An Evaluation of Osseointegration Outcomes Around Trabecular Metal Implants in Human Maxillaries Reconstructed with Allograft and Platelet-Rich Fibrin
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Trial Design, Eligibility Criteria and Ethical Considerations
2.2. Surgical Procedures
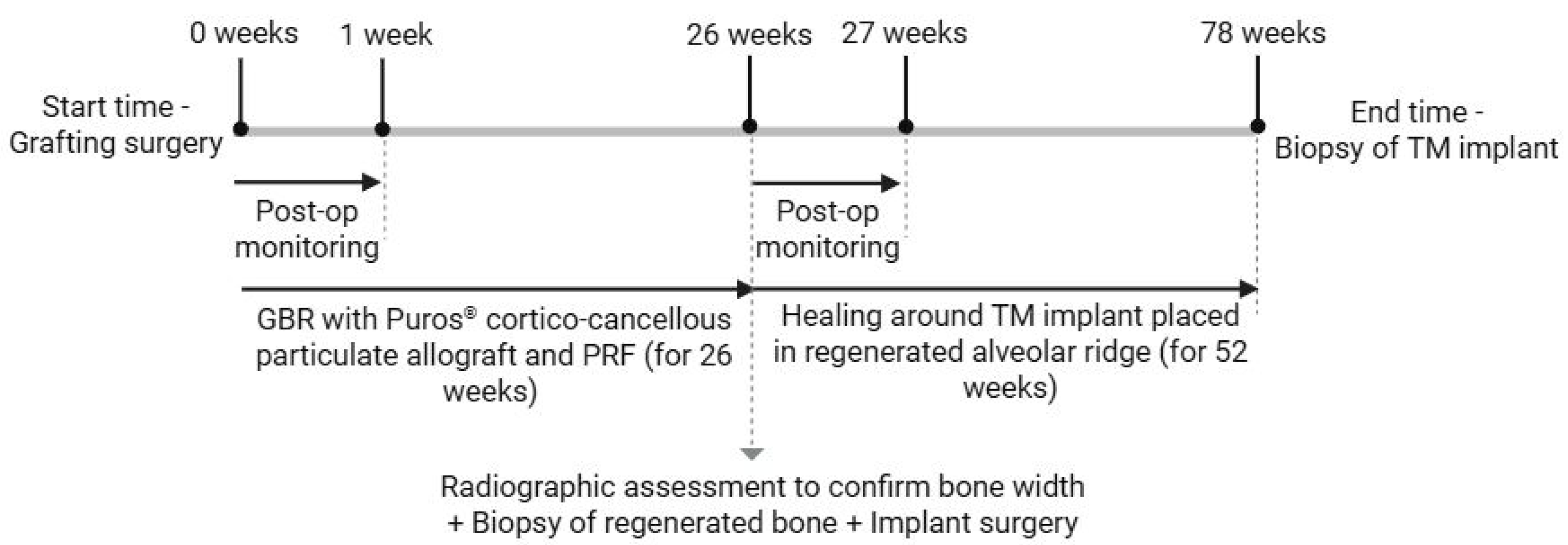
2.2.1. Grafting Surgery
2.2.2. Implant Surgery
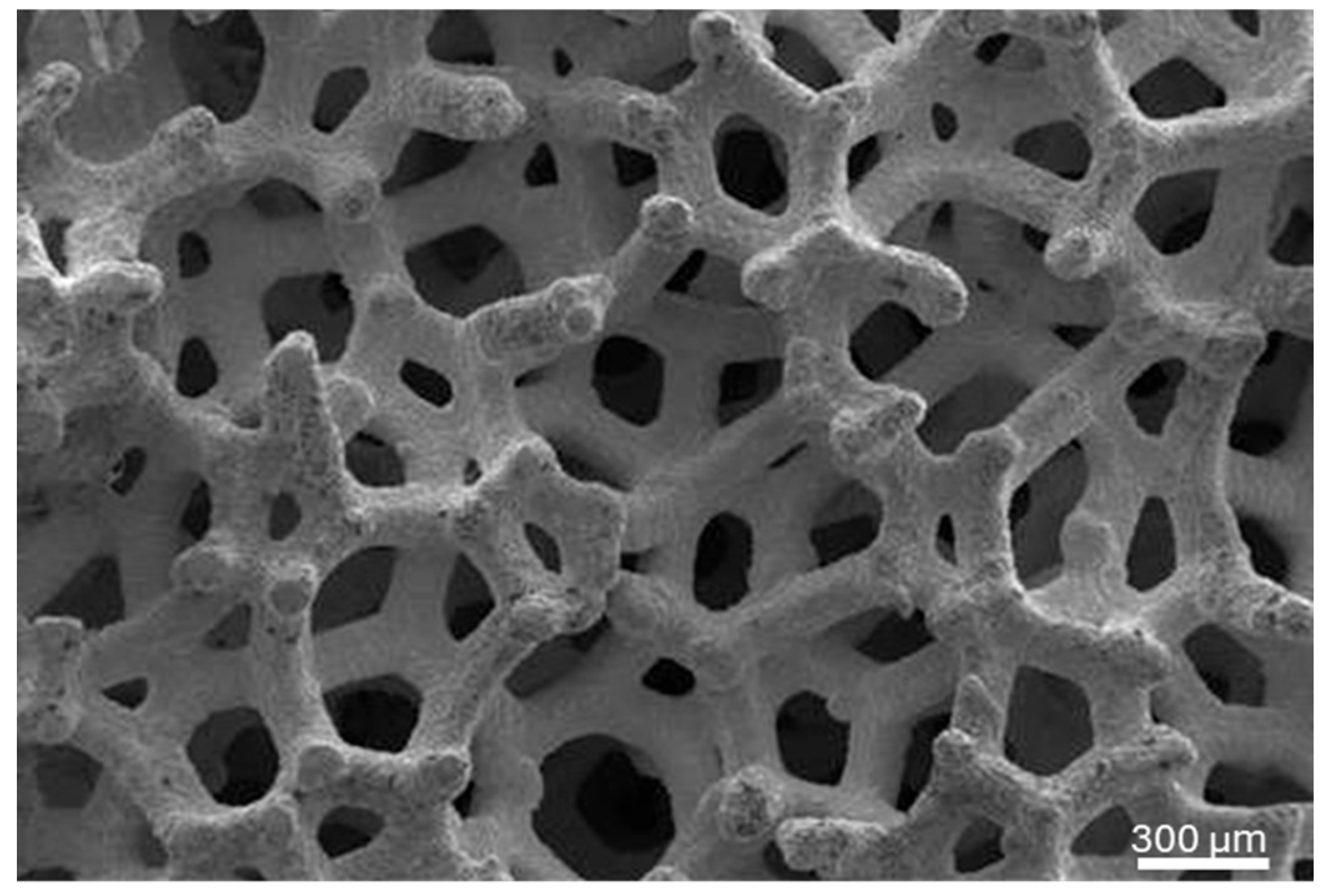
2.3. Histological Preparation
2.4. Histomorphometric Analysis
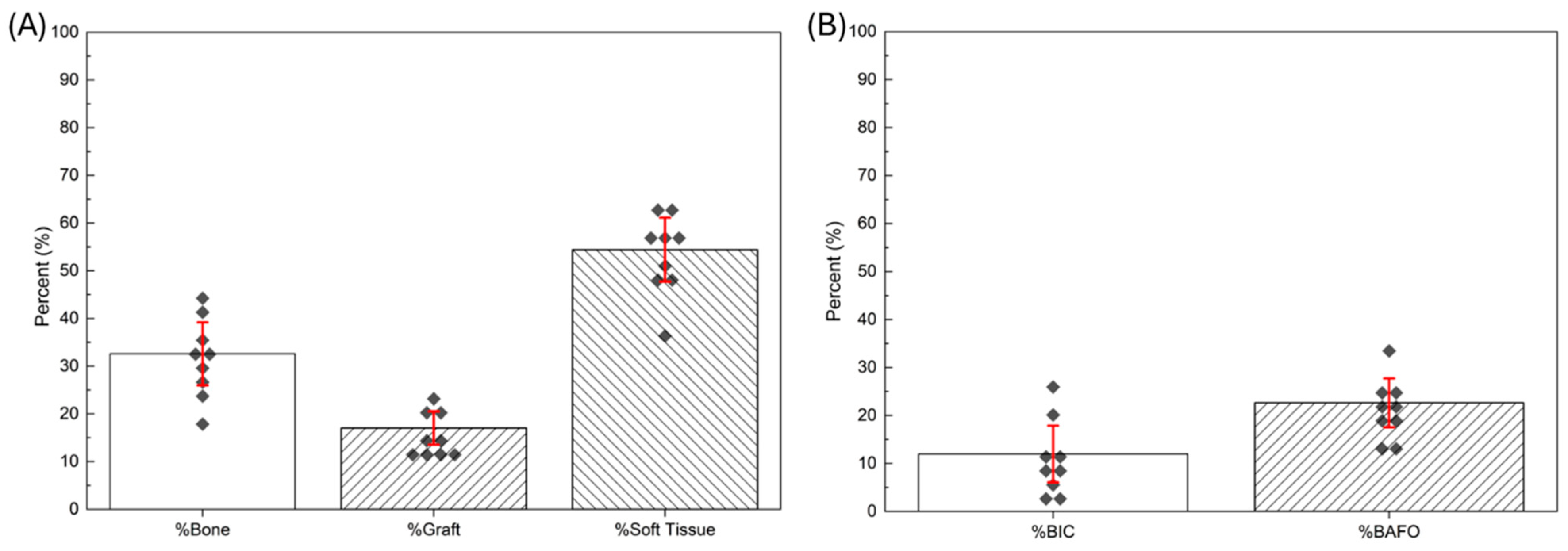
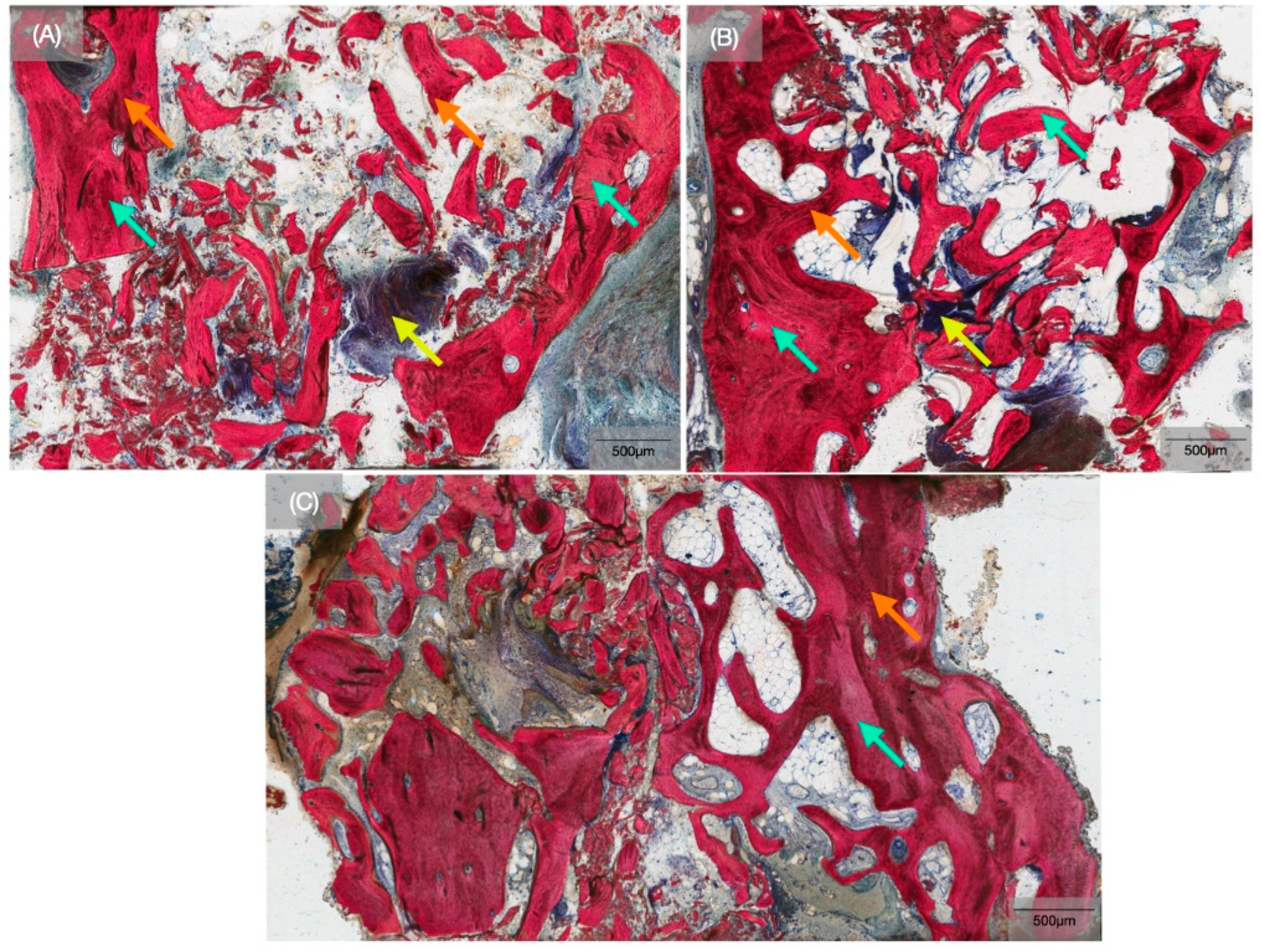
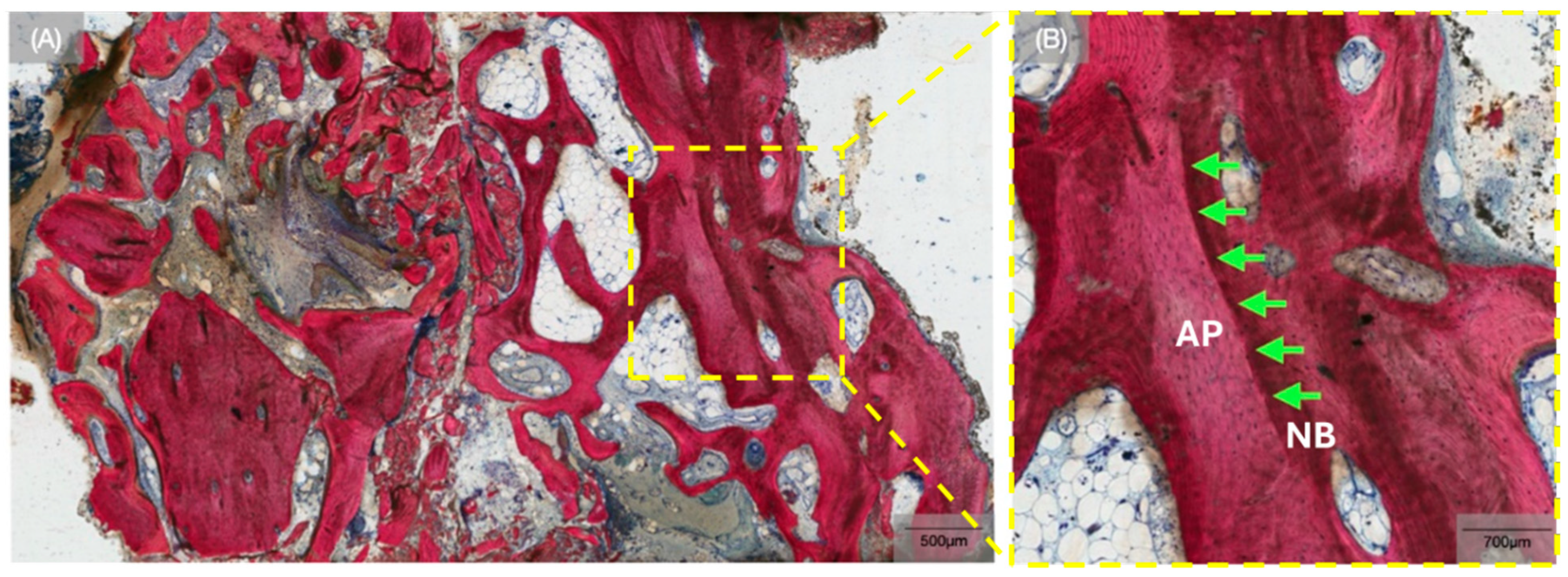
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tahmasebi, E.; Mohammadi, M.; Alam, M.; Abbasi, K.; Gharibian Bajestani, S.; Khanmohammad, R.; Haseli, M.; Yazdanian, M.; Esmaeili Fard Barzegar, P.; Tebyaniyan, H. The current regenerative medicine approaches of craniofacial diseases: A narrative review. Front. Cell Dev. Biol. 2023, 11, 1112378. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, S.-G. Advancements in alveolar bone grafting and ridge preservation: A narrative review on materials, techniques, and clinical outcomes. Maxillofac. Plast. Reconstr. Surg. 2024, 46, 14. [Google Scholar] [CrossRef]
- Kochar, S.P.; Reche, A.; Paul, P. The Etiology and Management of Dental Implant Failure: A Review. Cureus 2022, 14, e30455. [Google Scholar] [CrossRef]
- Bergamo, E.T.P.; Witek, L.; Ramalho, I.; Lopes, A.C.O.; Nayak, V.V.; Bonfante, E.A.; Tovar, N.; Torroni, A.; Coelho, P.G. Bone healing around implants placed in subjects with metabolically compromised systemic conditions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 1664–1671. [Google Scholar] [CrossRef]
