Advanced 3D In Vitro Liver Fibrosis Models: Spheroids, Organoids, and Liver-on-Chips
Abstract
1. Introduction
2. Pathogenesis of Liver Fibrosis
2.1. Hepatic Stellate Cell Activation and Transdifferentiation
2.2. Crosstalk with Other Cell Types
2.3. ECM Remodeling
3. Liver Fibrosis Modeling
3.1. Cell Lines
3.2. Spheroids
3.3. Organoids
3.4. Liver-on-Chips
4. Conclusions and Future Perspectives
| Platforms | Advantages | Disadvantages | References |
|---|---|---|---|
| Animal model |
|
| [87] |
| Monolayer |
|
| [88,89] |
| Spheroids |
|
| [86] |
| Organoids |
|
| [82,86] |
| Liver-on-chips |
|
| [85,86,90,91,92,93,94,95,96,97] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef]
- Ehrlich, A.; Duche, D.; Ouedraogo, G.; Nahmias, Y. Challenges and Opportunities in the Design of Liver-on-Chip Microdevices. Annu. Rev. Biomed. Eng. 2019, 21, 219–239. [Google Scholar] [CrossRef]
- Maharajan, N.; Kim, K.H.; Vijayakumar, K.A.; Cho, G.-W. Unlocking Therapeutic Potential: Camphorquinone’s Role in Alleviating Non-Alcoholic Fatty Liver Disease via SIRT1/LKB1/AMPK Pathway Activation. Tissue Eng. Regen. Med. 2025, 22, 129–144. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, Y.; Liu, Z. Reducing Nogo-B Improves Hepatic Fibrosis by Inhibiting BACe1-Mediated Autophagy. Tissue Eng. Regen. Med. 2024, 21, 777–789. [Google Scholar] [CrossRef]
- Somnay, K.; Wadgaonkar, P.; Sridhar, N.; Roshni, P.; Rao, N.; Wadgaonkar, R. Liver Fibrosis Leading to Cirrhosis: Basic Mechanisms and Clinical Perspectives. Biomedicines 2024, 12, 2229. [Google Scholar] [CrossRef]
- Akkız, H.; Gieseler, R.K.; Canbay, A. Liver fibrosis: From basic science towards clinical progress, focusing on the central role of hepatic stellate cells. Int. J. Mol. Sci. 2024, 25, 7873. [Google Scholar] [CrossRef] [PubMed]
- Milner, E.; Ainsworth, M.; McDonough, M.; Stevens, B.; Buehrer, J.; Delzell, R.; Wilson, C.; Barnhill, J. Emerging three-dimensional hepatic models in relation to traditional two-dimensional in vitro assays for evaluating drug metabolism and hepatoxicity. Med. Drug Discov. 2020, 8, 100060. [Google Scholar] [CrossRef]
- Ruoß, M.; Vosough, M.; Königsrainer, A.; Nadalin, S.; Wagner, S.; Sajadian, S.; Huber, D.; Heydari, Z.; Ehnert, S.; Hengstler, J.G. Towards improved hepatocyte cultures: Progress and limitations. Food Chem. Toxicol. 2020, 138, 111188. [Google Scholar] [CrossRef] [PubMed]
- Khetani, S.R.; Bhatia, S.N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2008, 26, 120–126. [Google Scholar] [CrossRef]
- Yao, T.; Zhang, Y.; Lv, M.; Zang, G.; Ng, S.S.; Chen, X. Advances in 3D cell culture for liver preclinical studies. Acta Biochim. Biophys. Sin. 2021, 53, 643–651. [Google Scholar] [CrossRef]
- Xu, A.-L.; Han, L.; Yan, J.; Liu, D.; Wang, W. Effects of Mesenchymal Stem Cells-Derived Extracellular Vesicles on Inhibition of Hepatic Fibrosis by Delivering miR-200a. Tissue Eng. Regen. Med. 2024, 21, 609–624. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef]
- Parola, M.; Pinzani, M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol. Asp. Med. 2019, 65, 37–55. [Google Scholar] [CrossRef]
- Zeng, X.; Huang, D.; Zhu, Z.; Cai, Q.; Yang, Y.; Lu, H.; Chen, J. Mechanism-guided drug development and treatment for liver fibrosis: A clinical perspective. Front. Pharmacol. 2025, 16, 1574385. [Google Scholar] [CrossRef]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef]
