Abstract
Pediatric asthma is the most common chronic childhood disease in the US and a major public health concern. It is considered to comprise multiple clinical variants or phenotypes with different etiologies and pathophysiologies. Former research has shown that airway bacteriomes vary in composition and structure across pediatric asthma phenotypes, but their functional diversity and bacterial interactions have hardly been investigated. A previous study of 163 children from Washington DC identified three statistically different asthma phenotypes, each with a unique nasopharyngeal bacterial composition and diversity. Here, I reanalyze 16S rRNA high-throughput sequences from the same cohort to characterize their bacterial metabolism and interactions. I detect 61 to 102 metabolic pathways (PICRUSt2; q ≤ 0.05) differentially expressed across the three asthma phenotypes. Most of those pathways are related to biosynthesis and degradation processes and statistically (p ≤ 0.0012) separated the three clinical groups. Co-occurrence networks also differ in connectivity across phenotypes, suggesting unique bacterial interactions in each group. Five to eight keystone taxa are detected across phenotypes. Insights from this and previous studies, hence, confirm the airway bacteriome heterogeneity across pediatric asthma, increasing our understanding of its etiology and pathophysiology, and provide new taxonomic and functional biomarkers of disease for targeted interventions and therapies.
Keywords:
16S rRNA; asthma; bacteriome; keystone taxa; metabolic pathways; microbiome; networks; phenotype 1. Introduction
Pediatric asthma is the leading chronic childhood disease in the US, affecting approximately five million children [1,2,3] and contributing to substantial healthcare costs and the caregiver burden [3,4,5]. The childhood asthma prevalence is particularly high (10.4%) in Washington DC, surpassing the national average (6.5%) and affecting ~16,000 youths under the age of 18 [2,3,6].
Pediatric asthma is recognized as a complex condition with differences in severity, natural history, comorbidities and treatment response [7,8,9,10]. Extensive evidence also indicates that it is likely to comprise multiple disease variants or phenotypes with different etiologies and pathophysiologies [7,8,11,12]. Disease phenotypes of asthma can be described by observable features (clinical, physiological, morphological, biochemical and treatment response) that can then be statistically analyzed using, for example, clustering analysis [13,14,15,16]. In 2018, Pérez-Losada et al. applied such an analysis to clinical, physiological and biochemical information collected from 163 children and adolescents from the Washington DC area (the Asthma Severity Modifying Polymorphisms 2 cohort—AsthMaP-2) and identified three statistically distinct phenotypes of pediatric asthma [17]. Furthermore, the authors collected 16S rRNA high-throughput sequencing data from the bacterial communities (i.e., bacteriomes) living in the nasopharynx of each participant to characterize their composition and diversity across those three asthma phenotypes. Their study, like others, demonstrated that nasopharyngeal bacteriomes differ across asthma phenotypes [18,19,20,21,22,23] and may play a significant role in the onset, development and severity of childhood asthma; it also showed that the nasopharynx can act as a major reservoir for opportunistic pathogens [24,25].
Nonetheless, the relationships between airway microbiota and asthma phenotypes are still poorly understood [26,27,28,29,30,31,32]. Very few studies have explored this interaction in children [17,33,34,35,36], and even fewer have addressed how bacterial functional diversity and microbe–microbe interactions may impact childhood asthma heterogeneity (phenotypes) [31,32,37]. Studying bacterial functional profiles is important for understanding asthma phenotypic diversity because the bacteriome plays a crucial role in modulating host immune responses, airway inflammation and disease severity [29,30,38,39,40]. For example, microbial short-chain fatty acid (SCFA) production, lipopolysaccharide (LPS) synthesis, oxidative stress resistance or protease activity can impact asthma exacerbations and progression and influence immune responses, hence ultimately driving asthma phenotypes. Similarly, SCFAs have anti-inflammatory properties, while bacterial proteases may damage the airway epithelium, contributing to tissue remodeling in severe asthma [41,42,43,44]. Understanding bacterial functional profiles also enables the identification of biomarkers for specific asthma subtypes, leading to targeted microbiome-based interventions, such as probiotics, prebiotics or microbiome-modulating therapies [45,46]. Moreover, by focusing on bacterial function rather than just composition, researchers can also uncover mechanistic links between the bacteriome and asthma heterogeneity, ultimately leading to better diagnostic tools and personalized treatments [40,47,48].
Similarly, since bacteria coexist in interactive networks, differences in co-occurrence patterns may also induce different manifestations of asthma [49,50,51]. For example, co-occurring clusters of pathogenic bacteria like Moraxella, Haemophilus, Pseudomonas and Streptococcus can lead to different inflammatory phenotypes of asthma [26,50]. Moreover, bacterial associations may also help to predict asthma exacerbations or treatment response and reveal protective roles against pathogens [48,52]. In fact, by combining functional and network analyses, we may be able to differentiate between pathogenic bacteria that contribute to airway dysfunction (e.g., through toxin production) and commensals that may have protective effects (e.g., by promoting epithelial barrier integrity or immune tolerance) [53,54,55].
Hence, to address this knowledge gap in asthma bacteriome research, here, I reanalyze the existing 16S rRNA nasopharyngeal bacterial profiles from a cohort of 163 asthmatic children and adolescents (the AsthMaP-2 project; [17]) representing three statistically distinct phenotypes of pediatric asthma. I characterize and compare the functional metabolic diversity of their bacteriomes and estimate networks of bacterial associations across phenotypic groups. I hypothesize that nasopharyngeal bacteriomes will vary in functionality and connectivity across pediatric asthma phenotypes.
2. Materials and Methods
2.1. Pediatric Asthma Phenotypes
The AsthMaP-2 project was a study of urban children and adolescents (ages 6 to 18 years) carried out at Children’s National Medical Center, Washington, DC, USA [17]. The study was designed to find associations among airway microbes, environmental exposures, allergic sensitivities, genetics and asthma. All participants were recruited from the metropolitan Washington DC area and were physician-diagnosed with asthma for at least one year before enrolment—see [17] for details.
