Effect of Photobiomodulation Therapy in an Experimental Model of Chronic Obstructive Pulmonary Disease: A Dosimetric Study
Abstract
1. Introduction
2. Objectives
2.1. Primary Objective
2.2. Secondary Objectives
- To evaluate the levels of cytokines secreted by BAL cells;
- To quantify the total and differential cells present in BAL and in the blood of animals;
- To analyze airway remodeling by histology;
- To evaluate pulmonary mechanics;
3. Materials and Methods
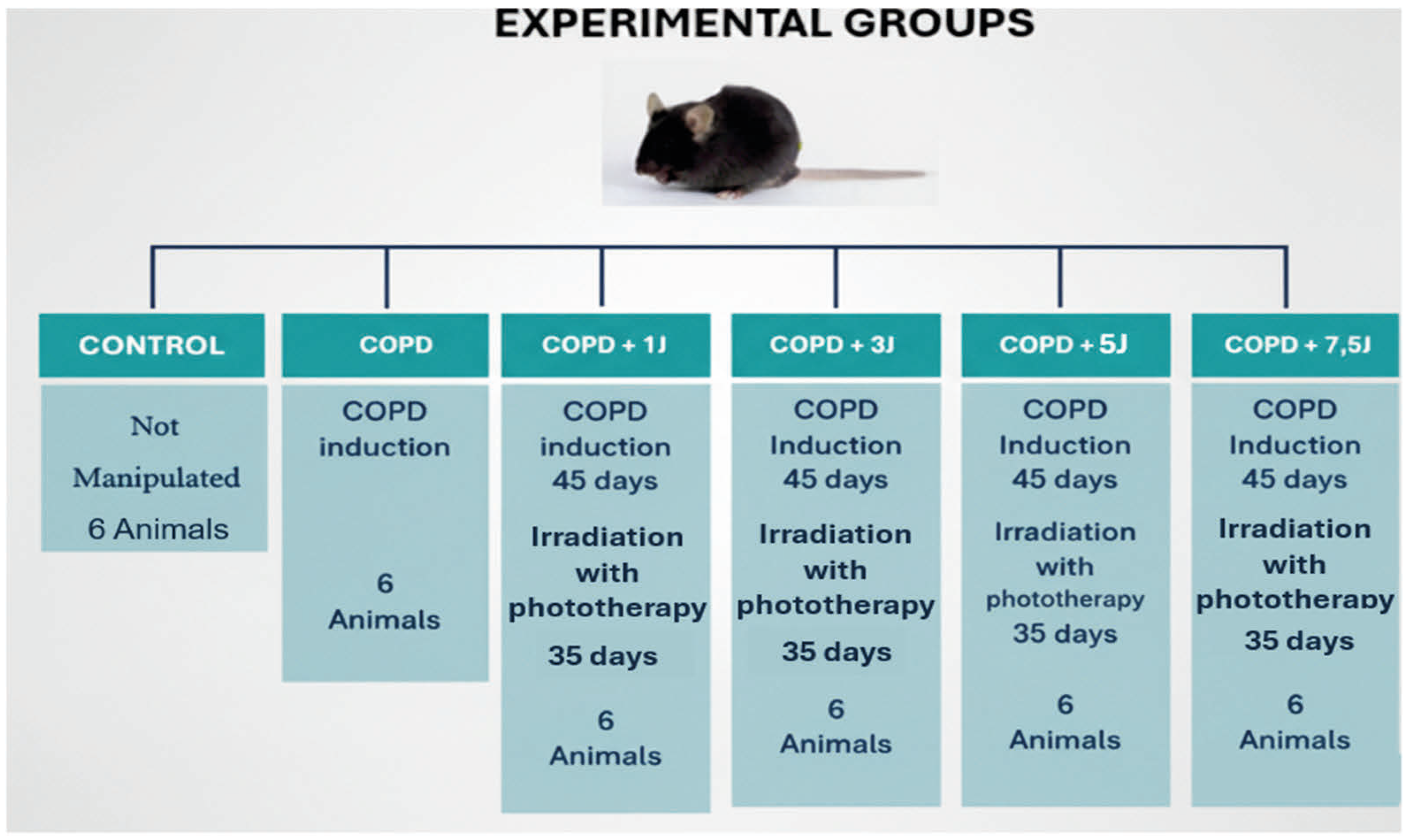
3.1. Study Design
3.2. Sample Size
3.3. Randomization
3.4. Blinding
3.5. Statistical Methods
3.6. Experimental Animals
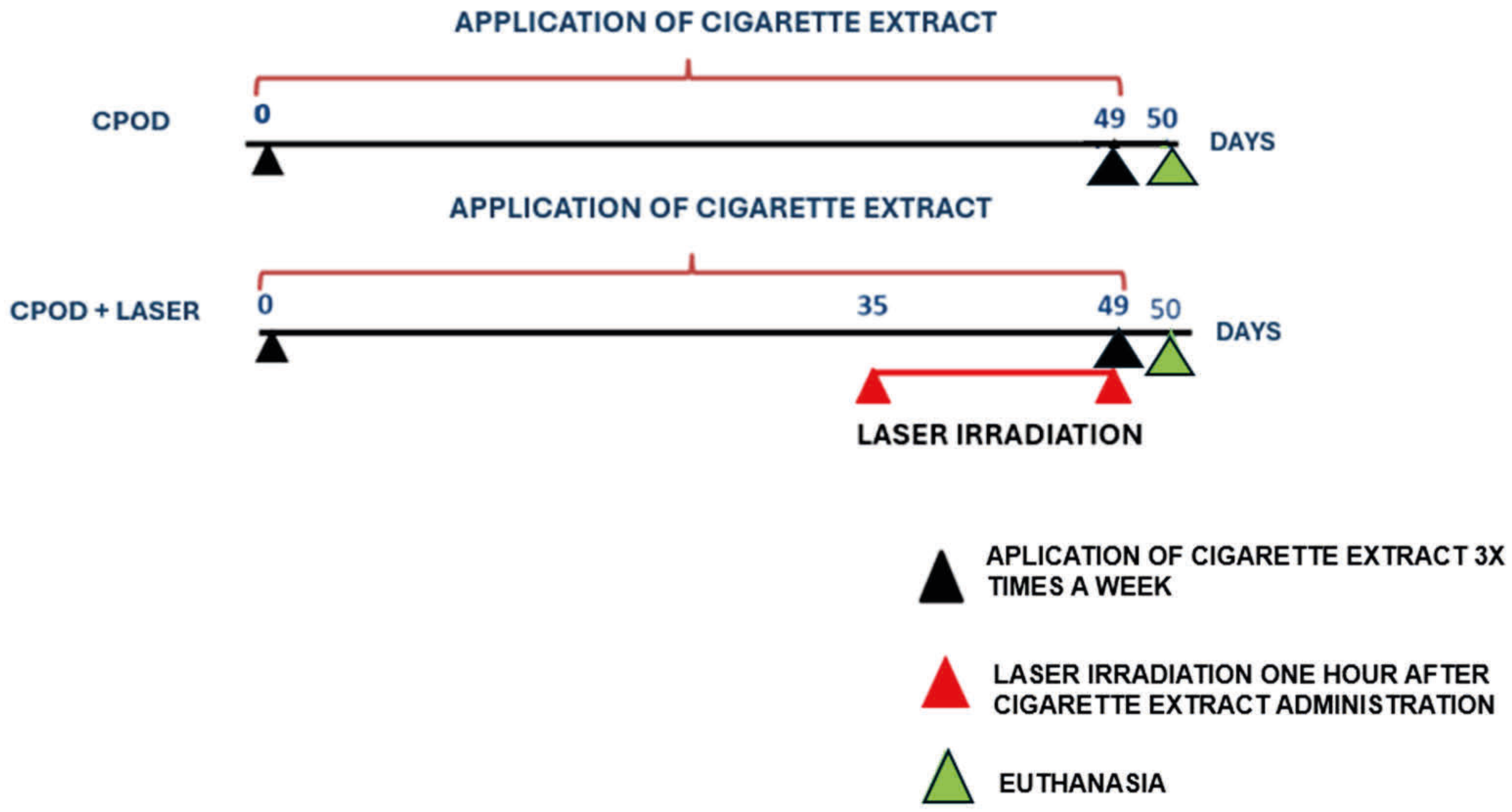
3.6.1. Cigarette Extract
3.6.2. Irradiation
3.7. Euthanasia
3.8. Assessment of Lung Inflammation in Bronchoalveolar Lavage (BAL)
3.9. Flow Cytometry
4. Results
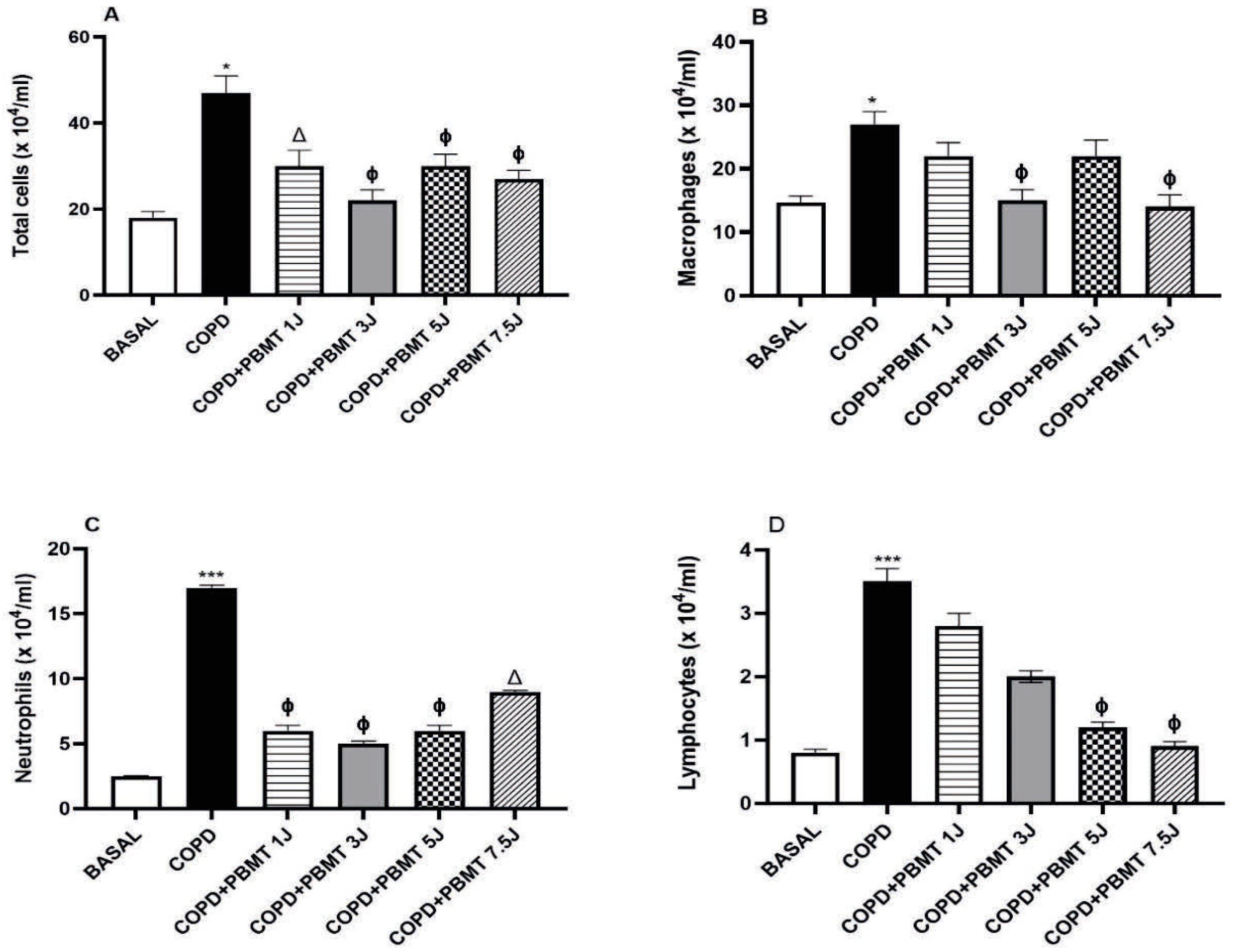
4.1. Effects of PBMT on the Number of Cells Present in BAL in a COPD Model
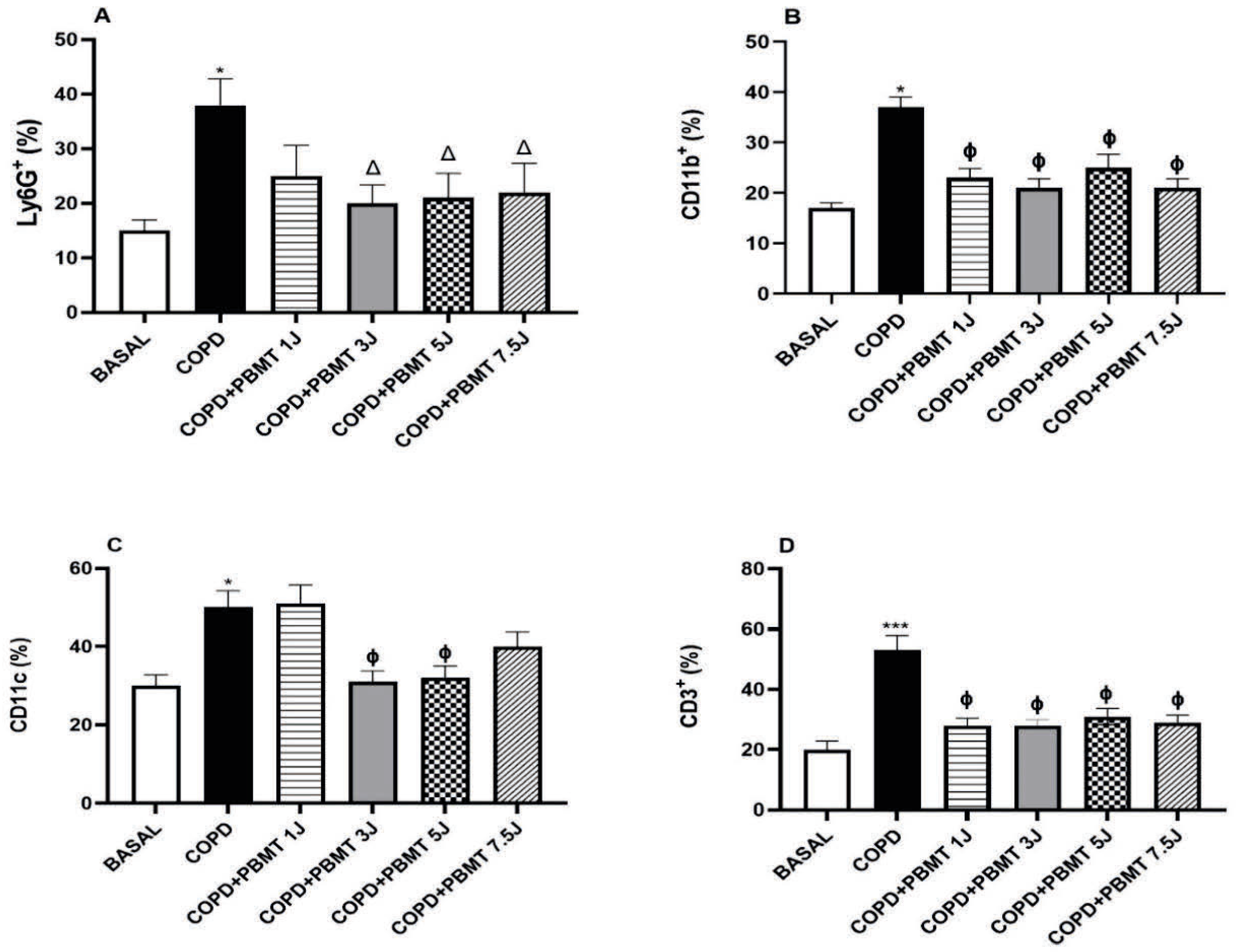
4.2. Effects of PBMT on the Quantification of Cells Present in the Lung in a COPD Model
4.3. Effects of PBMT on Necrosis, Apoptosis and Reactive Oxygen Species Production in Leukocytes in the Lung
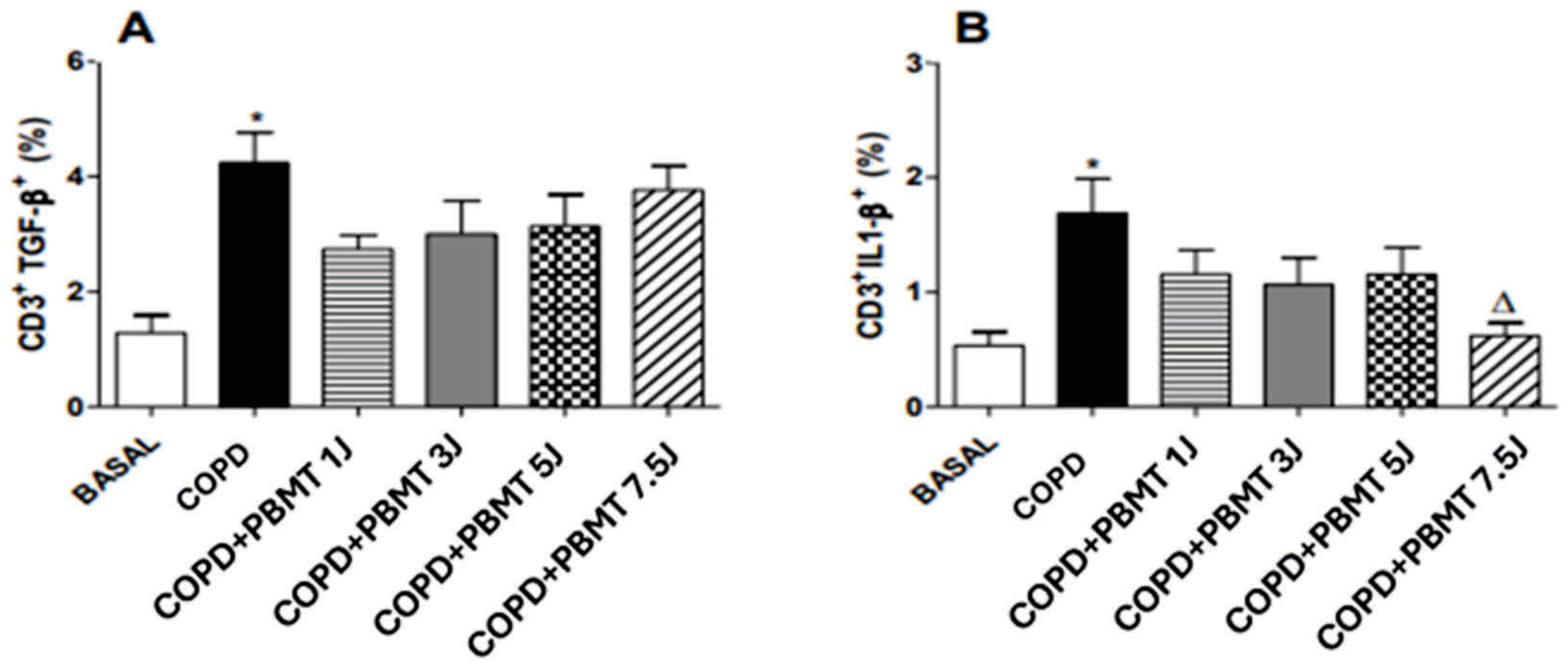
4.4. Effect of PBMT Therapy on the Percentage of CD3+TGF- β + (A) and CD3+IL-1 β+ (B) Lymphocytes in BAL in an Experimental Model of COPD