- Dooley, S.; Ten Dijke, P. TGF-β in progression of liver disease. Cell Tissue Res. 2012, 347, 245–256. [Google Scholar] [CrossRef]
- Flanders, K.; Sato, M.; Ooshima, A.; Russo, A.; Roberts, A. Smad-3 as a mediator of the fibrotic response. Int. J. Exp. Pathol. 2004, 85, A13. [Google Scholar] [CrossRef]
- Meng, X.-M.; Tang, P.M.-K.; Li, J.; Lan, H.Y. TGF-β/Smad signaling in renal fibrosis. Front. Physiol. 2015, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Fallowfield, J.A.; Mizuno, M.; Kendall, T.J.; Constandinou, C.M.; Benyon, R.C.; Duffield, J.S.; Iredale, J.P. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J. Immunol. 2007, 178, 5288–5295. [Google Scholar] [CrossRef]
- Kocabayoglu, P.; Lade, A.; Lee, Y.A.; Dragomir, A.C.; Sun, X.; Fiel, M.I.; Thung, S.; Aloman, C.; Soriano, P.; Hoshida, Y.; et al. β-PDGF receptor expressed by hepatic stellate cells regulates fibrosis in murine liver injury, but not carcinogenesis. J. Hepatol. 2015, 63, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Foglia, B.; Cannito, S.; Bocca, C.; Parola, M.; Novo, E. ERK Pathway in Activated, Myofibroblast-Like, Hepatic Stellate Cells: A Critical Signaling Crossroad Sustaining Liver Fibrosis. Int. J. Mol. Sci. 2019, 20, 2700. [Google Scholar] [CrossRef]
- Bonacchi, A.; Romagnani, P.; Romanelli, R.G.; Efsen, E.; Annunziato, F.; Lasagni, L.; Francalanci, M.; Serio, M.; Laffi, G.; Pinzani, M.; et al. Signal transduction by the chemokine receptor CXCR3: Activation of Ras/ERK, Src, and phosphatidylinositol 3-kinase/Akt controls cell migration and proliferation in human vascular pericytes. J. Biol. Chem. 2001, 276, 9945–9954. [Google Scholar] [CrossRef]
- Rennert, K.; Steinborn, S.; Gröger, M.; Ungerböck, B.; Jank, A.M.; Ehgartner, J.; Nietzsche, S.; Dinger, J.; Kiehntopf, M.; Funke, H.; et al. A microfluidically perfused three dimensional human liver model. Biomaterials 2015, 71, 119–131. [Google Scholar] [CrossRef]
- Pradere, J.-P.; Troeger, J.S.; Dapito, D.H.; Mencin, A.A.; Schwabe, R.F. Toll-like receptor 4 and hepatic fibrogenesis. Semin. Liver Dis. 2010, 30, 232–244. [Google Scholar] [CrossRef]
- Hammerich, L.; Tacke, F. Hepatic inflammatory responses in liver fibrosis. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.D.; Zhou, J.; Chen, E.Q. Molecular Mechanisms and Potential New Therapeutic Drugs for Liver Fibrosis. Front. Pharmacol. 2022, 13, 787748. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.A. Molecular pathogenesis of liver fibrosis. Trans. Am. Clin. Climatol. Assoc. 2009, 120, 361–368. [Google Scholar]
- Barron, L.; Wynn, T.A. Fibrosis is regulated by Th2 and Th17 responses and by dynamic interactions between fibroblasts and macrophages. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G723–G728. [Google Scholar] [CrossRef]
- Casari, M.; Siegl, D.; Deppermann, C.; Schuppan, D. Macrophages and platelets in liver fibrosis and hepatocellular carcinoma. Front. Immunol. 2023, 14, 1277808. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Peng, H.; Dong, Z.; Liangpunsakul, S.; Zuo, L.; Wang, H. Platelets in alcohol-associated liver disease: Interaction with neutrophils. Cell. Mol. Gastroenterol. Hepatol. 2024, 18, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Berumen, J.; Baglieri, J.; Kisseleva, T.; Mekeel, K. Liver fibrosis: Pathophysiology and clinical implications. WIREs Mech. Dis. 2021, 13, e1499. [Google Scholar] [CrossRef] [PubMed]