Twenty-nine variables comprising clinical, demographic, physiological and biochemical information were collected for 163 participants in AsthMaP-2 (see Table S1 in [17]). Then, using varimax rotation and hierarchical clustering, Pérez-Losada et al. identified three statistically distinct asthma phenotypes (APs), referred to here as AP1, AP2 and AP3. See [17] for details of the statistical analyses and phenotype characteristics. In this new study, I reanalyze the genomic data described below to compare bacterial functional profiles and network interactions across those three phenotypes.
2.2. Bacteriome Analyses
In the original study by Pérez-Losada et al. [17], a total of 205 nasal washes were collected from 163 AsthMaP-2 participants (42 of those patients yielded more than one sample). Samples were grouped for microbiome analyses into the three following asthma phenotypes: AP1 (68 samples/51 patients), AP2 (77/63) and AP3 (60/49). A detailed description of the molecular methods and bioinformatic and statistical analyses used to process those samples is presented in [17] and summarized here. Total DNA was extracted from each sample and sequenced using the Schloss’ MiSeq_WetLab_SOP protocol (09.2015) in [56]. Each DNA sample was amplified for the V4 region (~250 bp) of the 16S rRNA gene and libraries were sequenced in a single run of the Illumina MiSeq sequencing platform. Raw FASTQ files were processed in mothur v1.35.1 [57]. Clean paired-end sequences were joined into contigs of ~250 bp and then aligned to the SILVA bacterial reference alignment at www.mothur.org. Sequences were clustered into Operational Taxonomic Units (OTUs) at the 0.03 similarity threshold for downstream analyses. In the sections below, I describe the new analyses I performed here to characterize bacterial functional diversity and interactions across pediatric asthma phenotypes AP1, AP2 and AP3.
2.2.1. Functional Diversity
Bacterial metabolic pathways were predicted by coupling the Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt 2.0) software [58] with the Integrated Microbial Genomes & Microbiomes (IMG/M) database [59]. OTU abundances were normalized by the 16S rRNA gene copy number, and gene abundances were estimated by multiplying the normalized OTU counts by the predicted gene copy numbers. Metabolic pathways were determined in the MetaCyc database [60,61] using gene abundance predictions and PICRUSt2 default parameters. Differentially abundant metabolic pathways were identified using DESEq2 1.42.1 as implemented in the ggpicrust R package [62]. To correct for multiple hypotheses testing [63,64], p-values were adjusted via the Benjamini–Hochberg method (cutoff q = 0.05).
Functional diversity across bacterial communities (beta-diversity) was estimated using the Bray–Curtis and Jaccard distances. Dissimilarity between samples was then explored using principal coordinates analysis (PCoA). Beta-diversity indices were compared using permutational multivariate analysis of variance (adonis) as implemented in the vegan 2.6.4 R package [65] and significance determined through 10,000 permutations.
2.2.2. Bacterial Networks
Bacterial association networks for each asthma phenotype were created by first estimating a compositionally corrected correlation matrix using the CCREPE R package [66]. All strong significant correlations (Spearman’s r ≥ +/−0.6 and q ≤ 0.01) were then used in Cytoscape 3.10.3 to generate undirected networks [67]. The following network parameters were estimated in the igraph R package [68]: node and edge counts, node degree (number of edges a node has with other nodes within the network), neighborhood connectivity (degree of interconnectivity among immediate neighbors in the network), cohesion (how tightly linked the nodes are of a network), betweenness centrality (how often a specific node acts as a bridge between other nodes in the network), modularity (how well a network can be separated into distinct communities or modules) and keystone taxa (taxa considered key players in the network, as indicated by a hub score ≥ 0.5 and node scores in the top 20% percentile). OTUs were agglomerated into genera, and only those with relative mean abundances of ≤0.1% were included in the network analyses.
3. Results
In a previous study [17], the variable V4 region of the 16S rRNA gene (~250 bp) was sequenced in 205 nasal washes from children and adolescents belonging to three asthma phenotypes, rendering 6,386,235 sequences (sample mean = 25,932; sample median = 31,152.4) and 8034 OTUs. Bacterial taxonomic profiles were then generated for those samples, and significant differences in composition and structure were detected across asthma phenotypes. Here, I reanalyze those bacterial profiles to assess the functional diversity and network interactions of the nasopharyngeal bacteriomes across those three asthma phenotypes.
3.1. Functional Diversity
I predicted (PICRUSt2) bacterial functional profiles (Figure S1) for the three phenotypes of pediatric asthma (AP1, AP2 and AP3) and compared them using DESeq2 (Table S1). I detected 102, 67 and 61 differentially expressed metabolic pathways (q ≤ 0.05) up- or down-regulated in AP1 vs. AP2, AP1 vs. AP3 and AP2 vs. AP3, respectively. The top 30 most significantly different pathways for each pairwise comparison are shown in Figures S2–S4. Among the 423 metabolic pathways compared, 53 (52.0%), 34 (50.7%) and 9 (14.8%) pathways were only significant in AP1 vs. AP2, AP1 vs. AP3 and AP2 vs. AP3, respectively. Most of the pathways in all comparisons were primarily related to biosynthesis and secondly to degradation processes.