- Bergamo, E.T.P.; de Oliveira, P.G.F.P.; Campos, T.M.B.; Bonfante, E.A.; Tovar, N.; Boczar, D.; Nayak, V.V.; Coelho, P.G.; Witek, L. Osseointegration of implant surfaces in metabolic syndrome and type-2 diabetes mellitus. J. Biomed. Mater. Res. Part B Appl. Biomater. 2024, 112, e35382. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Yin, Y.; Sun, J.; Wang, Z.; Tang, Q.; Yang, C. Hormone and implant osseointegration: Elaboration of the relationship among function, preclinical, and clinical practice. Front. Mol. Biosci. 2022, 9, 965753. [Google Scholar] [CrossRef]
- Alanazi, S. Aesthetic problems related to dental implants in the aesthetic zone: A systematic review. Saudi Dent. J. 2024, 36, 9. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, S.; Nakayama, T. Recent Clinical Treatment and Basic Research on the Alveolar Bone. Biomedicines 2023, 11, 843. [Google Scholar] [CrossRef]
- De Elío Oliveros, J.; Del Canto Díaz, A.; Del Canto Díaz, M.; Orea, C.J.; Del Canto Pingarrón, M.; Calvo, J.S. Alveolar Bone Density and Width Affect Primary Implant Stability. J. Oral Implantol. 2020, 46, 389–395. [Google Scholar] [CrossRef]
- Chen, S.T.; Darby, I. Alveolar ridge preservation and early implant placement at maxillary central incisor sites: A prospective case series study. Clin. Oral Implant. Res. 2020, 31, 803–813. [Google Scholar] [CrossRef]
- Quisiguiña Salem, C.; Ruiz Delgado, E.; Crespo Reinoso, P.A.; Robalino, J.J. Alveolar ridge preservation: A review of concepts and controversies. Natl. J. Maxillofac. Surg. 2023, 14, 167–176. [Google Scholar] [CrossRef]
- Adams, R.J. Is there clinical evidence to support alveolar ridge preservation over extraction alone? A review of recent literature and case reports of late graft failure. Br. Dent. J. 2022, 233, 469–474. [Google Scholar] [CrossRef]
- Ferraz, M.P. Bone Grafts in Dental Medicine: An Overview of Autografts, Allografts and Synthetic Materials. Materials 2023, 16, 4117. [Google Scholar] [CrossRef]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef]
- Qiao, J.; An, N.; Ouyang, X. Quantification of growth factors in different platelet concentrates. Platelets 2017, 28, 774–778. [Google Scholar] [CrossRef]
