Comparative Analysis of Flexural and Compressive Strengths of Bioactive Alkasite Compared to Other Ion-Releasing Restorative Materials
Abstract
1. Introduction
2. Materials and Methods
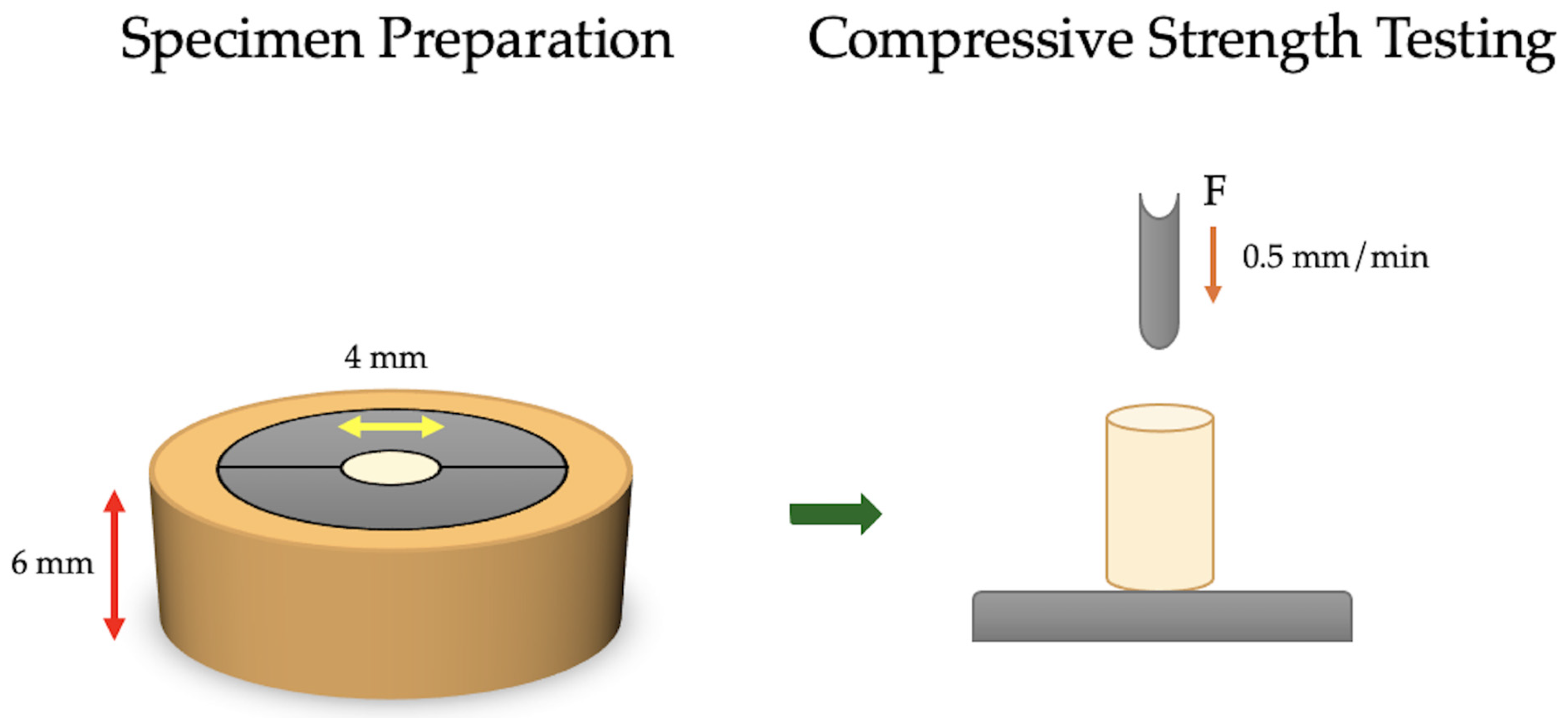
2.1. Compressive Strength Specimen Preparation and Testing
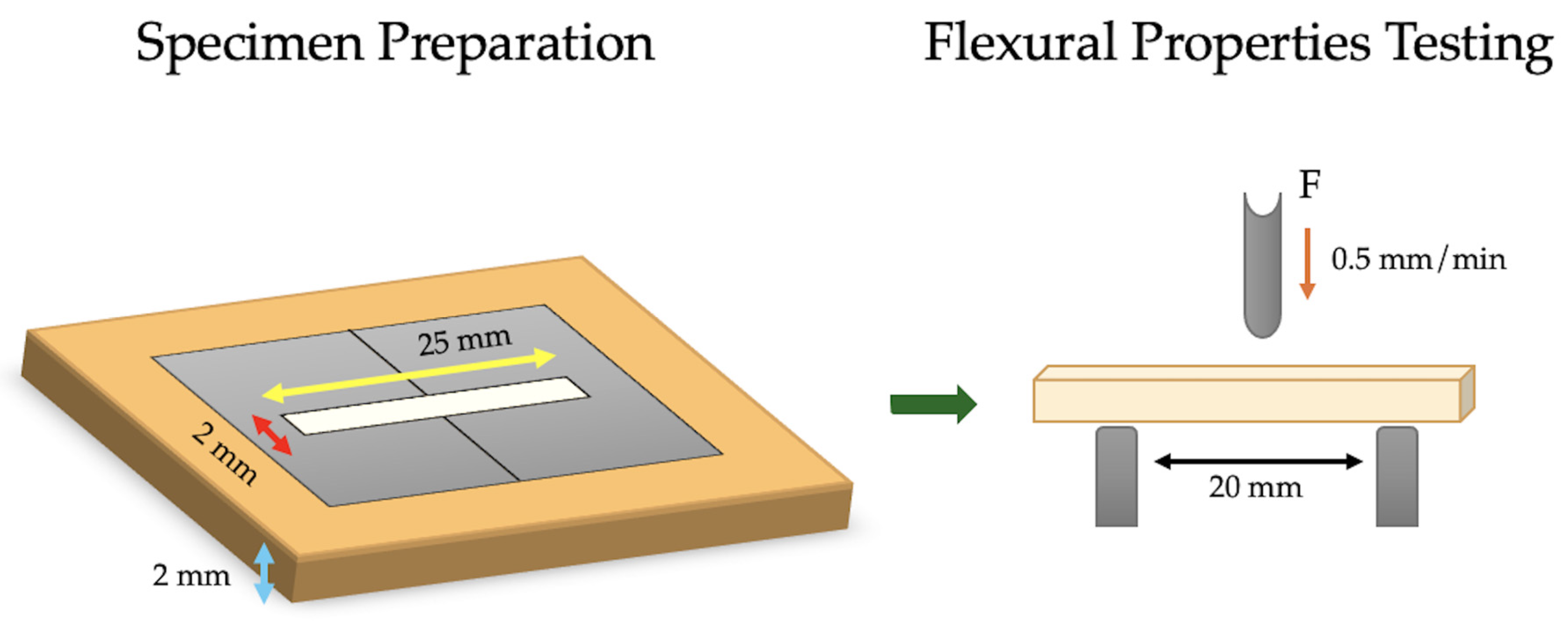
2.2. Flexural Properties’ Specimen Preparation and Testing
2.3. Statistical Analysis
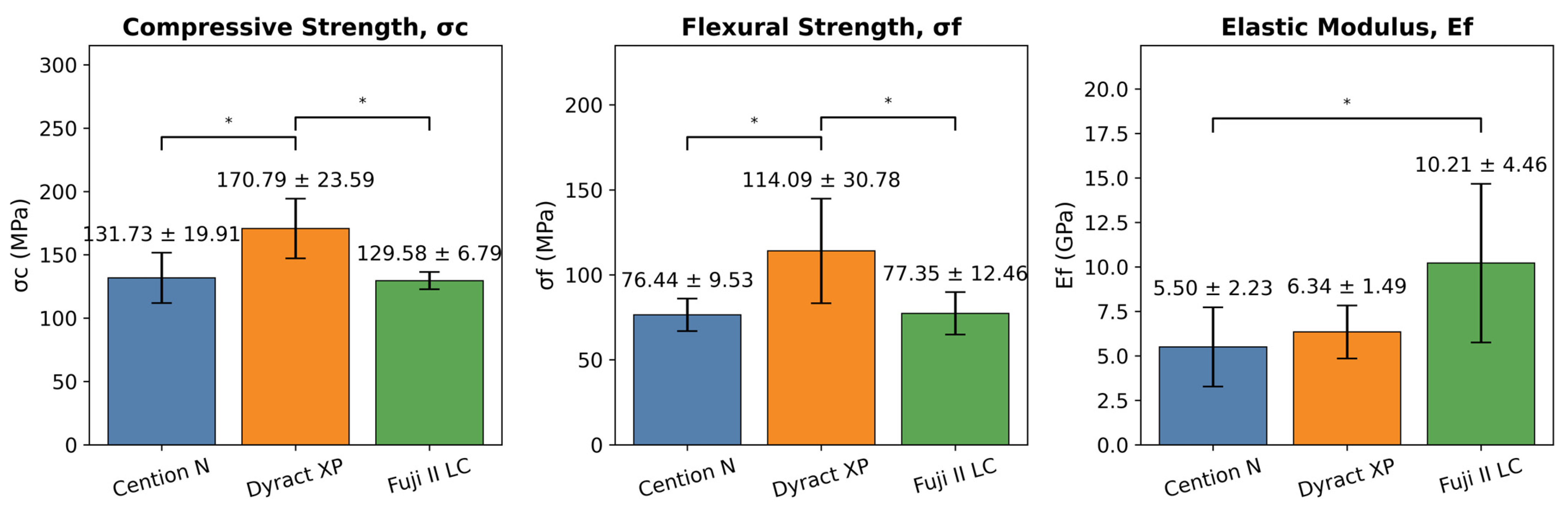
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| Bis-GMA | Bisphenol A-glycidyl methacrylate |
| CI | Confidence interval |
| DCP | Tricyclodecan-dimethanoldimethacrylate |
| DMA | Dimethacrylate |
| FAS | Fluoroaluminosilicate glass |
| GIC | Glass ionomer cement |
| HEMA | 2-Hydroxyethyl methacrylate |
| LED | Light emitting diode |
| RMGIC | Resin-modified glass ionomer cement |
| TEGDMA | Triethylene glycol dimethacrylate |
| TMPTMA | Trimethacrylate resin |
| UDMA | Urethane dimethacrylate |
References
- Hellyer, P. Dental caries. Br. Dent. J. 2025, 238, 36. [Google Scholar] [CrossRef]
- Featherstone, J.D.B.; Crystal, Y.O.; Alston, P.; Chaffee, B.W.; Doméjean, S.; Rechmann, P.; Zhan, L.; Ramos-Gomez, F. Evidence-Based Caries Management for All Ages-Practical Guidelines. Front. Oral Health 2021, 2, 657518. [Google Scholar] [CrossRef]
- Özcan, M.; Garcia, L.d.F.R.; Volpato, C.A.M. Bioactive Materials for Direct and Indirect Restorations: Concepts and Applications. Front. Dent. Med. 2021, 2, 647267. [Google Scholar] [CrossRef]
- Banerjee, A.; Splieth, C.; Breschi, L.; Fontana, M.; Paris, S.; Burrow, M.; Crombie, F.; Foster Page, L.; Gaton-Hernandez, P.; Giacaman, R.A.; et al. When to intervene in the caries process? A Delphi consensus statement. Br. Dent. J. 2020, 229, 474–482. [Google Scholar] [CrossRef]
- Singer, L.; Fouda, A.; Bourauel, C. Biomimetic approaches and materials in restorative and regenerative dentistry: Review article. BMC Oral Health 2023, 23, 105. [Google Scholar] [CrossRef]
- Pagano, S.; Lombardo, G.; Orso, M.; Abraha, I.; Capobianco, B.; Cianetti, S. Lasers to prevent dental caries: A systematic review. BMJ Open 2020, 10, e038638. [Google Scholar] [CrossRef] [PubMed]