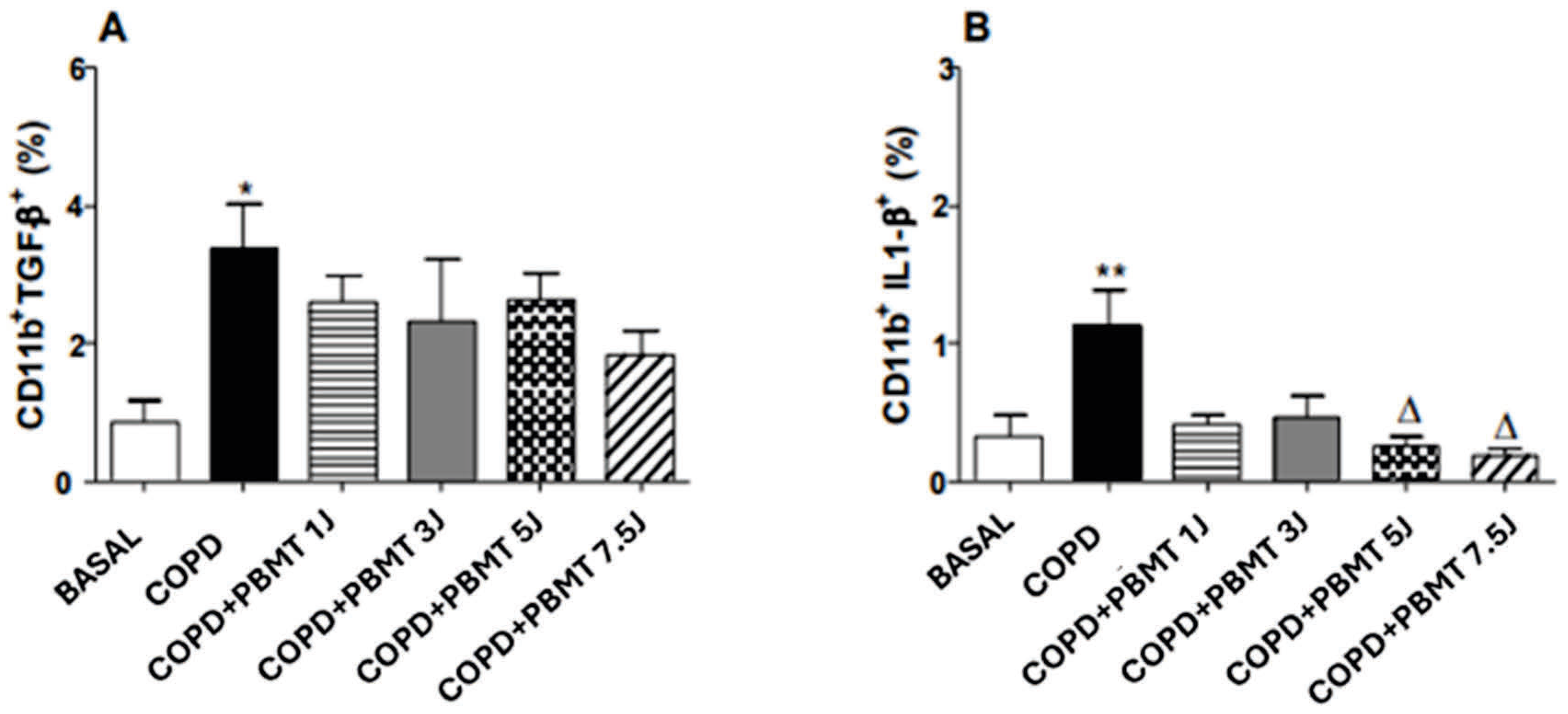
4.5. Effect of PBMT Therapy on the Quantification of CD11b+TGF- β+ (A) and CD11b+IL-1 β+ (B) Macrophages in BAL in an Experimental Model COPD
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Boers, E.; Barrett, M.; Su, J.G.; Benjafield, A.V.; Sinha, S.; Kaye, L.; Zar, H.J.; Vuong, V.; Tellez, D.; Gondalia, R.; et al. Global Burden of Chronic Obstructive Pulmonary Disease Through 2050. JAMA Netw. Open. 2023, 6, e2346598. [Google Scholar] [PubMed]
- Devereux, G. ABC of chronic obstructive pulmonary disease. Definition, epidemiology, and risk factors. BMJ 2006, 332, 1142–1144. [Google Scholar] [PubMed]
- Da Cunha Moraes, G.; Vitoretti, L.B.; de Brito, A.A.; Alves, C.E.; de Oliveira, N.C.R.; Dos Santos Dias, A.; Matos, Y.S.T.; Oliveira-Junior, M.C.; Oliveira, L.V.F.; da Palma, R.K.; et al. Low-Level Laser Therapy Reduces Lung Inflammation in an Experimental Model of Chronic Obstructive Pulmonary Disease Involving P2X7 Receptor. Oxid. Med. Cell Longev. 2018, 2018, 6798238. [Google Scholar]
- Lomas, D.A.; Silverman, E.K. The genetics of chronic obstructive pulmonary disease. Respir Res. 2001, 2, 20–26. [Google Scholar]
- Barnes, P.J. Oxidative Stress in Chronic Obstructive Pulmonary Disease. Antioxidants 2022, 11, 965. [Google Scholar] [CrossRef]
