Pruritus in Autoimmune Demyelinating Diseases of the Central Nervous System: A Review
Abstract
1. Introduction
2. Multiple Sclerosis (MS), Paroxysmal Symptoms (PSs) and Pruritus
3. Neuromyelitis Optica Spectrum Disorders (NMOSD), Paroxysmal Symptoms (PSs) and Pruritus
4. Pruritus and Pathogenetic Mechanisms
5. Spinal Cord and Pruritus
6. Brainstem and Pruritus
7. Cerebellum and Pruritus
8. Brain and Pruritus
9. The Role of Astrocytes in Pruritus in Demyelinating Diseases of CNS
10. Drugs and Pruritus
11. Pruritus in Other Autoimmune Demyelinating Disorders of the CNS
12. Management and Treatment of Pruritus in Demyelinating Diseases of the CNS
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajagopalan, M.; Saraswat, A.; Godse, K.; Shankar, D.S.K.; Kandhari, S.; Shenoi, S.D.; Tahiliani, S.; Zawar, V.V. Diagnosis and management of chronic pruritus: An expert consensus review. Indian J. Dermatol. 2017, 62, 7–17. [Google Scholar] [CrossRef]
- Huguen, J.; Brenaut, E.; Clerc, C.J.; Poizeau, F.; Marcorelles, P.; Quereux, G.; Dupuy, A.; Misery, L. Comparison of Characteristics of Neuropathic and Non-neuropathic Pruritus to Develop a Tool for the Diagnosis of Neuropathic Pruritus: The NP5. Front. Med. 2019, 6, 79. [Google Scholar] [CrossRef]
- Jakimovski, D.; Bittner, S.; Zivadinov, R.; Morrow, S.A.; Benedict, R.H.; Zipp, F.; Weinstock-Guttman, B. Multiple sclerosis. Lancet 2024, 403, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Siriratnam, P.; Huda, S.; Butzkueven, H.; van der Walt, A.; Jokubaitis, V.; Monif, M. A comprehensive review of the advances in neuromyelitis optica spectrum disorder. Autoimmun. Rev. 2023, 22, 103465. [Google Scholar] [CrossRef] [PubMed]
- Ingrasci, G.; Tornes, L.; Brown, A.; Delgado, S.; Hernandez, J.; Yap, Q.V.; Yosipovitch, G. Chronic pruritus in multiple sclerosis and clinical correlates. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Haki, M.; AL-Biati, H.A.; Al-Tameemi, Z.S.; Ali, I.S.; Al-hussaniy, H.A. Review of multiple sclerosis: Epidemiology, etiology, pathophysiology, and treatment. Medicine 2024, 103, e37297. [Google Scholar] [CrossRef]
- Correale, J.; Farez, M.F. The Role of Astrocytes in Multiple Sclerosis Progression. Front. Neurol. 2015, 6, 180. [Google Scholar] [CrossRef]