- Amirtharaj Mosas, K.K.; Chandrasekar, A.R.; Dasan, A.; Pakseresht, A.; Galusek, D. Recent Advancements in Materials and Coatings for Biomedical Implants. Gels 2022, 8, 323. [Google Scholar] [CrossRef] [PubMed]
- Sadananda, V.; Shetty, C.; Hegde, M.; S Bhat, G. Alkasite restorative material: Flexural and compressive strength evaluation. Res. J. Pharm. Biol. Chem. Sci. 2018, 9, 2179. [Google Scholar]
- Radwanski, M.; Zmyslowska-Polakowska, E.; Osica, K.; Krasowski, M.; Sauro, S.; Hardan, L.; Lukomska-Szymanska, M. Mechanical properties of modern restorative “bioactive” dental materials—An in vitro study. Sci. Rep. 2025, 15, 3552. [Google Scholar] [CrossRef]
- do Nascimento Santos, J.V.; Magalhães, G.d.A.P.; Costa Leite, J.V.; Pacheco, R.R.; Puppin-Rontani, R.M.; Ferracane, J.L.; Lima, R.B.W. From names to concepts: Unraveling bioactivity in restorative dental materials. J. Am. Dent. Assoc. 2025, 156, 355–373.e2. [Google Scholar] [CrossRef]
- Sajini, S.I. Effect of Staining and Bleaching protocol on Color Change of Bioactive Resin Composite Restorative Materials. J. Pharm. Bioallied Sci. 2025, 17 (Suppl. 2), S1960–S1965. [Google Scholar] [CrossRef] [PubMed]
- El-Adl, E.T.; Ebaya, M.M.; Habib, E.-S.E.; Zaghloul, N.M. Comparative measurement of short-term fluoride release and inhibition of caries around restoration by ion releasing restorative materials: An in vitro study. Sci. Rep. 2025, 15, 1600. [Google Scholar] [CrossRef]
- Alambiaga-Caravaca, A.M.; Chou, Y.F.; Moreno, D.; Aparicio, C.; López-Castellano, A.; Feitosa, V.P.; Tezvergil-Mutluay, A.; Sauro, S. Characterisation of experimental flowable composites containing fluoride-doped calcium phosphates as promising remineralising materials. J. Dent. 2024, 143, 104906. [Google Scholar] [CrossRef]
- Daabash, R.; Alshabib, A.; Alqahtani, M.Q.; Price, R.B.; Silikas, N.; Alshaafi, M.M. Ion releasing direct restorative materials: Key mechanical properties and wear. Dent. Mater. 2022, 38, 1866–1877. [Google Scholar] [CrossRef]
- Chistyakov, E.M.; Kolpinskaya, N.; Posokhova, V.; Chuev, V. Dental Composition Modified with Aryloxyphosphazene Containing Carboxyl Groups. Polymers 2020, 12, 1176. [Google Scholar] [CrossRef]
- Turkistani, A.; Yeslam, H.E. Comparative Evaluation of Color Stability in Bioactive and Conventional Resin Cements Under Thermal Stress Conditions. Biomimetics 2025, 10, 432. [Google Scholar] [CrossRef]
