Characterization of Gramicidin A in Triblock and Diblock Polymersomes and Hybrid Vesicles via Continuous Wave Electron Paramagnetic Resonance Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Polymersome Preparation
2.2. Hybrid Vesicle Preparation
2.3. Vesicle Preparation
2.4. Spin-Labeled Lipids
2.5. Transmission Electron Microscopy
2.6. Gramicidin A Preparation
2.7. CW-EPR Measurements
2.8. EPR Spectral Simulations
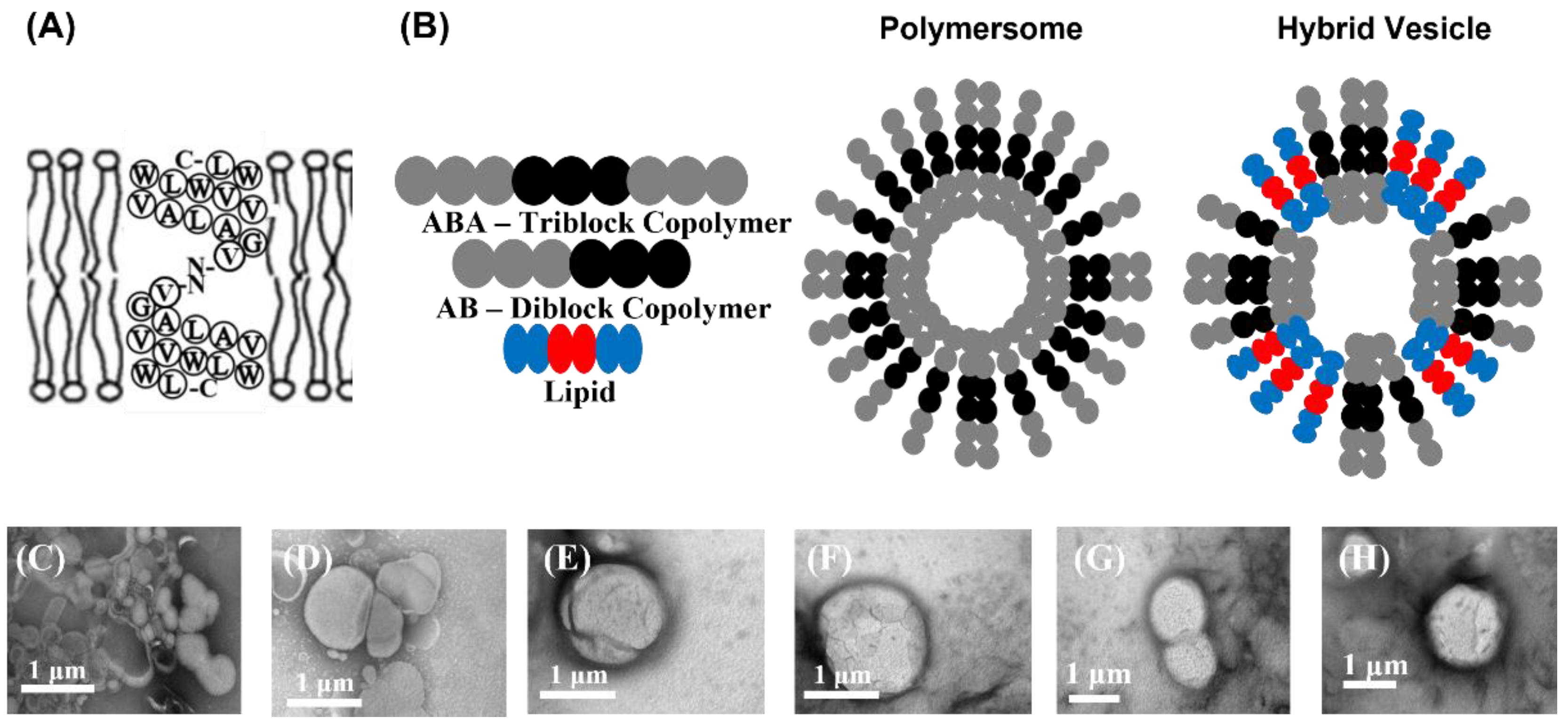
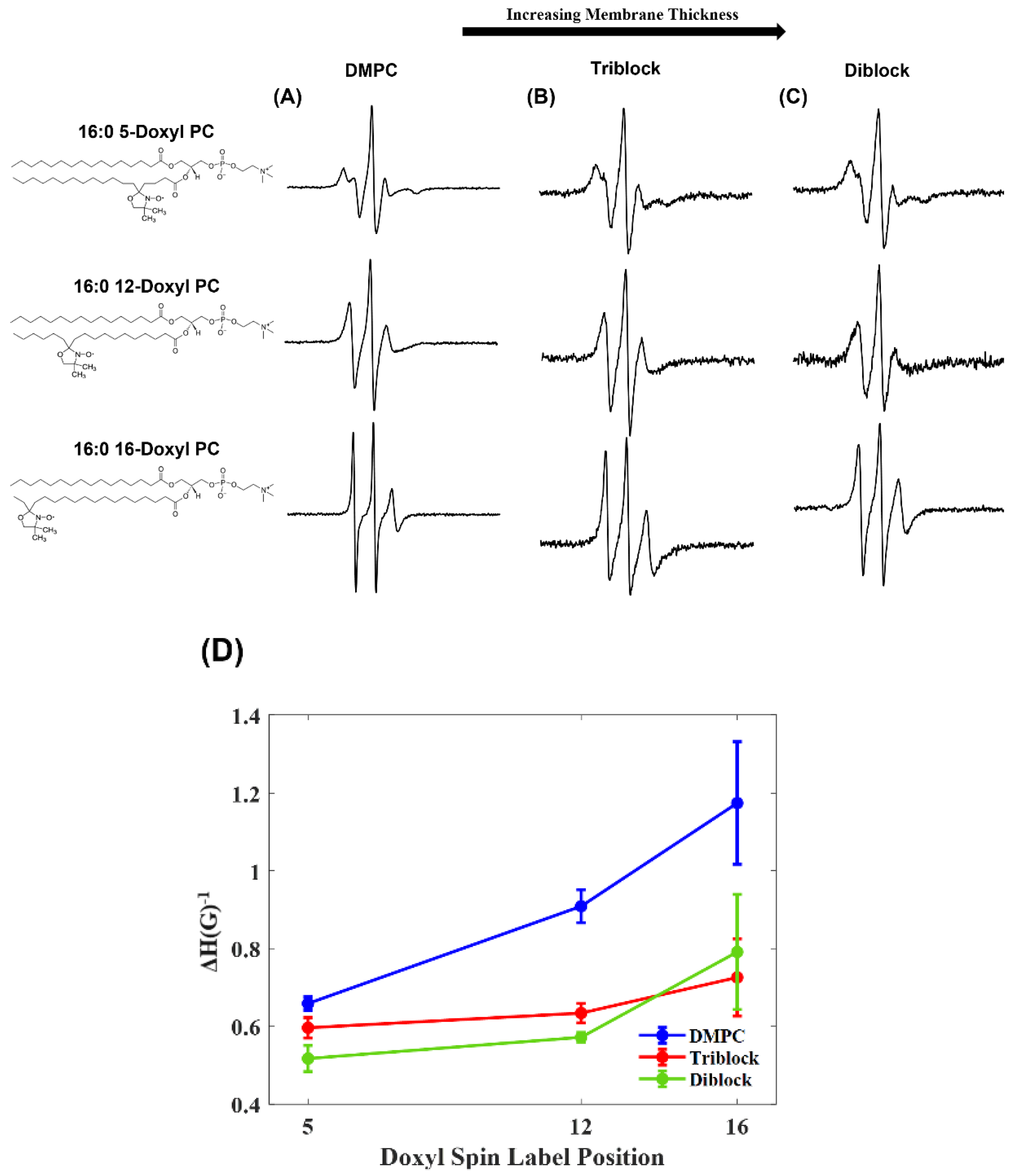
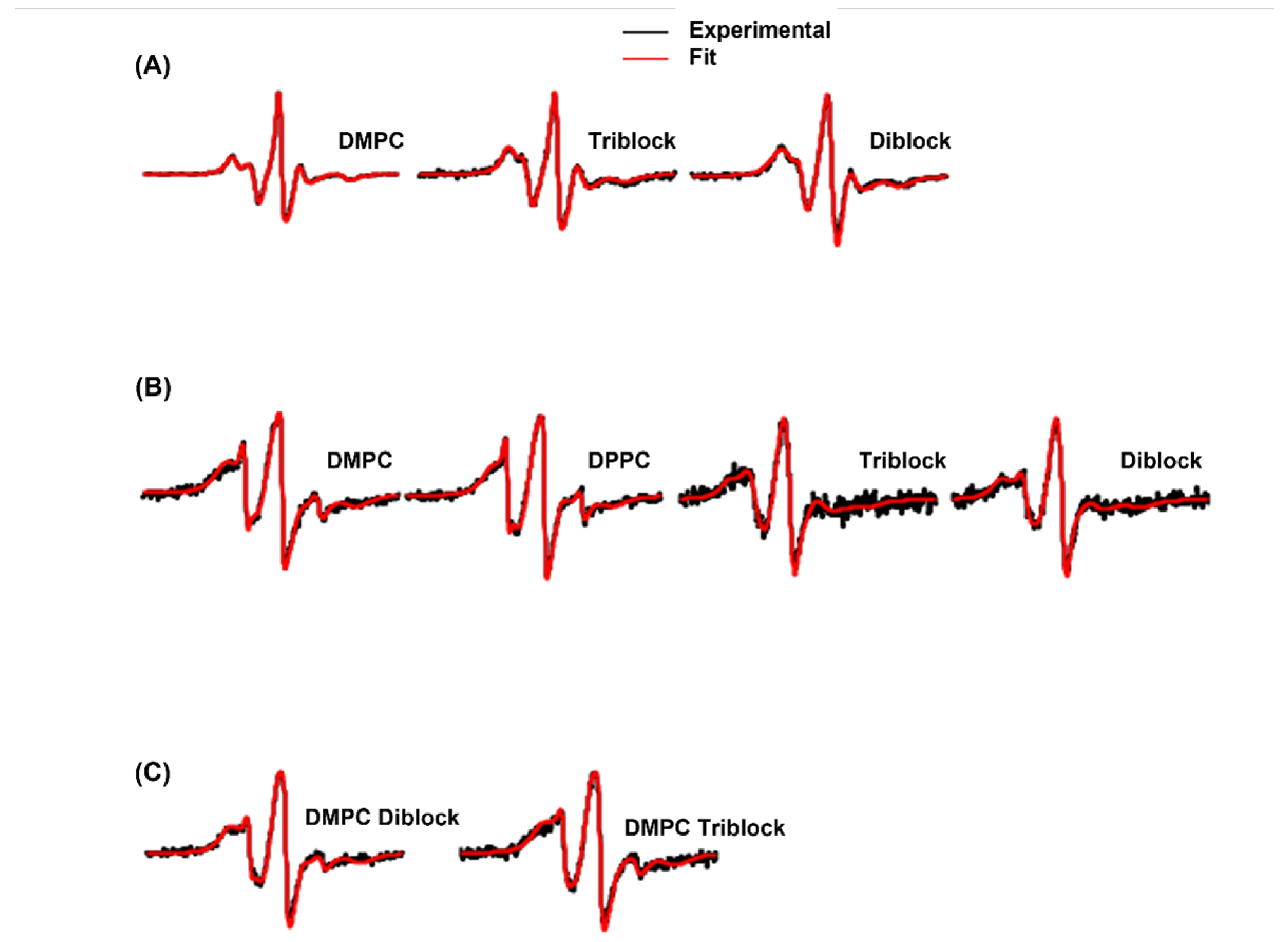
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Majeed, S.; Ahmad, A.B.; Sehar, U.; Georgieva, E.R. Lipid Membrane Mimetics in Functional and Structural Studies of Integral Membrane Proteins. Membranes 2021, 11, 685. [Google Scholar] [CrossRef]
- Yin, H.; Flynn, A.D. Drugging Membrane Protein Interactions. Annu. Rev. Biomed. Eng. 2016, 18, 51–76. [Google Scholar] [CrossRef]
- Beales, P.; Khan, S.; Muench, S.; Jeuken, L. Durable vesicles for reconstitution of membrane proteins in biotechnology. Biochem. Soc. Trans. 2017, 45, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Raschle, T.; Hiller, S.; Etzkorn, M.; Wagner, G. Nonmicellar systems for solution NMR spectroscopy of membrane proteins. Curr. Opin. Struct. Biol. 2010, 20, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Vold, R.R.; Prosser, R.S. Magnetically oriented phospholipid bilayered micelles for structural studies of polypeptides. Does the ideal bicelle exist? J. Magn. Reson. Ser. B 1996, 113, 267–271. [Google Scholar] [CrossRef]
- Duerr, U.H.N.; Gildenberg, M.; Ramamoorthy, A. The Magic of Bicelles Lights Up Membrane Protein Structure. Chem. Rev. 2012, 112, 6054–6074. [Google Scholar] [CrossRef] [PubMed]