- Rolvien, T.; Barbeck, M.; Wenisch, S.; Amling, M.; Krause, M. Cellular Mechanisms Responsible for Success and Failure of Bone Substitute Materials. Int. J. Mol. Sci. 2018, 19, 2893. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, X.; Yu, J.; Wang, J.; Zhai, P.; Chen, S.; Liu, M.; Zhou, Y. Platelet-Rich Fibrin as a Bone Graft Material in Oral and Maxillofacial Bone Regeneration: Classification and Summary for Better Application. BioMed Res. Int. 2019, 2019, 3295756. [Google Scholar] [CrossRef]
- Ehrenfest, D.M.D.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte-and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef]
- Mise-Omata, S.; Alles, N.; Fukazawa, T.; Aoki, K.; Ohya, K.; Jimi, E.; Obata, Y.; Doi, T. NF-κB RELA-deficient bone marrow macrophages fail to support bone formation and to maintain the hematopoietic niche after lethal irradiation and stem cell transplantation. Int. Immunol. 2014, 26, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Tsigkou, O.; Pomerantseva, I.; Spencer, J.A.; Redondo, P.A.; Hart, A.R.; O’Doherty, E.; Lin, Y.; Friedrich, C.C.; Daheron, L.; Lin, C.P.; et al. Engineered vascularized bone grafts. Proc. Natl. Acad. Sci. USA 2010, 107, 3311–3316. [Google Scholar] [CrossRef]
- Shiezadeh, F.; Taher, M.; Shooshtari, Z.; Arab, H.; Shafieian, R. Using Platelet-Rich Fibrin in Combination With Allograft Bone Particles Can Induce Bone Formation in Maxillary Sinus Augmentation. J. Oral Maxillofac. Surg. 2023, 81, 904–912. [Google Scholar] [CrossRef]
- Mudalal, M.; Wang, Z.; Mustafa, S.; Liu, Y.; Wang, Y.; Yu, J.; Wang, S.; Sun, X.; Zhou, Y. Effect of Leukocyte-Platelet Rich Fibrin (L-PRF) on Tissue Regeneration and Proliferation of Human Gingival Fibroblast Cells Cultured Using a Modified Method. Tissue Eng. Regen. Med. 2021, 18, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Miron, R.J.; Moraschini, V.; Zhang, Y.; Gruber, R.; Wang, H.-L. Efficacy of platelet-rich fibrin on socket healing after mandibular third molar extractions. J. Oral Maxillofac. Surg. Med. Pathol. 2021, 33, 379–388. [Google Scholar] [CrossRef]
