A Bionic Sensing Platform for Cell Separation: Simulation of a Dielectrophoretic Microfluidic Device That Leverages Dielectric Fingerprints
Abstract
1. Introduction
2. Methods
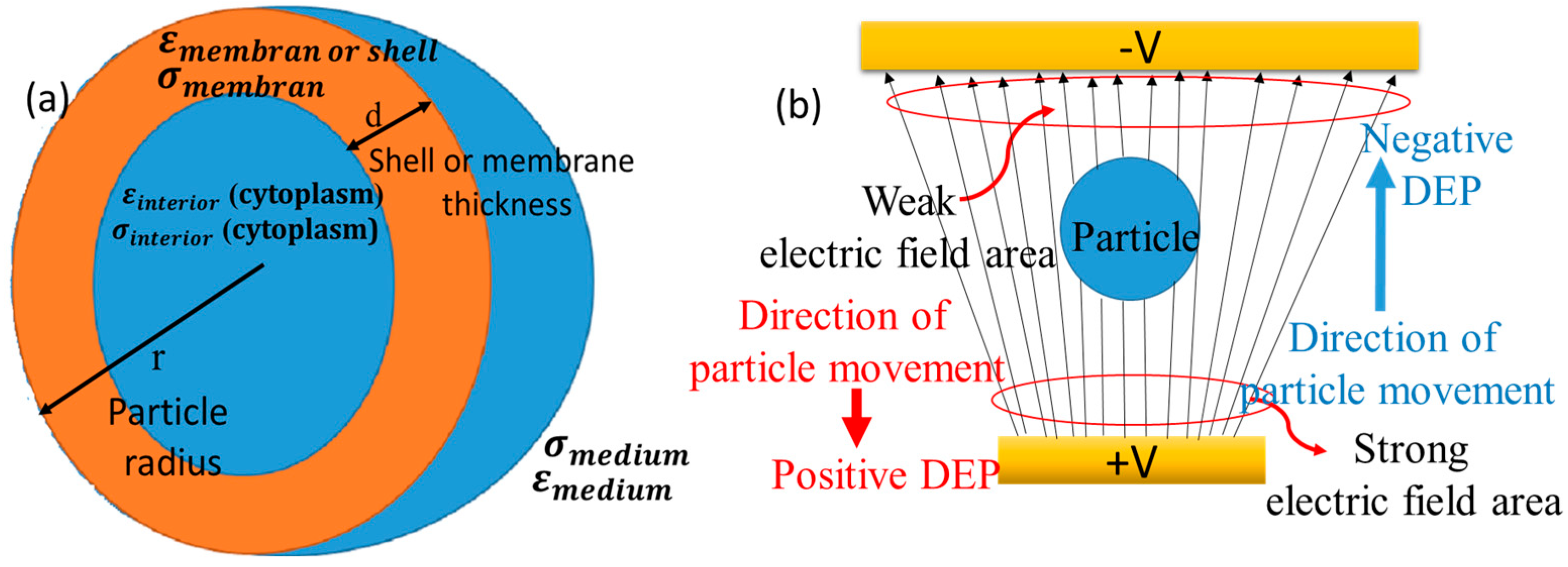
2.1. Theoretical Background of DEP
2.2. Physics for Cell Separation
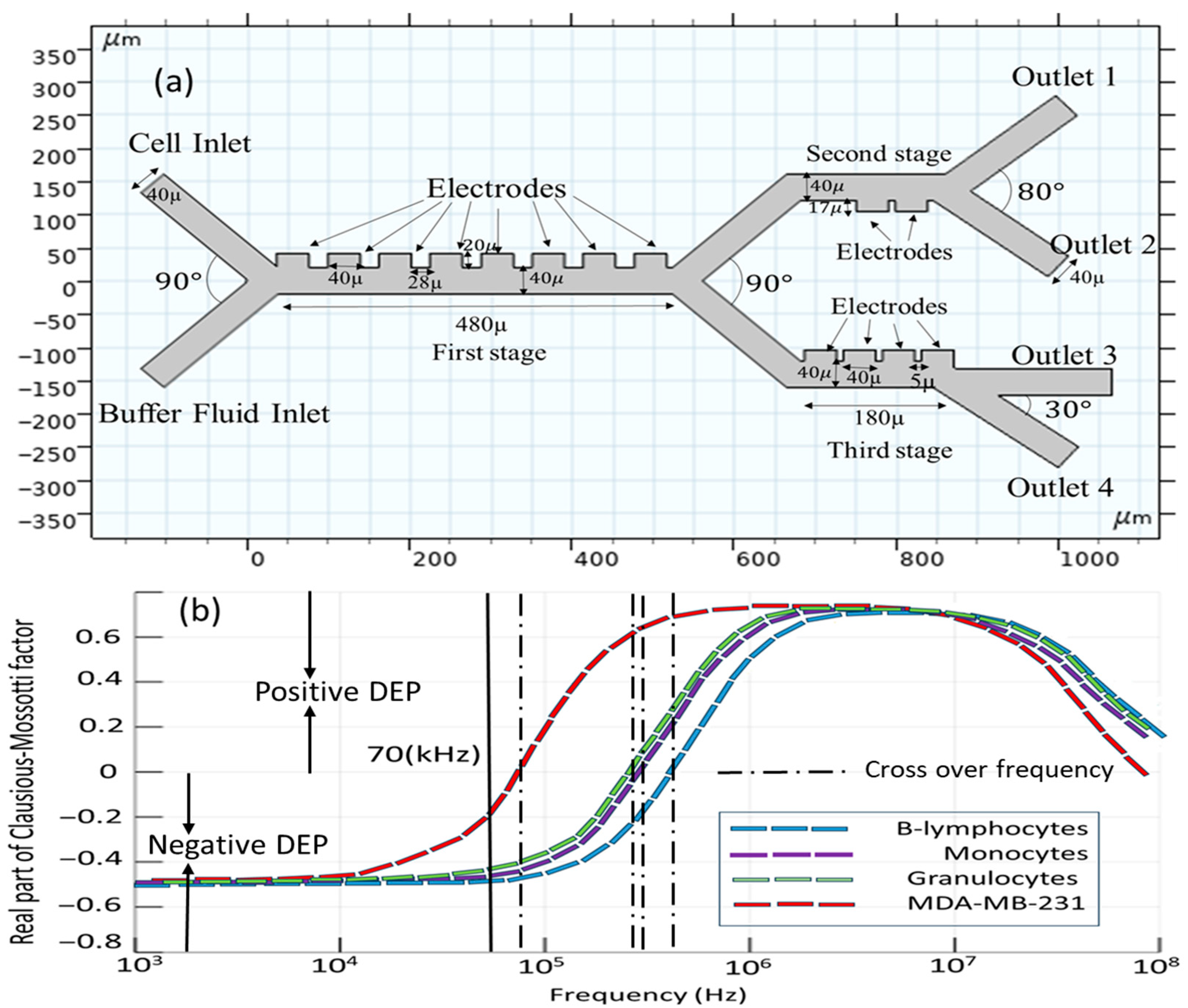
2.3. Geometry and Material Properties
2.4. Mesh Set-Up
3. Results and Discussion
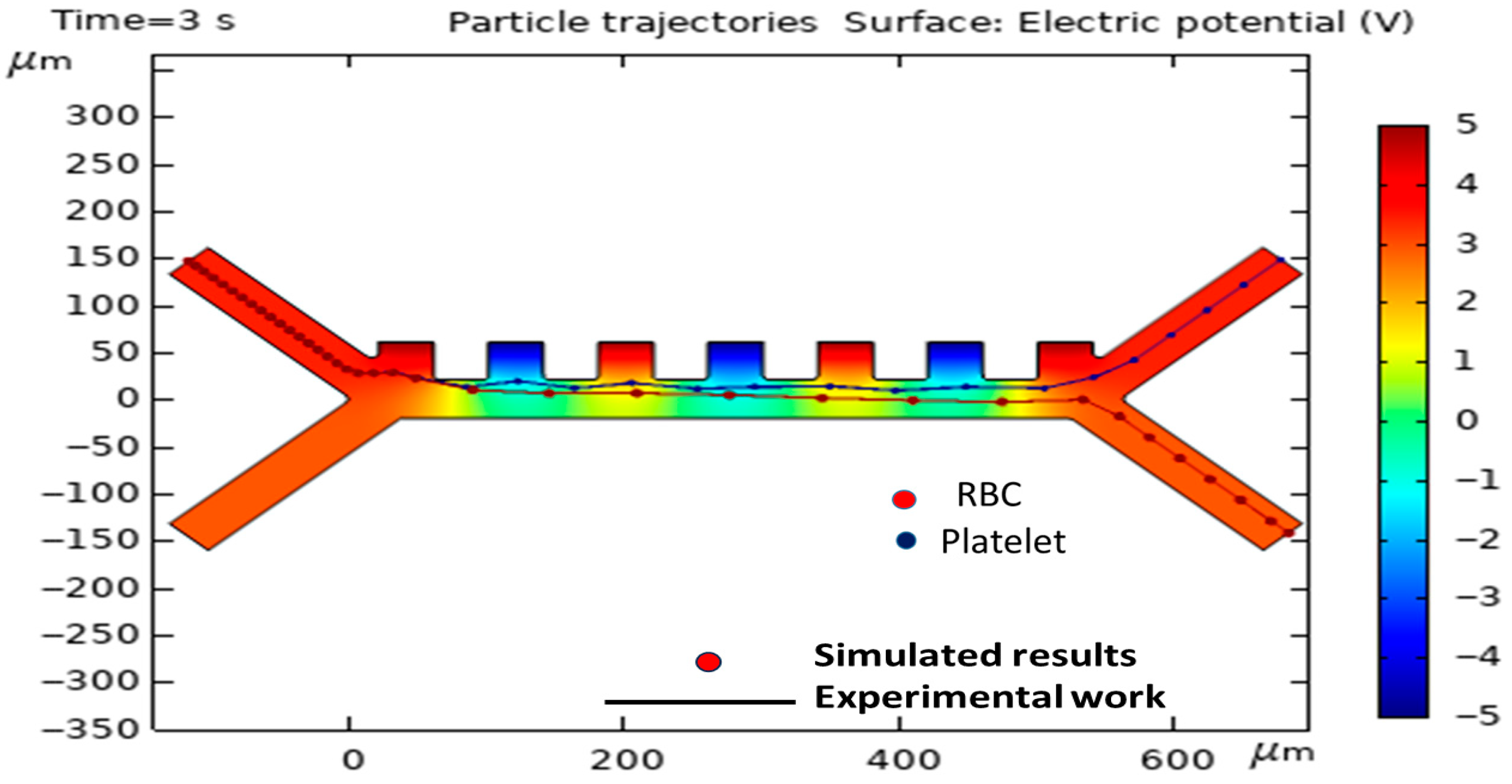
3.1. Validation and Assessment of the Model
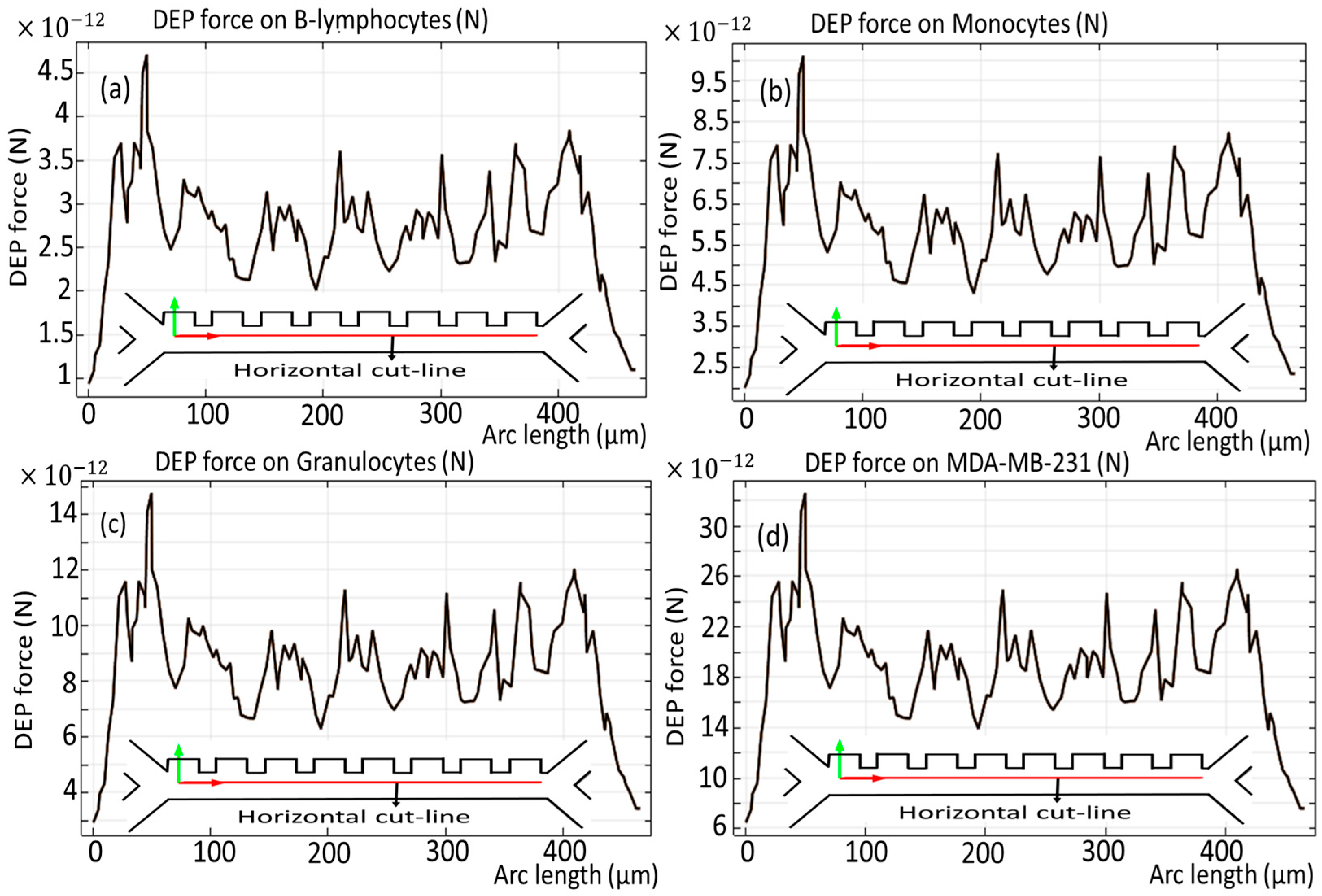
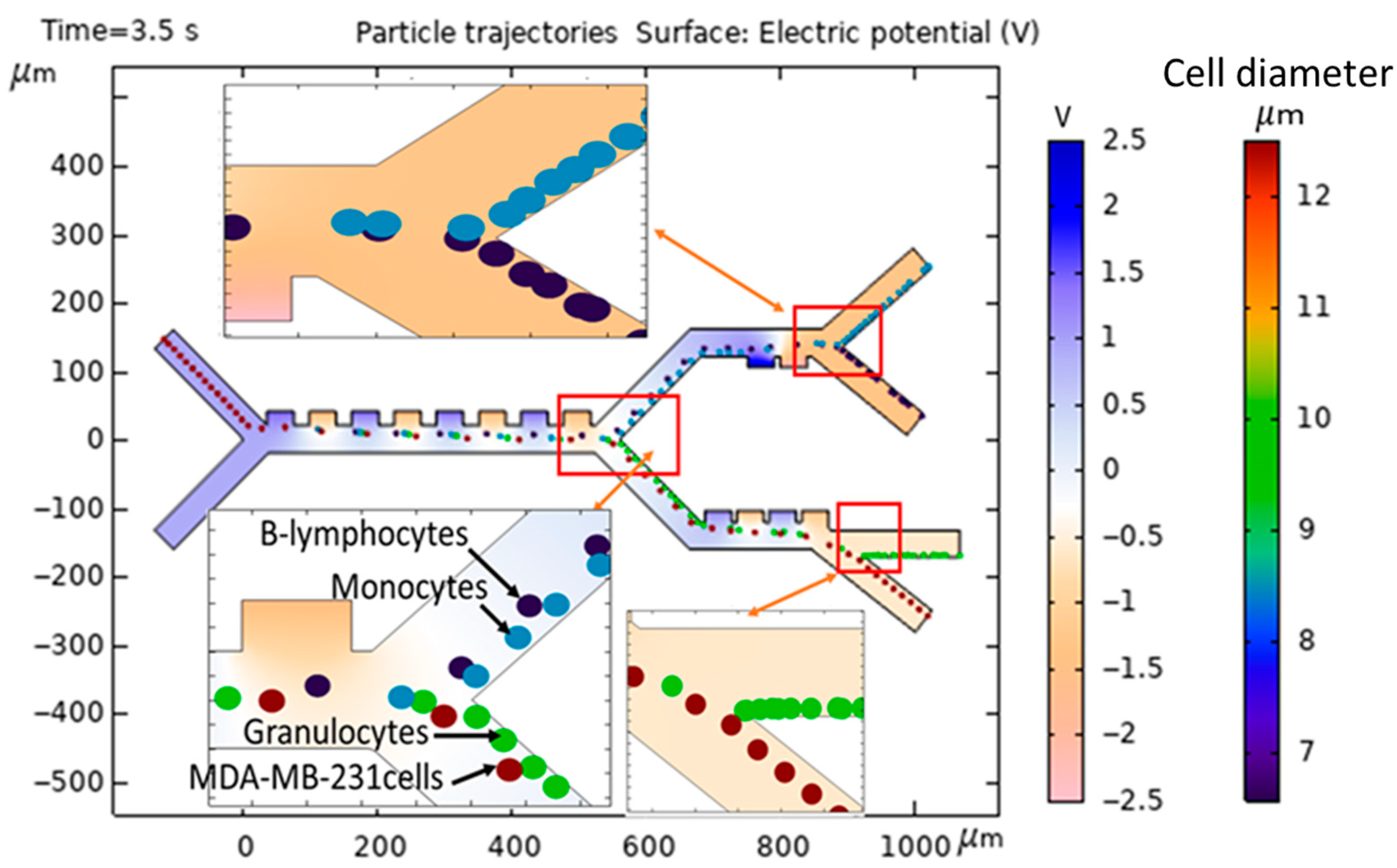
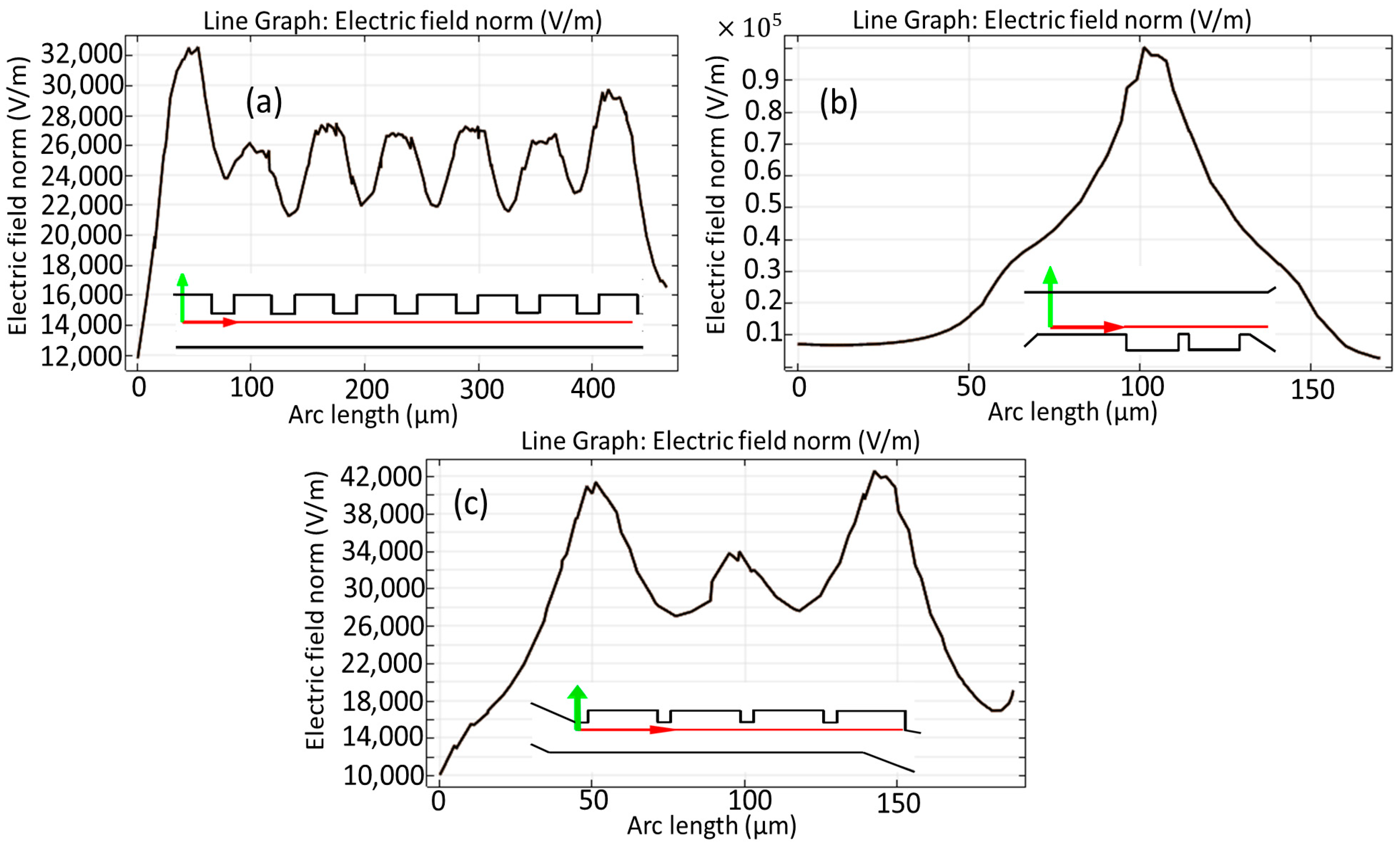
3.2. Simulated Physical Fields and Particle Dynamics: Spatial Electric Field, Flow Velocity, Pressure Path, and Force Due to Dielectrophoresis
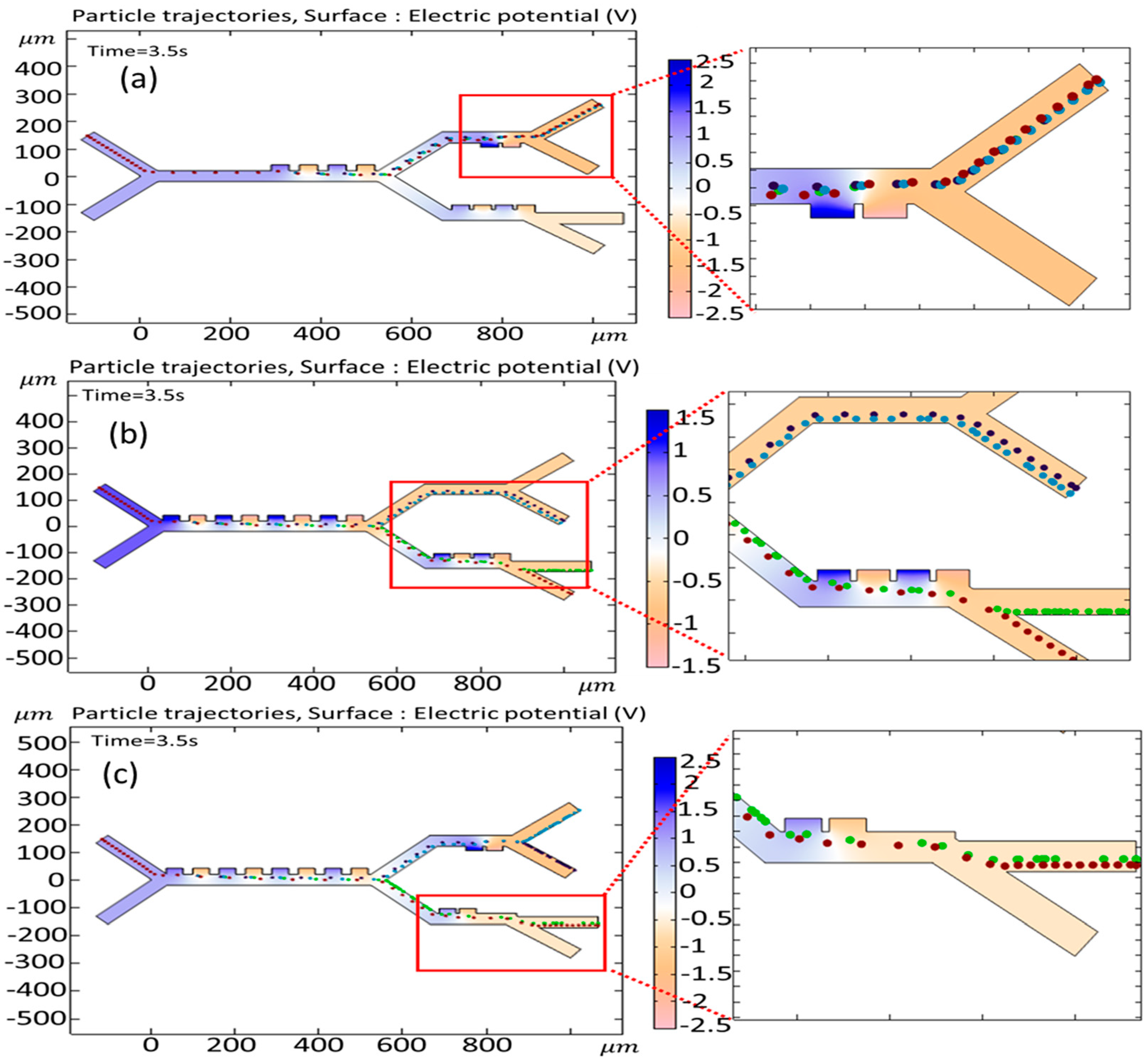
3.3. The Effect of Changing the Input Voltage on the Trajectories of the Cells
3.4. The Effect of Changing Velocity of Cells on the Cell Trajectories at the Outlet
3.5. The Effect of Changing the Number of Electrodes on the Trajectories of the Cells and DEP Force
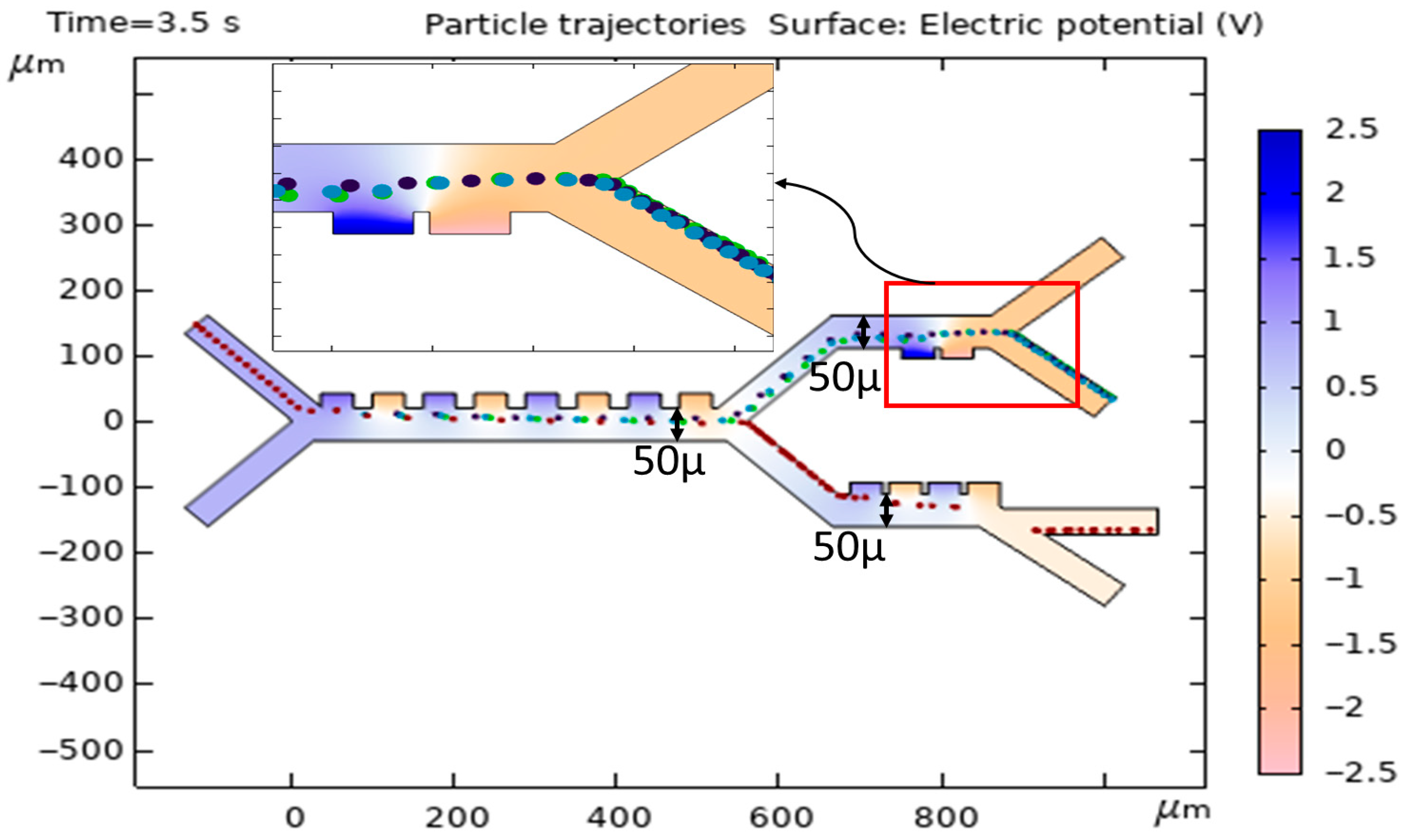
3.6. The Effect of Changing the Width of First, Second, and Third Stages on the Cell’s Trajectories and DEP Force
3.7. The Effect of Changing the Input Angle (α) and Output Angles (β, γ) on the Cell Trajectories
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Xu, Q.; Cai, Y.; Wang, Q.; Liu, Y.; Wang, D. Biological Particle Separation Techniques Based on Microfluidics. Interdiscip. Med. 2024, 2, e20240003. [Google Scholar] [CrossRef]
- Suresh, G.; Pearson, B.E.; Schreiner, R.; Lin, Y.; Rafii, S.; Rabbany, S.Y. Design and Development of a Real-Time Pressure-Driven Monitoring System for In Vitro Microvasculature Formation. Biomimetics 2025, 10, 501. [Google Scholar] [CrossRef] [PubMed]
- Hadjiaghaie Vafaie, R.; Fardi-Ilkhchy, A.; Sheykhivand, S.; Danishvar, S. Theoretical and Experimental Study of an Electrokinetic Micromanipulator for Biological Applications. Biomimetics 2025, 10, 56. [Google Scholar] [CrossRef]