- González-Pérez, A.; Stibius, K.; Vissing, T.; Nielsen, C.; Mouritsen, O. Biomimetic Triblock Copolymer Membrane Arrays: A Stable Template for Functional Membrane Proteins. Langmuir 2009, 25, 10447–10450. [Google Scholar] [CrossRef]
- Meyer, R.; Hussmann, G.; Peterson, N.; Santos, J.; Tuesca, A. A scalable and robust cationic lipid/polymer hybrid nanoparticle platform for mRNA delivery. Int. J. Pharm. 2022, 611, 121314. [Google Scholar] [CrossRef]
- Zhang, X.; Tanner, P.; Graff, A.; Palivan, C.; Meier, W. Mimicking the cell membrane with block copolymer membranes. J. Polym. Sci. Part A-Polym. Chem. 2012, 50, 2293–2318. [Google Scholar] [CrossRef]
- Zheng, C.; Han, L.; Yap, C.W.; Xie, B.; Chen, Y. Progress and problems in the exploration of therapeutic targets. Drug Discov. Today 2006, 11, 412–420. [Google Scholar] [CrossRef]
- Tonge, S.; Tighe, B. Responsive hydrophobically associating polymers: A review of structure and properties. Adv. Drug Deliv. Rev. 2001, 53, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef]
- Lo, C.; Zeng, J. Application of polymersomes in membrane protein study and drug discovery: Progress, strategies, and perspectives. Bioeng. Transl. Med. 2023, 8, e10350. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Dubbs, T.; Fried, J. Planar Bilayer Measurements of Alamethicin and Gramicidin Reconstituted in Biomimetic Block Copolymers. Langmuir 2017, 33, 1171–1179. [Google Scholar] [CrossRef]
- Dao, T.; Brûlet, A.; Fernandes, F.; Er-Rafik, M.; Ferji, K.; Schweins, R.; Chapel, J.; Schmutz, F.; Prieto, M.; Sandre, O.; et al. Mixing Block Copolymers with Phospholipids at the Nanoscale: From Hybrid Polymer/Lipid Wormlike Micelles to Vesicles Presenting Lipid Nanodomains. Langmuir 2017, 33, 1705–1715. [Google Scholar] [CrossRef]
- Le Meins, J.; Schatz, C.; Lecommandoux, S.; Sandre, O. Hybrid polymer/lipid vesicles: State of the art and future perspectives. Mater. Today 2013, 16, 397–402. [Google Scholar] [CrossRef]