- Bencharit, S.; Byrd, W.C.; Altarawneh, S.; Hosseini, B.; Leong, A.; Reside, G.; Morelli, T.; Offenbacher, S. Development and Applications of Porous Tantalum Trabecular Metal-Enhanced Titanium Dental Implants. Clin. Implant Dent. Relat. Res. 2014, 16, 817–826. [Google Scholar] [CrossRef]
- Schlee, M.; Pradies, G.; Mehmke, W.U.; Beneytout, A.; Stamm, M.; Meda, R.G.; Kamm, T.; Poiroux, F.; Weinlich, F.; Del Canto Pingarron, M.; et al. Prospective, Multicenter Evaluation of Trabecular Metal-Enhanced Titanium Dental Implants Placed in Routine Dental Practices: 1-Year Interim Report From the Development Period (2010 to 2011). Clin. Implant Dent. Relat. Res. 2015, 17, 1141–1153. [Google Scholar] [CrossRef]
- Al Deeb, M.; Aldosari, A.A.; Anil, S. Osseointegration of Tantalum Trabecular Metal in Titanium Dental Implants: Histological and Micro-CT Study. J. Funct. Biomater. 2023, 14, 355. [Google Scholar] [CrossRef]
- Bencharit, S.; Byrd, W.C.; Hosseini, B. Immediate placement of a porous-tantalum, trabecular metal-enhanced titanium dental implant with demineralized bone matrix into a socket with deficient buccal bone: A clinical report. J. Prosthet. Dent. 2015, 113, 262–269. [Google Scholar] [CrossRef]
- Jiao, J.; Hong, Q.; Zhang, D.; Wang, M.; Tang, H.; Yang, J.; Qu, X.; Yue, B. Influence of porosity on osteogenesis, bone growth and osteointegration in trabecular tantalum scaffolds fabricated by additive manufacturing. Front. Bioeng. Biotechnol. 2023, 11, 1117954. [Google Scholar] [CrossRef] [PubMed]
- Reveron, H.; Fornabaio, M.; Palmero, P.; Fürderer, T.; Adolfsson, E.; Lughi, V.; Bonifacio, A.; Sergo, V.; Montanaro, L.; Chevalier, J. Towards long lasting zirconia-based composites for dental implants: Transformation induced plasticity and its consequence on ceramic reliability. Acta Biomater. 2017, 48, 423–432. [Google Scholar] [CrossRef]