- Olaizola-Rodrigo, C.; Castro-Abril, H.; Perisé-Badía, I.; Pancorbo, L.; Ochoa, I.; Monge, R.; Oliván, S. Reducing Inert Materials for Optimal Cell–Cell and Cell–Matrix Interactions within Microphysiological Systems. Biomimetics 2024, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Zakertabrizi, M.; Bozorgmehrian, F.; Jeong, M.; Hosseini, E.; Ponce, V.; Fallahi, H.; Bahadorikhalili, S.; Nasrabadi, H.; Jarrahbashi, D.; Castaneda, H.; et al. Patterned Nanostructures on Cathodes: A Pathway to Stronger, High-Energy, High-Power Li-Ion Batteries. ACS Nano 2025. [Google Scholar] [CrossRef]
- Emmerich, M.E.P.; Sinnigen, A.-S.; Neubauer, P.; Birkholz, M. Dielectrophoretic Separation of Blood Cells. Biomed. Microdevices 2022, 24, 30. [Google Scholar] [CrossRef]
- Mathew, B.; Alazzam, A.; Destgeer, G.; Sung, H.J. Dielectrophoresis Based Cell Switching in Continuous Flow Microfluidic Devices. J. Electrostat. 2016, 84, 63–72. [Google Scholar] [CrossRef]
- Karimi, K.; Ghodratnama, A.; Tavakkoli-Moghaddam, R.; Ahmadirad, Z. Optimizing Knowledge Management Integrated with Industry 4.0: A Comprehensive Case Study of Smart Manufacturing Transformation. VINE J. Inf. Knowl. Manag. Syst. 2025, 1–24. [Google Scholar] [CrossRef]
- Poorreza, E.; Vafaie, R.H.; Mehdipoor, M.; Pourmand, A.; Ghavifekr, H.B. Microseparator Based on 4-Phase Travelling Wave Dielectrophoresis for Lab-on-a-Chip Applications. Indian J. Pure Appl. Phys. 2013, 51, 506–515. [Google Scholar]
- Tada, S. Numerical Simulation of the Electric Field Induced in a Contactless Dielectrophoretic Quadrupole Cell Separator. Eng. Comput. 2021, 38, 1076–1094. [Google Scholar] [CrossRef]
- Sajeesh, P.; Sen, A.K. Particle Separation and Sorting in Microfluidic Devices: A Review. Microfluid. Nanofluid. 2014, 17, 1–52. [Google Scholar] [CrossRef]
- Al-Fandi, M.; Al-Rousan, M.; Jaradat, M.A.; Al-Ebbini, L. New Design for the Separation of Microorganisms Using Microfluidic Deterministic Lateral Displacement. Robot. Comput.-Integr. Manuf. 2011, 27, 237–244. [Google Scholar] [CrossRef]
