Recent Advances in the Applications of Biomaterials in Ovarian Cancer
Abstract
1. Introduction
2. Applications of Biomaterials in Ovarian Cancer Diagnosis
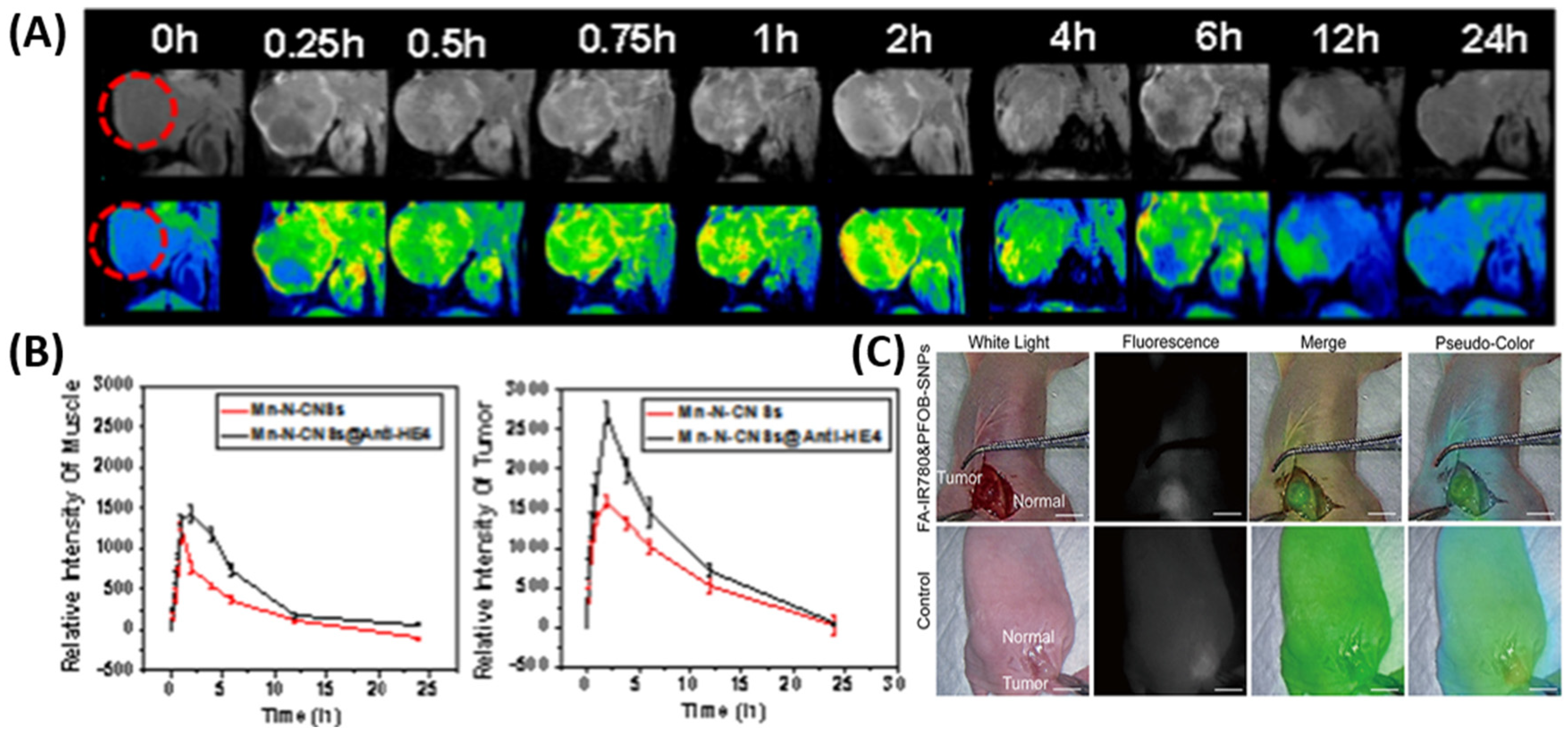
2.1. Nanomaterials for Enhanced Imaging
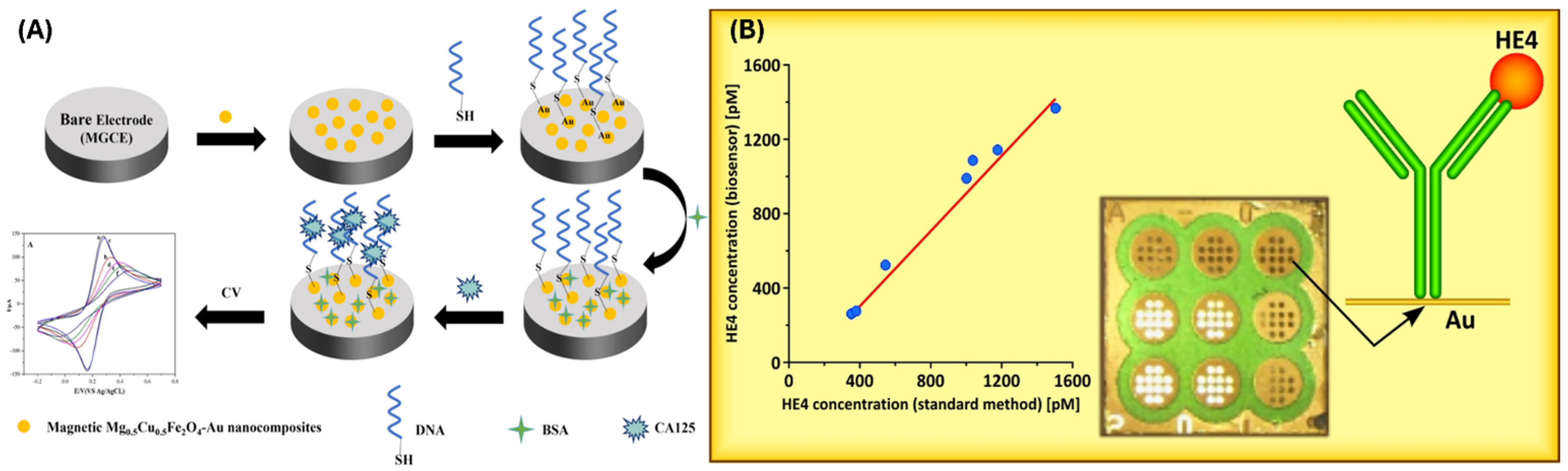
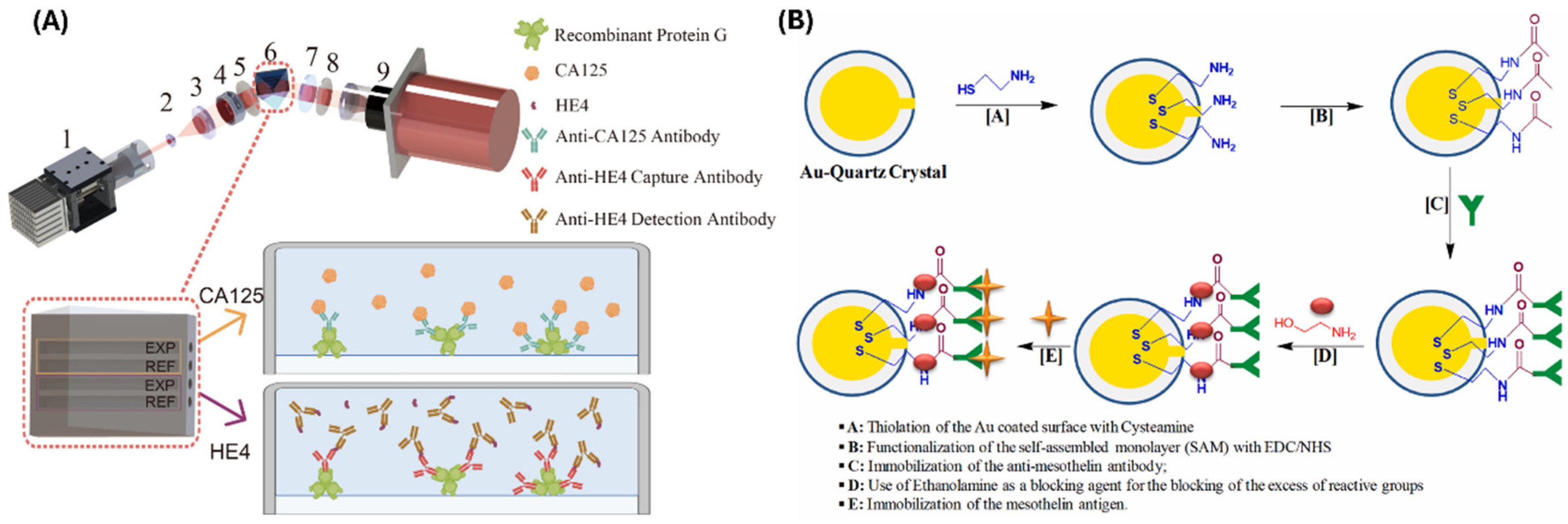
2.2. Biosensors for Detecting Ovarian Cancer Markers
| Sensor | Concentration Range | LOD | References |
|---|---|---|---|
| MPA/AuNPs@SiO2/QD/mAb | 0–0.1 U/mL | 0.0016 U/mL | [40] |
| Ag NPs-GQDs/Ab/BSA/Ag | 0.01 U/mL | 0.01–400 U/mL | [41] |
| Au-PB-PtNP-PANI hydrogel/GCE | 0.01–5000 U/mL | 4.4 mU/mL | [42] |
| Ab2–Ag–Ab1/Au-VBG/BDD/Ta | 0.09 mU/mL | 0.5–100 U/mL | [43] |
| BSA/Ab/Au NPs/Cys A/ERGO-P(DA)-GCE | 0.1 U/mL | 0.1–400 U/mL | [44] |
| FA@H-PANI@CS-HCl | 0.25 pg/mL | 0.001–25 ng/mL | [45] |
| CuO nanoflakes | 0.77–500 IU/mL | 0.77 IU/mL | [46] |
| MOF-808/CNT/GCE | 0.001–0.1 ng/mL & 0.1–30 ng/mL | 0.5 pg/mL | [47] |
| organohydrogels | 0.41–8.3 U/mL & 8.3–249 U/mL | 0.805 μU/mL | [39] |
| Co(bpy)33+/MWNTs-Nafion/GC | 1–30 U/mL & 30–150 U/mL | 0.36 U/mL | [48] |
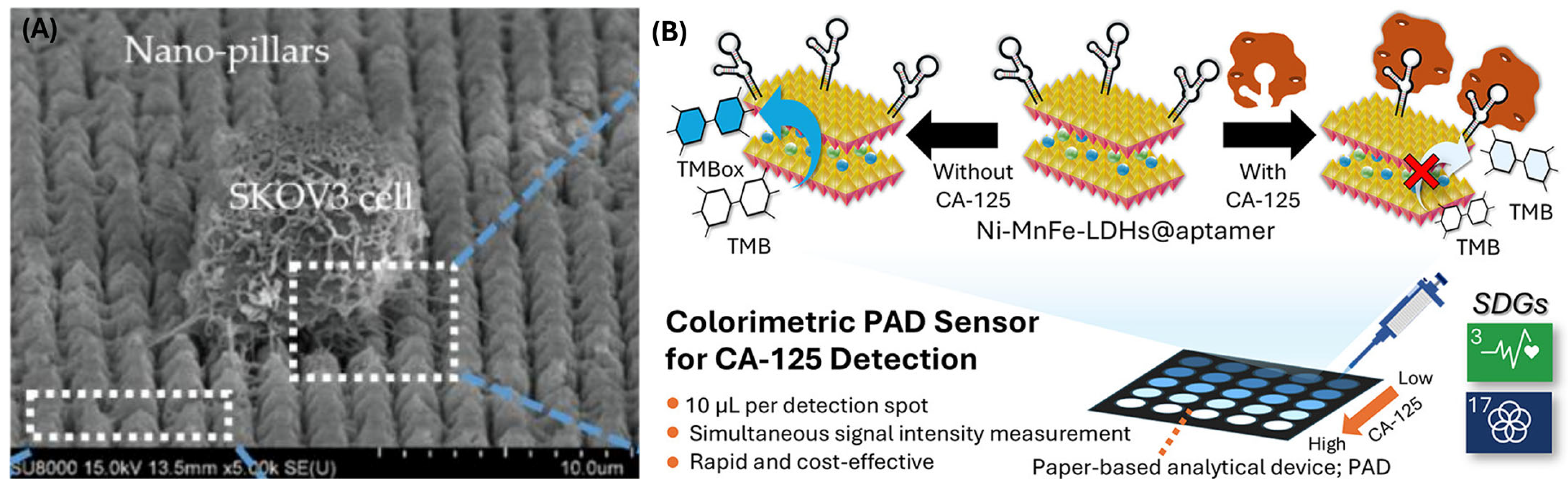
2.3. Microfluidic Devices & Ovarian Tissue Engineering
3. Applications of Biomaterials in Ovarian Cancer Treatment
3.1. Nanoscale Drug Delivery Systems (DDSs) for Ovarian Cancer
3.1.1. Targeted and Stimuli-Responsive Drug Delivery Systems
3.1.2. Combination Therapy and Overcoming Chemoresistance
3.1.3. Immunomodulatory and Gene-Based Therapies
3.1.4. Localized, Controlled, and Advanced Formulation Systems
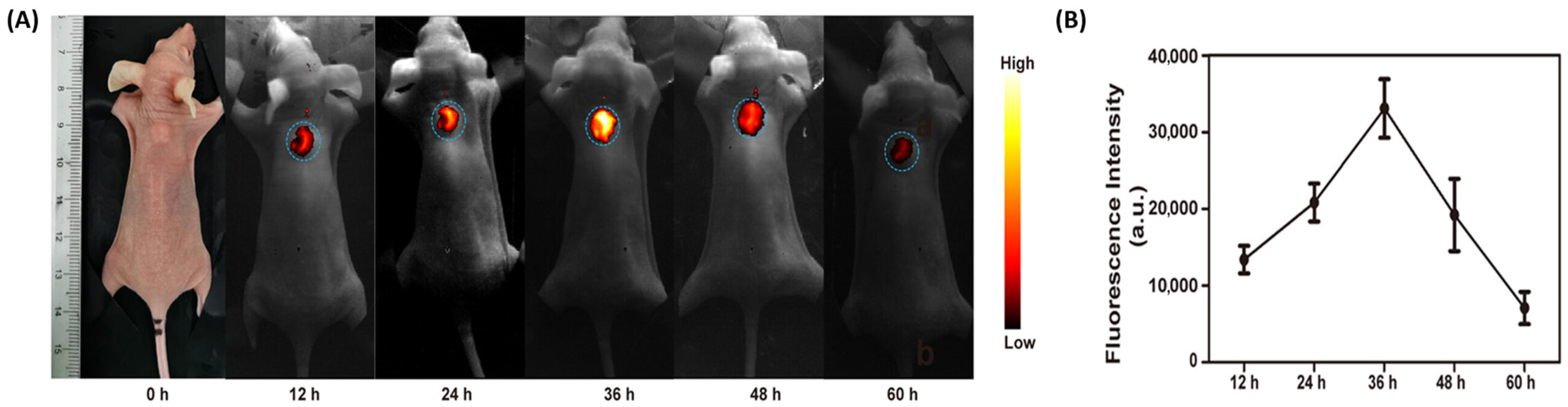
3.2. Photothermal, Photodynamic, and Sonodynamic Therapies for Ovarian Cancer
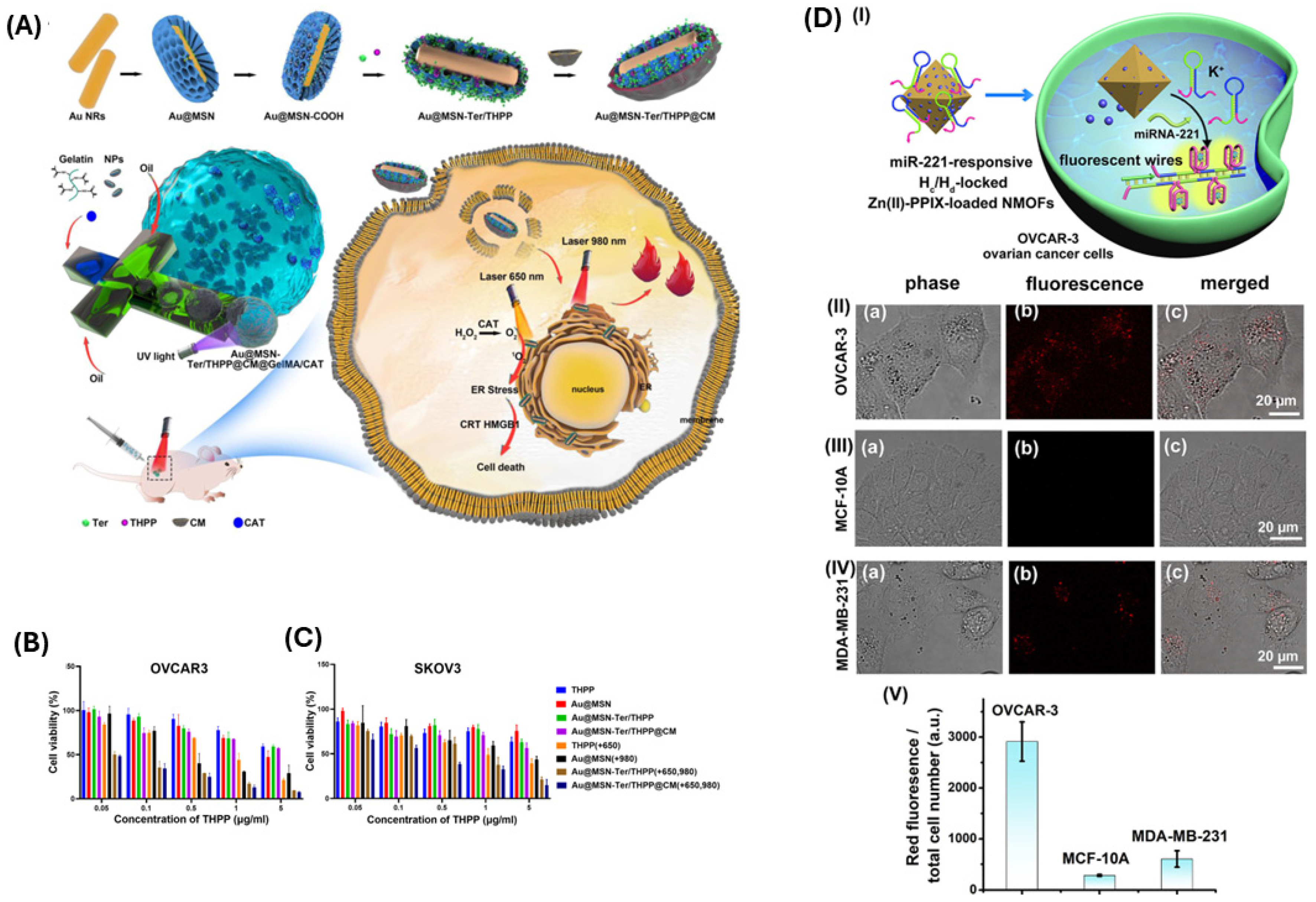
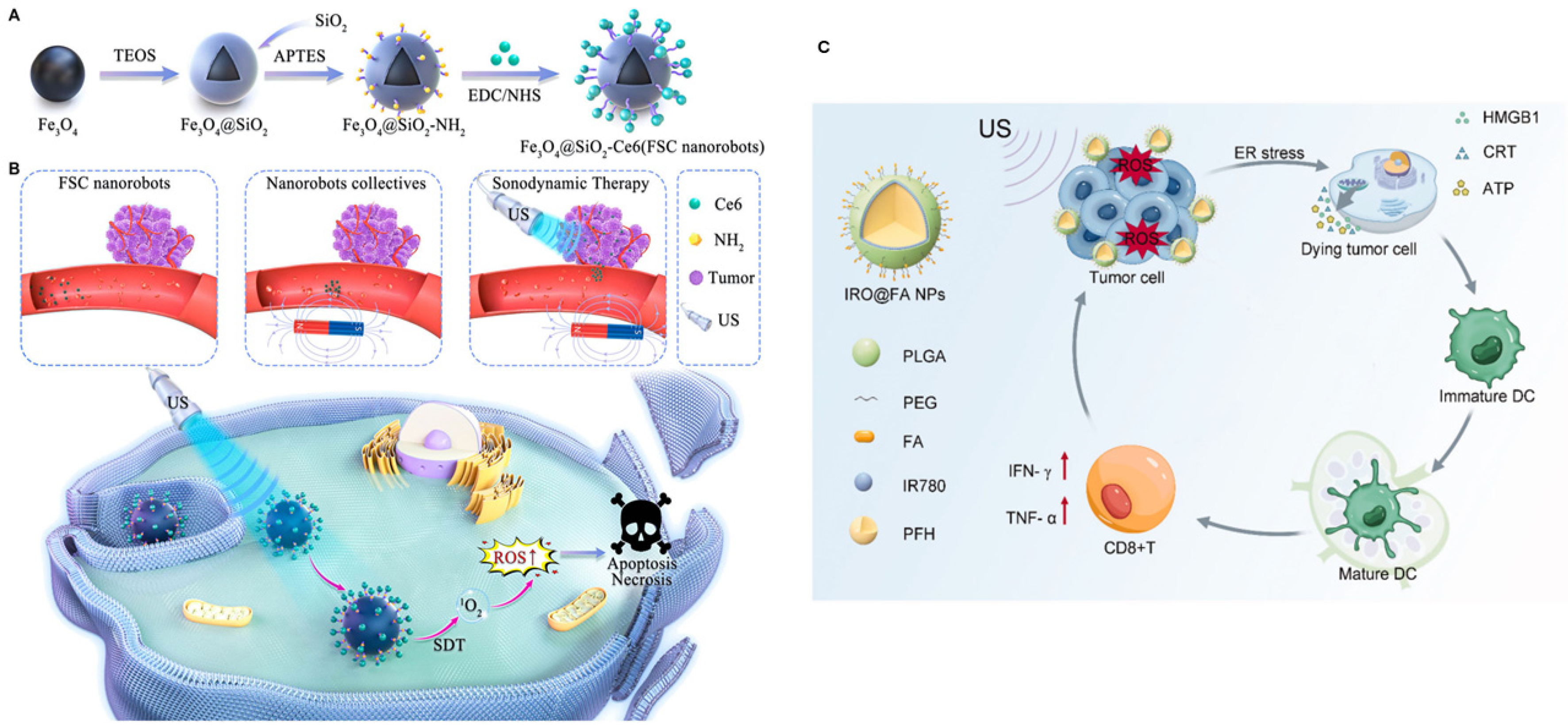
4. Current Status, Challenges, and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Elias, K.M.; Guo, J.; Bast, R.C. Early Detection of Ovarian Cancer. Hematol. Oncol. Clin. N. Am. 2018, 32, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Veneziani, A.C.; Gonzalez-Ochoa, E.; Alqaisi, H.; Madariaga, A.; Bhat, G.; Rouzbahman, M.; Sneha, S.; Oza, A.M. Heterogeneity and Treatment Landscape of Ovarian Carcinoma. Nat. Rev. Clin. Oncol. 2023, 20, 820–842. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xie, H.-J.; Li, Y.-Y.; Wang, X.; Liu, X.-X.; Mai, J. Molecular Mechanisms of Platinum-based Chemotherapy Resistance in Ovarian Cancer (Review). Oncol. Rep. 2022, 47, 82. [Google Scholar] [CrossRef] [PubMed]
- Höhn, A.K.; Brambs, C.E.; Hiller, G.G.R.; May, D.; Schmoeckel, E.; Horn, L.-C. 2020 WHO Classification of Female Genital Tumors. Geburtshilfe Frauenheilkd 2021, 81, 1145–1153. [Google Scholar] [CrossRef]
- Köbel, M.; Kang, E.Y. The Evolution of Ovarian Carcinoma Subclassification. Cancers 2022, 14, 416. [Google Scholar] [CrossRef]
- Brett, M.R.; Jennifer, B.P.; Thomas, A.S.; Brett, M.R.; Jennifer, B.P.; Thomas, A.S. Epidemiology of Ovarian Cancer: A Review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef]
- Dilley, J.; Burnell, M.; Gentry-Maharaj, A.; Ryan, A.; Neophytou, C.; Apostolidou, S.; Karpinskyj, C.; Kalsi, J.; Mould, T.; Woolas, R.; et al. Ovarian Cancer Symptoms, Routes to Diagnosis and Survival—Population Cohort Study in the ‘No Screen’ Arm of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Gynecol. Oncol. 2020, 158, 316–322. [Google Scholar] [CrossRef]
- Ali, A.T.; Al-ani, O.; Al-ani, F. Epidemiology and Risk Factors for Ovarian Cancer. Menopausal Rev. 2023, 22, 93–104. [Google Scholar] [CrossRef]
- Bowtell, D.D.; Böhm, S.; Ahmed, A.A.; Aspuria, P.-J.; Bast, R.C.; Beral, V.; Berek, J.S.; Birrer, M.J.; Blagden, S.; Bookman, M.A.; et al. Rethinking Ovarian Cancer II: Reducing Mortality from High-Grade Serous Ovarian Cancer. Nat. Rev. Cancer 2015, 15, 668–679. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih, I.-M. The Dualistic Model of Ovarian Carcinogenesis. Am. J. Pathol. 2016, 186, 733–747. [Google Scholar] [CrossRef]
- Prat, J. New Insights into Ovarian Cancer Pathology. Ann. Oncol. 2012, 23, x111–x117. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Integrated Genomic Analyses of Ovarian Carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Pignata, S.; Cannella, L.; Leopardo, D.; Pisano, C.; Bruni, G.S.; Facchini, G. Chemotherapy in Epithelial Ovarian Cancer. Cancer Lett. 2011, 303, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Leali, P.T.; Merolli, A. Fundamentals of Biomaterials. In Biomaterials in Hand Surgery; Springer: Milan, Italy, 2009; pp. 1–11. [Google Scholar]
- Trucillo, P. Biomaterials for Drug Delivery and Human Applications. Materials 2024, 17, 456. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hu, S.; Teng, Y.; Chen, J.; Wang, H.; Xu, Y.; Wang, K.; Xu, J.; Cheng, Y.; Gao, X. Current Advance of Nanotechnology in Diagnosis and Treatment for Malignant Tumors. Signal Transduct. Target. Ther. 2024, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Portella, L.; Bertolini, G.; Guardascione, G.; Di Febbraro, D.G.; Ieranò, C.; D’Alterio, C.; Rea, G.; Napolitano, M.; Santagata, S.; Trotta, A.M.; et al. CXCL12-Loaded-Hydrogel (CLG): A New Device for Metastatic Circulating Tumor Cells (CTCs) Capturing and Characterization. Heliyon 2024, 10, e35524. [Google Scholar] [CrossRef]
- Wu, X.; Wen, X.; Lin, X.; Wang, X.; Wan, Y.; Gao, R.; Zhang, Y.; Han, C. PH/Glutathione-Responsive Theranostic Nanoprobes for Chemoimmunotherapy and Magnetic Resonance Imaging of Ovarian Cancer Cells. Colloids Surf. B Biointerfaces 2024, 241, 114053. [Google Scholar] [CrossRef]
- Xu, L.; Gao, H.; Deng, Y.; Liu, X.; Zhan, W.; Sun, X.; Xu, J.-J.; Liang, G. β-Galactosidase-Activated near-Infrared AIEgen for Ovarian Cancer Imaging in Vivo. Biosens. Bioelectron. 2024, 255, 116207. [Google Scholar] [CrossRef]
- Quindoza, G.M.; Horimoto, R.; Nakagawa, Y.; Aida, Y.; Irawan, V.; Norimatsu, J.; Mizuno, H.L.; Anraku, Y.; Ikoma, T. Folic Acid-Mediated Enhancement of the Diagnostic Potential of Luminescent Europium-Doped Hydroxyapatite Nanocrystals for Cancer Biomaging. Colloids Surf. B Biointerfaces 2024, 239, 113975. [Google Scholar] [CrossRef]
- Dai, J.; Ouyang, H.; Wei, S.; Chen, B.; Dong, X.; Hu, J.; Wu, M.; Wang, S.; Xia, F.; Lou, X. Cancer-Associated Fibroblast Mimetic AIE Probe for Precision Imaging-Guided Full-Cycle Management of Ovarian Cancer Surgery. Anal. Chem. 2023, 95, 15068–15077. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Huang, W.; Li, N.; Shen, Y.; Wang, X.; Chen, T. Highly Specific Selenium Nanosystems for Fluorescent Image-Guided Rapid Diagnosis and Pathological Grading of Ovarian Malignant Tumors. Chin. Chem. Lett. 2023, 34, 107764. [Google Scholar] [CrossRef]
- Pu, T.; Liu, Y.; Pei, Y.; Peng, J.; Wang, Z.; Du, M.; Liu, Q.; Zhong, F.; Zhang, M.; Li, F.; et al. NIR-II Fluorescence Imaging for the Detection and Resection of Cancerous Foci and Lymph Nodes in Early-Stage Orthotopic and Advanced-Stage Metastatic Ovarian Cancer Models. ACS Appl. Mater. Interfaces 2023, 15, 32226–32239. [Google Scholar] [CrossRef]