- Meyer, C.E.; Abram, S.L.; Craciun, I.; Palivan, C.G. Biomolecule-polymer hybrid compartments: Combining the best of both worlds. Phys. Chem. Chem. Phys. 2020, 22, 11197–11218. [Google Scholar] [CrossRef] [PubMed]
- Paxton, W.; McAninch, P.; Achyuthan, K.; Shin, S.; Monteith, H. Monitoring and modulating ion traffic in hybrid lipid/polymer vesicles. Colloids Surf. B-Biointerfaces 2017, 159, 268–276. [Google Scholar] [CrossRef]
- Schulz, M.; Binder, W. Mixed Hybrid Lipid/Polymer Vesicles as a Novel Membrane Platform. Macromol. Rapid Commun. 2015, 36, 2031–2041. [Google Scholar] [CrossRef]
- Kelkar, D.; Chattopadhyay, A. The gramicidin ion channel: A model membrane protein. Biochim. Et Biophys. Acta-Biomembr. 2007, 1768, 2011–2025. [Google Scholar] [CrossRef]
- Qin, Z.; Tepper, H.; Voth, G. Effect of membrane environment on proton permeation through gramicidin a channels. J. Phys. Chem. B 2007, 111, 9931–9939. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fried, J. A hierarchical approach for predicting the transport properties of the gramicidin A channel. Soft Matter 2007, 3, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Ahammad, T.; Drew, D.L., Jr.; Sahu, I.D.; Serafin, R.A.; Clowes, K.R.; Lorigan, G.A. Continuous Wave Electron Paramagnetic Resonance Spectroscopy Reveals the Structural Topology and Dynamic Properties of Active Pinholin S2168 in a Lipid Bilayer. J. Phys. Chem. B 2019, 123, 8048–8056. [Google Scholar] [CrossRef]
- Sahu, I.D.; Lorigan, G.A. Site-Directed Spin Labeling EPR for Studying Membrane Proteins. Biomed Res. Int. 2018, 2018, 3248289. [Google Scholar] [CrossRef]
- Sahu, I.D.; Lorigan, G.A. Electron paramagnetic resonance as a tool for studying membrane proteins. Biomolecules 2020, 10, 763. [Google Scholar] [CrossRef]