- Freiha, J.; Riachi, N.; Chalah, M.A.; Zoghaib, R.; Ayache, S.S.; Ahdab, R. Paroxysmal Symptoms in Multiple Sclerosis—A Review of the Literature. J. Clin. Med. 2020, 9, 3100. [Google Scholar] [CrossRef]
- Osterman, P.O. Paroxysmal Itching in Multiple Sclerosis. Int. J. Dermatol. 1979, 18, 626–627. [Google Scholar] [CrossRef]
- Sandyk, R. Paroxysmal Itching in Multiple Sclerosis During Treatment with External Magnetic Fields. Int. J. Neurosci. 1994, 75, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Mutch, K.; Elsone, L.; Miller, J.; Jacob, A. An unusual case of ‘itchy paralysis’: Neuromyelitis optica presenting with severe neuropathic itch. Pract. Neurol. 2015, 15, 149–151. [Google Scholar] [CrossRef]
- Mora Cuervo, D.L.; Hansel, G.; Sato, D.K. Immunobiology of neuromyelitis optica spectrum disorders. Curr. Opin. Neurobiol. 2022, 76, 102618. [Google Scholar] [CrossRef]
- Lotan, I.; Bacon, T.; Kister, I.; Levy, M. Paroxysmal symptoms in neuromyelitis optica spectrum disorder: Results from an online patient survey. Mult. Scler. Relat. Disord. 2020, 46, 102578. [Google Scholar] [CrossRef]
- Ikoma, A.; Rukwied, R.; Ständer, S.; Steinhoff, M.; Miyachi, Y.; Schmelz, M. Neurophysiology of Pruritus. Arch. Dermatol. 2003, 139, 1475–1478. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xian, D.; Yang, L.; Xiong, X.; Lai, R.; Zhong, J. Pruritus: Progress toward Pathogenesis and Treatment. Biomed. Res. Int. 2018, 2018, 9625936. [Google Scholar] [CrossRef]
- Luo, J.; Feng, J.; Liu, S.; Walters, E.T.; Hu, H. Molecular and cellular mechanisms that initiate pain and itch. Cell. Mol. Life Sci. 2015, 72, 3201–3223. [Google Scholar] [CrossRef]
- Akiyama, T.; Carstens, E. Neural processing of itch. Neuroscience 2013, 250, 697–714. [Google Scholar] [CrossRef]
- Wilson, S.R.; Gerhold, K.A.; Bifolck-Fisher, A.; Liu, Q.; Patel, K.N.; Dong, X.; Bautista, D.M. TRPA1 is required for histamine-independent, Mas-related G protein–coupled receptor–mediated itch. Nat. Neurosci. 2011, 14, 595–602. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wu, L.; Huang, D.; Yau, V.; Yu, S. Pruritus in neuromyelitis optica spectrum disorders and multiple sclerosis. J. Clin. Neurosci. 2020, 79, 108–112. [Google Scholar] [CrossRef]