- Kumar, D.; Moghiseh, M.; Chitcholtan, K.; Mutreja, I.; Lowe, C.; Kaushik, A.; Butler, A.; Sykes, P.; Anderson, N.; Raja, A. LHRH Conjugated Gold Nanoparticles Assisted Efficient Ovarian Cancer Targeting Evaluated via Spectral Photon-Counting CT Imaging: A Proof-of-Concept Research. J. Mater. Chem. B 2023, 11, 1916–1928. [Google Scholar] [CrossRef]
- Song, J.; Ye, H.; Jiang, S.; Yang, Y.; Li, X. An Acid Response IR780-Based Targeted Nanoparticle for Intraoperative Near-Infrared Fluorescence Imaging of Ovarian Cancer. Int. J. Nanomed. 2022, 17, 4961–4974. [Google Scholar] [CrossRef]
- Shahdeo, D.; Roberts, A.; Kesarwani, V.; Horvat, M.; Chouhan, R.S.; Gandhi, S. Polymeric Biocompatible Iron Oxide Nanoparticles Labeled with Peptides for Imaging in Ovarian Cancer. Biosci. Rep. 2022, 42, BSR20212622. [Google Scholar] [CrossRef]
- Vankayala, R.; Bahena, E.; Guerrero, Y.; Singh, S.P.; Ravoori, M.K.; Kundra, V.; Anvari, B. Virus-Mimicking Nanoparticles for Targeted Near Infrared Fluorescence Imaging of Intraperitoneal Ovarian Tumors in Mice. Ann. Biomed. Eng. 2021, 49, 548–559. [Google Scholar] [CrossRef]
- Burns, J.M.; Shafer, E.; Vankayala, R.; Kundra, V.; Anvari, B. Near Infrared Fluorescence Imaging of Intraperitoneal Ovarian Tumors in Mice Using Erythrocyte-Derived Optical Nanoparticles and Spatially-Modulated Illumination. Cancers 2021, 13, 2544. [Google Scholar] [CrossRef] [PubMed]
- Hada, A.-M.; Craciun, A.-M.; Focsan, M.; Borlan, R.; Soritau, O.; Todea, M.; Astilean, S. Folic Acid Functionalized Gold Nanoclusters for Enabling Targeted Fluorescence Imaging of Human Ovarian Cancer Cells. Talanta 2021, 225, 121960. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Sakhare, N.; Das, S.; Kale, P.; Mathur, A.; Mirapurkar, S.; Muralidharan, S.; Chaudhari, P.; Mohanty, B.; Ballal, A.; et al. Development of Technetium-99m Labeled Ultrafine Gold Nanobioconjugates for Targeted Imaging of Folate Receptor Positive Cancers. Nucl. Med. Biol. 2021, 93, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, Y.; Fu, H.; Huang, H.; Wu, Z.; Zhao, M.; Yang, X.; Guo, Q.; Duan, Y.; Sun, Y. Multifunctional Tumor-Targeted PLGA Nanoparticles Delivering Pt(IV)/SiBIRC5 for US/MRI Imaging and Overcoming Ovarian Cancer Resistance. Biomaterials 2021, 269, 120478. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, K.C.; Zareba, J.K.; Toporkiewicz, M.; Chodaczek, G.; Wawrzynczyk, D.; Kulbacka, J.; Bazylinska, U.; Nyk, M. Polymeric Nanocarriers with Luminescent Colloidal Nanoplatelets as Hydrophilic and Non-Toxic Two-Photon Bioimaging Agents. Int. J. Nanomed. 2021, 16, 3649–3660. [Google Scholar] [CrossRef] [PubMed]
- Kantamneni, H.; Barkund, S.; Donzanti, M.; Martin, D.; Zhao, X.; He, S.; Riman, R.E.; Tan, M.C.; Pierce, M.C.; Roth, C.M.; et al. Shortwave Infrared Emitting Multicolored Nanoprobes for Biomarker-Specific Cancer Imaging in Vivo. BMC Cancer 2020, 20, 1082. [Google Scholar] [CrossRef] [PubMed]
- Asgari, M.; Khanahmad, H.; Motaghi, H.; Farzadniya, A.; Mehrgardi, M.A.; Shokrani, P. Aptamer Modified Nanoprobe for Multimodal Fluorescence/Magnetic Resonance Imaging of Human Ovarian Cancer Cells. Appl. Phys. A 2021, 127, 47. [Google Scholar] [CrossRef]
- Han, C.; Xie, T.; Wang, K.; Jin, S.; Li, K.; Dou, P.; Yu, N.; Xu, K. Development of Fluorescence/MR Dual-Modal Manganese-Nitrogen-Doped Carbon Nanosheets as an Efficient Contrast Agent for Targeted Ovarian Carcinoma Imaging. J. Nanobiotechnol. 2020, 18, 175. [Google Scholar] [CrossRef]
- Wu, F.; Gao, H.; Qiu, R.; Zhang, H.; Ren, X.; Qi, X.; Miao, M.; Rui, C.; Chang, D.; Pan, H. A Novel Ratiometric Electrochemical Immunosensor for the Detection of Cancer Antigen 125 Based on Three-Dimensional Carbon Nanomaterial and MOFs. Microchem. J. 2024, 200, 110372. [Google Scholar] [CrossRef]
- Gharehaghaji, Z.H.; Khalilzadeh, B.; Yousefi, H.; Mohammad-Rezaei, R. An Electrochemical Immunosensor Based on MXene-GQD/AuNPs for the Detection of Trace Amounts of CA-125 as Specific Tracer of Ovarian Cancer. Microchim. Acta 2024, 191, 418. [Google Scholar] [CrossRef]
- Er, O.F.; Kivrak, H.; Alpaslan, D.; Dudu, T.E. One-Step Electrochemical Sensing of CA-125 Using Onion Oil-Based Novel Organohydrogels as the Matrices. ACS Omega 2024, 9, 17919–17930. [Google Scholar] [CrossRef]
- Johari-Ahar, M.; Rashidi, M.R.; Barar, J.; Aghaie, M.; Mohammadnejad, D.; Ramazani, A.; Karami, P.; Coukos, G.; Omidi, Y. An Ultra-Sensitive Impedimetric Immunosensor for Detection of the Serum Oncomarker CA-125 in Ovarian Cancer Patients. Nanoscale 2015, 7, 3768–3779. [Google Scholar] [CrossRef]
- Jafari, M.; Hasanzadeh, M.; Solhi, E.; Hassanpour, S.; Shadjou, N.; Mokhtarzadeh, A.; Jouyban, A.; Mahboob, S. Ultrasensitive Bioassay of Epitope of Mucin-16 Protein (CA 125) in Human Plasma Samples Using a Novel Immunoassay Based on Silver Conductive Nano-Ink: A New Platform in Early Stage Diagnosis of Ovarian Cancer and Efficient Management. Int. J. Biol. Macromol. 2019, 126, 1255–1265. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, H.; Ma, Z. A Nanocomposite Containing Prussian Blue, Platinum Nanoparticles and Polyaniline for Multi-Amplification of the Signal of Voltammetric Immunosensors: Highly Sensitive Detection of Carcinoma Antigen 125. Microchim. Acta 2017, 184, 4269–4277. [Google Scholar] [CrossRef]
- Li, H.; Qin, J.; Li, M.; Li, C.; Xu, S.; Qian, L.; Yang, B. Gold-Nanoparticle-Decorated Boron-Doped Graphene/BDD Electrode for Tumor Marker Sensor. Sens. Actuators B Chem. 2020, 302, 127209. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Sahmani, R.; Solhi, E.; Mokhtarzadeh, A.; Shadjou, N.; Mahboob, S. Ultrasensitive Immunoassay of Carcinoma Antigen 125 in Untreated Human Plasma Samples Using Gold Nanoparticles with Flower like Morphology: A New Platform in Early Stage Diagnosis of Ovarian Cancer and Efficient Management. Int. J. Biol. Macromol. 2018, 119, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Wang, H.; Wu, D.; Fan, D.; Zhang, Y.; Du, B.; Wei, Q. Ultrasensitive Immunoassay for CA125 Detection Using Acid Site Compound as Signal and Enhancer. Talanta 2015, 144, 535–541. [Google Scholar] [CrossRef]
- Raghav, R.; Srivastava, S. Copper(II) Oxide Nanoflakes Based Impedimetric Immunosensor for Label Free Determination of Cancer Antigen-125. Sens. Lett. 2016, 14, 97–101. [Google Scholar] [CrossRef]
- Biswas, S.; Lan, Q.; Xie, Y.; Sun, X.; Wang, Y. Label-Free Electrochemical Immunosensor for Ultrasensitive Detection of Carbohydrate Antigen 125 Based on Antibody-Immobilized Biocompatible MOF-808/CNT. ACS Appl. Mater. Interfaces 2021, 13, 3295–3302. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, R.; Chai, Y.; Min, L.; Li, W.; Xu, Y. Electrochemical Sensing Platform Based on Tris(2,2′-Bipyridyl)Cobalt(III) and Multiwall Carbon Nanotubes–Nafion Composite for Immunoassay of Carcinoma Antigen-125. Electrochim. Acta 2009, 54, 7242–7247. [Google Scholar] [CrossRef]
- Yue, Y.; Chen, X.; Wang, J.; Ma, M.; He, A.; Liu, R. Label-Free Electrochemical Biosensor with Magnetically Induced Self-Assembly for the Detection of Cancer Antigen 125. Arab. J. Chem. 2023, 16, 105070. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, S.; Zhang, S.; Gao, L.; Lin, F.; Dai, H. Self-Reduced MXene-Metal Interaction Electrochemiluminescence Support with Synergistic Electrocatalytic and Photothermal Effects for the Bimodal Detection of Ovarian Cancer Biomarkers. J. Colloid Interface Sci. 2024, 661, 793–801. [Google Scholar] [CrossRef]