- Liu, J.; Kerns, D.G. Mechanisms of guided bone regeneration: A review. Open Dent. J. 2014, 8, 56–65. [Google Scholar] [CrossRef]
- Smith, J.O.; Sengers, B.G.; Aarvold, A.; Tayton, E.R.; Dunlop, D.G.; Oreffo, R.O.C. Tantalum trabecular metal—Addition of human skeletal cells to enhance bone implant interface strength and clinical application. J. Tissue Eng. Regen. Med. 2014, 8, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features—PubMed. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e45–e50. [Google Scholar] [CrossRef]
- Urban, I.A.; Mirsky, N.; Serroni, M.; Tovar, N.; Nayak, V.V.; Witek, L.; Marin, C.; Saleh, M.; Ravidà, A.; Baczko, I.; et al. Elucidating the Benefit of Perforated vs Non-Perforated Membranes in Guided Bone Regeneration: An in Vivo Histologic Evaluation and Histomorphometric Analysis. Int. J. Periodontics Restor. Dent. 2024, 45, 341–355. [Google Scholar] [CrossRef]
- Benalcázar-Jalkh, E.B.; Nayak, V.V.; Gory, C.; Marquez-Guzman, A.; Bergamo, E.T.; Tovar, N.; Coelho, P.G.; Bonfante, E.A.; Witek, L. Impact of implant thread design on insertion torque and osseointegration: A preclinical model—PubMed. Med. Oral Patol. Oral Y Cir. Bucal 2023, 28, e48–e55. [Google Scholar] [CrossRef]
- De Angelis, P.; De Rosa, G.; Manicone, P.F.; De Giorgi, A.; Cavalcanti, C.; Speranza, A.; Grassi, R.; D’Addona, A. Hard and soft tissue evaluation of alveolar ridge preservation compared to spontaneous healing: A retrospective clinical and volumetric analysis. Int. J. Implant Dent. 2022, 8, 62. [Google Scholar] [CrossRef]
- Guan, S.; Xiao, T.; Bai, J.; Ning, C.; Zhang, X.; Yang, L.; Li, X. Clinical application of platelet-rich fibrin to enhance dental implant stability: A systematic review and meta-analysis. Heliyon 2023, 9, e13196. [Google Scholar] [CrossRef]