- Dzikovski, B.G.; Borbat, P.P.; Freed, J.H. Spin-labeled gramicidin a: Channel formation and dissociation. Biophys. J. 2004, 87, 3504–3517. [Google Scholar] [CrossRef] [PubMed]
- GE, M.; Freed, J. An electron-spin-resonance study of interactions between gramicidin-A’ and phosphatidylcholine bilayers. Biophys. J. 1993, 65, 2106–2123. [Google Scholar] [CrossRef]
- Patyal, B.; Crepeau, R.; Freed, J. Lipid-gramicidin interactions using two-dimensional Fourier-transform electron spin resonance. Biophys. J. 1997, 73, 2201–2220. [Google Scholar] [CrossRef]
- Bouchard, M.; Auger, M. Solvent history dependence of gramicidin-lipid interactions—A raman and infrared spectroscopic STUDY. Biophys. J. 1993, 65, 2484–2492. [Google Scholar] [CrossRef]
- Strauba, J.; Nowotarskib, M.; Luc, J.; Shethd, T.; Jiaod, S.; Fishera, M.; Shelld, M.; Helgesond, M.; Jerschowc, A.; Han, S. Phosphates form spectroscopically dark state assemblies in common aqueous solutions. Proc. Natl. Acad. Sci. USA 2023, 120, e2206765120. [Google Scholar] [CrossRef]
- Lu, J.; Straub, J.; Nowotarski, M.; Han, S.; Xu, X.; Jerschow, A. Spectroscopically dark phosphate features revealed by chemical exchange saturation transfer. NMR Biomed. 2024, 37, e5057. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Peyear, T.; Bennett, W.; Andersen, O.; Lightstone, F.; Ingólfsson, H. Molecular Mechanism for Gramicidin Dimerization and Dissociation in Bilayers of Different Thickness. Biophys. J. 2019, 117, 1831–1844. [Google Scholar] [CrossRef]
- Itel, F.; Chami, M.; Najer, A.; Lörcher, S.; Wu, D.; Dinu, I.; Meier, W. Molecular Organization and Dynamics in Polymersome Membranes: A Lateral Diffusion Study. Macromolecules 2014, 47, 7588–7596. [Google Scholar] [CrossRef]