- Zoughi, S.; Faridbod, F.; Moradi, S. Rapid Enzyme-Free Detection of MiRNA-21 in Human Ovarian Cancerous Cells Using a Fluorescent Nanobiosensor Designed Based on Hairpin DNA-Templated Silver Nanoclusters. Anal. Chim. Acta 2024, 1320, 342968. [Google Scholar] [CrossRef]
- Ren, X.; Wu, F.; Wu, M.; Gao, H.; Wu, C.; Mu, W.; Liu, S.; Que, L.; Zhang, H.; Miao, M.; et al. Sandwich-Type Immunosensor Based on Aminated 3D-RGOF-NH2 and CMK-3-Fc-MgAl-LDH Multilayer Nanocomposites for Detection of CA125. Bioelectrochemistry 2024, 156, 108613. [Google Scholar] [CrossRef]
- Can, F.; Akkas, T.; Bekler, S.Y.; Takmakli, S.; Uzun, L.; Ozaydin Ince, G. Selective Determination of an Ovarian Cancer Biomarker at Low Concentrations with Surface Imprinted Nanotube Based Chemosensor. Bioelectrochemistry 2024, 157, 108655. [Google Scholar] [CrossRef]
- Lin, X.; Cai, F.; Lin, J.; Zhang, K.; Lin, Y. Digital Multimeter-Based Portable Photoelectrochemical Immunoassay with Enzyme-Catalyzed Precipitation for Screening Carbohydrate Antigen 125. Anal. Methods 2024, 16, 4619–4625. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yuan, J.; Wang, C.; Wang, T.; Zhao, F.; Zeng, B. CdS/Bi2S3/NiS Ternary Heterostructure-Based Photoelectrochemical Immunosensor for the Sensitive Detection of Carbohydrate Antigen 125. Anal. Chim. Acta 2024, 1312, 342765. [Google Scholar] [CrossRef] [PubMed]
- Maghiani, I.; Souza, L.V.; Bach-Toledo, L.; Faria, A.M.; Ortega, P.P.; Amoresi, R.A.C.; Simões, A.Z.; Mazon, T. Application of NiFe2O4 Nanoparticles towards the Detection of Ovarian Cancer Marker. Mater. Res. Bull. 2024, 177, 112835. [Google Scholar] [CrossRef]
- Yılmaz, M.; Bilgi, M. A Disposable Impedimetric Immunosensor for the Analysis of CA125 in Human Serum Samples. Biomed. Microdevices 2024, 26, 8. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Tang, L.; Yu, Y.; Zhang, J.; Zhou, X.; Zhou, T.; Xuan, C.; Tian, Q.; Pan, D. Cooperative Amplification of Prussian Blue as a Signal Indicator and Functionalized Metal-Organic Framework-Based Electrochemical Biosensor for an Ultrasensitive HE4 Assay. Biosens. Bioelectron. 2024, 262, 116541. [Google Scholar] [CrossRef]
- Szymanska, B.; Lukaszewski, Z.; Zelazowska-Rutkowska, B.; Hermanowicz-Szamatowicz, K.; Gorodkiewicz, E. An SPRi Biosensor for Determination of the Ovarian Cancer Marker HE4 in Human Plasma. Sensors 2021, 21, 3567. [Google Scholar] [CrossRef]
- Wang, B.; Liang, G.; Meng, L.; Li, H.; Song, Z.; Xu, Y.; He, Y.; Duan, D.; Shi, Q.; Guan, T.; et al. A Dual Immunosensor Based on Optical Weak Value Amplification for Simultaneous Detection of CA125 and HE4. Sensors 2025, 25, 3347. [Google Scholar] [CrossRef]
- Davoudian, K.; Spagnolo, S.; Lotay, N.; Satkauskas, M.; Mészáros, G.; Hianik, T.; Keresztes, Z.; Walker, G.; Thompson, M. Design and Characterization of a Dual-Protein Strategy for an Early-Stage Assay of Ovarian Cancer Biomarker Lysophosphatidic Acid. Biosensors 2024, 14, 287. [Google Scholar] [CrossRef]
- Joshi, H.C.; Kharkwal, H.; Kumar, A.; Gupta, P.K. Development of a Quartz Crystal Microbalance-Based Immunosensor for the Early Detection of Mesothelin in Cancer. Sens. Int. 2023, 4, 100248. [Google Scholar] [CrossRef]
- Saorin, A.; Saorin, G.; Duzagac, F.; Parisse, P.; Cao, N.; Corona, G.; Cavarzerani, E.; Rizzolio, F. Microfluidic Production of Amiodarone Loaded Nanoparticles and Application in Drug Repositioning in Ovarian Cancer. Sci. Rep. 2024, 14, 6280. [Google Scholar] [CrossRef]
- Giannitelli, S.M.; Limiti, E.; Mozetic, P.; Pinelli, F.; Han, X.; Abbruzzese, F.; Basoli, F.; Del Rio, D.; Scialla, S.; Rossi, F.; et al. Droplet-Based Microfluidic Synthesis of Nanogels for Controlled Drug Delivery: Tailoring Nanomaterial Properties via Pneumatically Actuated Flow-Focusing Junction. Nanoscale 2022, 14, 11415–11428. [Google Scholar] [CrossRef] [PubMed]
- Rios De La Rosa, J.M.; Spadea, A.; Donno, R.; Lallana, E.; Lu, Y.; Puri, S.; Caswell, P.; Lawrence, M.J.; Ashford, M.; Tirelli, N. Microfluidic-Assisted Preparation of RGD-Decorated Nanoparticles: Exploring Integrin-Facilitated Uptake in Cancer Cell Lines. Sci. Rep. 2020, 10, 14505. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.-Y.; Huang, C.-C.; Chen, Y.-S.; Hsu, K.-F.; Lee, G.-B. Isolation and Quantification of Extracellular Vesicle-Encapsulated MicroRNA on an Integrated Microfluidic Platform. Lab. Chip 2021, 21, 4660–4671. [Google Scholar] [CrossRef] [PubMed]
- Jou, H.-J.; Chou, L.-Y.; Chang, W.-C.; Ho, H.-C.; Zhang, W.-T.; Ling, P.-Y.; Tsai, K.-H.; Chen, S.-H.; Chen, T.-H.; Lo, P.-H.; et al. An Automatic Platform Based on Nanostructured Microfluidic Chip for Isolating and Identification of Circulating Tumor Cells. Micromachines 2021, 12, 473. [Google Scholar] [CrossRef]
- Chang, T.-Y.; Chen, S.-W.; Lin, W.-H.; Huang, C.-E.; Evans, M.I.; Chung, I.-F.; Wu, J.-W.; Ma, G.-C.; Chen, M. Comparison of Genetic Profiling between Primary Tumor and Circulating Tumor Cells Captured by Microfluidics in Epithelial Ovarian Cancer: Tumor Heterogeneity or Allele Dropout? Diagnostics 2021, 11, 1102. [Google Scholar] [CrossRef]
- Saadati, A.; Hassanpour, S.; Bahavarnia, F.; Hasanzadeh, M. A Novel Biosensor for the Monitoring of Ovarian Cancer Tumor Protein CA 125 in Untreated Human Plasma Samples Using a Novel Nano-Ink: A New Platform for Efficient Diagnosis of Cancer Using Paper Based Microfluidic Technology. Anal. Methods 2020, 12, 1639–1649. [Google Scholar] [CrossRef]
- Bahavarnia, F.; Saadati, A.; Hassanpour, S.; Hasanzadeh, M.; Shadjou, N.; Hassanzadeh, A. Paper Based Immunosensing of Ovarian Cancer Tumor Protein CA 125 Using Novel Nano-Ink: A New Platform for Efficient Diagnosis of Cancer and Biomedical Analysis Using Microfluidic Paper-Based Analytical Devices (ΜPAD). Int. J. Biol. Macromol. 2019, 138, 744–754. [Google Scholar] [CrossRef]
- Prakobkij, A.; Kitchawengkul, N.; Anutrasakda, W.; Vilaivan, T.; Wanram, S.; Amatatongchai, M.; Citterio, D.; Jarujamrus, P. A Paper-Based Colorimetric Sensor for Tumor Marker CA-125 Using Aptamer-Enhanced Ni–MnFe Layered Double Hydroxide Nanozymes. ACS Appl. Nano Mater. 2025, 8, 14556–14566. [Google Scholar] [CrossRef]
- Wimalachandra, D.C.; Li, Y.; Liu, J.; Shikha, S.; Zhang, J.; Lim, Y.-C.; Zhang, Y. Microfluidic-Based Immunomodulation of Immune Cells Using Upconversion Nanoparticles in Simulated Blood Vessel–Tumor System. ACS Appl. Mater. Interfaces 2019, 11, 37513–37523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhou, X.; He, M.; Shang, Y.; Tetlow, A.L.; Godwin, A.K.; Zeng, Y. Ultrasensitive Detection of Circulating Exosomes with a 3D-Nanopatterned Microfluidic Chip. Nat. Biomed. Eng. 2019, 3, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Hisey, C.L.; Dorayappan, K.D.P.; Cohn, D.E.; Selvendiran, K.; Hansford, D.J. Microfluidic Affinity Separation Chip for Selective Capture and Release of Label-Free Ovarian Cancer Exosomes. Lab. Chip 2018, 18, 3144–3153. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, J.; Mu, B.; Shi, H. A Design of Quartz Crystal Microbalance Immune Sensing System for Ovarian Cancer Tumor Markers Detection. In Proceedings of the 2024 18th Symposium on Piezoelectricity, Acoustic Waves, and Device Applications (SPAWDA), Dongguan, China, 8–11 November 2024; pp. 538–542. [Google Scholar]