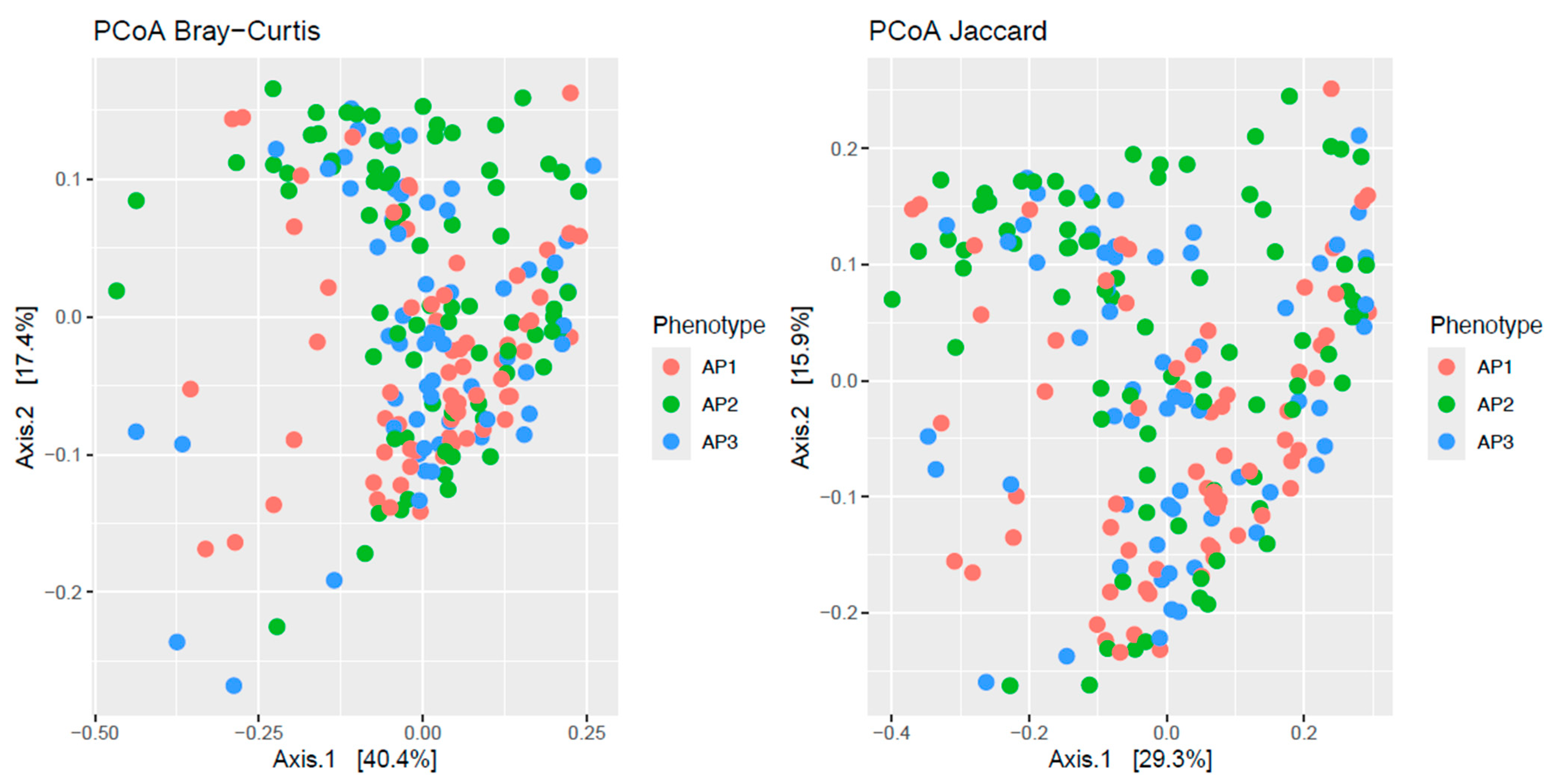
PCoAs of the Bray–Curtis and Jaccard distances of the 423 metabolic pathways showed partial segregation of the bacteriomes across asthma phenotypes (Figure 1). This observation was statistically confirmed by the adonis analyses, which detected significant differences (p ≤ 0.012) in beta-diversity across phenotypes for both distances.
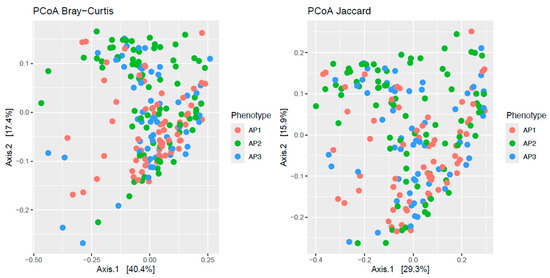
Figure 1.
Principal coordinate analysis (PCoA) plots of beta-diversity estimates (Bray–Curtis and Jaccard indices) of nasopharyngeal bacteriome metabolic profiles from children and adolescents clustered into three asthma phenotypes (AP1, AP2 and AP3).