- Lomora, M.; Garni, M.; Itel, F.; Tanner, P.; Spulber, M.; Palivan, C. Polymersomes with engineered ion selective permeability as stimuli-responsive nanocompartments with preserved architecture. Biomaterials 2015, 53, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Steinkühler, J.; Jacobs, M.; Boyd, M.; Villaseñor, C.; Loverde, S.; Kamat, N. PEO-b-PBD Diblock Copolymers Induce Packing Defects in Lipid/Hybrid Membranes and Improve Insertion Rates of Natively Folded Peptides. Biomacromolecules 2022, 23, 4756–4765. [Google Scholar] [CrossRef] [PubMed]
- Itel, F.; Najer, A.; Palivan, C.; Meier, W. Dynamics of Membrane Proteins within Synthetic Polymer Membranes with Large Hydrophobic Mismatch. Nano Lett. 2015, 15, 3871–3878. [Google Scholar] [CrossRef]
- Bali, A.P.; Sahu, I.D.; Craig, A.F.; Clark, E.E.; Burridge, K.M.; Dolan, M.T.; Dabney-Smith, C.; Konkolewicz, D.; Lorigan, G.A. Structural characterization of styrene-maleic acid copolymer-lipid nanoparticles (SMALPs) using EPR spectroscopy. Chem. Phys. Lipids 2019, 220, 6–13. [Google Scholar] [CrossRef]
- Rizzolo, F.; Sabatino, G.; Chelli, M.; Rovero, P.; Papini, A. A convenient microwave-enhanced solid-phase synthesis of difficult peptide sequences: Case study of Gramicidin A and CSF114(Glc). Int. J. Pept. Res. Ther. 2007, 13, 203–208. [Google Scholar] [CrossRef]
- Schneider, D.J.; Freed, J.H. Calculating slow motional magnetic resonance spectra: A user’s guide. In Biological Magnetic Resonance; Berlinger, L.J., Ed.; Plenum Publishing: New York, NY, USA, 1989. [Google Scholar]
- Budil, D.E.; Lee, S.; Saxena, S.; Freed, J.H. Nonlinear-Least-Squares Analysis of Slow-Motion EPR Spectra in One and Two Dimensions Using a Modified Levenberg–Marquardt Algorithm. J. Magn. Reson. Ser. A 1996, 120, 155–189. [Google Scholar] [CrossRef]