- Borkham-Kamphorst, E.; van Roeyen, C.R.; Van de Leur, E.; Floege, J.; Weiskirchen, R. CCN3/NOV small interfering RNA enhances fibrogenic gene expression in primary hepatic stellate cells and cirrhotic fat storing cell line CFSC. J. Cell Commun. Signal 2012, 6, 11–25. [Google Scholar] [CrossRef]
- Palma, E.; Doornebal, E.J.; Chokshi, S. Precision-cut liver slices: A versatile tool to advance liver research. Hepatol. Int. 2019, 13, 51–57. [Google Scholar] [CrossRef]
- Ramboer, E.; De Craene, B.; De Kock, J.; Vanhaecke, T.; Berx, G.; Rogiers, V.; Vinken, M. Strategies for immortalization of primary hepatocytes. J. Hepatol. 2014, 61, 925–943. [Google Scholar] [CrossRef]
- Javitt, N.B. Hep G2 cells as a resource for metabolic studies: Lipoprotein, cholesterol, and bile acids. Faseb J. 1990, 4, 161–168. [Google Scholar] [CrossRef]
- Zeilinger, K.; Freyer, N.; Damm, G.; Seehofer, D.; Knöspel, F. Cell sources for in vitro human liver cell culture models. Exp. Biol. Med. 2016, 241, 1684–1698. [Google Scholar] [CrossRef]
- Xu, L.; Hui, A.; Albanis, E.; Arthur, M.; O’byrne, S.; Blaner, W.; Mukherjee, P.; Friedman, S.; Eng, F. Human hepatic stellate cell lines, LX-1 and LX-2: New tools for analysis of hepatic fibrosis. Gut 2005, 54, 142–151. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Weimer, J.; Meurer, S.K.; Kron, A.; Seipel, B.; Vater, I.; Arnold, N.; Siebert, R.; Xu, L.; Friedman, S.L. Genetic characteristics of the human hepatic stellate cell line LX-2. PLoS ONE 2013, 8, e75692. [Google Scholar] [CrossRef]
- Ramos, M.J.; Bandiera, L.; Menolascina, F.; Fallowfield, J.A. In vitro models for non-alcoholic fatty liver disease: Emerging platforms and their applications. iScience 2022, 25, 103549. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.M.; Bansal, R.; Barrias, C.C.; Sarmento, B. The Material World of 3D-Bioprinted and Microfluidic-Chip Models of Human Liver Fibrosis. Adv. Mater. 2024, 36, e2307673. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Fang, J.; Shen, Y.; Li, M.; Wang, B.; Xu, Z.; Hu, W. Advances of naturally derived biomedical polymers in tissue engineering. Front. Chem. 2024, 12, 1469183. [Google Scholar] [CrossRef]
- Farazin, A.; Darghiasi, S.F. Advanced polymeric scaffolds for bone tissue regeneration. Explor. BioMat-X 2025, 2, 101340. [Google Scholar] [CrossRef]
- Bell, C.C.; Hendriks, D.F.; Moro, S.M.; Ellis, E.; Walsh, J.; Renblom, A.; Fredriksson Puigvert, L.; Dankers, A.C.; Jacobs, F.; Snoeys, J.; et al. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci. Rep. 2016, 6, 25187. [Google Scholar] [CrossRef]
- Abu-Absi, S.F.; Friend, J.R.; Hansen, L.K.; Hu, W.-S. Structural polarity and functional bile canaliculi in rat hepatocyte spheroids. Exp. Cell Res. 2002, 274, 56–67. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Romeo, S.; Valenti, L. Genetic Factors in the Pathogenesis of Nonalcoholic Fatty Liver and Steatohepatitis. Biomed. Res. Int. 2015, 2015, 460190. [Google Scholar] [CrossRef] [PubMed]