- Dekhuijzen, P.N.; Aben, K.K.; Dekker, I.; Aarts, L.P.; Wielders, P.L.; van Herwaarden, C.L.; Bast, A. Increased exhalation of hydrogen peroxide in patients with stable and unstable chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1996, 154 Pt 1, 813–816. [Google Scholar] [CrossRef]
- Rahman, I.; Adcock, I.M. Oxidative stress and redox regulation of lung inflammation in COPD. Eur. Respir. J. 2006, 28, 219–242. [Google Scholar] [CrossRef]
- Noguera, A.; Batle, S.; Miralles, C.; Iglesias, J.; Busquets, X.; Macnee, W. Agusti AG Resposta aumentada de neutrófilos na doença pulmonar obstrutiva crônica. Thorax 2001, 56, 432–437. [Google Scholar]
- Barnes, P.J. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin. Chest Med. 2014, 35, 71–86. [Google Scholar] [CrossRef]
- Chung, K.F. Cytokines in chronic obstructive pulmonary disease. Eur. Respir. J. 2001, 18 (Suppl. 34), 50s–59s. [Google Scholar] [CrossRef]
- Farrell, L.A.; O’Rourke, M.B.; Padula, M.P.; Souza-Fonseca-Guimaraes, F.; Caramori, G.; Wark, P.A.B.; Dharmage, S.C.; Hansbro, P.M. The Current Molecular and Cellular Landscape of Chronic Obstructive Pulmonary Disease (COPD): A Review of Therapies and Efforts towards Personalized Treatment. Proteomes 2024, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Celli, B.R. Systemic manifestations and comorbidities of COPD. Eur. Respir. J. 2009, 33, 1165–1185. [Google Scholar] [CrossRef]