- Attik, N.; Richert, R.; Garoushi, S. Biomechanics, Bioactive and Biomimetic philosophy in restorative dentistry—Quo vadis? J. Dent. 2024, 148, 105036. [Google Scholar] [CrossRef]
- Lowe, R.A. Focus On “Bioactive Dental Materials”. Dent. Today 2017, 36, 16. [Google Scholar]
- Schmalz, G.; Hickel, R.; Price, R.B.; Platt, J.A. Bioactivity of Dental Restorative Materials: FDI Policy Statement. Int. Dent. J. 2023, 73, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Yadav, A.K.; Rathee, G.; Dhingra, K.; Das Mukherjee, M.; Solanki, P.R. Review-Prospects of Nanomaterial-Based Biosensors: A Smart Approach for Bisphenol-A Detection in Dental Sealants. J. Electrochem. Soc. 2022, 169, 027516. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, L.; Bai, R.; Zhuang, Z.; Zhang, Y.; Yu, T.; Peng, L.; Xin, T.; Chen, S.; Han, B. Recent Progress in Antimicrobial Strategies for Resin-Based Restoratives. Polymers 2021, 13, 1590. [Google Scholar] [CrossRef]
- Neti, B.; Sayana, G.; Muddala, L.; Raju, S.; Yarram, A.; Gvd, H. Fluoride releasing restorative materials: A review. Int. J. Dent. Mater. 2020, 02, 19–23. [Google Scholar] [CrossRef]
- Llancari-Alonzo, P.; Alvítez-Temoche, D.; Ladera-Castañeda, M.; Castro-Ramirez, L.; López-Gurreonero, C.; Cayo-Rojas, C. Fluoride release and flexural strength of four ion-releasing restorative materials: An in vitro comparative study. J. Clin. Exp. Dent. 2024, 16, e1207–e1216. [Google Scholar] [CrossRef]
- Hill, R. Glass ionomer polyalkenoate cements and related materials: Past, present and future. Br. Dent. J. 2022, 232, 653–657. [Google Scholar] [CrossRef]
- Puttipanampai, O.; Panpisut, P.; Sitthisettapong, T. Assessment of Fluoride-Releasing Materials in Remineralization of Adjacent Demineralized Enamel. Appl. Sci. 2025, 15, 2077. [Google Scholar] [CrossRef]
- Sajini, S.I.; Mushayt, A.B.; Almutairi, T.A.; Abuljadayel, R. Color Stability of Bioactive Restorative Materials After Immersion in Various Media. J. Int. Soc. Prev. Community Dent. 2022, 12, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.K.; Nicholson, J.W. A Review of Glass-Ionomer Cements for Clinical Dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Vaid, D.S.; Shah, N.C.; Bilgi, P.S. One year comparative clinical evaluation of EQUIA with resin-modified glass ionomer and a nanohybrid composite in noncarious cervical lesions. J. Conserv. Dent. JCD 2015, 18, 449. [Google Scholar] [CrossRef]