- Salloum, S.; Jeyarajan, A.J.; Kruger, A.J.; Holmes, J.A.; Shao, T.; Sojoodi, M.; Kim, M.H.; Zhuo, Z.; Shroff, S.G.; Kassa, A.; et al. Fatty Acids Activate the Transcriptional Coactivator YAP1 to Promote Liver Fibrosis via p38 Mitogen-Activated Protein Kinase. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 1297–1310. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, T.; Kastrinou-Lampou, V.; Fardellas, A.; Hendriks, D.F.G.; Nordling, Å.; Johansson, I.; Baze, A.; Parmentier, C.; Richert, L.; Ingelman-Sundberg, M. Human Liver Spheroids as a Model to Study Aetiology and Treatment of Hepatic Fibrosis. Cells 2020, 9, 964. [Google Scholar] [CrossRef]
- Bell, C.C.; Dankers, A.C.; Lauschke, V.M.; Sison-Young, R.; Jenkins, R.; Rowe, C.; Goldring, C.E.; Park, K.; Regan, S.L.; Walker, T. Comparison of hepatic 2D sandwich cultures and 3D spheroids for long-term toxicity applications: A multicenter study. Toxicol. Sci. 2018, 162, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Mizoi, K.; Arakawa, H.; Yano, K.; Koyama, S.; Kojima, H.; Ogihara, T. Utility of three-dimensional cultures of primary human hepatocytes (spheroids) as pharmacokinetic models. Biomedicines 2020, 8, 374. [Google Scholar] [CrossRef]
- Järvinen, E.; Hammer, H.S.; Pötz, O.; Ingelman-Sundberg, M.; Stage, T.B. 3D spheroid primary human hepatocytes for prediction of cytochrome P450 and drug transporter induction. Clin. Pharmacol. Ther. 2023, 113, 1284–1294. [Google Scholar] [CrossRef]
- Tanaka, Y.; Shimanaka, Y.; Caddeo, A.; Kubo, T.; Mao, Y.; Kubota, T.; Kubota, N.; Yamauchi, T.; Mancina, R.M.; Baselli, G.; et al. LPIAT1/MBOAT7 depletion increases triglyceride synthesis fueled by high phosphatidylinositol turnover. Gut 2021, 70, 180–193. [Google Scholar] [CrossRef]
- Schwartz, B.E.; Rajagopal, V.; Smith, C.; Cohick, E.; Whissell, G.; Gamboa, M.; Pai, R.; Sigova, A.; Grossman, I.; Bumcrot, D.; et al. Discovery and Targeting of the Signaling Controls of PNPLA3 to Effectively Reduce Transcription, Expression, and Function in Pre-Clinical NAFLD/NASH Settings. Cells 2020, 9, 2247. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Kim, T.-H. Recent advances in multicellular tumor spheroid generation for drug screening. Biosensors 2021, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Liang, Z.; Wang, Y.; Yu, Y.; Zhou, X.; Geng, X.; Li, B. A 3D spheroid model of quadruple cell co-culture with improved liver functions for hepatotoxicity prediction. Toxicology 2024, 505, 153829. [Google Scholar] [CrossRef]
- Broutier, L.; Andersson-Rolf, A.; Hindley, C.J.; Boj, S.F.; Clevers, H.; Koo, B.K.; Huch, M. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 2016, 11, 1724–1743. [Google Scholar] [CrossRef]
- Elbadawy, M.; Yamanaka, M.; Goto, Y.; Hayashi, K.; Tsunedomi, R.; Hazama, S.; Nagano, H.; Yoshida, T.; Shibutani, M.; Ichikawa, R.; et al. Efficacy of primary liver organoid culture from different stages of non-alcoholic steatohepatitis (NASH) mouse model. Biomaterials 2020, 237, 119823. [Google Scholar] [CrossRef]
- Ramos Pittol, J.M.; Milona, A.; Morris, I.; Willemsen, E.C.L.; van der Veen, S.W.; Kalkhoven, E.; van Mil, S.W.C. FXR Isoforms Control Different Metabolic Functions in Liver Cells via Binding to Specific DNA Motifs. Gastroenterology 2020, 159, 1853–1865.e1810. [Google Scholar] [CrossRef]