3.2. Network Interactions
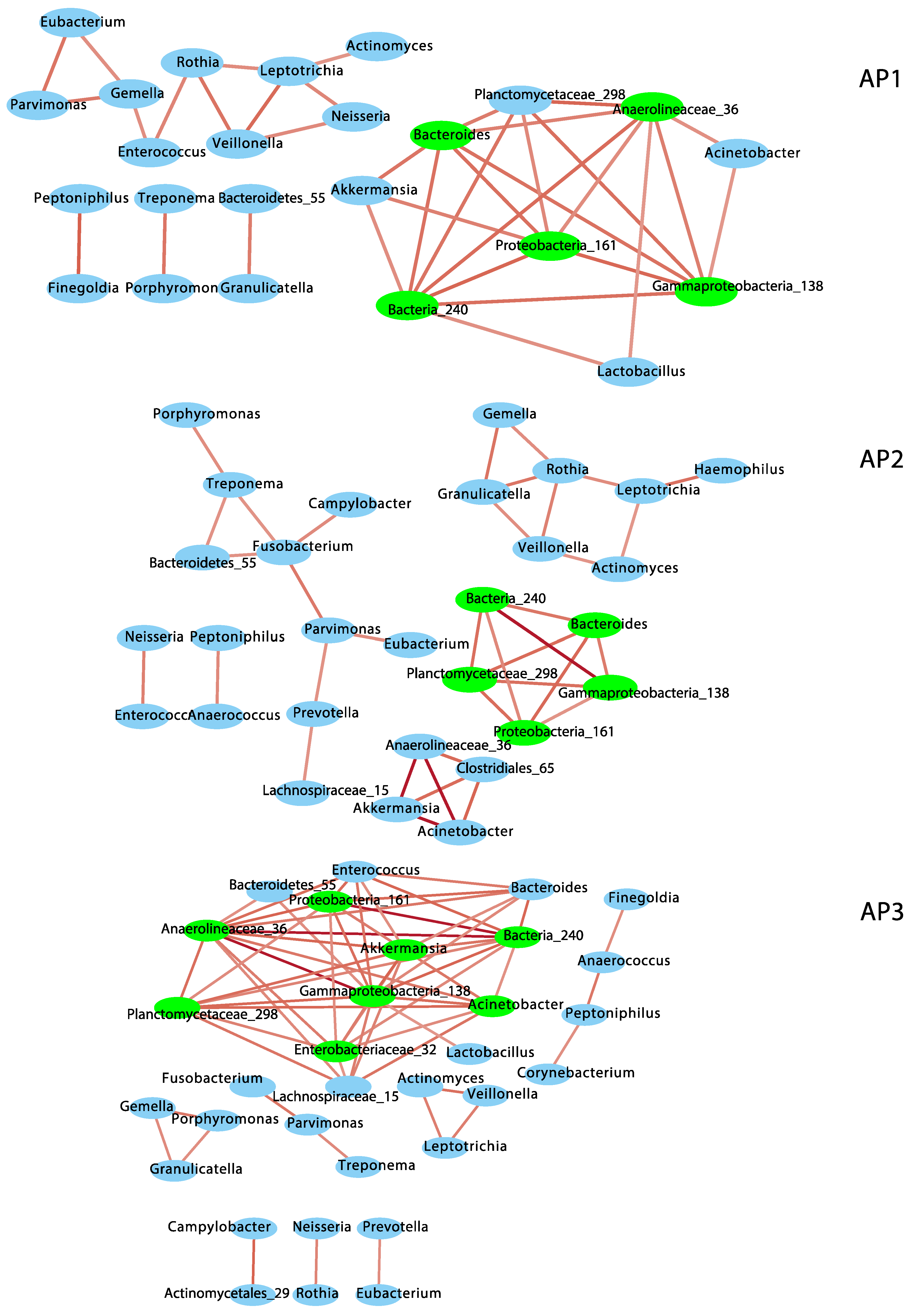
To understand the nature of bacterial associations in each phenotypic group, I performed a co-occurrence network analysis based on compositionally corrected correlations among bacterial genera (Figure 2 and Table S2). Only positive correlations were observed at r ≥ +/−0.6 and q ≤ 0.01. AP3 showed the most complex network, including eight subnetworks, with the highest number of nodes (32) and edges (61), node degree, neighborhood connectivity, cohesion and number of keystone taxa or hub nodes (8 hubs). However, AP3 showed the lowest betweenness centrality (which suggests the structure and function of this bacterial community are maintained by fewer bacterial taxa) and lowest modularity (0.55), which indicates a lesser-organized network (compared to AP1 and AP2), with not so clearly defined functional groups of bacteria (see Table S2). AP2 included six subnetworks, 29 nodes and 36 edges and 5 keystone taxa and showed the lowest node degree, neighborhood connectivity and cohesion (i.e., sparsest or less complex network), but the highest modularity (0.77). AP1 included five subnetworks with 24 nodes and 36 edges and 5 keystone taxa and showed the highest betweenness centrality but intermediate scores for all the other parameters (Table S2), including modularity (0.55). A total of 22 to 27 nodes and 17 to 21 edges intersected between AP network pairs. Similarly, four keystone taxa were shared between AP network pairs, and three genera (Gammaproteobacteria_138, Bacteria_240 and Proteobacteria_161) were present in all of them.
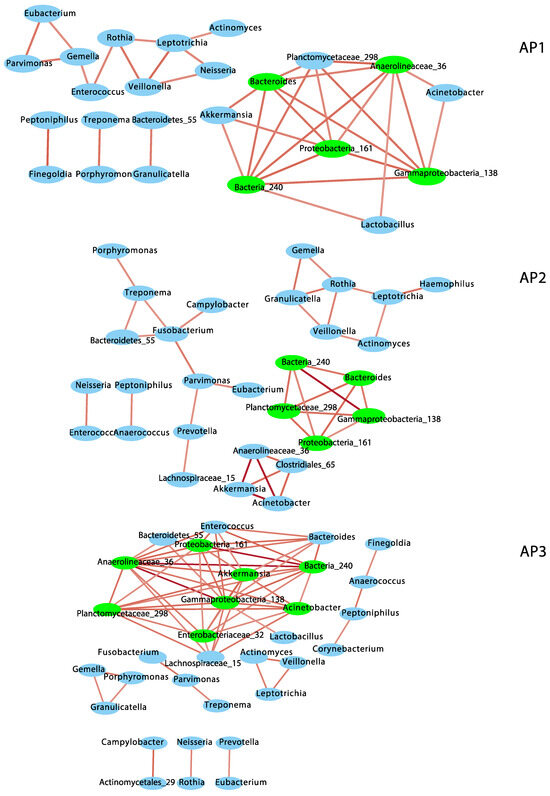
Figure 2.
Co-occurrence networks of bacterial genera in the nasopharyngeal bacteriomes from children and adolescents clustered into three asthma phenotypes (AP1, AP2 and AP3). Red edges indicate positive correlations (r = 0.6 to 1). Keystone taxa are indicated in green.
Of the ten dominant genera observed in the nasopharyngeal bacteriome [17], six (Corynebacterium, Prevotella, Fusobacterium, Haemophilus, Neisseria and Peptoniphilus) were assembled into subnetworks in different AP phenotypes, but only Fusobacterium was a keystone in AP2. Four of these genera (Prevotella, Fusobacterium, Neisseria and Haemophilus) include common pathogenic and opportunistic species of the upper airways and were associated with other commensal taxa of the nasopharynx.