- Dadashzadeh, A.; Moghassemi, S.; Amorim, C.A. Evaluation of PEGylated Fibrin as a Three-Dimensional Biodegradable Scaffold for Ovarian Tissue Engineering. Mater. Today Chem. 2021, 22, 100626. [Google Scholar] [CrossRef]
- Kim, J.; Perez, A.S.; Claflin, J.; David, A.; Zhou, H.; Shikanov, A. Synthetic Hydrogel Supports the Function and Regeneration of Artificial Ovarian Tissue in Mice. NPJ Regen. Med. 2016, 1, 16010. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, Y.; Yang, B.; Li, Y. Nanocomposite Hydrogel Bioinks for 3D Bioprinting of Tumor Models. Biomacromolecules 2024, 25, 5288–5299. [Google Scholar] [CrossRef]
- Giordano, G.; Ferioli, E.; Tafuni, A. The Role of Mesothelin Expression in Serous Ovarian Carcinoma: Impacts on Diagnosis, Prognosis, and Therapeutic Targets. Cancers 2022, 14, 2283. [Google Scholar] [CrossRef]
- Spizzo, G.; Fong, D.; Wurm, M.; Ensinger, C.; Obrist, P.; Hofer, C.; Mazzoleni, G.; Gastl, G.; Went, P. EpCAM Expression in Primary Tumour Tissues and Metastases: An Immunohistochemical Analysis. J. Clin. Pathol. 2011, 64, 415–420. [Google Scholar] [CrossRef]
- Charkhchi, P.; Cybulski, C.; Gronwald, J.; Wong, F.O.; Narod, S.A.; Akbari, M.R. CA125 and Ovarian Cancer: A Comprehensive Review. Cancers 2020, 12, 3730. [Google Scholar] [CrossRef]
- Leung, Y.Y.; Llaurado-Fernandez, M.; Cameron, A.; Da-Anoy, A.; Cook, L.C.; Hoenisch, J.; Ghesquiere, C.; Gaillard, S.; Schmid, J.; Dawson, A.; et al. FOLR1 as a Therapeutic Target in Platinum-Resistant Ovarian Carcinoma: Unique Expression Patterns across Ovarian Carcinoma Histotypes and Molecular Subtypes of Low-Grade Serous Carcinoma. J. Gynecol. Oncol. 2025, 36, e74. [Google Scholar] [CrossRef]
- Kim, Y.-N.; Chung, Y.S.; Park, E.; Lee, S.T.; Lee, J.-Y. Human Epidermal Growth Factor Receptor-2 Expression and Subsequent Dynamic Changes in Patients with Ovarian Cancer. Sci. Rep. 2024, 14, 7992. [Google Scholar] [CrossRef]
- Nasioudis, D.; Gysler, S.; Latif, N.; Cory, L.; Giuntoli, R.L.; Kim, S.H.; Simpkins, F.; Martin, L.; Ko, E.M. Molecular Landscape of ERBB2/HER2 Gene Amplification among Patients with Gynecologic Malignancies; Clinical Implications and Future Directions. Gynecol. Oncol. 2024, 180, 1–5. [Google Scholar] [CrossRef]
- Wang, L.; Lin, X.; Sun, P. UPAR, beyond Regulating Physiological Functions, Has Orchestrated Roles in Cancer (Review). Int. J. Oncol. 2022, 61, 151. [Google Scholar] [CrossRef]
- Al-Hassan, N.N.; Behzadian, A.; Caldwell, R.; Ivanova, V.S.; Syed, V.; Motamed, K.; Said, N.A. Differential Roles of UPAR in Peritoneal Ovarian Carcinomatosis. Neoplasia 2012, 14, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Figueras, A.; Alsina-Sanchís, E.; Lahiguera, Á.; Abreu, M.; Muinelo-Romay, L.; Moreno-Bueno, G.; Casanovas, O.; Graupera, M.; Matias-Guiu, X.; Vidal, A.; et al. A Role for CXCR4 in Peritoneal and Hematogenous Ovarian Cancer Dissemination. Mol. Cancer Ther. 2018, 17, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Si, J.; Li, S.; Yu, J.; Ma, D.; Li, T.; Yu, Y.; Kong, L.; Li, X.; et al. Octreotide Modified Liposomes That Co-Deliver Paclitaxel and Neferine Effectively Inhibit Ovarian Cancer Metastasis by Specifically Binding to the SSTR2 Receptors. J. Drug Deliv. Sci. Technol. 2024, 98, 105851. [Google Scholar] [CrossRef]
- Tang, S.; Liu, Q.; Song, M.; Li, X.; Ji, D.; Yang, Y.-W.; Yu, H. A Silica Nanobean Carrier Utilizing Lysosomal and Mitochondrial Autophagy to Kill Ovarian Cancer Cell. APL Mater. 2024, 12, 051114. [Google Scholar] [CrossRef]
- Colli, C.; Masi, I.; Jacchetti, E.; Santoni, S.; Sponchioni, M.; Colosimo, B.M.; Rosanò, L.; Raimondi, M.T.; Mauri, E.; Moscatelli, D. Zwitterionic Nanoparticles for Thermally Activated Drug Delivery in Hyperthermia Cancer Treatment. Nanoscale 2024, 16, 12635–12649. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, H.; Zhang, L.; Zhu, C.; Du, X.; Wang, L.; Chen, H.; Liu, J. Lysophosphatidic Acid Responsive Photosensitive Supramolecular Organic Frameworks for Tumor Imaging, Drug Loading, and Photodynamic Therapy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 310, 123923. [Google Scholar] [CrossRef]
- Xia, X.; Yang, X.; Gao, W.; Huang, W.; Xia, X.; Yan, D. A Novel HER2 Targeting Nanoagent Self-Assembled from Affibody-Epothilone B Conjugate for Cancer Therapy. J. Nanobiotechnol. 2024, 22, 502. [Google Scholar] [CrossRef]
- Yi, Y.; Zhong, L.; Chu, X.; Wan, Q.; Hu, A.; Liao, B. Ginsenoside RG3-Loaded Microneedles for in Situ Treatment of Ovarian Cancer. J. Drug Deliv. Sci. Technol. 2024, 97, 105643. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Q.; Shen, J.; Liu, Z.; Cui, X.; Ma, L.; Zheng, Y.; Wang, L.; Ying, T. Magnetically Actuated Cisplatin-Loaded Nanoparticle Collectives Enhance Drug Penetration for Potentiated Ovarian Cancer Chemotherapy. J. Colloid Interface Sci. 2025, 678, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Saddam Hussain, M.; Khetan, R.; Albrecht, H.; Krasowska, M.; Blencowe, A. Oligoelectrolyte-Mediated, PH-Triggered Release of Hydrophobic Drugs from Non-Responsive Micelles: Influence of Oligo(2-Vinyl Pyridine)-Loading on Drug-Loading, Release and Cytotoxicity. Int. J. Pharm. 2024, 661, 124368. [Google Scholar] [CrossRef]
- Juul, C.A.; Engel, T.B.; Fliedner, F.P.; Ringgaard, L.; Eliasen, R.; Melander, F.; Bak, M.; Kjær, A.; Henriksen, J.R.; Elema, D.R.; et al. HER2-Targeted, Enzyme-Activated Liposomes Show Superior in Vivo Efficacy in an Ovarian Cancer Model. J. Control. Release 2024, 371, 288–297. [Google Scholar] [CrossRef]
- Alharbi, M.; Lai, A.; Godbole, N.; Guanzon, D.; Nair, S.; Zuñiga, F.; Quinn, A.; Yang, M.; Wu, S.Y.; Salomon, C. Enhancing Precision Targeting of Ovarian Cancer Tumor Cells in Vivo through Extracellular Vesicle Engineering. Int. J. Cancer 2024, 155, 1510–1523. [Google Scholar] [CrossRef]
- Zhao, C.; Qiu, L.; Wu, D.; Zhang, M.; Xia, W.; Lv, H.; Cheng, L. Targeted Reversal of Multidrug Resistance in Ovarian Cancer Cells Using Exosome-encapsulated Tetramethylpyrazine. Mol. Med. Rep. 2023, 29, 25. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; Song, K.; Wang, W.; Zhang, N.; Dai, L.; Di, W. Nanoparticle Co-Delivery of Carboplatin and PF543 Restores Platinum Sensitivity in Ovarian Cancer Models through Inhibiting Platinum-Induced pro-Survival Pathway Activation. Nanoscale Adv. 2024, 6, 4082–4093. [Google Scholar] [CrossRef]
- Gaikwad, N.M.; Chaudhari, P.D.; Shaikh, K.S.; Chaudhari, S.Y.; Pathare, S.S.; Shaikh, A.A.; Aljarba, N.H.; Kumer, A.; Dhara, B. Dual Drug-loaded Polymeric Mixed Micelles for Ovarian Cancer: Approach to Enhanced Therapeutic Efficacy of Albendazole and Paclitaxel. J. Cell Mol. Med. 2024, 28, e18389. [Google Scholar] [CrossRef]