- Sano, A.; Kakazu, E.; Hamada, S.; Inoue, J.; Ninomiya, M.; Iwata, T.; Tsuruoka, M.; Sato, K.; Masamune, A. Steatotic Hepatocytes Release Mature VLDL Through Methionine and Tyrosine Metabolism in a Keap1-Nrf2-Dependent Manner. Hepatology 2021, 74, 1271–1286. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, C.; Zhai, Z.; Deng, Z.Y.; De Jonge, H.R.; Wu, X.; Ruan, Z. Uridine attenuates obesity, ameliorates hepatic lipid accumulation and modifies the gut microbiota composition in mice fed with a high-fat diet. Food Funct. 2021, 12, 1829–1840. [Google Scholar] [CrossRef]
- Kim, J.; Koo, B.K.; Knoblich, J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Leite, S.B.; Roosens, T.; El Taghdouini, A.; Mannaerts, I.; Smout, A.J.; Najimi, M.; Sokal, E.; Noor, F.; Chesne, C.; van Grunsven, L.A. Novel human hepatic organoid model enables testing of drug-induced liver fibrosis in vitro. Biomaterials 2016, 78, 1–10. [Google Scholar] [CrossRef]
- Ströbel, S.; Kostadinova, R.; Fiaschetti-Egli, K.; Rupp, J.; Bieri, M.; Pawlowska, A.; Busler, D.; Hofstetter, T.; Sanchez, K.; Grepper, S. A 3D primary human cell-based in vitro model of non-alcoholic steatohepatitis for efficacy testing of clinical drug candidates. Sci. Rep. 2021, 11, 22765. [Google Scholar] [CrossRef] [PubMed]
- Chiabotto, G.; Ceccotti, E.; Bruno, S. Narrative review of in vitro experimental models of hepatic fibrogenesis. Dig. Med. Res. 2022, 5, 8238. [Google Scholar] [CrossRef]
- Xu, Q. Human three-dimensional hepatic models: Cell type variety and corresponding applications. Front. Bioeng. Biotechnol. 2021, 9, 730008. [Google Scholar] [CrossRef]
- Coll, M.; Perea, L.; Boon, R.; Leite, S.B.; Vallverdú, J.; Mannaerts, I.; Smout, A.; El Taghdouini, A.; Blaya, D.; Rodrigo-Torres, D.; et al. Generation of Hepatic Stellate Cells from Human Pluripotent Stem Cells Enables In Vitro Modeling of Liver Fibrosis. Cell Stem Cell 2018, 23, 101–113.e107. [Google Scholar] [CrossRef]
- Ouchi, R.; Togo, S.; Kimura, M.; Shinozawa, T.; Koido, M.; Koike, H.; Thompson, W.; Karns, R.A.; Mayhew, C.N.; McGrath, P.S.; et al. Modeling Steatohepatitis in Humans with Pluripotent Stem Cell-Derived Organoids. Cell Metab. 2019, 30, 374–384.e376. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, D.; Yang, Y.; Li, S.; Ding, Q. Modeling drug-induced liver injury and screening for anti-hepatofibrotic compounds using human PSC-derived organoids. Cell Regen. 2023, 12, 6. [Google Scholar] [CrossRef]
- Underhill, G.H.; Khetani, S.R. Bioengineered Liver Models for Drug Testing and Cell Differentiation Studies. Cell Mol. Gastroenterol. Hepatol. 2018, 5, 426–439.e421. [Google Scholar] [CrossRef]
- Eckstrum, K.; Striz, A.; Ferguson, M.; Zhao, Y.; Welch, B.; Solomotis, N.; Olejnik, N.; Sprando, R. Utilization of a model hepatotoxic compound, diglycolic acid, to evaluate liver Organ-Chip performance and in vitro to in vivo concordance. Food Chem. Toxicol. 2020, 146, 111850. [Google Scholar] [CrossRef] [PubMed]
- Gori, M.; Simonelli, M.C.; Giannitelli, S.M.; Businaro, L.; Trombetta, M.; Rainer, A. Investigating Nonalcoholic Fatty Liver Disease in a Liver-on-a-Chip Microfluidic Device. PLoS ONE 2016, 11, e0159729. [Google Scholar] [CrossRef]
- Suurmond, C.E.; Lasli, S.; van den Dolder, F.W.; Ung, A.; Kim, H.J.; Bandaru, P.; Lee, K.; Cho, H.J.; Ahadian, S.; Ashammakhi, N.; et al. In Vitro Human Liver Model of Nonalcoholic Steatohepatitis by Coculturing Hepatocytes, Endothelial Cells, and Kupffer Cells. Adv. Healthc. Mater. 2019, 8, e1901379. [Google Scholar] [CrossRef]