4. Discussion
Pediatric asthma is the most common chronic childhood disease in the US, showing a particularly high prevalence in Washington DC. Significant progress has been made in characterizing the bacterial composition and structure of the airway bacteriome during asthma, but little is still known about their metabolic diversity and bacterial interactions across asthma phenotypes. To address this knowledge gap, here, I have reanalyzed 16S rRNA amplicon sequence data generated in [17] for 205 nasopharyngeal samples representing three distinct phenotypes of pediatric asthma.
Airway microbiota can impact epithelial cell growth and repair and host inflammatory and immune responses, thereby affecting respiratory disease onset and progression [18,20,41,42,43,44,69,70,71,72,73,74,75,76,77]. The PICRUSt2 analyses predicted 61 to 102 (14.4% to 24.1%) pathways significantly up- and down-regulated in the nasopharyngeal bacteriomes of the AP phenotypes compared in this study (Table S1 and Figures S2–S4). No other studies so far have compared metabolic profiles across similar phenotypes in pediatric asthma, but a previous study of the oral bacteriome across inflammatory phenotypes in asthmatic adults showed a great overlap (86%) in differentially expressed pathways with those reported here [50]. Similarly, previous research in the airways has suggested that some of the microbial metabolic pathways predicted here involved in amino acids (e.g., pyrimidine, leucine and arginine), carbohydrates (e.g., peptidoglycan complexes and mannan), lipopolysaccharide synthesis, oxidative stress resistance (e.g., pentose phosphate pathway) and fatty acid biosynthesis, degradation and metabolism (e.g., glycolysis and TCA cycle) may be associated with Th2, allergic sensitization, IgE sensitivity, inflammation of the airways, the host immune response (e.g., superpathway of UDP-N-acetylglucosamine-derived O-antigen building blocks’ biosynthesis) and bacterial oxidative stress resistance [18,29,30,38,39,40,49,54,55,61,78,79,80,81,82,83,84]. The same studies have also indicated that those bacterial pathways may play a more direct role in asthma exacerbations and chronic disease progression, asthma etiology and pathophysiology, and ultimately may help to unravel mechanistic links between the bacteriome and asthma. Additionally, since those pathways and many others in Table S1 varied significantly across asthma phenotypes and led to significant differences in bacterial functional diversity (as indicated by the adonis tests), they could be used as prognostic markers (i.e., asthma biomarkers) of disease, as indicated in previous studies of asthma [18,20], and help to identify and redefine phenotypes in this study as in other asthma cohorts. Moreover, the outcomes of the functional analysis presented here validate previously observed significant differences in taxonomic profiles (bacterial composition and structure) among children and adolescents with different asthma characteristics [17,55,85,86].
Deciphering interactions among microorganisms in a community is paramount to understanding host–microbiome homeostasis and microbial dysbiosis, performing pathogen identification and control, informing treatment strategies and designing novel therapeutics [87,88,89]. I investigated correlation-based bacteriome networks across three phenotypes of childhood asthma and found differences in bacterial interactions across groups (Figure 2 and Table S2). The AP3 network exhibited more connections and higher connectivity among bacterial nodes, but lower functional organization. These may have important implications for the individuals of this phenotype, since such a bacterial network suggests a more pathological or dysfunctional state with enhanced virulence, resilience to treatment and asthma severity [87,90,91,92].
The three AP networks overlapped largely in taxonomic membership and keystone taxa (five to eight keystone genera) (Figure 2). These taxa maintain the connections, stability and functionality of microbial networks. Moreover, since these same keystone taxa are consistently linked to multiple disease phenotypes, they might serve as therapeutic targets through microbiome-based interventions and, if absent in healthy individuals, serve as biomarkers of disease.
Most of the keystone taxa showed lower relative abundances compared to other genera in a previous study (Moraxella, Staphylococcus or Corynebacterium) [17]. This supports the “rare taxa” concept, which postulates that the abundance of a species is not necessarily the best determinant for its importance within the microbial community structure [93,94]. Hence, studying the airway microbiome in a more system-centric context may provide further insight into the importance and roles of lesser-known microbes and help to identify microbial genera for potential intervention targets [95].
This study has several limitations. Bacteriome analyses of 16S rRNA amplicon sequences have limited taxonomic resolution (genus or above) and may experience PCR biases [96,97]. Metabolic profiles were predicted from 16S bacterial abundances via PICRUSt2 instead of inferred from bacterial genomes using the more powerful (but also costly) metagenome or metatranscriptomic approaches. The upper airway microbiomes may not be stable over time, and since this was a cross-sectional study, potential longitudinal variation may have impacted the outcomes presented here. Similarly, potential confounding factors, such as antibiotic use, environmental exposures or host genetic factors, could also influence observed bacterial functional profiles and interactions. This study did not include healthy controls; hence, I could not test how functional profiles and bacterial networks in asthmatics compare to healthy children of a similar age. Finally, although this study revealed significant differences in bacterial functioning and connectivity, I could not determine whether specific bacteria here were drivers or bystanders (i.e., causality) in pediatric asthma.