- Gong, K.; Liao, J.; Lin, J.; Wang, Q.; Wu, Z.; Wang, L.; Zhang, J.; Dong, Y.; Duan, Y.; Chen, J. Mitochondria-Targeted Nanoparticles Overcome Chemoresistance via Downregulating BACH1/CD47 Axis in Ovarian Carcinoma. Chin. Chem. Lett. 2024, 35, 108888. [Google Scholar] [CrossRef]
- Lu, Q.; Gao, W.; Chen, Z.; Liu, Z.; Wang, J.; Zeng, L.; Hu, X.; Zheng, E.; Zhang, Q.; Song, H. Co-Delivery of Paclitaxel/Atovaquone/Quercetin to Regulate Energy Metabolism to Reverse Multidrug Resistance in Ovarian Cancer by PLGA-PEG Nanoparticles. Int. J. Pharm. 2024, 655, 124028. [Google Scholar] [CrossRef]
- Tang, L.; Wang, Y.-J.; Wang, Y.-Y.; Li, S.-T.; Kong, L.; Li, X.-T.; Ma, L.-L.; Liu, X.-X. Construction of ROS-Responsive Hyaluronic Acid Modified Paclitaxel and Diosgenin Liposomes and Study on Synergistic Enhancement of Anti-Ovarian Cancer Efficacy. Int. J. Nanomed. 2024, 19, 5193–5211. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, Y.; Yang, C.; Li, F.; Yang, B.; Li, R.; Qiu, B.; Li, Y.; Liu, L. Aptamer-Modified Zinc Ferrite Nanoparticles for Autophagy-Mediated Chemotherapy and MR Imaging of Ovarian Cancer. ACS Appl. Nano Mater. 2024, 7, 1055–1066. [Google Scholar] [CrossRef]
- Bian, X.; Guo, T.; Chen, G.; Nie, D.; Yue, M.; Zhu, Y.; Lin, M. The Therapeutic Effect and MR Molecular Imaging of FA-PEG-FePt/DDP Nanoliposomes in AMF on Ovarian Cancer. Int. J. Nanomed. 2024, 19, 5227–5243. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Li, H.; Yu, D.; Liu, W.; Dong, S.; Zhang, X.; Xu, K.; Guo, W.; Li, W.; Wang, T. Apoptosis-Induced Treatment for Ovarian Cancer from Self-Assembled Nanocomposites of Fe3O4 Nanoparticles and Porous Organic Cages. Colloids Surf. A Physicochem. Eng. Asp. 2024, 695, 134070. [Google Scholar] [CrossRef]
- Xu, L.; Ma, S.; Fan, B.; Yuan, Z.; Yin, P. Bufalin-Loaded Vitamin E Succinate-Grafted Chitosan Oligosaccharide/RGD-Conjugated TPGS Mixed Micelles Inhibit Intraperitoneal Metastasis of Ovarian Cancer. Cancer Nanotechnol. 2023, 14, 25. [Google Scholar] [CrossRef]
- Chen, T.; Wang, Y.; Zhu, L.; Wu, J.; Lin, J.; Huang, W.; Yan, D. Hybrid Membrane Camouflaged Chemodrug-Gene Nanoparticles for Enhanced Combination Therapy of Ovarian Cancer. ACS Appl. Mater. Interfaces 2023, 15, 58067–58078. [Google Scholar] [CrossRef]
- Han, L.; Song, T.; Wang, X.; Luo, Y.; Gu, C.; Li, X.; Wen, J.; Wen, Z.; Shi, X. MiR-21 Responsive Nanocarrier Targeting Ovarian Cancer Cells. Comput. Struct. Biotechnol. J. 2024, 24, 196–204. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, S.; Sienkiewicz, J.; Zhou, H.; Berahovich, R.; Sun, J.; Li, M.; Ocampo, A.; Liu, X.; Huang, Y.; et al. HER2-CD3-Fc Bispecific Antibody-Encoding MRNA Delivered by Lipid Nanoparticles Suppresses HER2-Positive Tumor Growth. Vaccines 2024, 12, 808. [Google Scholar] [CrossRef]
- Xi, M.; Deng, Z.; Zhang, C.; Wu, X.; Zhang, L.; Zhang, Y.; Sun, X.; Zhou, J.; Yang, G. FeS-Based Cascade Bioreactors Driven by Shewanella Oneidensis MR-1 for Efficient Chemodynamic Therapy with Augmented Antitumor Immunity. Nano Today 2024, 55, 102165. [Google Scholar] [CrossRef]
- Wang, S.; Mao, Y.; Rong, S.; Liu, G.; Cao, Y.; Yang, Z.; Yu, H.; Zhang, X.; Fang, H.; Cai, Z.; et al. Engineering Magnetic Extracellular Vesicles Mimetics for Enhanced Targeting Chemodynamic Therapy to Overcome Ovary Cancer. ACS Appl. Mater. Interfaces 2024, 16, 39021–39034. [Google Scholar] [CrossRef]
- Liang, S.; Xiao, L.; Chen, T.; Roa, P.; Cocco, E.; Peng, Z.; Yu, L.; Wu, M.; Liu, J.; Zhao, X.; et al. Injectable Nanocomposite Hydrogels Improve Intraperitoneal Co-Delivery of Chemotherapeutics and Immune Checkpoint Inhibitors for Enhanced Peritoneal Metastasis Therapy. ACS Nano 2024, 18, 18963–18979. [Google Scholar] [CrossRef]
- Wu, H.; Ding, X.; Chen, Y.; Cai, Y.; Yang, Z.; Jin, J. EGFR-Targeted Humanized Single Chain Antibody Fragment Functionalized Silica Nanoparticles for Precision Therapy of Cancer. Int. J. Biol. Macromol. 2023, 253, 127538. [Google Scholar] [CrossRef]
- Li, Y.; Tong, F.; Wang, Y.; Wang, J.; Wu, M.; Li, H.; Guo, H.; Gao, H. In Situ Tumor Vaccine with Optimized Nanoadjuvants and Lymph Node Targeting Capacity to Treat Ovarian Cancer and Metastases. Acta Pharm. Sin. B 2024, 14, 4102–4117. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiang, Y.; Koellhoffer, E.C.; Shukla, S.; Fiering, S.; Chen, S.; Steinmetz, N.F. 3D Bioprinting Cowpea Mosaic Virus as an Immunotherapy Depot for Ovarian Cancer Prevention in a Preclinical Mouse Model. Mater. Adv. 2024, 5, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Voznyuk, A.A.; Makarets, Y.A.; Advakhova, D.Y.; Khafizov, K.A.; Lugovoi, M.E.; Zakharova, V.A.; Senatov, F.S.; Koudan, E.V. Biodegradable Local Chemotherapy Platform with Prolonged and Controlled Release of Doxorubicin for the Prevention of Local Tumor Recurrence. ACS Appl. Bio Mater. 2024, 7, 2472–2487. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.V.; Nepacina, M.R.; Hsu, Y.-C. The Study of Designing a Controlled Drug Release Using Oxaliplatin-Loaded Hydrogel for Ovarian Cancer Treatment. J. Taiwan Inst. Chem. Eng. 2024, 163, 105326. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, L.; Guo, T.; Huang, L.; Yang, Y.; Ye, R.; Zhang, Y.; Lin, X.; Fan, Y.; Gong, C.; et al. Cationic Liposomes Overcome Neutralizing Antibodies and Enhance Reovirus Efficacy in Ovarian Cancer. Virology 2024, 598, 110196. [Google Scholar] [CrossRef]
- Kalogera, E.; Nevala, W.K.; Finnes, H.D.; Suman, V.J.; Schimke, J.M.; Strand, C.A.; Kottschade, L.A.; Kudgus, R.A.; Buhrow, S.A.; Becher, L.R.; et al. A Phase I Trial of Nab-Paclitaxel/Bevacizumab (AB160) Nano-Immunoconjugate Therapy for Gynecologic Malignancies. Clin. Cancer Res. 2024, 30, 2623–2635. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Y.; Tong, H.; Sun, X.; Lv, Z.; Yong, J.; Wu, Y.; Xiang, X.; Ding, F.; Zuo, X.; et al. Programmable DNA Hydrogel Assisting Microcrystal Formulations for Sustained Locoregional Drug Delivery in Surgical Residual Tumor Lesions and Lymph Node Metastasis. Adv. Healthc. Mater. 2024, 13, e2303762. [Google Scholar] [CrossRef]
- Han, X.; Li, G.; You, S.; Shen, M.; Xu, Y.; Yang, H.; Lu, C.; Zhang, M.; Fang, J.; Zhou, Q.; et al. Injectable Bio-Multifunctional Hyaluronic Acid-Based Hydrogels Loaded with Poly ADP-Ribose Polymerase Inhibitors for Ovarian Cancer Therapy. Int. J. Biol. Macromol. 2024, 270, 132275. [Google Scholar] [CrossRef]
- Chen, J.; Huang, L.; Yang, X.; Li, Y.; Lin, T.; Zhang, C.; Li, J.; Luo, X. Development of Receptor-Targeted and PH-Responsive Zeolitic Imidazole Framework-90 Nanoplatform for Anti-Ovarian Cancer. Inorg. Chem. Commun. 2024, 167, 112715. [Google Scholar] [CrossRef]
- Panebianco, R.; Viale, M.; Giglio, V.; Vecchio, G. Investigating the Anticancer Properties of Novel Functionalized Platinum(II)–Terpyridine Complexes. Inorganics 2024, 12, 167. [Google Scholar] [CrossRef]
- Nosrati, S.; Javid, H.; Amiri, H.; Jafari, N.; Hashemy, S.I. Investigating the Anticancer Effects of Chitosan-NLC-Folate Nanohybrid Loaded with Auraptene on A2780 Ovarian Cancer Cells. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 398, 1895–1903. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Du, Y.; Zhao, J.; Sha, S.; Wang, J. 177Lu-FA-DOTA-PEG-PLGA Nanoparticles Show Antitumor Efficiency in Targeting Ovarian Cancer. Clin. Exp. Obstet. Gynecol. 2024, 51, 118. [Google Scholar] [CrossRef]