- Zhao, Q.; Yuan, D.; Zhang, J.; Li, W. A Review of Secondary Flow in Inertial Microfluidics. Micromachines 2020, 11, 461. [Google Scholar] [CrossRef]
- Sivaramakrishnan, M.; Kothandan, R.; Govindarajan, D.K.; Meganathan, Y.; Kandaswamy, K. Active Microfluidic Systems for Cell Sorting and Separation. Curr. Opin. Biomed. Eng. 2020, 13, 60–68. [Google Scholar] [CrossRef]
- Cha, H.; Fallahi, H.; Dai, Y.; Yuan, D.; An, H.; Nguyen, N.-T.; Zhang, J. Multiphysics Microfluidics for Cell Manipulation and Separation: A Review. Lab Chip 2022, 22, 423–444. [Google Scholar] [CrossRef]
- Chu, P.-Y.; Wu, A.-Y.; Tsai, K.-Y.; Hsieh, C.-H.; Wu, M.-H. Combination of an Optically Induced Dielectrophoresis (ODEP) Mechanism and a Laminar Flow Pattern in a Microfluidic System for the Continuous Size-Based Sorting and Separation of Microparticles. Biosensors 2024, 14, 297. [Google Scholar] [CrossRef]
- Tabarhoseini, S.M.; Kale, A.S.; Koniers, P.M.; Boone, A.C.; Bentor, J.; Boies, A.; Zhao, H.; Xuan, X. Charge-Based Separation of Microparticles Using AC Insulator-Based Dielectrophoresis. Anal. Chem. 2024, 96, 13672–13678. [Google Scholar] [CrossRef]
- Chu, P.-Y.; Nguyen, T.N.A.; Wu, A.-Y.; Huang, P.-S.; Huang, K.-L.; Liao, C.-J.; Hsieh, C.-H.; Wu, M.-H. The Utilization of Optically Induced Dielectrophoresis (ODEP)-Based Cell Manipulation in a Microfluidic System for the Purification and Sorting of Circulating Tumor Cells (CTCs) with Different Sizes. Micromachines 2023, 14, 2170. [Google Scholar] [CrossRef]
- Shim, S.; Stemke-Hale, K.; Noshari, J.; Becker, F.F.; Gascoyne, P.R.C. Dielectrophoresis Has Broad Applicability to Marker-Free Isolation of Tumor Cells from Blood by Microfluidic Systems. Biomicrofluidics 2013, 7, 011808. [Google Scholar] [CrossRef]
- Qian, C.; Huang, H.; Chen, L.; Li, X.; Ge, Z.; Chen, T.; Yang, Z.; Sun, L. Dielectrophoresis for Bioparticle Manipulation. Int. J. Mol. Sci. 2014, 15, 18281–18309. [Google Scholar] [CrossRef] [PubMed]
- Varmazyari, V.; Habibiyan, H.; Ghafoorifard, H.; Ebrahimi, M.; Ghafouri-Fard, S. A Dielectrophoresis-Based Microfluidic System Having Double-Sided Optimized 3D Electrodes for Label-Free Cancer Cell Separation with Preserving Cell Viability. Sci. Rep. 2022, 12, 12100. [Google Scholar] [CrossRef]