- Elsone, L.; Townsend, T.; Mutch, K.; Das, K.; Boggild, M.; Nurmikko, T.; Jacob, A. Neuropathic pruritus (itch) in neuromyelitis optica. Mult. Scler. J. 2013, 19, 475–479. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kawazawa, S.; Takase, Y.; Fukusako, T.; Morimatsu, M. Paroxysmal itching and magnetic resonance imaging of the spinal cord in multiple sclerosis. Rinsho Shinkeigaku 1989, 29, 1345–1351. [Google Scholar]
- Akiyama, T.; Nguyen, T.; Curtis, E.; Nishida, K.; Devireddy, J.; Delahanty, J.; Carstens, M.L.; Carstens, E. A central role for spinal dorsal horn neurons that express neurokinin-1 receptors in chronic itch. Pain 2015, 156, 1240–1246. [Google Scholar] [CrossRef]
- Davidson, S.; Zhang, X.; Khasabov, S.G.; Moser, H.R.; Honda, C.N.; Simone, D.A.; Giesler, G.J., Jr. Pruriceptive spinothalamic tract neurons: Physiological properties and projection targets in the primate. J. Neurophysiol. 2012, 108, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Qiu, W.; Lu, Z.; Li, R.; Hu, X. Intractable pruritus in neuromyelitis optica. Neurol. Sci. 2016, 37, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Saini, L.; Gunasekaran, P.K.; Tiwari, S.; Krishna, D.; Laxmi, V.; Jindal, P.; Kumar, P. Paroxysmal Neuropathic Pruritus in Patients With Chiari Malformation Type I: A Rare Phenotype. Pediatr. Neurol. 2023, 140, 65–67. [Google Scholar] [CrossRef]
- Misery, L.; Genestet, S.; Zagnoli, F. Neuromyelitis Optica and Skin. Dermatology 2022, 238, 823–828. [Google Scholar] [CrossRef]
- Kremer, L.; Mealy, M.; Jacob, A.; Nakashima, I.; Cabre, P.; Bigi, S.; Paul, F.; Jarius, S.; Aktas, O.; Elsone, L.; et al. Brainstem manifestations in neuromyelitis optica: A multicenter study of 258 patients. Mult. Scler. J. 2014, 20, 843–847. [Google Scholar] [CrossRef]
- Matsuura, J.; Kimura, A.; Kasai, T.; Yoshida, T.; Nakagawa, M.; Mizuno, T. A Case of Neuromyelitis Optica with Relapse Symptoms from Paroxysmal Pruritus. Brain Nerve 2015, 67, 1057–1060. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Ishiuji, Y.; Patel, T.S.; Hicks, M.I.; Oshiro, Y.; Kraft, R.A.; Winnicki, E.; Coghill, R.C. The Brain Processing of Scratching. J. Investig. Dermatol. 2008, 128, 1806–1811. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Kakigi, R. Central mechanisms of itch. Clin. Neurophysiol. 2015, 126, 1650–1660. [Google Scholar] [CrossRef]