- Varshosaz, J.; Ghassami, E.; Noorbakhsh, A.; Jahanian-Najafabadi, A.; Minaiyan, M.; Behzadi, R. Poly (Butylene Adipate-Co-Butylene Terephthalate) Nanoparticles Prepared by Electrospraying Technique for Docetaxel Delivery in Ovarian Cancer Induced Mice. Drug Dev. Ind. Pharm. 2018, 44, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Sun, Z.; Wang, B.; Liu, X.; Hu, B.; Chen, N.; Zhang, S.; Yu, Z. Suicide Gene Delivery by Morphology-Adaptable Enantiomeric Peptide Assemblies for Combined Ovarian Cancer Therapy. Acta Biomater. 2024, 175, 250–261. [Google Scholar] [CrossRef]
- Wen, A.; Mei, X.; Feng, C.; Shen, C.; Wang, B.; Zhang, X. Electrosprayed Nanoparticles of Poly(p-Dioxanone-Co-Melphalan) Macromolecular Prodrugs for Treatment of Xenograft Ovarian Carcinoma. Mater. Sci. Eng. C 2020, 111, 110759. [Google Scholar] [CrossRef]
- Yan, E.; Fan, Y.; Sun, Z.; Gao, J.; Hao, X.; Pei, S.; Wang, C.; Sun, L.; Zhang, D. Biocompatible Core–Shell Electrospun Nanofibers as Potential Application for Chemotherapy against Ovary Cancer. Mater. Sci. Eng. C 2014, 41, 217–223. [Google Scholar] [CrossRef]
- Teja Surikutchi, B.; Obenza-Otero, R.; Russo, E.; Zelzer, M.; Golán Cancela, I.; Costoya, J.A.; Crecente Campo, J.; José Alonso, M.; Marlow, M. Development of a Nanocapsule-Loaded Hydrogel for Drug Delivery for Intraperitoneal Administration. Int. J. Pharm. 2022, 622, 121828. [Google Scholar] [CrossRef]
- Shin, D.H.; Kwon, G.S. Pre-Clinical Evaluation of a Themosensitive Gel Containing Epothilone B and MTOR/Hsp90 Targeted Agents in an Ovarian Tumor Model. J. Control. Release 2017, 268, 176–183. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, J.; Guo, R.; Chen, G.; Shen, Y.; Wu, Y.; Wang, J.; Lin, Z.; Wang, K.; Chen, J.; et al. Enhancing Antitumor Efficacy of NIR-I Region Zinc Phthalocyanine@upconversion Nanoparticle through Lysosomal Escape and Mitochondria Targeting. J. Photochem. Photobiol. B 2024, 255, 112923. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, G.; Chen, Z.; Yang, X.; Zhang, B.; Wang, S.; Li, Z.; Yang, Y.; Wu, Y.; Liu, Z.; et al. Mitochondria-Targeted Polyprodrug Nanoparticles Induce Mitochondrial Stress for Immunogenic Chemo-Photodynamic Therapy of Ovarian Cancer. J. Control. Release 2024, 371, 470–483. [Google Scholar] [CrossRef]
- Fan, Y.; Wen, J.; Shi, L.; Dai, Y. Engineering of Smart Nanostructured Lipid Carriers Encapsulated in Gold Nanocomposites and Camptothecin for Synergistic Photothermal and Chemotherapy Treatment of Ovarian Cancer. J. Mater. Sci. 2024, 59, 5518–5537. [Google Scholar] [CrossRef]
- Yu, H.; He, X.; Zhou, L.; Chen, L.; Lu, H.; Wang, J.; Gao, L. Exploring the Potential of Carbon-Coated MoSe2 Nanoparticles as a Photothermal Therapy for Ovarian Cancer. Arab. J. Chem. 2024, 17, 105495. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, W.; Zhang, R.; Zhang, C.; Yan, J.; Feng, J.; Rosenholm, J.M.; Shi, T.; Shen, X.; Zhang, H. Minimally Invasive Injection of Biomimetic Nano@Microgel for in Situ Ovarian Cancer Treatment through Enhanced Photodynamic Reactions and Photothermal Combined Therapy. Mater. Today Bio 2023, 20, 100663. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Davaa, E.; Jiang, Y.; Shin, K.-J.; Kim, M.H.; An, H.; Kim, J.; Cho, S.K.; Yang, S.-G. Pheophorbide A and SN38 Conjugated Hyaluronan Nanoparticles for Photodynamic- and Cascadic Chemotherapy of Cancer Stem-like Ovarian Cancer. Carbohydr. Polym. 2022, 289, 119455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ouyang, Y.; Sohn, Y.S.; Fadeev, M.; Karmi, O.; Nechushtai, R.; Stein, I.; Pikarsky, E.; Willner, I. MiRNA-Guided Imaging and Photodynamic Therapy Treatment of Cancer Cells Using Zn(II)-Protoporphyrin IX-Loaded Metal–Organic Framework Nanoparticles. ACS Nano 2022, 16, 1791–1801. [Google Scholar] [CrossRef]
- Zhou, Y.; Cao, Z.; Jiang, L.; Chen, Y.; Cui, X.; Wu, J.; Xie, X.; Wang, L.; Ying, T. Magnetically Actuated Sonodynamic Nanorobot Collectives for Potentiated Ovarian Cancer Therapy. Front. Bioeng. Biotechnol. 2024, 12, 1374423. [Google Scholar] [CrossRef]
- Yue, S.; He, Y.; Wang, M.; Liu, X.; Li, X.; Zhao, B.; Yi, Q.; Li, Q.; Yu, Q.; Yang, Z. Enhancement of Sonodynamic Treatment of Ovarian Cancer Based on Pt-B-P Ternary Nanoparticles. Nanomedicine 2023, 51, 102686. [Google Scholar] [CrossRef]
- Lee, H.R.; Kim, D.W.; Jones, V.O.; Choi, Y.; Ferry, V.E.; Geller, M.A.; Azarin, S.M. Sonosensitizer-Functionalized Graphene Nanoribbons for Adhesion Blocking and Sonodynamic Ablation of Ovarian Cancer Spheroids. Adv. Healthc. Mater. 2021, 10, 2001368. [Google Scholar] [CrossRef]
- Zheng, J.; Sun, J.; Chen, J.; Zhu, S.; Chen, S.; Liu, Y.; Hao, L.; Wang, Z.; Chang, S. Oxygen and Oxaliplatin-Loaded Nanoparticles Combined with Photo-Sonodynamic Inducing Enhanced Immunogenic Cell Death in Syngeneic Mouse Models of Ovarian Cancer. J. Control. Release 2021, 332, 448–459. [Google Scholar] [CrossRef]
- Zheng, J.; Sun, Y.; Long, T.; Yuan, D.; Yue, S.; Zhang, N.; Yang, Z. Sonosensitizer Nanoplatform-Mediated Sonodynamic Therapy Induced Immunogenic Cell Death and Tumor Immune Microenvironment Variation. Drug Deliv. 2022, 29, 1164–1175. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, L.; Liu, S.; Chen, Q.; Zeng, L.; Chen, X.; Zhang, Q. Moderating Hypoxia and Promoting Immunogenic Photodynamic Therapy by HER-2 Nanobody Conjugate Nanoparticles for Ovarian Cancer Treatment. Nanotechnology 2021, 32, 425101. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; Marangon, I.; Méreaux, J.; Nicolás-Boluda, A.; Lavieu, G.; Wilhelm, C.; Sarda-Mantel, L.; Silva, A.K.A.; Pocard, M.; Gazeau, F. Immune Reprogramming Precision Photodynamic Therapy of Peritoneal Metastasis by Scalable Stem-Cell-Derived Extracellular Vesicles. ACS Nano 2021, 15, 3251–3263. [Google Scholar] [CrossRef] [PubMed]
- Luan, S.; Tran, N.T.; Xue, H.-Y.; Wong, H.-L. Development of a High Payload, Cancer-Targeting Liposomes of Methyl Aminolevulinate for Intraoperative Photodynamic Diagnosis/Therapy of Peritoneal Carcinomatosis. Int. J. Pharm. 2021, 602, 120612. [Google Scholar] [CrossRef] [PubMed]
- Potara, M.; Nagy-Simon, T.; Focsan, M.; Licarete, E.; Soritau, O.; Vulpoi, A.; Astilean, S. Folate-Targeted Pluronic-Chitosan Nanocapsules Loaded with IR780 for near-Infrared Fluorescence Imaging and Photothermal-Photodynamic Therapy of Ovarian Cancer. Colloids Surf. B Biointerfaces 2021, 203, 111755. [Google Scholar] [CrossRef]
- Rakowski, J.A.; Ahmad, S.; Holloway, R.W. Use of Pegylated Liposomal Doxorubicin in the Management of Platinum-Sensitive Recurrent Ovarian Cancer: Current Concepts. Expert. Rev. Anticancer. Ther. 2012, 12, 31–40. [Google Scholar] [CrossRef]
- Nam, S.H.; Lee, S.-W.; Lee, Y.-J.; Kim, Y.M. Safety and Tolerability of Weekly Genexol-PM, a Cremophor-Free Polymeric Micelle Formulation of Paclitaxel, with Carboplatin in Gynecologic Cancer: A Phase I Study. Cancer Res. Treat. 2023, 55, 1346–1354. [Google Scholar] [CrossRef]