- Uddin, M.R.; Chen, X. Design of a Hybrid-Inertial Device for the Separation of Circulating Tumor Cells. In Frontiers in Biomedical Devices; ASME: New York, NY, USA, 2023. [Google Scholar]
- Sarowar, M.T.; Islam, M.S.; Chen, X. Separation of CTCs from Blood Cells Using Curved Contraction-Expansion Microchannel Equipped with DEP Force. In ASME International Mechanical Engineering Congress and Exposition; ASME: New York, NY, USA, 2023. [Google Scholar]
- Nguyen, T.H.; Nguyen, H.T.; Nguyen, M.C.; Quang, L.D.; Ducrée, J.; Duc, T.C.; Bui, T.T. Circulating Tumor Cell (CTC) Separation from Blood Constituents Using a Dielectrophoresis-Based Microdevice. In Proceedings of the 2024 Tenth International Conference on Communications and Electronics (ICCE), Da Nang, Vietnam, 31 July–2 August 2024. [Google Scholar]
- Ayash, A.A.; Al-Moameri, H.H.; Salman, A.A.; Lubguban, A.A.; Malaluan, R.M. Analysis and Simulation of Blood Cells Separation in a Polymeric Serpentine Microchannel under Dielectrophoresis Effect. Sustainability 2023, 15, 3444. [Google Scholar] [CrossRef]
- Pethig, R.; Markx, G.H. Applications of Dielectrophoresis in Biotechnology. Trends Biotechnol. 1997, 15, 426–432. [Google Scholar] [CrossRef]
- Lan, M.; Yang, F. Applications of Dielectrophoresis in Microfluidic-Based Exosome Separation and Detection. Chem. Eng. J. 2024, 491, 152067. [Google Scholar] [CrossRef]
- Pakhira, W.; Kumar, R.; Ibrahimi, K.M. Design and Numerical Simulation of a Microfluidic Lab-on-a-Chip Utilizing Positive and Negative Dielectrophoresis Technique for Separation of Multiple CTCs Distinctly. Comput. Part. Mech. 2024, 11, 2869–2882. [Google Scholar] [CrossRef]
- Valijam, S.; Salehi, A.; Andersson, M. Design of a Low-Voltage Dielectrophoresis Lab-on-the-Chip to Separate Tumor and Blood Cells. Microfluid. Nanofluid. 2023, 27, 22. [Google Scholar] [CrossRef]