- Ritchie, A.I.; Wedzicha, J.A. Definition, Causes, Pathogenesis, and Consequences of Chronic Obstructive Pulmonary Disease Exacerbations. Clin. Chest Med. 2020, 41, 421–438. [Google Scholar] [CrossRef]
- Dourado, V.Z.; Tanni, S.E.; Vale, S.A.; Faganello, M.M.; Sanchez, F.F.; Godoy, I. Manifestações sistêmicas na doença pulmonar obstrutiva crônica. J. Bras. Pneumol. 2006, 32, 161–171. [Google Scholar]
- MacIntyre, N.; Huang, Y.C. Acute exacerbations and respiratory failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008, 5, 530–535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drummond, M.B.; Dasenbrook, E.C.; Pitz, M.W.; Murphy, D.J.; Fan, E. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: A systematic review and meta-analysis. JAMA 2008, 300, 2407–2416. [Google Scholar]
- Singh, S.; Loke, Y.K.; Furberg, C.D. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. JAMA 2008, 300, 1439–1450. [Google Scholar] [CrossRef]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Leśniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation-Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar] [CrossRef]
- Amaroli, A.; Clemente Vargas, M.R.; Pasquale, C.; Raffetto, M.; Ravera, S. Photobiomodulation on isolated mitochondria at 810 nm: First results on the efficiency of the energy conversion process. Sci Rep. 2024, 14, 11060. [Google Scholar] [PubMed]