- Lee, S.-W.; Kim, Y.-M.; Cho, C.H.; Kim, Y.T.; Kim, S.M.; Hur, S.Y.; Kim, J.-H.; Kim, B.-G.; Kim, S.-C.; Ryu, H.-S.; et al. An Open-Label, Randomized, Parallel, Phase II Trial to Evaluate the Efficacy and Safety of a Cremophor-Free Polymeric Micelle Formulation of Paclitaxel as First-Line Treatment for Ovarian Cancer: A Korean Gynecologic Oncology Group Study (KGOG-3021). Cancer Res. Treat. 2018, 50, 195–203. [Google Scholar] [CrossRef]
- Coleman, R.L.; Brady, W.E.; McMeekin, D.S.; Rose, P.G.; Soper, J.T.; Lentz, S.S.; Hoffman, J.S.; Shahin, M.S. A Phase II Evaluation of Nanoparticle, Albumin-Bound (Nab) Paclitaxel in the Treatment of Recurrent or Persistent Platinum-Resistant Ovarian, Fallopian Tube, or Primary Peritoneal Cancer: A Gynecologic Oncology Group Study. Gynecol. Oncol. 2011, 122, 111–115. [Google Scholar] [CrossRef]
- Benigno, B.B.; Burrell, M.O.; Daugherty, P.; Hernandez, P. A Phase II Nonrandomized Study of Nab-Paclitaxel plus Carboplatin in Patients with Recurrent Platinum-Sensitive Ovarian or Primary Peritoneal Cancer. J. Clin. Oncol. 2010, 28, 5011. [Google Scholar] [CrossRef]
- Narayana, R.V.L.; Gupta, R. Exploring the Therapeutic Use and Outcome of Antibody-Drug Conjugates in Ovarian Cancer Treatment. Oncogene 2025, 44, 2343–2356. [Google Scholar] [CrossRef]
- Weiss, G.J.; Chao, J.; Neidhart, J.D.; Ramanathan, R.K.; Bassett, D.; Neidhart, J.A.; Choi, C.H.J.; Chow, W.; Chung, V.; Forman, S.J.; et al. First-in-Human Phase 1/2a Trial of CRLX101, a Cyclodextrin-Containing Polymer-Camptothecin Nanopharmaceutical in Patients with Advanced Solid Tumor Malignancies. Investig. New Drugs 2013, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, A.H.; Shaikh, S.; Kundaikar, G.; Furtado, S. Toxicological Aspects of Nanomaterials in Biomedical Research. In Advances in Nano and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2023; pp. 369–391. [Google Scholar]
- Aljabali, A.A.; Obeid, M.A.; Bashatwah, R.M.; Serrano-Aroca, Á.; Mishra, V.; Mishra, Y.; El-Tanani, M.; Hromić-Jahjefendić, A.; Kapoor, D.N.; Goyal, R.; et al. Nanomaterials and Their Impact on the Immune System. Int. J. Mol. Sci. 2023, 24, 2008. [Google Scholar] [CrossRef] [PubMed]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Baudis, S.; Roch, T.; Balk, M.; Wischke, C.; Lendlein, A.; Behl, M. Multivariate Analysis of Cellular Uptake Characteristics for a (Co)Polymer Particle Library. ACS Biomater. Sci. Eng. 2024, 10, 1481–1493. [Google Scholar] [CrossRef]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.; Huang, R. Nanoparticles-induced Potential Toxicity on Human Health: Applications, Toxicity Mechanisms, and Evaluation Models. MedComm 2023, 4, e327. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. P T 2017, 42, 742–755. [Google Scholar]
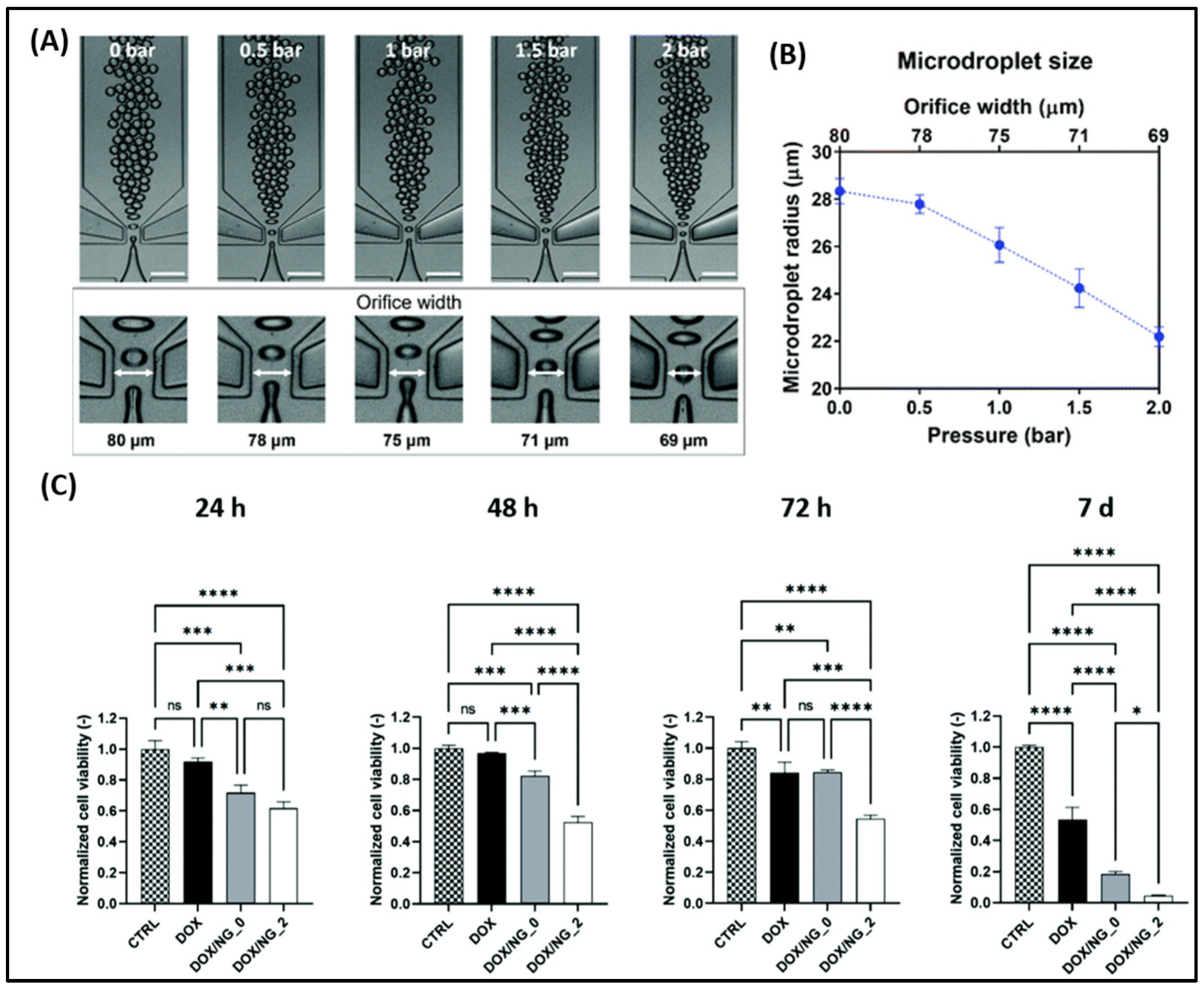

| Delivery System | Drug(s) | Targeting Mechanism | Stimuli/Trigger | Key Findings | Reference |
|---|---|---|---|---|---|
| Octreotide-modified liposome | Paclitaxel, Neferine | SSTR2-mediated endocytosis | - | Enhanced targeting via somatostatin receptor binding; extended circulation time | [88] |
| Dual-functionalized mesoporous silica nanobean | Doxorubicin | Lysosomal/mitochondrial localization | Elevated GSH levels, reduced pH | Disulfide bond cleavage activates fluorescence and drug release; mitochondrial dysfunction and autophagy induction | [89] |
| Thermoresponsive polymer nanoparticles made from (p(SB-co-ZB)) copolymers and vinyl oligoesters | Paclitaxel | Temperature-triggered release | Hyperthermia (43 °C) | Nearly complete drug release at 43 °C while retaining >95% at physiological temperature (37 °C) | [90] |
| Supramolecular organic framework | Doxorubicin | cRGD peptide targeting | Binding with lysophosphatidic acid (LPA) | SOF disassembly and LPA binding; combined chemotherapy and photodynamic therapy | [91] |
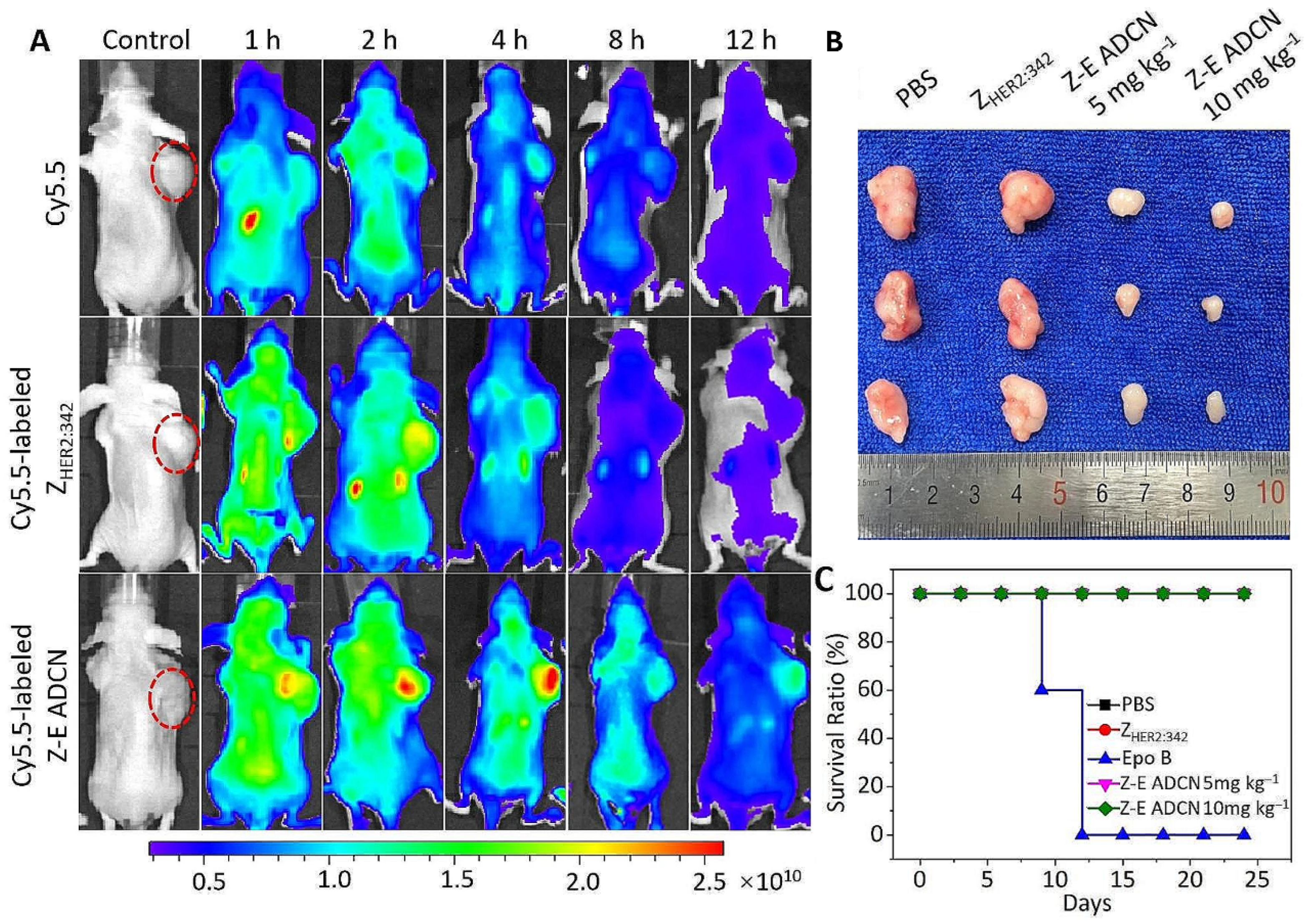
| ZHER2:342-Epo B Affibody-Drug Conjugate Nanoagent | Epothilone B | HER2 receptor targeting | Elevated reactive oxygen species | ROS-sensitive thioketal group enables drug release at the target site | [92] |
| Methacryloyl gelatin microneedle | Ginsenoside RG3 | Direct tumor penetration | Mechanical insertion | Bypasses first-pass metabolism; optimal release kinetics with enhanced bioavailability | [93] |
| Magnetic mesoporous silica nanoparticle | Cisplatin | External magnetic field guidance | Acidic tumor environment | Deep tumor penetration; pH-responsive drug release with reduced systemic toxicity | [94] |
| pH-responsive polymeric micelle | Doxorubicin, Paclitaxel, Gossypol, SN38 | pH-responsive release | pH changes (<6.5) | Rapid drug release triggered by acidic pH; enhanced cytotoxicity compared to non-responsive systems | [95] |
| Trastuzumab-modified liposome | Oxaliplatin | HER2 receptor targeting | Peptide linker cleavage by Matrix metalloproteinases | PEG layer removal leads to liposome destabilization and drug release | [96] |
| Ephrin-B2 and LAMP2b modified extracellular vesicle (EV) | - | Ephrin-B4 receptor targeting | Cellular internalization | Enhanced EV internalization compared to unmodified vesicles in vitro and in vivo | [97] |
| Tetramethylpyrazine-loaded exosome | Paclitaxel | Natural EV-mediated delivery | Cellular uptake | Overcame drug resistance by downregulating MDR proteins; enhanced apoptosis in resistant cells | [98] |
| PLGA nanoparticle | PF543 + Carboplatin | SphK1 inhibition | Targets pro-survival pathways regulated by sphingosine kinase 1 | Sensitized platinum-resistant SKOV3 cells to carboplatin in vitro and in vivo | [99] |
| TPGS-Soluplus® nanomicelle | Albendazole + Paclitaxel | Cellular entry via folic acid receptor | Dual drug delivery with sustained release profile | Superior cytotoxicity compared with free drugs; effective tumor targeting and penetration over 90 h | [100] |
| Folic acid receptor-targeted nanoparticle | Cisplatin | BACH1/CD47 inhibition | Inhibits BACH1 and CD47, promotes apoptosis, enhances macrophage phagocytosis | Increased M1 macrophages in tumors; potential for platinum-resistant ovarian cancer | [101] |
| PLGA-PEG nanoparticle | Paclitaxel + Atovaquone + Quercetin | ATP depletion | Inhibits oxidative phosphorylation and glycolysis to deplete ATP | Suppressed P-gp activity, increased drug accumulation, halted tumor growth in in vivo A2780/Taxol model | [102] |
| PEG-thioketal-hyaluronic acid- paclitaxel and diosgenin liposome | Paclitaxel + Diosgenin | CD44 targeting via hyaluronic acid | Long circulation time with targeted accumulation | Prolonged circulation time with enhanced drug delivery to tumor site | [103] |
| Nucleic acid aptamer-modified zinc ferrite nanoparticle | Zinc ferrite + Cisplatin | Tumor-targeting aptamer (NucA) | Induces autophagy via zinc ion release and ROS generation | Enhanced cisplatin sensitivity; dual capabilities (therapeutic and MRI contrast) | [104] |
| Ferroplatinum nanoliposome | Cisplatin | Folic acid receptor targeting | Dual therapeutic and diagnostic approach | Combined chemotherapy with imaging capabilities | [105] |
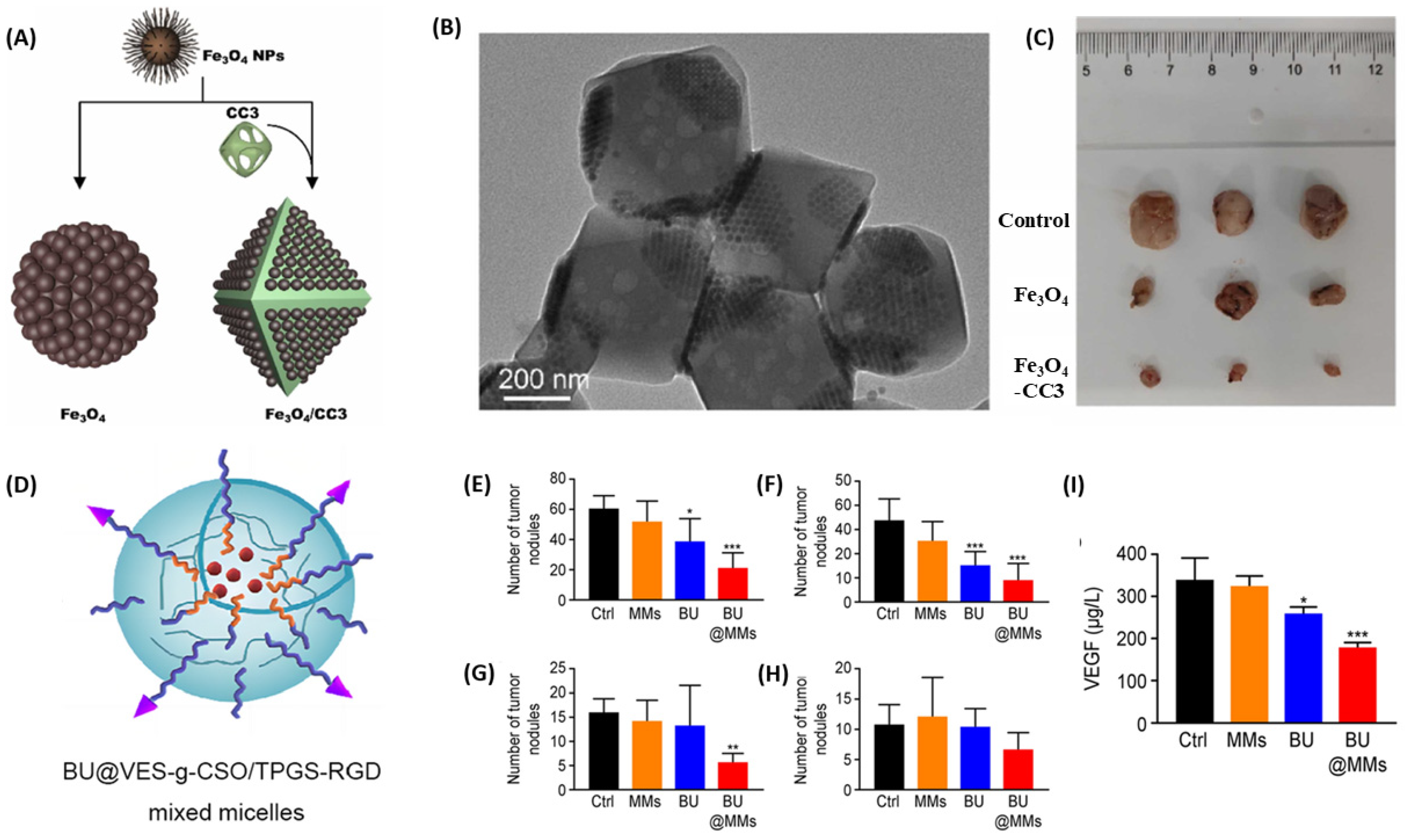