5. Conclusions
Microbiome function and interactions have seldomly been considered in the study of asthma phenotypes. This study identifies significant differences in metabolic pathway expression and co-occurrence in the nasopharyngeal bacteriomes of children and adolescents with different asthma characteristics (i.e., phenotypic clusters). These insights, coupled with previous bacterial taxonomic profiles and clinical, physiological and biochemical factors, confirm the existence of unique disease variants of pediatric asthma with potentially different etiologies and pathophysiologies. They also pinpoint both bacterial keystone taxa and specific metabolic pathways that could serve as prognostic markers (i.e., asthma biomarkers) of disease [15,26], aid efforts to redefine current asthma classifications and support the design of better treatments for asthmatic patients. New studies of host–bacteriome associations in other cohorts are desirable to further validate the outcomes from this study.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/allergies5020014/s1. Table S1: Differential abundance analysis (DESeq2) of predicted metabolic pathways (PICRUSt2) in the nasopharyngeal bacteriomes of children and adolescents clustered into three asthma phenotypes (AP1, AP2 and AP3). Table S2: Parameters estimated for co-occurrence networks of nasopharyngeal bacteriomes in children and adolescents clustered into three asthma phenotypes (AP1, AP2 and AP3). Figure S1: Relative mean abundance of 423 predicted metabolic pathways (PICRUSt2) in the nasopharyngeal bacteriomes of children and adolescents clustered into three asthma phenotypes (AP1, AP2 and AP3). Pathways names are listed in Table S1. Figure S2: Top 30 most significantly different pathways between AP1 and AP2. Figure S3: Top 30 most significantly different pathways between AP1 and AP3. Figure S4: Top 30 most significantly different pathways between AP2 and AP3.
Funding
M.P.-L. was funded in part by a K12 Career Development Program, NIH, K12HL119994 award, the Milken Institute School of Public Health Pilot Fund Program and the Margaret Q. Landenberger Research Foundation.
Institutional Review Board Statement
All participants in this study were part of the AsthMaP-2 Project. AsthMaP-2 and the work presented here were approved by the Children’s National Medical Center Institutional Review Board (approval code: IRB No PRO00002517; approval date: 9/20/2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Sequence data have been deposited in GenBank under SRA accession number SRP069020.
Acknowledgments
I thank the GWU Colonial One High Performance Computing Cluster for allowing my computational time.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Johnson, C.C.; Chandran, A.; Havstad, S.; Li, X.; McEvoy, C.T.; Ownby, D.R.; Litonjua, A.A.; Karagas, M.R.; Camargo, C.A., Jr.; Gern, J.E.; et al. US Childhood Asthma Incidence Rate Patterns From the ECHO Consortium to Identify High-risk Groups for Primary Prevention. JAMA Pediatr. 2021, 175, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Asthma Surveillance in the United States, 2001–2021. 2023. Available online: https://www.cdc.gov/asthma/asthma-prevalence-us-2023-508.pdf (accessed on 12 February 2025).
- American Lung Association. Asthma Trends Brief. 2024. Available online: https://www.lung.org/research/trends-in-lung-disease/asthma-trends-brief (accessed on 12 February 2025).
- Naja, A.S.; Permaul, P.; Phipatanakul, W. Taming Asthma in School-Aged Children: A Comprehensive Review. J. Allergy Clin. Immunol. Pr. 2018, 6, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.; Qin, X.; Beavers, S.F.; Mirabelli, M.C. Asthma-Related School Absenteeism, Morbidity, and Modifiable Factors. Am. J. Prev. Med. 2016, 51, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Children’s National. IMPACT DC: 2019 Annual Report; Children’s National: Washington, DC, USA, 2019. [Google Scholar]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef]
- Borish, L.; Culp, J.A. Asthma: A syndrome composed of heterogeneous diseases. Ann. Allerg. Asthma Im. 2008, 101, 1–8. [Google Scholar] [CrossRef]
- Martinez, F.D. Definition of pediatric asthma and associated risk factors. Pediatr. Pulm. 1997, 24, 9–12. [Google Scholar] [CrossRef]
- Cowan, K.; Guilbert, T.W. Pediatric asthma phenotypes. Curr. Opin. Pediatr. 2012, 24, 344–351. [Google Scholar] [CrossRef]
- Aujla, S.J.; Ross, K.R.; Chmiel, J.F.; Holguin, F. Airway molecular phenotypes in pediatric asthma. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 122–126. [Google Scholar] [CrossRef]
- Wenzel, S.E. Asthma: Defining of the persistent adult phenotypes. Lancet 2006, 368, 804–813. [Google Scholar] [CrossRef]
- Haldar, P.; Pavord, I.D.; Shaw, D.E.; Berry, M.A.; Thomas, M.; Brightling, C.E.; Wardlaw, A.J.; Green, R.H. Cluster analysis and clinical asthma phenotypes. Am. J. Respir. Crit. Care Med. 2008, 178, 218–224. [Google Scholar] [CrossRef]