- Oleniacz-Trawińska, M.; Kotela, A.; Kensy, J.; Kiryk, S.; Dobrzyński, W.; Kiryk, J.; Gerber, H.; Fast, M.; Matys, J.; Dobrzyński, M. Evaluation of Factors Affecting Fluoride Release from Compomer Restorative Materials: A Systematic Review. Materials 2025, 18, 1627. [Google Scholar] [CrossRef] [PubMed]
- Kaptan, A.; Oznurhan, F.; Candan, M. In Vitro Comparison of Surface Roughness, Flexural, and Microtensile Strength of Various Glass-Ionomer-Based Materials and a New Alkasite Restorative Material. Polymers 2023, 15, 650. [Google Scholar] [CrossRef]
- Piwowarczyk, A.; Ottl, P.; Lauer, H.C.; Büchler, A. Laboratory strength of glass ionomer cement, compomers, and resin composites. J. Prosthodont. 2002, 11, 86–91. [Google Scholar] [CrossRef]
- El-Kalla, I.; Garcia-Godoy, F. Mechanical properties of compomer restorative materials. Oper. Dent. 1999, 24, 2–8. [Google Scholar] [PubMed]
- Bonta, D.; Tofan, S.; Todor, L.; Miron, M.; Talpos, C.; Cosroaba, R.; Borcan, F.; Popovici, R. In vitro Study on Mechanical Properties of Polyacid-modified Composite Resins (Compomers). Mater. Plast. 2022, 59, 90–98. [Google Scholar] [CrossRef]
- Yenidunya, O.G.; Misilli, T.; Yilmaz, E. Gastric acid challenge: Mechanical proficiency and surface gloss of tooth-colored restorative materials. BMC Oral Health 2025, 25, 614. [Google Scholar] [CrossRef]
- Bonchev, A.; Bogovska-Gigova, R. Alkasites in restorative dentistry: A review of their performance and properties. J. Dent. 2025, 160, 105916. [Google Scholar] [CrossRef] [PubMed]
- Chole, D.; Shah, H.K.; Kundoor, S.; Bakle, S.; Gandhi, N.; Hatte, N. In vitro comparison of flexural strength of cention-n, bulkFill composites, light-cure nanocomposites and resin-modified glass ionomer cement. J. Dent. Med. Sci. 2018, 17, 79â. [Google Scholar]
- Di Lauro, A.; Di Duca, F.; Montuori, P.; Dal Piva, A.M.O.; Tribst, J.P.M.; Borges, A.L.S.; Ausiello, P. Fluoride and Calcium Release from Alkasite and Glass Ionomer Restorative Dental Materials: In Vitro Study. J. Funct. Biomater. 2023, 14, 109. [Google Scholar] [CrossRef]