- Wilkening, S.; Stahl, F.; Bader, A. Comparison of primary human hepatocytes and hepatoma cell line Hepg2 with regard to their biotransformation properties. Drug Metab. Dispos. 2003, 31, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Gerets, H.H.; Tilmant, K.; Gerin, B.; Chanteux, H.; Depelchin, B.; Dhalluin, S.; Atienzar, F. Characterization of primary human hepatocytes, HepG2 cells, and HepaRG cells at the mRNA level and CYP activity in response to inducers and their predictivity for the detection of human hepatotoxins. Cell Biol. Toxicol. 2012, 28, 69–87. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Sasaki, Y.; Terasaki, N.; Kawataki, T.; Takekawa, K.; Iwase, Y.; Shimizu, T.; Sanoh, S.; Ohta, S. Comparison of drug metabolism and its related hepatotoxic effects in HepaRG, cryopreserved human hepatocytes, and HepG2 cell cultures. Biol. Pharm. Bull. 2018, 41, 722–732. [Google Scholar] [CrossRef]
- Tolosa, L.; Gómez-Lechón, M.J.; Jiménez, N.; Hervás, D.; Jover, R.; Donato, M.T. Advantageous use of HepaRG cells for the screening and mechanistic study of drug-induced steatosis. Toxicol. Appl. Pharmacol. 2016, 302, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Kim, H.J.; Lee, K.; Lasli, S.; Ung, A.; Hoffman, T.; Nasiri, R.; Bandaru, P.; Ahadian, S.; Dokmeci, M.R.; et al. Bioengineered Multicellular Liver Microtissues for Modeling Advanced Hepatic Fibrosis Driven Through Non-Alcoholic Fatty Liver Disease. Small 2021, 17, e2007425. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Aleman, J.; Forsythe, S.; Rajan, S.; Murphy, S.; Devarasetty, M.; Pourhabibi Zarandi, N.; Nzou, G.; Wicks, R.; Sadri-Ardekani, H.; et al. Drug compound screening in single and integrated multi-organoid body-on-a-chip systems. Biofabrication 2020, 12, 025017. [Google Scholar] [CrossRef]
- Lee, S.Y.; Sung, J.H. Gut-liver on a chip toward an in vitro model of hepatic steatosis. Biotechnol. Bioeng. 2018, 115, 2817–2827. [Google Scholar] [CrossRef]
- Vinci, M.; Gowan, S.; Boxall, F.; Patterson, L.; Zimmermann, M.; Court, W.; Lomas, C.; Mendiola, M.; Hardisson, D.; Eccles, S.A. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 2012, 10, 29. [Google Scholar] [CrossRef]
- Nath, S.; Devi, G.R. Three-dimensional culture systems in cancer research: Focus on tumor spheroid model. Pharmacol. Ther. 2016, 163, 94–108. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Murphy, W.L. Synthetic alternatives to Matrigel. Nat. Rev. Mater. 2020, 5, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Picollet-D’hahan, N.; Zuchowska, A.; Lemeunier, I.; Le Gac, S. Multiorgan-on-a-Chip: A Systemic Approach To Model and Decipher Inter-Organ Communication. Trends Biotechnol. 2021, 39, 788–810. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Y.; Ueno, H.; Esch, M.B. Body-in-a-Cube: A microphysiological system for multi-tissue co-culture with near-physiological amounts of blood surrogate. Microphysiol. Syst. 2020, 4, 6050. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wei, W.; Chen, Z.; Lin, B.; Zhao, W.; Luo, Y.; Zhang, X. Engineered Liver-on-a-Chip Platform to Mimic Liver Functions and Its Biomedical Applications: A Review. Micromachines 2019, 10, 676. [Google Scholar] [CrossRef]