- Camargos, H.S.; Alonso, A. Electron paramagnetic resonance (epr) spectral components of spin-labeled lipids in saturated phospholipid bilayers. effect of cholesterol. Quim. Nova 2013, 36, 815–U152. [Google Scholar] [CrossRef]
- Sahu, I.D.; Zhang, R.; Dunagan, M.M.; Craig, A.F.; Lorigan, G.A. Characterization of KCNE1 inside lipodisq nanoparticles for EPR spectroscopic studies of membrane proteins. J Phys. Chem. B 2017, 121, 5312–5321. [Google Scholar] [CrossRef] [PubMed]
- Weil, J.A.; Bolton, J.R. Electron Paramagnetic Resonance: Elementary Theory and Practical Applications; Wiley-Interscience; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gordon, E.A.; Sahu, I.D.; Fried, J.R.; Lorigan, G.A. Characterization of Gramicidin A in Triblock and Diblock Polymersomes and Hybrid Vesicles via Continuous Wave Electron Paramagnetic Resonance Spectroscopy. Biomimetics 2025, 10, 154. https://doi.org/10.3390/biomimetics10030154
Gordon EA, Sahu ID, Fried JR, Lorigan GA. Characterization of Gramicidin A in Triblock and Diblock Polymersomes and Hybrid Vesicles via Continuous Wave Electron Paramagnetic Resonance Spectroscopy. Biomimetics. 2025; 10(3):154. https://doi.org/10.3390/biomimetics10030154
Chicago/Turabian StyleGordon, Emma A., Indra D. Sahu, Joel R. Fried, and Gary A. Lorigan. 2025. "Characterization of Gramicidin A in Triblock and Diblock Polymersomes and Hybrid Vesicles via Continuous Wave Electron Paramagnetic Resonance Spectroscopy" Biomimetics 10, no. 3: 154. https://doi.org/10.3390/biomimetics10030154
APA StyleGordon, E. A., Sahu, I. D., Fried, J. R., & Lorigan, G. A. (2025). Characterization of Gramicidin A in Triblock and Diblock Polymersomes and Hybrid Vesicles via Continuous Wave Electron Paramagnetic Resonance Spectroscopy. Biomimetics, 10(3), 154. https://doi.org/10.3390/biomimetics10030154