- Russo, G.I.; Musso, N.; Romano, A.; Caruso, G.; Petralia, S.; Lanzanò, L.; Broggi, G.; Camarda, M. The Role of Dielectrophoresis for Cancer Diagnosis and Prognosis. Cancers 2021, 14, 198. [Google Scholar] [CrossRef]
- Abdul Nasir, N.S.; Deivasigamani, R.; Rahim, M.K.A.; Nashruddin, S.N.A.M.; Hamzah, A.A.; Wee, M.F.M.R.; Buyong, M.R. Preliminary Dielectrophoresis Study: Manipulation of Protein Albumin and Electrical Quantification by Using Cyclic Voltammetry Technique. Microelectron. Int. 2021, 38, 162–171. [Google Scholar] [CrossRef]
- Buyong, M.R.; Larki, F.; Zainal, M.I.H.; Almahi, A.Y.A.; Ismail, A.G.; Hamzah, A.A.; Kayani, A.A.K.; Caille, C.E.; Majlis, B.Y. Implementation of Capacitance as Simultaneous Sensing and Actuating Tool in Tapered Microelectrode Arrays for Dielectrophoresis-on-a-Chip Application. Microelectron. Int. 2020, 37, 215–224. [Google Scholar] [CrossRef]
- Buyong, M.R.; Yunas, J.; Hamzah, A.A.; Yeop Majlis, B.; Larki, F.; Abd Aziz, N. Design, Fabrication and Characterization of Dielectrophoretic Microelectrode Array for Particle Capture. Microelectron. Int. 2015, 32, 96–102. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X. Blood Cells Separation Microfluidic Chip Based on Dielectrophoretic Force. J. Braz. Soc. Mech. Sci. Eng. 2020, 42, 1–11. [Google Scholar] [CrossRef]
- Pakhira, W.; Kumar, R.; Panwala, F.C.; Ibrahimi, K.M. Microfluidic Design for Continuous Separation of Blood Particles and Plasma Using Dielectrophoretic Force Principle. Comput. Assist. Methods Eng. Sci. 2023, 30, 323–345. [Google Scholar]
- Zhao, K.; Zhao, P.; Dong, J.; Wei, Y.; Chen, B.; Wang, Y.; Pan, X.; Wang, J. Implementation of an Integrated Dielectrophoretic and Magnetophoretic Microfluidic Chip for CTC Isolation. Biosensors 2022, 12, 757. [Google Scholar] [CrossRef]
- Poorreza, E. Computer-Assisted Modeling and Simulation of a Dielectrophoresis-Based Microseparator for Blood Cells Separation Applications. Chromatographia 2025, 88, 225–242. [Google Scholar] [CrossRef]
- Alkhaiyat, A.M.; Badran, M. Numerical Simulation of a Lab-on-Chip for Dielectrophoretic Separation of Circulating Tumor Cells. Micromachines 2023, 14, 1769. [Google Scholar] [CrossRef] [PubMed]