- Yu, F.; Hunziker, W.; Choudhury, D. Engineering Microfluidic Organoid-on-a-Chip Platforms. Micromachines 2019, 10, 165. [Google Scholar] [CrossRef]
- Heydari, Z.; Moeinvaziri, F.; Agarwal, T.; Pooyan, P.; Shpichka, A.; Maiti, T.K.; Timashev, P.; Baharvand, H.; Vosough, M. Organoids: A novel modality in disease modeling. Biodes Manuf. 2021, 4, 689–716. [Google Scholar] [CrossRef]
- Tomlinson, L.; Hyndman, L.; Firman, J.W.; Bentley, R.; Kyffin, J.A.; Webb, S.D.; McGinty, S.; Sharma, P. In vitro Liver Zonation of Primary Rat Hepatocytes. Front. Bioeng. Biotechnol. 2019, 7, 17. [Google Scholar] [CrossRef]
- Moravcová, A.; Červinková, Z.; Kučera, O.; Mezera, V.; Rychtrmoc, D.; Lotková, H. The effect of oleic and palmitic acid on induction of steatosis and cytotoxicity on rat hepatocytes in primary culture. Physiol. Res. 2015, 64, S627–S636. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.S.; Yuan, S.; Zhang, H.; Liu, J.; Wang, P.; Lai, C.S.; Zanella, F.; et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Prieto, A.M.; Skelton, J.K.; Wai, S.N.; Large, E.; Lussignol, M.; Vizcay-Barrena, G.; Hughes, D.; Fleck, R.A.; Thursz, M.; Catanese, M.T.; et al. 3D microfluidic liver cultures as a physiological preclinical tool for hepatitis B virus infection. Nat. Commun. 2018, 9, 682. [Google Scholar] [CrossRef] [PubMed]
- Frey, O.; Misun, P.M.; Fluri, D.A.; Hengstler, J.G.; Hierlemann, A. Reconfigurable microfluidic hanging drop network for multi-tissue interaction and analysis. Nat. Commun. 2014, 5, 4250. [Google Scholar] [CrossRef]
- Bavli, D.; Prill, S.; Ezra, E.; Levy, G.; Cohen, M.; Vinken, M.; Vanfleteren, J.; Jaeger, M.; Nahmias, Y. Real-time monitoring of metabolic function in liver-on-chip microdevices tracks the dynamics of mitochondrial dysfunction. Proc. Natl. Acad. Sci. USA 2016, 113, E2231–E2240. [Google Scholar] [CrossRef]
- Delalat, B.; Cozzi, C.; Rasi Ghaemi, S.; Polito, G.; Kriel, F.H.; Michl, T.D.; Harding, F.J.; Priest, C.; Barillaro, G.; Voelcker, N.H. Microengineered Bioartificial Liver Chip for Drug Toxicity Screening. Adv. Funct. Mater. 2018, 28, 1801825. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, L.; Zhou, E.M.; Xu, J.; Shen, S.; Wang, J. On-Chip Construction of Liver Lobule-like Microtissue and Its Application for Adverse Drug Reaction Assay. Anal. Chem. 2016, 88, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; George, S.M.; Vernetti, L.; Gough, A.H.; Taylor, D.L. A glass-based, continuously zonated and vascularized human liver acinus microphysiological system (vLAMPS) designed for experimental modeling of diseases and ADME/TOX. Lab A Chip 2018, 18, 2614–2631. [Google Scholar] [CrossRef]
- Schepers, A.; Li, C.; Chhabra, A.; Seney, B.T.; Bhatia, S. Engineering a perfusable 3D human liver platform from iPS cells. Lab A Chip 2016, 16, 2644–2653. [Google Scholar] [CrossRef] [PubMed]






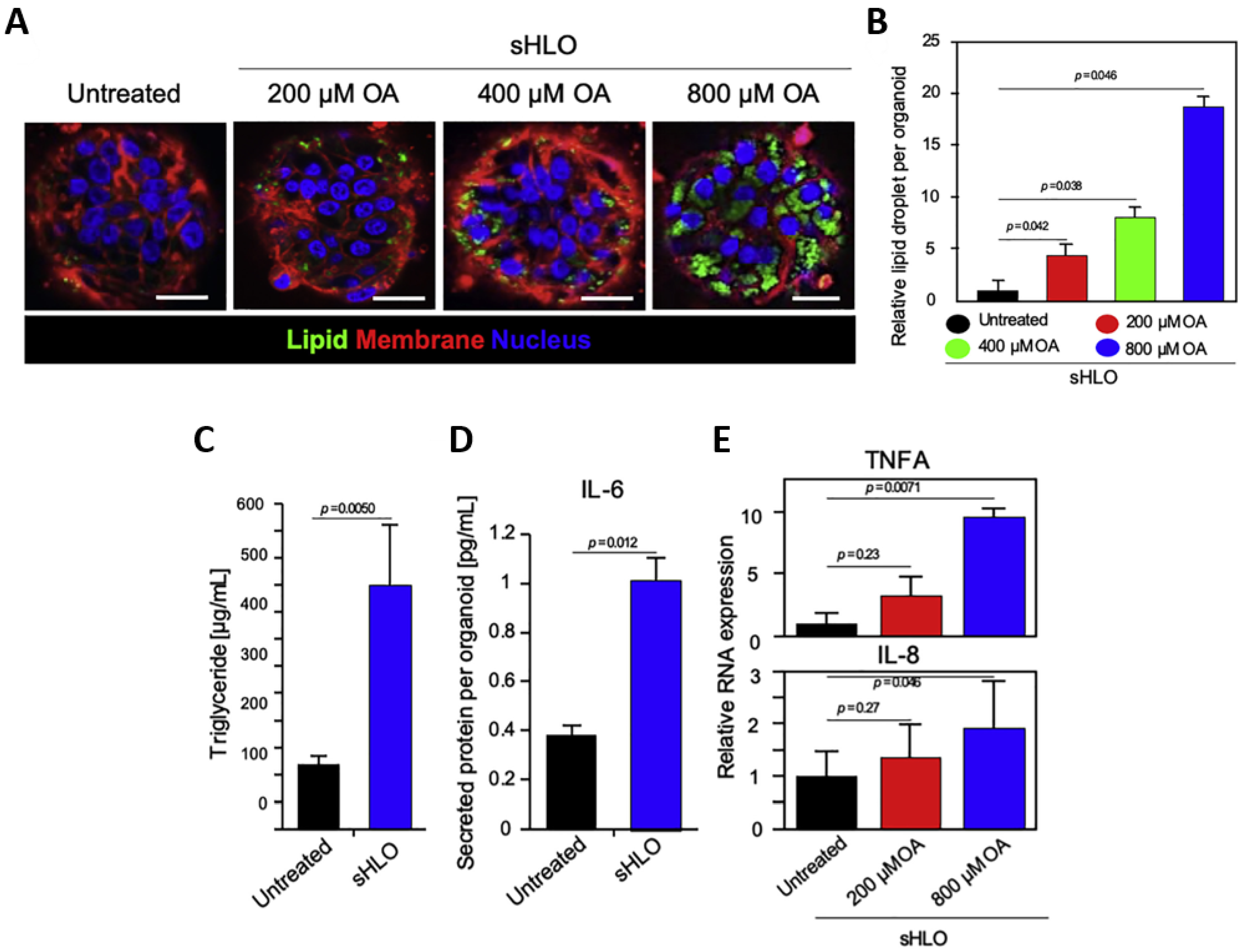




| Platforms | Cell Sources | Method to Induce Fibrosis | Methodological Strengths | Observations | Ref |
|---|---|---|---|---|---|
| Spheroid | PHH + HSCs | TGF-β1 stimulation | Enables multicellular fibrogenic signaling | ↑COL1A1, TIMP1, ACTA2 (fibrosis markers) | [47] |