- Ballal, N.V.; Jalan, P.; Rai, N.; Al-Haj Husain, N.; Ozcan, M. Evaluation of New Alkasite Based Restorative Material for Restoring Non-Carious Cervical Lesions-Randomized Controlled Clinical Trial. Eur. J. Prosthodont. Restor. Dent. 2023, 31, 72–77. [Google Scholar]
- Abdallah, R.M.; Aref, N.S. Development of Newly Formulated Nanoalumina-/Alkasite-Based Restorative Material. Int. J. Dent. 2021, 2021, 9944909. [Google Scholar] [CrossRef]
- Fousiya, K.; Balagopal, V.R.; Suresh, K.J.; Kumaran, P.; Xavier, A.M.; Menon, M.M. Comparative Evaluation of Compressive Strength and Flexural Strength of Self-cured Cention N with Dual-cured Cention N: An In Vitro Study. Int. J. Clin. Pediatr. Dent. 2022, 15, 210–214. [Google Scholar]
- da Cunha, C.; Wambier, L.M.; Matos, T.P.; Malaquias, P.; Reis, A.; Loguercio, A.D.; Wambier, D.S.; Chibinski, A.C.R. New Dual-cure Resin-based Material in Occlusal and Occluso-proximal Restorations of Primary Teeth: Results of a Randomized Clinical Trial. Int. J. Clin. Pediatr. Dent. 2022, 15, 38–46. [Google Scholar] [CrossRef]
- Vaithiyalingam, A.; Mathew, M.; Jayakumar, S.; Arumugam, K.; Ponnusamy, P.; Narasimman, T. Evaluation of Compressive and Flexural Strengths of Two Resin-based Core Materials with an Alkasite Material: An In Vitro Study. J. Contemp. Dent. Pract. 2023, 24, 385–389. [Google Scholar]
- ISO 9917-1:2025; Dentistry—Water-Based Cements Part 1: Acid-Base Cements. ISO: Geneva, Switzerland, 2025.
- DatatabTeam. DATAtab: Online Statistics Calculator; DATAtab e.U.: Seiersberg, Styria, Austria, 2025. [Google Scholar]
- Mustafa, K.; Alfakhry, G.; Milly, H. Periodontal Evaluation for a New Alkasite Restorative Material in Noncarious Cervical Lesions: A Randomized-Controlled Clinical Trial. Clin. Exp. Dent. Res. 2024, 10, e70025. [Google Scholar] [CrossRef]
- Pires, P.M.; Neves, A.d.A.; Makeeva, I.M.; Schwendicke, F.; Faus-Matoses, V.; Yoshihara, K.; Banerjee, A.; Sauro, S. Contemporary restorative ion-releasing materials: Current status, interfacial properties and operative approaches. Br. Dent. J. 2020, 229, 450–458. [Google Scholar] [CrossRef] [PubMed]
- ISO 4049:201; Dentistry—Polymer-Based Restorative Materials. International Organization for Standardization: Geneva, Switzerland, 2019.