- Hamblin, M.R. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Bisht, D.; Gupta, S.C.; Misra, V.; Mital, V.P.; Sharma, P. Effect of low intensity laser radiation on healing of open skin wounds in rats. Indian J. Med. Res. 1994, 100, 43–46. [Google Scholar] [PubMed]
- Cios, A.; Cieplak, M.; Szymański, Ł.; Lewicka, A.; Cierniak, S.; Stankiewicz, W.; Mendrycka, M.; Lewicki, S. Effect of Different Wavelengths of Laser Irradiation on the Skin Cells. Int. J. Mol. Sci. 2021, 22, 2437. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.; Nijkamp, F.P.; Folkerts, G. Nitric oxide synthase (NOS) as therapeutic target for asthma and chronic obstructive pulmonary disease. Curr. Drug Targets 2006, 7, 721–735. [Google Scholar] [CrossRef]
- Li, C.L.; Liu, J.F.; Liu, S.F. Mitochondrial Dysfunction in Chronic Obstructive Pulmonary Disease: Unraveling the Molecular Nexus. Biomedicines 2024, 12, 814. [Google Scholar] [CrossRef]
- Wang, X.Y.; Ma, W.J.; Liu, C.S.; Li, Y.X. Effect of low-level laser therapy on allergic asthma in rats. Lasers Med. Sci. 2014, 29, 1043–1050. [Google Scholar] [CrossRef]
- Oliveira, N.C.R.; Soares, J.M.; Cordeiro, J.M.; Paiva, M.S.; Pinheiro, A.L.B. Effect of low-level laser therapy in pulmonary inflammation in asthma induced by house dust mite: A dosimetry study. Int. J. Inflamm. 2018, 2018, 1–10. [Google Scholar]