- Mochizuki, H.; Tanaka, S.; Morita, T.; Wasaka, T.; Sadato, N.; Kakigi, R. The cerebral representation of scratching-induced pleasantness. J. Neurophysiol. 2014, 111, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zou, Z.; Yu, S.; Xiao, X.; Shi, Y.; Cao, W.; Liu, Y.; Zheng, H.; Zheng, Q.; Zhou, S.; et al. Functional connectivity impairment of thalamus-cerebellum-scratching neural circuits in pruritus of chronic spontaneous urticaria. Front. Neurosci. 2022, 16, 1026200. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, O.; Oladipo, O.; Mahmoud, R.H.; Yosipovitch, G. Itch: From the skin to the brain—Peripheral and central neural sensitization in chronic itch. Front. Mol. Neurosci. 2023, 16, 1272230. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Rosen, J.D.; Hashimoto, T. Itch: From mechanism to (novel) therapeutic approaches. J. Allergy Clin. Immunol. 2018, 142, 1375–1390. [Google Scholar] [CrossRef]
- Kataria, S.; Neupane, K.; Ahmed, Z.; Rehman, U.; Asif, S. An Unusual Presentation of Multiple Sclerosis in a Middle-Aged Woman: A Case Report and Literature Review. Cureus 2020, 12, e11017. [Google Scholar] [CrossRef]
- Hadad, R.; Mandelli, M.L.; Rankin, K.P.; Toohey, C.; Sturm, V.E.; Javandel, S.; Milicic, A.; Knudtson, M.; Allen, I.E.; Hoffmann, N.; et al. Itching Frequency and Neuroanatomic Correlates in Frontotemporal Lobar Degeneration. JAMA Neurol. 2024, 81, 977–984. [Google Scholar] [CrossRef]
- Wong, L.-S.; Wu, T.; Lee, C.-H. Inflammatory and Noninflammatory Itch: Implications in Pathophysiology-Directed Treatments. Int. J. Mol. Sci. 2017, 18, 1485. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, J.L.; Cui, B.; Liu, J.; Song, P.; Lang, C.; Bao, Y.; Sum, R.; Xu, C.; Ding, X.; et al. The functional and structural alterations of the striatum in chronic spontaneous urticaria. Sci. Rep. 2018, 8, 1725. [Google Scholar] [CrossRef]
- Harte, S.E.; Harris, R.E.; Clauw, D.J. The neurobiology of central sensitization. J. Appl. Biobehav. Res. 2018, 23, e12137. [Google Scholar] [CrossRef]
- Tsuda, M. Astrocytes in the spinal dorsal horn and chronic itch. Neurosci. Res. 2018, 126, 9–14. [Google Scholar] [CrossRef]