- Hiremath, G.; Horati, P.; Naik, B. Evaluation and comparison of flexural strength of Cention N with resin-modified glass-ionomer cement and composite—An in vitro study. J. Conserv. Dent. Endod. 2022, 25, 288–291. [Google Scholar]
- Chung, S.M.; Yap, A.U.; Chandra, S.P.; Lim, C.T. Flexural strength of dental composite restoratives: Comparison of biaxial and three-point bending test. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71, 278–283. [Google Scholar] [CrossRef]
- Mishra, A.; Singh, G.; Singh, S.; Agarwal, M.; Qureshi, R.; Khurana, N. Comparative Evaluation of Mechanical Properties of Cention N with Conventionally used Restorative Materials—An In Vitro Study. Int. J. Prosthodont. Restor. Dent. 2018, 8, 120–124. [Google Scholar] [CrossRef]
- Rajaraman, G.; Senthil Eagappan, A.R.; Bhavani, S.; Vijayaraghavan, R.; Harishma, S.; Jeyapreetha, P. Comparative Evaluation of Fracture Resistance of Fiber-Reinforced Composite and Alkasite Restoration in Class I Cavity. Contemp. Clin. Dent. 2022, 13, 56–60. [Google Scholar] [CrossRef]
- Kovarik, R.E.; Muncy, M.V. Fracture toughness of resin-modified glass ionomers. Am. J. Dent. 1995, 8, 145–148. [Google Scholar] [PubMed]
- Bepu, D.A.N.; Scatolin, R.S.; Franco, N.S.J.; Sanchez, L.P.; Souza-Gabriel, A.E.; Corona, S.A.M. Alkasite restorative material for endodontically treated teeth: A randomized controlled pilot study. Restor. Dent. Endod. 2024, 49, e24. [Google Scholar] [CrossRef]
- Kaur, G.; Shetty, C.; Hegde, M.N. Comparative Evaluation of Compressive Strength and Fracture Resistance of Posterior Restorative Materials Alkasite and Newer Glass Ionomers with Amalgam: An In Vitro Study. J. Int. Oral Health 2022, 14, 566–573. [Google Scholar] [CrossRef]
- Verma, V.; Mathur, S.; Sachdev, V.; Singh, D. Evaluation of compressive strength, shear bond strength, and microhardness values of glass-ionomer cement Type IX and Cention N. J. Conserv. Dent. Endod. 2020, 23, 550–553. [Google Scholar] [CrossRef]
- Justen, M.; Scheck, D.; Münchow, E.A.; Jardim, J.J. Is Cention-N comparable to other direct dental restorative materials? A systematic review with network meta-analysis of in vitro studies. Dent. Mater. 2024, 40, 1341–1352. [Google Scholar] [CrossRef]
- Safy, R.; Aboalazm, E. Comparative Evaluation of Microhardness and Compressive Strength of Cention N, Bulk Fill Resin Composite and Glass Ionomer Cement. Egypt. Dent. J. 2021, 67, 1657–1662. [Google Scholar] [CrossRef]
- Sulimany, A.M.; Aldowsari, M.K.; Bin Saleh, S.; Alotaibi, S.S.; Alhelal, B.M.; Hamdan, H.M. An In Vitro Assessment of the Shear Bond Strength of Alkasite Restorative Material in Primary Molars Compared with Glass Ionomer and Resin-Modified Glass Ionomer Restorations. Materials 2024, 17, 6230. [Google Scholar] [CrossRef] [PubMed]
- Ausiello, P.; Dal Piva, A.M.d.O.; Di Lauro, A.E.; Garcia-Godoy, F.; Testarelli, L.; Tribst, J.P.M. Mechanical behavior of Alkasite posterior restorations in comparison to polymeric materials: A 3D-FEA study. Polymers 2022, 14, 1502. [Google Scholar] [CrossRef]
- Huang, C.; Tay, F.R.; Cheung, G.S.; Kei, L.H.; Wei, S.H.; Pashley, D.H. Hygroscopic expansion of a compomer and a composite on artificial gap reduction. J. Dent. 2002, 30, 11–19. [Google Scholar] [CrossRef]
- Nicholson, J.W.; Alsarheed, M. Changes on storage of polyacid-modified composite resins. J. Oral Rehabil. 1998, 25, 616–620. [Google Scholar] [CrossRef]
- Nakade, P.; Thaore, S.; Bangar, B.; Grover, I.; Alharethi, N.; Adsure, G.; Kulkarni, D. Comparative Evaluation of Fracture Toughness and Flexural Strength of Four Different Core Build-up Materials: An In Vitro Study. J. Contemp. Dent. Pract. 2024, 25, 191–195. [Google Scholar]