- Moore, W.C.; Meyers, D.A.; Wenzel, S.E.; Teague, W.G.; Li, H.; Li, X.; D’Agostino, R., Jr.; Castro, M.; Curran-Everett, D.; Fitzpatrick, A.M.; et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2010, 181, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Benton, A.S.; Wang, Z.; Lerner, J.; Foerster, M.; Teach, S.J.; Freishtat, R.J. Overcoming heterogeneity in pediatric asthma: Tobacco smoke and asthma characteristics within phenotypic clusters in an African American cohort. J. Asthma Off. J. Assoc. Care Asthma 2010, 47, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.C.; Bleecker, E.R.; Curran-Everett, D.; Erzurum, S.C.; Ameredes, B.T.; Bacharier, L.; Calhoun, W.J.; Castro, M.; Chung, K.F.; Clark, M.P.; et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J. Allergy Clin. Immunol. 2007, 119, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Perez-Losada, M.; Authelet, K.J.; Hoptay, C.E.; Kwak, C.; Crandall, K.A.; Freishtat, R.J. Pediatric asthma comprises different phenotypic clusters with unique nasal microbiotas. Microbiome 2018, 6, 179. [Google Scholar] [CrossRef]
- Pérez-Losada, M.; Castro-Nallar, E.; Bendall, M.L.; Freishtat, R.J.; Crandall, K.A. Dual Transcriptomic Profiling of Host and Microbiota during Health and Disease in Pediatric Asthma. PLoS ONE 2015, 10, e0131819. [Google Scholar] [CrossRef]
- Pérez-Losada, M.; Crandall, K.A.; Freishtat, R.J. Two sampling methods yield distinct microbial signatures in the nasopharynges of asthmatic children. Microbiome 2016, 4, 25. [Google Scholar] [CrossRef]
- Castro-Nallar, E.; Shen, Y.; Freishtat, R.J.; Pérez-Losada, M.; Manimaran, S.; Liu, G.; Spira, A.; Johnson, W.E.; Crandall, K.A. Integrating metagenomics and host gene expression to characterize asthma-associated microbial communities. BMC Med. Genom. 2015, 8, 50. [Google Scholar] [CrossRef]
- Teo, S.M.; Mok, D.; Pham, K.; Kusel, M.; Serralha, M.; Troy, N.; Holt, B.J.; Hales, B.J.; Walker, M.L.; Hollams, E.; et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 2015, 17, 704–715. [Google Scholar] [CrossRef]
- Bogaert, D.; Keijser, B.; Huse, S.; Rossen, J.; Veenhoven, R.; van Gils, E.; Bruin, J.; Montijn, R.; Bonten, M.; Sanders, E. Variability and diversity of nasopharyngeal microbiota in children: A metagenomic analysis. PLoS ONE 2011, 6, e17035. [Google Scholar] [CrossRef]
- Pérez-Losada, M.; Alamri, L.; Crandall, K.A.; Freishtat, R.J. Nasopharyngeal Microbiome Diversity Changes over Time in Children with Asthma. PLoS ONE 2017, 12, e0170543. [Google Scholar] [CrossRef]
- Esposito, S.; Principi, N. Impact of nasopharyngeal microbiota on the development of respiratory tract diseases. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2018, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J. Nasopharyngeal Microbiota: Gatekeepers or Fortune Tellers of Susceptibility to Respiratory Tract Infections? Am. J. Respir. Crit. Care Med. 2017, 196, 1504–1505. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.L.; Leong, L.E.X.; Choo, J.M.; Wesselingh, S.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; et al. Inflammatory phenotypes in patients with severe asthma are associated with distinct airway microbiology. J. Allergy Clin. Immunol. 2018, 141, 94–103.e15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cox, M.; Liang, Z.; Brinkmann, F.; Cardenas, P.A.; Duff, R.; Bhavsar, P.; Cookson, W.; Moffatt, M.; Chung, K.F. Airway Microbiota in Severe Asthma and Relationship to Asthma Severity and Phenotypes. PLoS ONE 2016, 11, e0152724. [Google Scholar] [CrossRef]
- Chung, K.F. Potential Role of the Lung Microbiome in Shaping Asthma Phenotypes. Ann. Am. Thorac. Soc. 2017, 14, S326–S331. [Google Scholar] [CrossRef]
- Kozik, A.J.; Huang, Y.J. The microbiome in asthma: Role in pathogenesis, phenotype, and response to treatment. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2019, 122, 270–275. [Google Scholar] [CrossRef]
- Liu, C.; Makrinioti, H.; Saglani, S.; Bowman, M.; Lin, L.L.; Camargo, C.A., Jr.; Hasegawa, K.; Zhu, Z. Microbial dysbiosis and childhood asthma development: Integrated role of the airway and gut microbiome, environmental exposures, and host metabolic and immune response. Front. Immunol. 2022, 13, 1028209. [Google Scholar] [CrossRef]
- van Beveren, G.J.; Said, H.; van Houten, M.A.; Bogaert, D. The respiratory microbiome in childhood asthma. J. Allergy Clin. Immunol. 2023, 152, 1352–1367. [Google Scholar] [CrossRef]
- Pijnenburg, M.W.; Frey, U.; De Jongste, J.C.; Saglani, S. Childhood asthma: Pathogenesis and phenotypes. Eur. Respir. J. 2022, 59, 2100731. [Google Scholar] [CrossRef]
- Shibata, R.; Zhu, Z.; Ooka, T.; Freishtat, R.J.; Mansbach, J.M.; Perez-Losada, M.; Ramos-Tapia, I.; Teach, S.; Camargo, C.A., Jr.; Hasegawa, K. Immunoglobulin E-virus phenotypes of infant bronchiolitis and risk of childhood asthma. Front. Immunol. 2023, 14, 1187065. [Google Scholar] [CrossRef]
- Tsai, M.H.; Shih, H.J.; Su, K.W.; Liao, S.L.; Hua, M.C.; Yao, T.C.; Lai, S.H.; Yeh, K.W.; Chen, L.C.; Huang, J.L.; et al. Nasopharyngeal microbial profiles associated with the risk of airway allergies in early childhood. J. Microbiol. Immunol. Infect. 2022, 55, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, M.I.; Thorsen, J.; Hashimoto, S.; Vijverberg, S.J.H.; Neerincx, A.H.; Brinkman, P.; van Aalderen, W.; Stokholm, J.; Rasmussen, M.A.; Roggenbuck-Wedemeyer, M.; et al. Oropharyngeal Microbiota Clusters in Children with Asthma or Wheeze Associate with Allergy, Blood Transcriptomic Immune Pathways, and Exacerbation Risk. Am. J. Respir. Crit. Care Med. 2023, 208, 142–154. [Google Scholar] [CrossRef] [PubMed]