- Shiratori-Hayashi, M.; Tsuda, M. Role of reactive astrocytes in the spinal dorsal horn under chronic itch conditions. J. Pharmacol. Sci. 2020, 144, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Maurelli, M.; Bergamaschi, R.; Antonini, A.; Fargnoli, M.C.; Puma, E.; Mallucci, G.; Totaro, R.; Girolomoni, G. Interferon-beta injection site reactions in patients with multiple sclerosis. J. Dermatol. Treat. 2018, 29, 831–834. [Google Scholar] [CrossRef]
- Piqué-Duran, E.; Eguía, P.; García-Vázquez, O. Acquired perforating dermatosis associated with natalizumab. J. Am. Acad. Dermatol. 2013, 68, e185–e187. [Google Scholar] [CrossRef]
- Fisher, R.M.; Hadley, G.; Ieremia, E.; Moswela, O.; Zaki, F.; DeLuca, G.C.; McPherson, T. Natalizumab-induced acquired perforating dermatosis. Clin. Exp. Dermatol. 2021, 46, 1373–1375. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.A.; Sorbara, E.E.; Battaglia, A.; Cicala, G.; Rizzo, V.; Spina, E.; Cutroneo, P.M. Adverse Drug Reactions with Drugs Used in Multiple Sclerosis: An Analysis from the Italian Pharmacovigilance Database. Front. Pharmacol. 2022, 13, 808370. [Google Scholar] [CrossRef]
- He, D.; Guo, R.; Zhang, F.; Zhang, C.; Dong, S.; Zhou, H. Rituximab for relapsing-remitting multiple sclerosis. Cochrane Database Syst. Rev. 2013, 2013, CD009130. [Google Scholar] [CrossRef]
- Liang, G.; Chai, J.; Ng, H.S.; Tremlett, H. Safety of dimethyl fumarate for multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2020, 46, 102566. [Google Scholar] [CrossRef]
- Fateen, T.; Sultana, N.; Sarwar, M.; Saqlain, N. Complications of Therapeutic Plasma Exchange in pediatric patients: An experience at a tertiary care hospital. Pak. J. Med. Sci. 2023, 39, 994. [Google Scholar] [CrossRef]
- Moreno Escobosa, M.C.; Cruz Granados, S.; Moya Quesada, M.C.; Amat López, J. Anaphylaxis due to methylprednisolone. J. Investig. Allergol. Clin. Immunol. 2008, 18, 407–408. [Google Scholar] [PubMed]
- Pujari, S.S.; Kulkarni, R.V.; Nadgir, D.B.; Ojha, P.K.; Nagendra, S.; Aglave, V.; Nadgir, R.D.; Sant, H.; Palasdeokar, N.; Nirhale, S.; et al. Myelin Oligodendrocyte Glycoprotein (MOG)-IgG Associated Demyelinating Disease. Ann. Indian Acad. Neurol. 2021, 24, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R. Influenza Vaccine-Induced CNS Demyelination in a 50-Year-Old Male. Am. J. Case Rep. 2014, 15, 368–373. [Google Scholar] [CrossRef]
- Steinhoff, M.; Schmelz, M.; Szabó, I.L.; Oaklander, A.L. Clinical presentation, management, and pathophysiology of neuropathic itch. Lancet Neurol. 2018, 17, 709–720. [Google Scholar] [CrossRef]