- Gascoyne, P.R.C.; Shim, S.; Noshari, J.; Becker, F.F.; Stemke-Hale, K. Correlations between the Dielectric Properties and Exterior Morphology of Cells Revealed by Dielectrophoretic Field-Flow Fractionation. Electrophoresis 2013, 34, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, N.; Mernier, G.; Tornay, R.; Renaud, P. Separation of Platelets from Other Blood Cells in Continuous-Flow by Dielectrophoresis Field-Flow-Fractionation. Biomicrofluidics 2011, 5, 034122. [Google Scholar] [CrossRef]
- Gascoyne, P.R.C.; Noshari, J.; Anderson, T.J.; Becker, F.F. Isolation of Rare Cells from Cell Mixtures by Dielectrophoresis. Electrophoresis 2009, 30, 1388–1398. [Google Scholar] [CrossRef]
- Gao, J.; Riahi, R.; Sin, M.L.Y.; Zhang, S.; Wong, P.K. Electrokinetic Focusing and Separation of Mammalian Cells in Conductive Biological Fluids. Analyst 2012, 137, 5215–5221. [Google Scholar] [CrossRef]
- Nguyen, N.-V.; Le Manh, T.; Nguyen, T.S.; Le, V.T.; Van Hieu, N. Applied Electric Field Analysis and Numerical Investigations of the Continuous Cell Separation in a Dielectrophoresis-Based Microfluidic Channel. J. Sci. Adv. Mater. Devices 2021, 6, 11–18. [Google Scholar] [CrossRef]
- Golkarieh, A.; Razmara, P.; Lagzian, A.; Dolatabadi, A.; Mousavirad, S. Semi-supervised GAN with hybrid regularization and evolutionary hyperparameter tuning for accurate melanoma detection. Sci. Rep. 2025, 15, 31977. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi Mohammadabadi, S.M.; Seyedkhamoushi, F.; Mostafavi, M.; Borhani Peikani, M. Examination of AI’s role in Diagnosis, Treatment, and Patient care. In Transforming Gender-Based Healthcare with AI and Machine Learning; Gupta, M., Kumar, R., Lu, Z., Eds.; CRC Press: Boca Raton, FL, USA, 2024; pp. 221–238. [Google Scholar] [CrossRef]
- Entezami, Z.; Davis, C.H.; Entezami, M. An AI-Assisted Topic Model of the Media Literacy Research Literature. Media Lit. Acad. Res. 2025, 8, 5–28. [Google Scholar] [CrossRef]