- Chadda, H.; Satapathy, B.K.; Patnaik, A.; Ray, A.R. Mechanistic interpretations of fracture toughness and correlations to wear behavior of hydroxyapatite and silica/hydroxyapatite filled bis-GMA/TEGDMA micro/hybrid dental restorative composites. Compos. Part B Eng. 2017, 130, 132–146. [Google Scholar] [CrossRef]
- Kiran, N.; Chowdhary, N.; John, D.; Reddy, R.; Shidhara, A.; Pavana, M. Comparative Evaluation of Mechanical Properties of Cention-N And Type IX GIC-An In Vitro Study. Int. J. Curr. Adv. Res. 2019, 8, 20498–20501. [Google Scholar]
- Panpisut, P.; Toneluck, A. Monomer conversion, dimensional stability, biaxial flexural strength, and fluoride release of resin-based restorative material containing alkaline fillers. Dent. Mater. J. 2020, 39, 608–615. [Google Scholar] [CrossRef]
- Rêgo, H.M.C.; Butler, S.; Santos, M.J.C. Evaluation of the Mechanical Properties of Three Resin-Modified Glass-Ionomer Materials. Biomed. Res. Int. 2022, 2022, 4690656. [Google Scholar] [CrossRef]
- Moberg, M.; Brewster, J.; Nicholson, J.; Roberts, H. Physical property investigation of contemporary glass ionomer and resin-modified glass ionomer restorative materials. Clin. Oral Investig. 2019, 23, 1295–1308. [Google Scholar] [CrossRef]
- Li, Y.; Lin, H.; Zheng, G.; Zhang, X.; Xu, Y. A comparison study on the flexural strength and compressive strength of four resin-modified luting glass ionomer cements. Bio Med. Mater. Eng. 2015, 26 (Suppl. 1), S9–S17. [Google Scholar] [CrossRef]
- Magne, P.; Silva, S.; Andrada, M.; Maia, H. Fatigue resistance and crack propensity of novel “super-closed” sandwich composite resin restorations in large MOD defects. Int. J. Esthet. Dent. 2016, 11, 82–97. [Google Scholar]
- Ruengrungsom, C.; Burrow, M.F.; Parashos, P.; Palamara, J.E.A. Comprehensive characterisation of flexural mechanical properties and a new classification for porosity of 11 contemporary ion-leaching dental restorative materials. J. Mech. Behav. Biomed. Mater. 2021, 121, 104615. [Google Scholar] [CrossRef]
- Baskar, H.; Hari, A.; Anirudhan, S. Comparative Evaluation of Flexural Strength, Modulus of Elasticity, and Microleakage of Three Different Glass Ionomer Restorative Materials in Class V Preparations—An In vitro Study. Indian J. Dent. Sci. 2023, 15, 67–71. [Google Scholar] [CrossRef]
- Yilmaz, F.; Ozturk, Z.; Demirbas, A.; Kursun, S. Evaluation of the clinical success of direct restorations of endodontically treated posterior teeth in the presence of parafunction: A 12-month pilot study. Head Face Med. 2025, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- El-Salamouny, N.A.; El Mahy, W.A.E.M.; Holiel, A.A. Clinical performance of an alkasite based restorative material (a split mouth randomized controlled clinical trial). Alex. Dent. J. 2025, 50, 217–224. [Google Scholar] [CrossRef]
| Name | Material | Manufacturer | Composition |
|---|---|---|---|
| Cention N | Alkasite | Ivoclar Vivadent, Schaan, Liechtenstein | Powder: 25–35% Calcium fluorosilicate glass (alkaline), 20–30% Barium-aluminum silicate glass, 10–20% fluorosilicate glass, Barium glass, 5–10% Ytterbium trifluoride, 15–25% isofiller (Copolymer), <1% initiator (self-cure initiator: copper salt & thiocarbamide, and light cure initiator: ivocerin and acyl phosphine oxidephotoinitiator), <0.1 Pigment. Liquid: 95–97% Urethane dimethacrylate (UDMA), Tricyclodecan-dimethanoldimethacrylate (DCP), Tetramethylxylylen-diurethanedimethacrylate (aromatic-aliphatic UDMA), and Polyethylene glycol 400 dimethacrylate (PEG-400 DMA). 1–2% additives, 2–3% self-cure initiator (hydroperoxide), and <1% stabilizer. Particle size: 0.15–0.17 μm. (58–59 vol% inorganic fillers) |