- Yamamoto, M.; Yabuki, S.; Hayabara, T.; Otsuki, S. Paroxysmal itching in multiple sclerosis: A report of three cases. J. Neurol. Neurosurg. Psychiatry 1981, 44, 19–22. [Google Scholar] [CrossRef]
- Portugal, D.M.; Ferreira, E.F.; Camões-Barbosa, A. Botulinum toxin type A therapy for bilateral focal neuropathic pruritus in multiple sclerosis: A case report. Int. J. Rehabil. Res. 2021, 44, 382–383. [Google Scholar] [CrossRef] [PubMed]
- Dhand, A.; Aminoff, M.J. The neurology of itch. Brain 2014, 137, 313–322. [Google Scholar] [CrossRef] [PubMed]
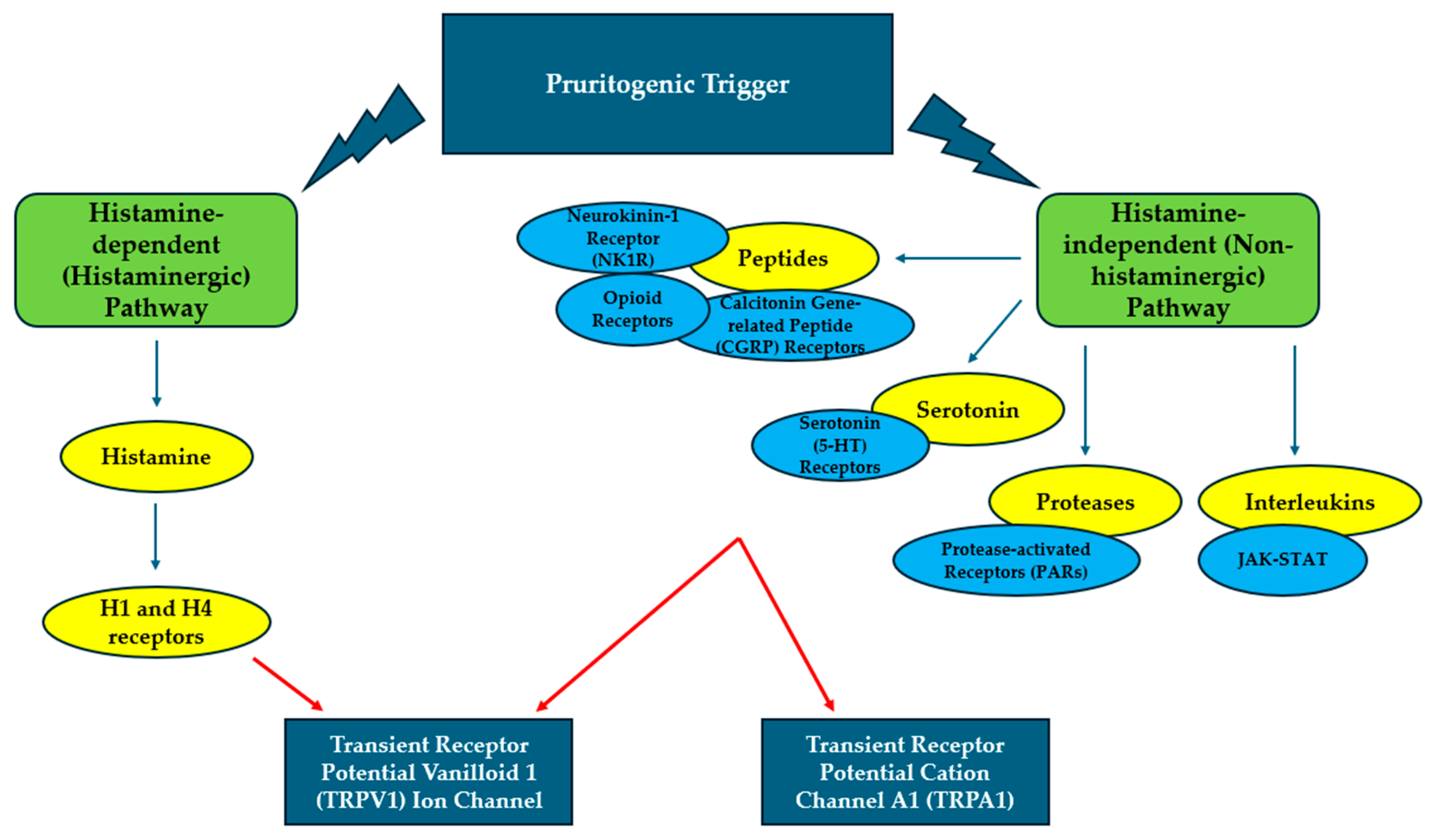
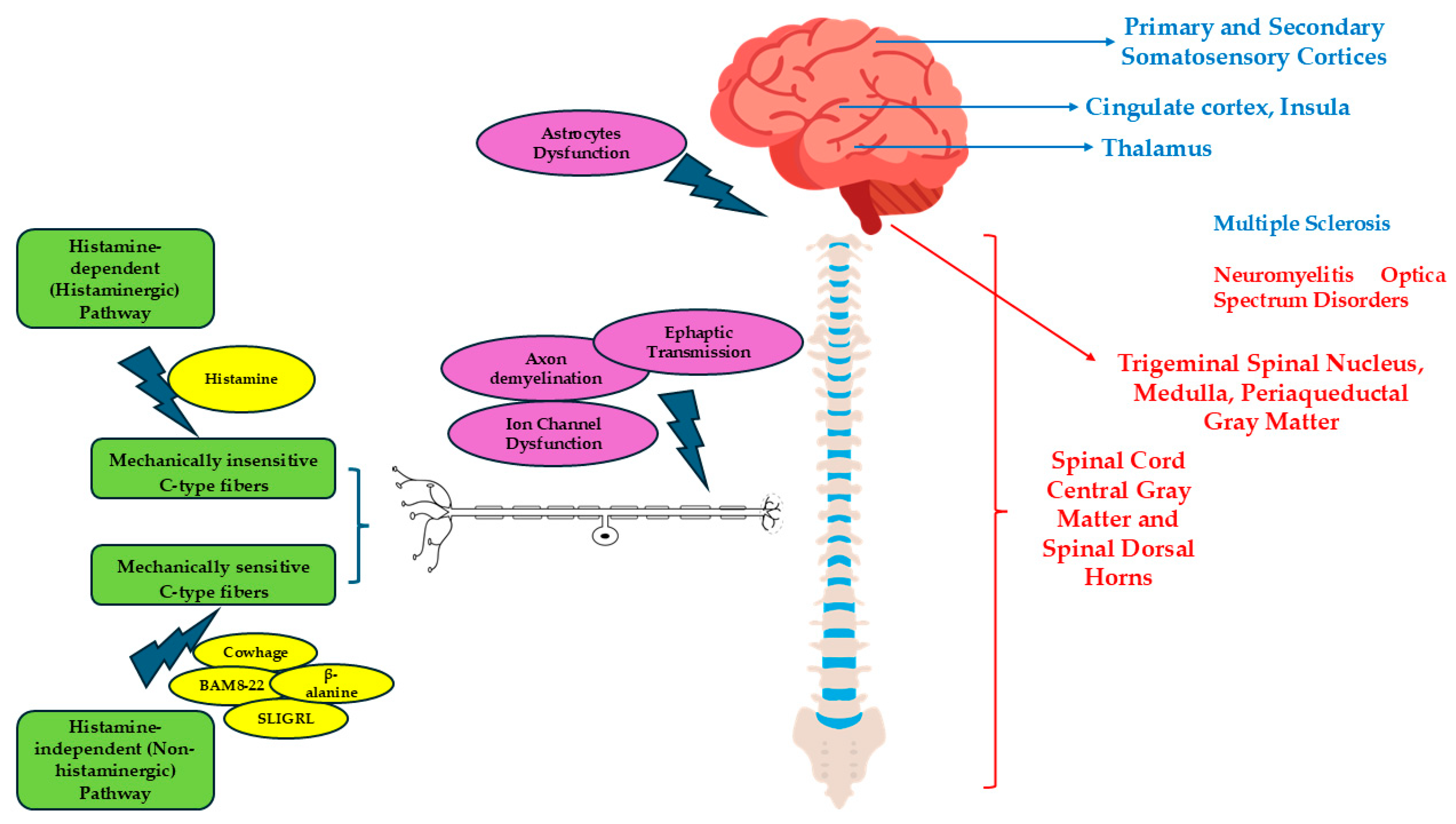
| MS | NMOSD | |
|---|---|---|
| Frequency of neuropathic pruritus | 4.5% of patients | 12–27% of patients |
| Clinical features of pruritus | Sudden onset, localized, intense, lasting from a few seconds to several minutes, often accompanied by superficial sensory disturbances and/or pain | Sudden onset, localized, intense, lasting from a few seconds to several minutes, often accompanied by superficial sensory disturbances and/or pain |
| Clinical significance of pruritus | Initial presenting symptom, or a manifestation during disease relapses. Rarely, it occurs during remissions | Initial presenting symptom, or a manifestation during disease relapses |
| Dermatomal/Non-dermatomal Distribution | Predominantly dermatomal distribution | Both dermatomal and non-dermatomal distribution |
| Location of lesions | Potentially throughout the CNS |
|
| Neuronal pathways involved |
|
|
| Response to treatment | Good response to therapy | Partial or insufficient response to therapy |
| Effectiveness | Notes | |
|---|---|---|
| Systemic drugs | ||
| High Dose Methylprednisolone | Good | To be administered in case of acute attack or contrast-enhancing lesions |
| H1-antihistamines | High in non-neuropathic pruritus, limited in neuropathic pruritus |
|
| Systemic sodium channel blockers (Carbamazepine, Oxcarbazepine) | High (considered the first-line therapy) | Recommended in cases of neuropathic pruritus in MS |
| Calcium channel (α2δ subunit) modulators (Pregabalin, Gabapentin) | High |
|
| Antidepressants (Amitriptyline, Paroxetine, Sertraline) | Good | Recommended in case of anxiety/depression coexistence |