- Rahman, I. Oxidative stress in pathogenesis of chronic obstructive pulmonary disease: Cellular and molecular mechanisms. Cell. Biochem. Biophys. 2005, 43, 167–188. [Google Scholar] [CrossRef]
- Marciniuk, D.; Nana, A.; Rabe, K.; Zar, H.; Ferkol, T.; MontesdeOca, M. Doenças respiratórias no mundo: Realidades de hoje, oportunidades para amanhã. Afr. J. Resp. Dis. 2014, 6, 4–13. [Google Scholar]
- Pauwels, R.A.; Löfdahl, C.-G.; Laitinen, L.A.; Schouten, J.P.; Postma, D.S.; Pride, N.B.; Ohlsson, S.V. Tratamento de longo prazo com budesonida inalada em pessoas com doença pulmonar obstrutiva crônica leve que continuam fumando. Estudo da Sociedade Respiratória Europeia sobre Doença Pulmonar Obstrutiva Crônica. N. Engl. J. Med. 1999, 340, 1948–1953. [Google Scholar] [CrossRef] [PubMed]
- Vestbo, J.; Sorensen, T.; Lange, P.; Brix, A.; Torre, P.; Viskum, K. Efeito de longo prazo da budesonida inalada na doença pulmonar obstrutiva crônica leve e moderada: Um ensaio clínico randomizado. Lancet 1999, 353, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, R.D.S.; Atum, A.L.B.; Ruiz, É.G.D.S.; Serra, A.J.; Antônio, E.L.; Manchini, M.T.; Silva, J.M.A.; Tucci, P.J.F.; Nathanson, L.; Morris, M.; et al. Photobiomodulation Therapy on Myocardial Infarction in Rats: Transcriptional and Posttranscriptional Implications to Cardiac Remodeling. Lasers Surg. Med. 2021, 53, 1247–1257. [Google Scholar] [CrossRef]