- Kizildağ, A.; Taşdemir, U.; Arabaci, T.; Özmen, Ö.; Kizildağ, C.A.; Iyilikci, B. Evaluation of New Bone Formation Using Autogenous Tooth Bone Graft Combined with Platelet-Rich Fibrin in Calvarial Defects—PubMed. J. Craniofacial. Surg. 2019, 30, 1662–1666. [Google Scholar] [CrossRef] [PubMed]
- Kyyak, S.; Blatt, S.; Pabst, A.; Thiem, D.; Al-Nawas, B.; Kämmerer, P.W. Combination of an allogenic and a xenogenic bone substitute material with injectable platelet-rich fibrin—A comparative in vitro study. J. Biomater. Appl. 2020, 35, 83–96. [Google Scholar] [CrossRef]
- Ucer, C.; Khan, R.S. Alveolar Ridge Preservation with Autologous Platelet-Rich Fibrin (PRF): Case Reports and the Rationale. Dent. J. 2023, 11, 244. [Google Scholar] [CrossRef]
- Sculean, A.; Gruber, R.; Bosshardt, D.D. Soft tissue wound healing around teeth and dental implants. J. Clin. Periodontol. 2014, 41, S6–S22. [Google Scholar] [CrossRef]
- Solakoğlu, Ö.; Ofluoğlu, D.; Schwarzenbach, H.; Heydecke, G.; Reißmann, D.; Ergun, S.; Götz, W. A 3-year prospective randomized clinical trial of alveolar bone crest response and clinical parameters through 1, 2, and 3 years of clinical function of implants placed 4 months after alveolar ridge preservation using two different allogeneic bone-graftin. Int. J. Implant Dent. 2022, 8, 5. [Google Scholar] [CrossRef]
- Edelmann, A.R.; Patel, D.; Allen, R.K.; Gibson, C.J.; Best, A.M.; Bencharit, S. Retrospective analysis of porous tantalum trabecular metal–enhanced titanium dental implants. J. Prosthet. Dent. 2019, 121, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Trimmel, B.; Gyulai-Gaál, S.; Kivovics, M.; Jákob, N.P.; Hegedűs, C.; Szabó, B.T.; Dobó-Nagy, C.; Szabó, G. Evaluation of the Histomorphometric and Micromorphometric Performance of a Serum Albumin-Coated Bone Allograft Combined with A-PRF for Early and Conventional Healing Protocols after Maxillary Sinus Augmentation: A Randomized Clinical Trial. Materials 2021, 14, 1810. [Google Scholar] [CrossRef]