| Dyract XP | Compomer | Dentsply Sirona, Konstanz, Germany | UDMA, carboxylic acid modified dimethacrylate, TEGDMA, trimethacrylate resin (TMPTMA), dimethacrylate resins, camphorquinone, ethyl-4 (dimethylamino) benzoate, butylated hydroxy toluene (BHT), strontium-alumino-sodium-fluoro phosphor-silicate glass, highly dispersed silicon dioxide, strontium fluoride, iron oxide pigments and titanium oxide pigments. Particle size: 0.8 μm. (50 vol% inorganic fillers). |
| Fuji II LC | Resin Modified Glass-ionomer | GC Europe N.V, Leuven, Belgium | Powder: 100% fluoroaluminosilicate glass (FAS) Liquid: 35% 2-Hydroxyethyl methacrylate (HEMA), 25% distilled water, 24% polyacrylic acid, 6% tartaric acid and 0.10% camphorquinone (photoinitiator), bisphenol A-glycidyl methacrylate (Bis-GMA), and traces of triethylene glycol dimethacrylate (TEGDMA). Particle size: 5.9 μm. (47.0 vol% inorganic fillers) |
| Compressive Strength (σc) | |||||||
|---|---|---|---|---|---|---|---|
| ANOVA | Sum of Squares | df | Mean Square | F | η2 | Cohens f2 | p |
| Material | 6457.68 | 2 | 3228.84 | 9.69 | 0.56 | 1.14 | 0.002 * |
| Residual | 4995.89 | 15 | 333.06 | (Power = 97.9%) | |||
| Total | 11,453.57 | 17 | |||||
| Tukey’s HSD | Mean difference | 95% CI lower limit | 95% CI upper limit | p | |||
| Cention N-Dyract XP | 39.06 | 11.69 | 66.43 | 0.006 * | |||
| Cention N-Fuji II LC | 2.15 | −25.22 | 29.52 | 0.977 | |||
| Dyract XP-Fuji II LC | 41.21 | 13.84 | 68.58 | 0.004 * | |||
| Flexural Strength (σf) | |||||||
| ANOVA | Sum of Squares | df | Mean Square | F | η2 | Cohens f2 | p |
| Material | 5535.03 | 2 | 2767.52 | 6.96 | 0.48 | 0.96 | 0.007 * |
| Residual | 5966.76 | 15 | 397.78 | (large) | (Power = 91.9%) | ||
| Total | 11,501.8 | 17 | |||||
| Tukey’s HSD | Mean difference | 95% CI lower limit | 95% CI upper limit | p | |||
| Cention N-Dyract XP | 37.64 | 7.73 | 67.55 | 0.013 * | |||
| Cention N-Fuji II LC | 0.91 | −29 | 30.82 | 0.997 | |||
| Dyract XP-Fuji II LC | 36.74 | 6.83 | 66.65 | 0.016 * | |||
| Flexural modulus (Ef) | |||||||
| Analysis of variance | Sum of Squares | df | Mean Square | F | η2 | Cohens f2 | p |
| Material | 75.82 | 2 | 37.91 | 4.21 | 0.36 | 0.75 | 0.035 * |
| Residual | 135.21 | 15 | 9.01 | (large) | (Power = 73%) | ||
| Total | 211.02 | 17 | |||||
| Tukey’s HSD | Mean difference | 95% CI lower limit | 95% CI upper limit | p | |||
| Cention N-Dyract XP | 0.84 | −3.66 | 5.35 | 0.879 | |||
| Cention N-Fuji II LC | 4.71 | 0.21 | 9.22 | 0.04 * | |||
| Dyract XP-Fuji II LC | 3.87 | −0.63 | 8.37 | 0.098 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeslam, H.E.; Hasanain, F.A. Comparative Analysis of Flexural and Compressive Strengths of Bioactive Alkasite Compared to Other Ion-Releasing Restorative Materials. Biomimetics 2025, 10, 751. https://doi.org/10.3390/biomimetics10110751
Yeslam HE, Hasanain FA. Comparative Analysis of Flexural and Compressive Strengths of Bioactive Alkasite Compared to Other Ion-Releasing Restorative Materials. Biomimetics. 2025; 10(11):751. https://doi.org/10.3390/biomimetics10110751
Chicago/Turabian StyleYeslam, Hanin E., and Fatin A. Hasanain. 2025. "Comparative Analysis of Flexural and Compressive Strengths of Bioactive Alkasite Compared to Other Ion-Releasing Restorative Materials" Biomimetics 10, no. 11: 751. https://doi.org/10.3390/biomimetics10110751
APA StyleYeslam, H. E., & Hasanain, F. A. (2025). Comparative Analysis of Flexural and Compressive Strengths of Bioactive Alkasite Compared to Other Ion-Releasing Restorative Materials. Biomimetics, 10(11), 751. https://doi.org/10.3390/biomimetics10110751