| PHH + NPCs (KCs, biliary cells) | Cytokine exposure | Maintains cell-specific phenotypes in 3D | ↑IL-6 secretion; preserved CD68, VIM, CK19 | [48] | |
| Hepa1-6 + HSCs | MB0AT7 silencing | Captures genetic drivers of NAFLD | ↑lipid accumulation, ↑collagen production | [52] | |
| Hepa1-6 + HSCs | PNPLA3 I148M knockdown | Models NAFLD-associated genetic variants | ↑lipid storage, ↑collagen secretion | [53] | |
| HepG2 + HUVEC + NIH3T3 (with collagen) | ECM scaffold modulation | Provides ECM-based microenvironmental control | Reduced necrotic core; enlarged spheroid size | [54] | |
| Organoid | Murine hepatocytes + activated HSCs | High-fat diet (in vivo) → organoid culture | Captures stage-specific fibrogenic responses | Early organoids: ↑IL-1β; stage-dependent lipid metabolism changes | [57] |
| Murine tissue-derived organoids | Oxidative/electrophilic stress (FAAs) | Captures metabolic/ detox pathways | FXR isoform analysis | [58] | |
| Murine tissue-derived organoids | Oxidative/electrophilic stress (methionine and tyrosine metabolism) | Models stress responses during NAFLD progression | Functional detoxification assays | [59] | |
| Murine tissue-derived organoids | Lipotoxic/oxidative stress (Keap1–Nrf2 antioxidant pathway) | Enables antioxidant pathway analysis | Identified uridine effect in alleviating lipotoxic stress | [60] | |
| Human HepaRG + primary HSCs | Compound exposure | Provides parenchymal–stromal interaction | HSC activation under hepatocellular injury | [61] | |
| iPSC-derived HSCs + HepaRG | APAP (acetaminophen) exposure | Models hepatocyte injury-driven HSC activation cascade | ↑pro-collagen, ↑α-SMA, ↑retinol storage | [65] | |
| Liver-on-a-chip | HepG2 in parallel microchannels | FFA perfusion | Provides endothelial barrier mimicry under flow | Modeled FFA-induced steatosis | [70] |
| GelMA-encapsulated HepG2 + HUVEC | FFA exposure | ECM-like GelMA supports adhesion and remodeling | Lipid accumulation; ↑ROS and ↑pro-inflammatory cytokines (with KCs) | [71] | |
| PHHs | Drug metabolism assays | Provides in vivo-like enzyme activity | Improved biotransformation and toxicant sensitivity | [72] | |
| HepaRG instead of HepG2 | FFA exposure | Enhanced metabolic competence (↑CYP activity) | ↑inflammatory cytokine secretion; improved steatohepatitis modeling | [73] | |
| HepaRG + HSCs + KCs + HUVECs | FFA treatment | Integrates multicell crosstalk under perfusion | ↑collagen I, fibronectin, α-SMA expression (fibrogenesis recapitulated) | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.E.; Lee, Y.-J.; Yoon, J.-K. Advanced 3D In Vitro Liver Fibrosis Models: Spheroids, Organoids, and Liver-on-Chips. Biomimetics 2025, 10, 639. https://doi.org/10.3390/biomimetics10100639
Lee JE, Lee Y-J, Yoon J-K. Advanced 3D In Vitro Liver Fibrosis Models: Spheroids, Organoids, and Liver-on-Chips. Biomimetics. 2025; 10(10):639. https://doi.org/10.3390/biomimetics10100639
Chicago/Turabian StyleLee, Jae Eun, Yu-Jeong Lee, and Jeong-Kee Yoon. 2025. "Advanced 3D In Vitro Liver Fibrosis Models: Spheroids, Organoids, and Liver-on-Chips" Biomimetics 10, no. 10: 639. https://doi.org/10.3390/biomimetics10100639
APA StyleLee, J. E., Lee, Y.-J., & Yoon, J.-K. (2025). Advanced 3D In Vitro Liver Fibrosis Models: Spheroids, Organoids, and Liver-on-Chips. Biomimetics, 10(10), 639. https://doi.org/10.3390/biomimetics10100639