- Grandinetti, V.; Carlos, F.P.; Antonio, E.L.; de Oliveira, H.A.; Dos Santos, L.F.N.; Yoshizaki, A.; Mansano, B.S.D.M.; Silva, F.A.; Porte, L.A.; Albuquerque-Pontes, G.M.; et al. Photobiomodulation therapy combined with carvedilol attenuates post-infarction heart failure by suppressing excessive inflammation and oxidative stress in rats. Sci. Rep. 2019, 9, 9425. [Google Scholar] [CrossRef]
- Matsunaga, K.; Harada, M.; Suizu, J.; Oishi, K.; Asami-Noyama, M.; Hirano, T. Comorbid Conditions in Chronic Obstructive Pulmonary Disease: Potential Therapeutic Targets for Unmet Needs. J. Clin. Med. 2020, 9, 3078. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Sharma, S.K.; Carroll, J.; Hamblin, M.R. Biphasic dose response in low level light therapy—An update. Dose Response 2011, 9, 602–618. [Google Scholar] [CrossRef]
- Bjordal, J.M.; Couppe, C.; Ljunggren, A.E. Low level laser therapy for tendinopathy: Evidence of a dose-response pattern. In Database of Abstracts of Reviews of Effects (DARE): Quality-Assessed Reviews [Internet]; Centre for Reviews and Dissemination (UK): York, UK, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK68932/ (accessed on 26 May 2025).