- Piglionico, S.; Bousquet, J.; Fatima, N.; Renaud, M.; Collart-Dutilleul, P.-Y.; Bousquet, P. Porous Tantalum vs. Titanium Implants: Enhanced Mineralized Matrix Formation after Stem Cells Proliferation and Differentiation. J. Clin. Med. 2020, 9, 3657. [Google Scholar] [CrossRef]
- Lee, J.W.; Wen, H.B.; Gubbi, P.; Romanos, G.E. New bone formation and trabecular bone microarchitecture of highly porous tantalum compared to titanium implant threads: A pilot canine study. Clin. Oral Implant. Res. 2018, 29, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Bobyn, J.D.; Stackpool, G.J.; Hacking, S.A.; Tanzer, M.; Krygier, J.J. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial—PubMed. J. Bone Jt. Surgery Br. Vol. 1999, 81, 907–914. [Google Scholar] [CrossRef]
- Fraser, D.; Mendonca, G.; Sartori, E.; Funkenbusch, P.; Ercoli, C.; Meirelles, L. Bone response to porous tantalum implants in a gap-healing model. Clin. Oral Implant. Res. 2019, 30, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Dimaira, M. Immediate Placement of Trabecular Implants in Sites of Failed Implants. Int. J. Oral Maxillofac. Implant. 2019, 34, e77–e83. [Google Scholar] [CrossRef] [PubMed]
- Witek, L.; Alifarag, A.M.; Tovar, N.; Lopez, C.; Gil, L.F.; Gorbonosov, M.; Hannan, K.; Neiva, R.; Coelho, P.G. Osteogenic parameters surrounding trabecular tantalum metal implants in osteotomies prepared via osseodensification drilling. Med. Oral Patol. Oral Y Cir. Bucal 2019, 24, e764–e769. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, R.; Kovács, A.; Berkó, S.; Budai-Szűcs, M. Developments in Alloplastic Bone Grafts and Barrier Membrane Biomaterials for Periodontal Guided Tissue and Bone Regeneration Therapy. Int. J. Mol. Sci. 2024, 25, 7746. [Google Scholar] [CrossRef]
- Lin, I.P.; Chen, S.H.; Chang, C.C.; Chang, J.Z.; Sun, J.S.; Chang, C.H. Morphology of Peri-Implant Tissues Around Permanent Prostheses with Various Emergence Angles Following Free Gingival Grafting. J. Prosthodont. 2022, 31, 681–688. [Google Scholar] [CrossRef]