| Phenytoin | Variable | |
| Phenobarbital | Variable | |
| Synachten | Variable | |
| Local drugs | ||
| Intradermal administration of botulinum toxin type A | Effective in one case | Employed in refractory bilateral focal pruritus |
| Topical Steroids | Low |
|
| Topical Tacrolimus | Low |
|
| TRPV1 receptor desensitization (Capsaicin cream) | Good | Burning on initial application |
| Effectiveness | Notes | |
|---|---|---|
| Trimming the fingernails | High | |
| Thermoplastic mesh dressings | High | It may also promote wound healing |
| Cognitive behavioral therapy | High | It can assist patients in resisting the urge to scratch, alleviate depression or aggression, and reduce self-inflicted injury |
| Physiotherapy | High | It can assist patients in resisting the urge to scratch, alleviate depression or aggression, and reduce self-inflicted injury |
| Meditation | High | It can assist patients in resisting the urge to scratch, alleviate depression or aggression, and reduce self-inflicted injury |
| Occlusive therapy | Good | Covering the itchy areas to reduce visual triggers and limit scratching could protect the skin from further damage and sun exposure, enhance the effectiveness of topical treatments, help relieve itch |
| Decompressive neurosurgery | Variable | Recommended in case of refractory neuropathic itch |
| Transcranial direct stimulation | Effective in one case | It does not relieve pain |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messina, C.; Zuccarello, M. Pruritus in Autoimmune Demyelinating Diseases of the Central Nervous System: A Review. Allergies 2025, 5, 32. https://doi.org/10.3390/allergies5040032
Messina C, Zuccarello M. Pruritus in Autoimmune Demyelinating Diseases of the Central Nervous System: A Review. Allergies. 2025; 5(4):32. https://doi.org/10.3390/allergies5040032
Chicago/Turabian StyleMessina, Christian, and Mariateresa Zuccarello. 2025. "Pruritus in Autoimmune Demyelinating Diseases of the Central Nervous System: A Review" Allergies 5, no. 4: 32. https://doi.org/10.3390/allergies5040032
APA StyleMessina, C., & Zuccarello, M. (2025). Pruritus in Autoimmune Demyelinating Diseases of the Central Nervous System: A Review. Allergies, 5(4), 32. https://doi.org/10.3390/allergies5040032