- Ahmadkhan, K.; Ahmadirad, Z.; Karaminezhad, K.; SeyedKhamoushi, F.; Karimi, K.; Khakpash, F. A novel blockchain-based approach for enhanced food supply chain traceability and waste mitigation. Br. Food J. 2025, 1–41. [Google Scholar] [CrossRef]
- Mohaghegh, S.; Mohaghegh, A. Machine Learning in Aircraft Design: A Comprehensive Review of Optimization, Aerodynamics, and Structural Applications. IEEE Access 2025, 13, 105642–105653. [Google Scholar] [CrossRef]
- Talebian, S.; Golkarieh, A.; Eshraghi, S.; Naseri, M.; Naseri, S. Artificial Intelligence Impacts on Architecture and Smart Built Environments: A Comprehensive Review. Adv. Civ. Eng. Environ. Sci. 2025, 2, 45–56. [Google Scholar] [CrossRef]
- Nawaser, K.; Jafarkhani, F.; Khamoushi, S.; Yazdi, A.; Mohsenifard, H.; Gharleghi, B. The Dark Side of Digitalization: A Visual Journey of Research through Digital Game Addiction and Mental Health. IEEE Eng. Manag. Rev. 2024, 1–27. [Google Scholar] [CrossRef]
- Seifi, N.; Ghoodjani, E.; Majd, S.S.; Maleki, A.; Khamoushi, S. Evaluation and prioritization of artificial intelligence integrated block chain factors in healthcare supply chain: A hybrid Decision Making Approach. Comput. Decis. Mak. Int. J. 2025, 2, 374–405. [Google Scholar] [CrossRef]
- Basirat, S.; Raoufi, S.; Bazmandeh, D.; Khamoushi, S.; Entezami, M. Ranking of AI-Based Criteria in Health Tourism Using Fuzzy SWARA Method. Comput. Decis. Mak. Int. J. 2025, 2, 530–545. [Google Scholar] [CrossRef]
- Mehrabi Jorshary, K.; Sassani, M.; Seyed Khamoushi, F.S.; Raoufi, S.; Khamoushi, S. Ranking of AI-Driven Strategies for Optimizing the Health Tourism Supply Chain Using Stratified BWM. Knowl. Decis. Syst. Appl. 2025, 1, 295–315. [Google Scholar] [CrossRef]
- Seyedkhamoushi, F.; Sassani Asl, M.; Seyedkhamooshi, R.; Abdollahi, H.R.; Sharifnia, M. Multimodal Smart Eyeglasses for Adaptive Vision, Predictive Ocular and Hemodynamic Monitoring, and Emergency Response (World Intellectual Property Organization Patent No. WO2025163631A1). 2025. Available online: https://patents.google.com/patent/WO2025163631A1/en/ (accessed on 4 November 2025).