- De Brito, A.A.; Herculano, K.Z.; de Alvarenga-Nascimento, C.R.; Estefano-Alves, C.; Duran, C.C.G.; Marcos, R.L.; Silva Junior, J.A.; Chavantes, M.C.; Zamuner, S.R.; Aimbire, F.; et al. Effect of photobiomodulation in the balance between effector and regulatory T cells in an experimental model of COPD. Front. Med. 2024, 11, 1347517. [Google Scholar] [CrossRef]
- Fonseca, K.M.; de Melo Rodrigues, L.; Ferreira, D.C.L.; Vilaça, M.M.O.; Galvão, S.M.P.; dos Santos Alves, W. Efeitos da fotobiomodulação nas alterações extrapulmonares ocasionadas pelo enfisema experimental em rattus norvegicus. Saúde Pesquisa 2016, 9, 143–152. [Google Scholar] [CrossRef]
- Secchi, T.M.; Oliveira, T.L.P.; Pereira, T.I.M.; Araújo, P.F.; Sganzerla, J.T. Photobiomodulation for the prevention and treatment of bisphosphonate-induced osteonecrosis: A pilot study in an animal model. Rev. Cereus. 2023, 15, 120–132. [Google Scholar]
- Cosio, M.G.; Saetta, M.; Agusti, A. Immunologic aspects of chronic obstructive pulmonary disease. N. Engl. J. Med. 2009, 360, 2445–2454. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.S. Terapia de Fotobiomodulação Regula a Produção de Espécies Reativas de Oxigênio (ROS) em Modelo Experimental de Doença Pulmonar Obstrutiva Crônica (DPOC). Ph.D. Thesis, Universidade Nove de Julho, São Paulo, Brazil, 2021. [Google Scholar]
- Boschi, E.S. Efeito Antiinflamatório da Terapia Laser de Baixa Potência (660 nm) na Pleurisia em Ratos. Ph.D. Thesis, Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, Brazil, 2007. [Google Scholar]
- De Brito, A.A.; da Silveira, E.C.; Ligeiro de Oliveira, A.P. Low-level laser therapy attenuates lung inflammation and airway remodeling in a murine model of idiopathic pulmonary fibrosis: Relevance to cytokines secretion from lung structural cells. J. Photochem. Photobiol. B Biol. 2020, 203, 111731. [Google Scholar] [CrossRef] [PubMed]
- Asnaashari, M.; Shojaeian, S.; Mesgharani, A.; Mehrabinia, P. The effect of low-level laser therapy on the viability of human dental pulp stem cells. J. Lasers Med. Sci. 2022, 13, e60. [Google Scholar] [CrossRef] [PubMed]
- Bonvicini, J.F.S.; de Souza, G.L.; Basso, F.G.; de Souza Costa, C.A.; Moura, C.C.G.; Silva, M.J.B.; Turrioni, A.P. The effect of infrared LED irradiation on the oxidative stress induced in human dental pulp cells by lipopolysaccharide (LPS). Int. Endod. J. 2014, 47, 747–755. [Google Scholar]
- Balbi, M.; Lai, R.; Stigliani, S.; Massarotti, C.; Bozzo, M.; Scaruffi, P.; Ravera, S.; Amaroli, A. Efficacy and Safety of Visible and Near-Infrared Photobiomodulation Therapy on Astenospermic Human Sperm: Wavelength-Dependent Regulation of Nitric Oxide Levels and Mitochondrial Energetics. Biology 2025, 14, 491. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Group 1 | Group 3 | Group 5 | Group 7,5 |
|---|---|---|---|---|
| Radiant Energy (J) | 1 J | 3 J | 5 J | 7.5 J |
| Wavelength (nm) | 660 | 660 | 660 | 660 |
| Spectral width (nm) | ±20 | ±20 | ±20 | ±20 |
| Operating mode | Continuous | Continuous | Continuous | Continuous |
| Average radiant power (mW) | 100 | 100 | 100 | 100 |
| Radiant exposure (J/cm2) | 22 | 66 | 111 | 116 |
| Beam area on target (cm2) | 0.045 | 0.045 | 0.045 | 0.045 |
| Exposure time (s) | 10 | 30 | 50 | 75 |
| Frequency (Hz) | Unique | Unique | Unique | Unique |
| Application points | 3 points | 3 points | 3 points | 3 points |
| Application technique | Contact | Contact | Contact | Contact |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, C.E.; Santos, T.G.; Vitoretti, L.B.; Gutierrez Duran, C.C.; Zamuner, S.; Labat, R.; Silva, J.A., Jr.; Chavantes, M.C.; Aimbire, F.; da Palma, R.K.; et al. Effect of Photobiomodulation Therapy in an Experimental Model of Chronic Obstructive Pulmonary Disease: A Dosimetric Study. Allergies 2025, 5, 33. https://doi.org/10.3390/allergies5040033
Alves CE, Santos TG, Vitoretti LB, Gutierrez Duran CC, Zamuner S, Labat R, Silva JA Jr., Chavantes MC, Aimbire F, da Palma RK, et al. Effect of Photobiomodulation Therapy in an Experimental Model of Chronic Obstructive Pulmonary Disease: A Dosimetric Study. Allergies. 2025; 5(4):33. https://doi.org/10.3390/allergies5040033
Chicago/Turabian StyleAlves, Cintia Estefano, Tawany Gonçalves Santos, Luana Beatriz Vitoretti, Cinthya Cosme Gutierrez Duran, Stella Zamuner, Rodrigo Labat, José Antonio Silva, Jr., Maria Cristina Chavantes, Flavio Aimbire, Renata Kelly da Palma, and et al. 2025. "Effect of Photobiomodulation Therapy in an Experimental Model of Chronic Obstructive Pulmonary Disease: A Dosimetric Study" Allergies 5, no. 4: 33. https://doi.org/10.3390/allergies5040033
APA StyleAlves, C. E., Santos, T. G., Vitoretti, L. B., Gutierrez Duran, C. C., Zamuner, S., Labat, R., Silva, J. A., Jr., Chavantes, M. C., Aimbire, F., da Palma, R. K., & de Oliveira, A. P. L. (2025). Effect of Photobiomodulation Therapy in an Experimental Model of Chronic Obstructive Pulmonary Disease: A Dosimetric Study. Allergies, 5(4), 33. https://doi.org/10.3390/allergies5040033