- El Chaar, E.; Castaño, A. A Retrospective Survival Study of Trabecular Tantalum Implants Immediately Placed in Posterior Extraction Sockets Using a Flapless Technique. J. Oral Implantol. 2017, 43, 114–124. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oroumieh, S.I.; Shah, H.; Nordlund, A.; Ignacio De Bellis Tulle, L.; Souza, B.M.d.; Desai, A.; Nayak, V.V.; Carlos Carvajal Herrera, J.; Witek, L.; Coelho, P.G. An Evaluation of Osseointegration Outcomes Around Trabecular Metal Implants in Human Maxillaries Reconstructed with Allograft and Platelet-Rich Fibrin. Biomimetics 2025, 10, 789. https://doi.org/10.3390/biomimetics10110789
Oroumieh SI, Shah H, Nordlund A, Ignacio De Bellis Tulle L, Souza BMd, Desai A, Nayak VV, Carlos Carvajal Herrera J, Witek L, Coelho PG. An Evaluation of Osseointegration Outcomes Around Trabecular Metal Implants in Human Maxillaries Reconstructed with Allograft and Platelet-Rich Fibrin. Biomimetics. 2025; 10(11):789. https://doi.org/10.3390/biomimetics10110789
Chicago/Turabian StyleOroumieh, Sana Imani, Hana Shah, Andrew Nordlund, Luis Ignacio De Bellis Tulle, Bruno Martins de Souza, Anshumi Desai, Vasudev Vivekanand Nayak, Juan Carlos Carvajal Herrera, Lukasz Witek, and Paulo G. Coelho. 2025. "An Evaluation of Osseointegration Outcomes Around Trabecular Metal Implants in Human Maxillaries Reconstructed with Allograft and Platelet-Rich Fibrin" Biomimetics 10, no. 11: 789. https://doi.org/10.3390/biomimetics10110789
APA StyleOroumieh, S. I., Shah, H., Nordlund, A., Ignacio De Bellis Tulle, L., Souza, B. M. d., Desai, A., Nayak, V. V., Carlos Carvajal Herrera, J., Witek, L., & Coelho, P. G. (2025). An Evaluation of Osseointegration Outcomes Around Trabecular Metal Implants in Human Maxillaries Reconstructed with Allograft and Platelet-Rich Fibrin. Biomimetics, 10(11), 789. https://doi.org/10.3390/biomimetics10110789