- Lin, M.H.; Sassani, M.; Golchin, N.; Jabbari, Y.; Boymatova, Z.; Rustambekovich, J.U.; Ugli, Y.J.E.; Atajanova, S.; Turdiyeva, Y. Optimal Planning and Operation of the Smart Electrical Distribution Network Considering Stochastic Optimization Modeling and Energy Storage Systems. Oper. Res. Forum 2025, 6, 123. [Google Scholar] [CrossRef]








| Parameter | Value |
|---|---|
| Frequency | 70 [kHz] |
| Electrical conductivity of the suspension medium | 55 [mS/m] |
| Electrical permittivity of the suspension medium | 80 |
| Fluid density | 1000 [kg/m3] |
| Viscosity of the fluid | [Pa*s] |
| Particle density | 1050 [kg/m3] |
| Cell radius: Granulocytes | 4.97 [µm] |
| Cell radius: B-lymphocytes | 3.28 [µm] |
| Cell radius: Monocytes | 4.23 [µm] |
| Cell radius: MDA-MB-231 | 6.2 [µm] |
| Cell conductivity: Granulocytes | 0.6 [S/m] |
| Cell conductivity: B-lymphocytes | 0.73 [S/m] |
| Cell conductivity: Monocytes | 0.56 [S/m] |
| Cell conductivity: MDA-MB-231 | 0.6 [S/m] |
| Cell dielectric constant: Granulocytes | 151 |
| Cell dielectric constant: B-lymphocytes | 154 |
| Cell dielectric constant: Monocytes | 127 |
| Cell dielectric constant: MDA-MB-231 | 52 |
| Conductivity of shell: Granulocytes | [S/m] |
| Conductivity of shell: B-lymphocytes | [S/m] |
| Conductivity of shell: Monocytes | [S/m] |
| Conductivity of shell: MDA-MB-231 | [S/m] |
| Shell dielectric constant: Granulocytes | 5 |
| Shell dielectric constant: B-lymphocytes | 5 |
| Shell dielectric constant: Monocytes | 5 |
| Shell dielectric constant: MDA-MB-231 | 5 |
| Shell thickness: Granulocytes | 4 [nm] |
| Shell thickness: B-lymphocytes | 4 [nm] |
| Shell thickness: Monocytes | 4 [nm] |
| Shell thickness: MDA-MB-231 | 4 [nm] |
| Cell | Diameter | Conductivity | Relative Permittivity | Shell Conductivity | Shell Permittivity | Shell Thickness |
|---|---|---|---|---|---|---|
| PLT | 1.8 [µm] | 0.25 [S/m] | 50 | [S/m] | 6 | 8 [nm] |
| RBC | 5 [µm] | 0.31 [S/m] | 59 | [S/m] | 4.44 | 9 [nm] |
| Selected Cell Type | Buffer Conductivity (mS/m) | Frequency | Voltage (V) | Efficiency | References |
|---|---|---|---|---|---|
| MDA-MB-435, MDA-MB-468, MDA-MB-231 | 30 | 15–60 kHz | 10 (Vpp) | 90 | [41] |
| MDA-MB-231, HeLa | 1 | 100 kHz | 7 (Vpp) | 98 | [42] |
| MDA-MB-231 | 55 | 1 kHz | 10 (Vpp) | 90 | [43] |
| MDA-MB-231 | 55 | 125 kHz | 3.52 (Vp) | 95 | [21] |
| MDA-MB-231 | 55 | 70 kHz | 2.5 (Vp) | 92 | This study |
| Parameter | Condition/Value | Separation Efficiency |
|---|---|---|
| Number of Electrodes (Stage 1) | 4 electrodes | 2% |
| 8 electrodes | 92% | |
| Number of Electrodes (Stage 2) | 0 electrodes | 90% |
| 2 electrodes | 91% | |
| Number of Electrodes (Stage 3) | 2 electrodes | 5% |
| 4 electrodes | 92% | |
| Applied Voltage (Stage 1) | 1.0 V | 2% |
| 2.0 V | 62% | |
| Applied Voltage (Stage 2) | 2.0 V | 91% |
| 3.0 V | 92% | |
| Applied Voltage (Stage 3) | 1.0 V | 11% |
| 1.5 V | 82% | |
| Flow Velocity | 80 µm/s | 81% |
| 280 µm/s | 90% | |
| 380 µm/s | 91% | |
| Channel Thickness | 40 µm | 92% |
| 50 µm | 5% | |
| Input Angle (α) | α > 90° | 91% |
| α < 90° | 90% | |
| Output Angle (β) | β > 80° | 92% |
| β < 80° | 92% | |
| Output Angle (γ) | γ > 30° | 90% |
| γ < 30° | 92% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadjiaghaie Vafaie, R.; Poorreza, E.; Sheykhivand, S.; Danishvar, S. A Bionic Sensing Platform for Cell Separation: Simulation of a Dielectrophoretic Microfluidic Device That Leverages Dielectric Fingerprints. Biomimetics 2025, 10, 753. https://doi.org/10.3390/biomimetics10110753
Hadjiaghaie Vafaie R, Poorreza E, Sheykhivand S, Danishvar S. A Bionic Sensing Platform for Cell Separation: Simulation of a Dielectrophoretic Microfluidic Device That Leverages Dielectric Fingerprints. Biomimetics. 2025; 10(11):753. https://doi.org/10.3390/biomimetics10110753
Chicago/Turabian StyleHadjiaghaie Vafaie, Reza, Elnaz Poorreza, Sobhan Sheykhivand, and Sebelan Danishvar. 2025. "A Bionic Sensing Platform for Cell Separation: Simulation of a Dielectrophoretic Microfluidic Device That Leverages Dielectric Fingerprints" Biomimetics 10, no. 11: 753. https://doi.org/10.3390/biomimetics10110753
APA StyleHadjiaghaie Vafaie, R., Poorreza, E., Sheykhivand, S., & Danishvar, S. (2025). A Bionic Sensing Platform for Cell Separation: Simulation of a Dielectrophoretic Microfluidic Device That Leverages Dielectric Fingerprints. Biomimetics, 10(11), 753. https://doi.org/10.3390/biomimetics10110753








