [66] | |
| I− | No solution [66] | 1–2 wt% [66] | |||
| SCN− | 5–7 wt% [64] | ||||
| TsO− | 1 wt% [66] | ||||
| AcO− | 8 wt% [66] | 12 wt% [66] | |||
| R2PO4− | 10 wt% [66] | 12–14 wt% [66] | No solution [66] |
| Study/Researcher | Cellulose Modification | Drug Delivered | Release Duration | Application |
|---|---|---|---|---|
| [2] | CMC blended with PVA and PEO | Ciprofloxacin hydrochloride (antibiotic) | 10 h | Daily wound dressing changes are recommended |
| [3] | Sodium CMC | Silver nitrate, sulphacetamide sodium, neomycin trisulphate | Best effect with neomycin trisulfate | Antimicrobial wound dressing for mucosal wounds |
| [4] | 2,3-dialdehyde modification | Chloramphenicol (antibacterial) | Sustained release for 24 h | Sustained antibacterial effect for 3 days |
| [5] | Cellulose nanocrystals | Curcumin (antimicrobial) | 98.9% release within 36 h | Potential agent for diabetic wound healing |
| [6] | Silica particles (mesoporous) | Chloramphenicol (antibacterial) | Two-stage release, sustained for 270 h | Long-term antimicrobial dressing (144 h) |
| [7] | BC without modification | Vaccarin (epithelial growth promoter) | Promoted epithelialization and regeneration | Enhanced wound healing via epithelial growth |
| [8] | Cellulose-based thin film | Diclofenac (analgesic) | Controlled release with additional cellulose layers | Prolonged pain relief via cellulose layering |
| [9] | Cellulose nanofibrils with chitosan | Ketorolac tromethamine (analgesic) | Controlled release till 10 h | Transdermal drug-delivery system |
| Scaffold Sample | Cellulose Nanofiber Content (%) | Salt Content (%) | Young’s Modulus (MPa) | Tensile Strength (MPa) |
|---|---|---|---|---|
| S/70 | 0 | 70 | 24 ± 5.5 | 2.6 ± 0.41 |
| SC5/70 | 5 | 70 | 66 ± 3.9 | 3.61 ± 0.32 |
| SC10/70 | 10 | 70 | 82 ± 7.1 | 3.82 ± 0.55 |
| SC15/70 | 15 | 70 | 93 ± 3.6 | 4.03 ± 0.48 |
| S/90 | 0 | 90 | 20 ± 4.2 | 2.48 ± 0.33 |
| SC5/90 | 5 | 90 | 63 ± 2.9 | 3.58 ± 0.74 |
| SC10/90 | 10 | 90 | 81 ± 6.5 | 3.66 ± 0.42 |
| SC15/90 | 15 | 90 | 85 ± 7.5 | 3.97 ± 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masoud, A.R.; Velisdeh, Z.J.; Bappy, M.J.P.; Pandey, G.; Saberian, E.; Mills, D.K. Cellulose-Based Nanofibers in Wound Dressing. Biomimetics 2025, 10, 344. https://doi.org/10.3390/biomimetics10060344
Masoud AR, Velisdeh ZJ, Bappy MJP, Pandey G, Saberian E, Mills DK. Cellulose-Based Nanofibers in Wound Dressing. Biomimetics. 2025; 10(6):344. https://doi.org/10.3390/biomimetics10060344
Chicago/Turabian StyleMasoud, Abdul Razak, Zeinab Jabbari Velisdeh, Mohammad Jabed Perves Bappy, Gaurav Pandey, Elham Saberian, and David K. Mills. 2025. "Cellulose-Based Nanofibers in Wound Dressing" Biomimetics 10, no. 6: 344. https://doi.org/10.3390/biomimetics10060344
APA StyleMasoud, A. R., Velisdeh, Z. J., Bappy, M. J. P., Pandey, G., Saberian, E., & Mills, D. K. (2025). Cellulose-Based Nanofibers in Wound Dressing. Biomimetics, 10(6), 344. https://doi.org/10.3390/biomimetics10060344