| Fe3O4-porous organic cage CC3 nanocomposite | Fe3O4 + CC3 cage | Direct tumor targeting | CytC/caspase-3 apoptotic pathway activation | Significant inhibition of SKOV3 subcutaneous tumors in nude mice | [106] |
| chitosan oligosaccharide—d-alpha-tocopheryl polyethylene glycol 1000 succinate micelle | Bufalin | RGD peptide-mediated targeting | Targets integrin αvβ3 receptors | Reduced migration/invasion of cancerous cells, induction of apoptosis, decreased tumor burden in intraperitoneal metastasis model | [107] |
| Delivery System | Therapeutic Agent | Mechanism/Target | Key Findings | Reference |
|---|---|---|---|---|
| Cell membrane-camouflaged nanoparticle | Mitoxantrone, Her2 antisense oligonucleotides | RBC membrane for immune evasion, cancer cell membrane for targeting | 75.7% apoptosis in vitro, 83.3% tumor suppression in vivo | [108] |
| DNA Tubular Origami | Fluorescent-labeled DNA strands | miR-21 downregulation | Significant decrease in miR-21 expression; effective cargo protection | [109] |
| Lipid nanoparticle | HER2-CD3-Fc bispecific antibody | HER2 and CD3 dual targeting caused T-cell activation | Strong T cell-directed cytotoxicity against HER2-positive cells | [110] |
| FeS@MR-1 bacterial nanoparticle | FeS present on Shewanella oneidensis MR-1 | Chemodynamic therapy via Fenton reaction | H2S and Fe(II) release; systemic antitumor responses | [111] |
| Extracellular vesicle mimetic nanovesicle | β-Lapachone, iron oxide nanoparticles | •OH generation and GSH depletion | H2O2 generation and enhanced oxidative stress via GSH depletion | [112] |
| Injectable nanohydrogel | Paclitaxel, anti-PD-1 antibody | Sustained release of chemotherapy and immunotherapy agents from hydrogel | Sustained release over 7 days; suppression of metastasis | [113] |
| EGFR-targeted mesoporous silica nanoparticle | Doxorubicin | EGFR targeting with husA modification of mesoporous silica nanoparticles | Very high drug-to-antibody ratio (11.8) for an antibody-drug conjugate; enzyme/pH-responsive release | [114] |
| Smart nanogel vaccine system | CpG@Man-P, trametinib, PD-1 antibody | TLR9 activation and tumor antigen generation | 40 nm particles with superior lymph node accumulation, tumor growth and metastasis suppression | [115] |
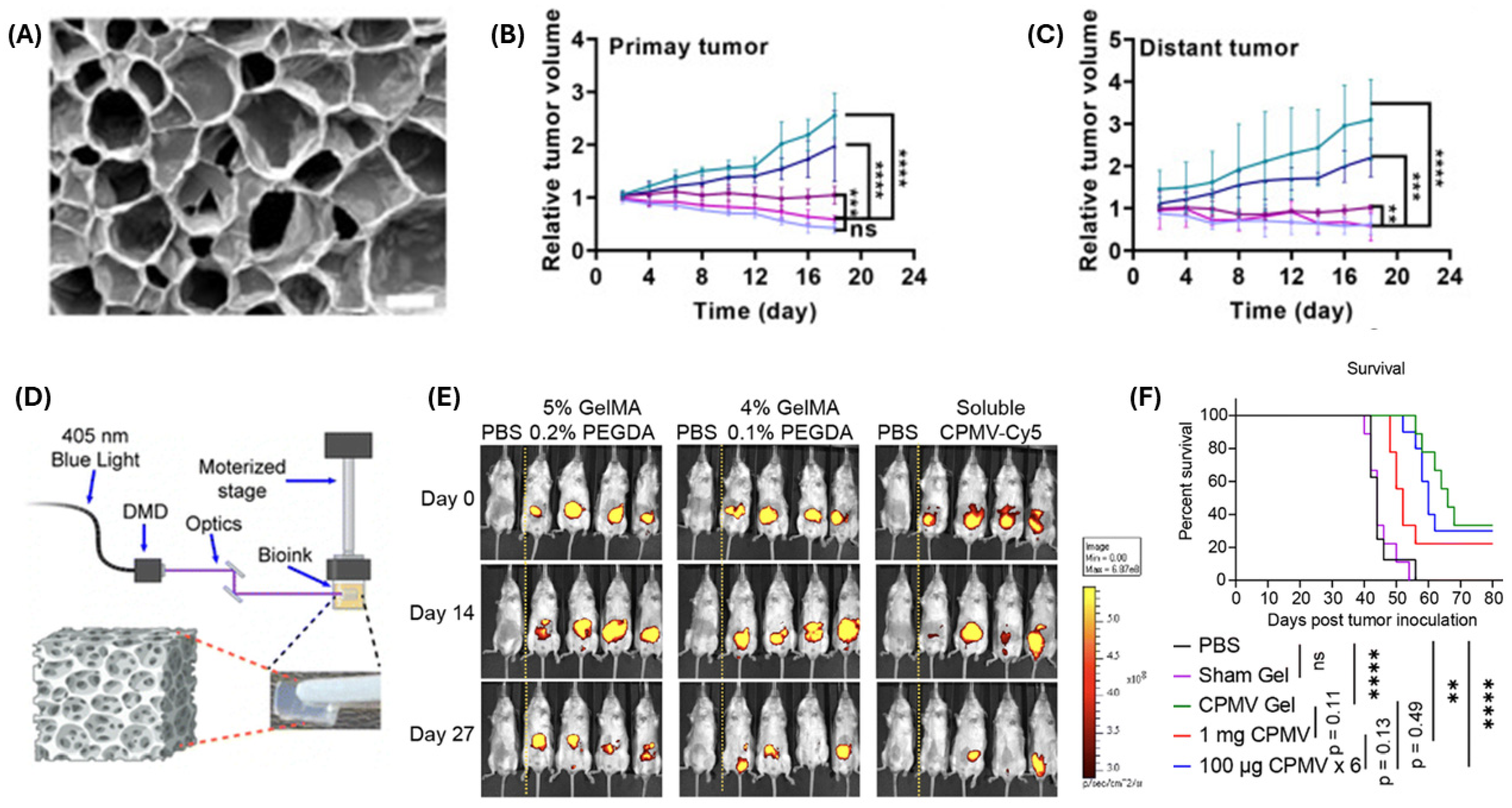
| CPMV-loaded hydrogel depot | Cowpea mosaic virus | TLR agonist (TLR 2, 4, 7) and innate immune cell activation | Sustained immunogenicity; improved survival with slow-release depots | [116] |
| Biodegradable polymer platform | Doxorubicin | PCL core with multilayer coating of γ-PGA | Controlled release > 150 days | [117] |
| Diels-Alder hydrogel | Oxaliplatin | Crosslinking between furan-modified alginate and maleimido-PLGA-PEG triblock copolymer | 69.9% entrapment efficiency; 78.8% release over one week | [118] |
| Cationic liposome | Reovirus | Caveolin-mediated endocytosis | Enhanced reovirus uptake despite the presence of neutralizing antibodies | [119] |
| AB160 antibody-drug conjugate | Paclitaxel | Bevacizumab-paclitaxel noncovalent binding | Phase I trial has been completed | [120] |
| DNA hydrogel | Elimusertib | DNA strand-derived hydrogel for drug delivery | Microcrystallization of radiosensitizer elimusertib and its sustained release | [121] |
| Injectable hydrogel | Niraparib | Hydrogel made from hyaluronic acid and pluronic127 | Good injectability; 20-day biodegradation; anti-proliferative and anti-migratory effects | [122] |
| ZIF-90 nanoparticle | Carboplatin | Folate-PEG-NH2 modification with acid-responsive Schiff base bond | 6.84% release at physiological pH; 92.89% in acidic tumor environment | [123] |
| Platinum(II)-terpyridine complex | Platinum derivatives | 4′ carbon modification with amine, glucose, biotin, and hyaluronic acid (one at a time) | Platinum-terpyridine amino derivative compound outperformed cisplatin in cytotoxicity | [124] |
| Nanostructured lipid carrier | Auraptene, methylene blue | Folic acid-conjugated chitosan surface modification | P53 upregulation; Bax and Bcl-2 downregulation | [125] |
| Radiolabeled PLGA-PEG nanoparticle | 177Lutetium | Folic acid receptor targeting | 72 h blood circulation; low renal accumulation; comparable to chemotherapy | [126] |
| Electrosprayed nanoparticle | Docetaxel | Nanoparticles prepared from poly(butylene adipate-co-terephthalate) | Optimized conditions: 20 kV, 12 cm distance, 1:3 drug:polymer ratio | [127] |
| Enantiomeric peptide nanoparticle | Suicidal gene, paclitaxel | GSH-responsive disulfide bond cleavage | Transformation from particle to fiber upon GSH contact; dual therapeutic release | [128] |
| Melphalan prodrug nanoparticle | Melphalan-p-dioxanone copolymer | Lysosomal activation of electrosprayed prodrug | Enhanced cytotoxicity compared to free melphalan | [129] |
| Coaxial electrospun fiber | Doxorubicin | Core-PVA, shell-chitosan | Controlled drug delivery from electrospun fibers | [130] |
| National Clinical Trial (NCT) Number | Study Title | Study Status | Interventions |
|---|---|---|---|
| NCT04778839 | Study of Paclitaxel Micelles for Injection in Chinese Patients With Advanced Solid Tumors. | RECRUITING | Drug: Paclitaxel Micelles for Injection |
| NCT04669002 | EP0057 in Combination With Olaparib in Advanced Ovarian Cancer | COMPLETED | Drug: EP0057 (cyclodextrin-camptothecin nanoparticles) Drug: Olaparib tablets |
| NCT06048367 | Carbon Nanoparticle-Loaded Iron [CNSI-Fe(II)] in the Treatment of Advanced Solid Tumor | COMPLETED | Drug: CNSI-Fe(II) nanoparticles 30/60/90/120/150 mg |
| NCT05001282 | A Study to Evaluate ELU001 in Patients With Solid Tumors That Overexpress Folate Receptor Alpha (FRα) | TERMINATED | Drug: ELU001 nanoparticles (consist of ~13 folic acid targeting moieties and a payload of ~22 molecules of the topoisomerase-1 inhibitor, exatecan) |
| NCT05969041 | Study of MT-302 in Adults With Advanced or Metastatic Epithelial Tumors | RECRUITING | Drug: MT-302 (TROP2-targeting mRNA-based CAR therapy) |
| NCT05092373 | Phase I Study of Tumor Treating Fields (TTF) in Combination With Cabozantinib or With Pembrolizumab and Nab-Paclitaxel in Patients With Advanced Solid Tumors Involving the Abdomen or Thorax | RECRUITING | Biological: Atezolizumab Drug: Cabozantinib S-malate Drug: Nab-paclitaxel |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahfuz, A.M.U.B.; Janorkar, A.V.; Rocconi, R.P.; Duan, Y. Recent Advances in the Applications of Biomaterials in Ovarian Cancer. Biomimetics 2025, 10, 768. https://doi.org/10.3390/biomimetics10110768
Mahfuz AMUB, Janorkar AV, Rocconi RP, Duan Y. Recent Advances in the Applications of Biomaterials in Ovarian Cancer. Biomimetics. 2025; 10(11):768. https://doi.org/10.3390/biomimetics10110768
Chicago/Turabian StyleMahfuz, A M U B, Amol V. Janorkar, Rodney P. Rocconi, and Yuanyuan Duan. 2025. "Recent Advances in the Applications of Biomaterials in Ovarian Cancer" Biomimetics 10, no. 11: 768. https://doi.org/10.3390/biomimetics10110768
APA StyleMahfuz, A. M. U. B., Janorkar, A. V., Rocconi, R. P., & Duan, Y. (2025). Recent Advances in the Applications of Biomaterials in Ovarian Cancer. Biomimetics, 10(11), 768. https://doi.org/10.3390/biomimetics10110768