- McCauley, K.E.; Durack, J.; Lynch, K.V.; Fadrosh, D.W.; Fujimura, K.E.; Vundla, F.; Ozcam, M.; LeBeau, P.; Caltroni, A.; Burns, P.; et al. Early-life nasal microbiota dynamics relate to longitudinal respiratory phenotypes in urban children. J. Allergy Clin. Immunol. 2024, 153, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Aldriwesh, M.G.; Al-Mutairi, A.M.; Alharbi, A.S.; Aljohani, H.Y.; Alzahrani, N.A.; Ajina, R.; Alanazi, A.M. Paediatric Asthma and the Microbiome: A Systematic Review. Microorganisms 2023, 11, 939. [Google Scholar] [CrossRef]
- Logon, K.; Swirkosz, G.; Nowak, M.; Wrzesniewska, M.; Szczygiel, A.; Gomulka, K. The Role of the Microbiome in the Pathogenesis and Treatment of Asthma. Biomedicines 2023, 11, 1618. [Google Scholar] [CrossRef]
- Valverde-Molina, J.; Garcia-Marcos, L. Microbiome and Asthma: Microbial Dysbiosis and the Origins, Phenotypes, Persistence, and Severity of Asthma. Nutrients 2023, 15, 486. [Google Scholar] [CrossRef]
- Santacroce, L.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Luperto, P.; De Nitto, E.; Topi, S. The Human Respiratory System and its Microbiome at a Glimpse. Biology 2020, 9, 318. [Google Scholar] [CrossRef]
- Liu, X.F.; Shao, J.H.; Liao, Y.T.; Wang, L.N.; Jia, Y.; Dong, P.J.; Liu, Z.Z.; He, D.D.; Li, C.; Zhang, X. Regulation of short-chain fatty acids in the immune system. Front. Immunol. 2023, 14, 1186892. [Google Scholar] [CrossRef]
- Liu, T.; Sun, Z.; Yang, Z.; Qiao, X. Microbiota-derived short-chain fatty acids and modulation of host-derived peptides formation: Focused on host defense peptides. Biomed. Pharmacother. 2023, 162, 114586. [Google Scholar] [CrossRef]
- Qian, G.; Jiang, W.; Zou, B.; Feng, J.; Cheng, X.; Gu, J.; Chu, T.; Niu, C.; He, R.; Chu, Y.; et al. LPS inactivation by a host lipase allows lung epithelial cell sensitization for allergic asthma. J. Exp. Med. 2018, 215, 2397–2412. [Google Scholar] [CrossRef]
- Dipak, G.V.; Nami, S.P.; Harissios, V. Protease-activated receptor-2: Role in asthma pathogenesis and utility as a biomarker of disease severity. Front. Med. 2022, 9, 954990. [Google Scholar]
- Gulliver, E.L.; Young, R.B.; Chonwerawong, M.; D’Adamo, G.L.; Thomason, T.; Widdop, J.T.; Rutten, E.L.; Rossetto Marcelino, V.; Bryant, R.V.; Costello, S.P.; et al. Review article: The future of microbiome-based therapeutics. Aliment. Pharmacol. Ther. 2022, 56, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Logotheti, M.; Agioutantis, P.; Katsaounou, P.; Loutrari, H. Microbiome Research and Multi-Omics Integration for Personalized Medicine in Asthma. J. Pers. Med. 2021, 11, 1299. [Google Scholar] [CrossRef]
- Campbell, C.D.; Gleeson, M.; Sulaiman, I. The role of the respiratory microbiome in asthma. Front. Allergy 2023, 4, 1120999. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, J.; Zhu, J.; Yang, F.; Wu, H.; Ba, Y.; Cui, L.; Chen, R.; Chen, S. Bacterial composition and community structure of the oropharynx of adults with asthma are associated with environmental factors. Microb. Pathog. 2020, 149, 104505. [Google Scholar] [CrossRef]
- Li, Y.; Zou, C.; Li, J.; Wang, W.; Wang, F.; Guo, Y. Airway Microbiome Composition and Co-Occurrence Network Are Associated with Inflammatory Phenotypes of Asthma. Int. Arch. Allergy Immunol. 2023, 184, 1254–1263. [Google Scholar] [CrossRef]
- Huang, C.; Yu, Y.; Du, W.; Liu, Y.; Dai, R.; Tang, W.; Wang, P.; Zhang, C.; Shi, G. Fungal and bacterial microbiome dysbiosis and imbalance of trans-kingdom network in asthma. Clin. Transl. Allergy 2020, 10, 42. [Google Scholar] [CrossRef]
- Earl, C.S.; An, S.Q.; Ryan, R.P. The changing face of asthma and its relation with microbes. Trends Microbiol. 2015, 23, 408–418. [Google Scholar] [CrossRef]
- Littman, D.R.; Pamer, E.G. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe 2011, 10, 311–323. [Google Scholar] [CrossRef]
- Perez-Losada, M.; Castro-Nallar, E.; Laerte Boechat, J.; Delgado, L.; Azenha Rama, T.; Berrios-Farias, V.; Oliveira, M. The oral bacteriomes of patients with allergic rhinitis and asthma differ from that of healthy controls. Front. Microbiol. 2023, 14, 1197135. [Google Scholar] [CrossRef] [PubMed]
- Perez-Losada, M.; Castro-Nallar, E.; Laerte Boechat, J.; Delgado, L.; Azenha Rama, T.; Berrios-Farias, V.; Oliveira, M. Nasal Bacteriomes of Patients with Asthma and Allergic Rhinitis Show Unique Composition, Structure, Function and Interactions. Microorganisms 2023, 11, 683. [Google Scholar] [CrossRef] [PubMed]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microb. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Chen, I.A.; Chu, K.; Palaniappan, K.; Ratner, A.; Huang, J.; Huntemann, M.; Hajek, P.; Ritter, S.; Varghese, N.; Seshadri, R.; et al. The IMG/M data management and analysis system v.6.0: New tools and advanced capabilities. Nucleic Acids Res. 2021, 49, D751–D763. [Google Scholar] [CrossRef]
- Caspi, R.; Billington, R.; Fulcher, C.A.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Midford, P.E.; Ong, Q.; Ong, W.K.; et al. The MetaCyc database of metabolic pathways and enzymes. Nucleic Acids Res. 2018, 46, D633–D639. [Google Scholar] [CrossRef]
- Caspi, R.; Billington, R.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Midford, P.E.; Ong, W.K.; Paley, S.; Subhraveti, P.; Karp, P.D. The MetaCyc database of metabolic pathways and enzymes—A 2019 update. Nucleic Acids Res. 2020, 48, D445–D453. [Google Scholar] [CrossRef]
- Yang, C.; Mai, J.; Cao, X.; Burberry, A.; Cominelli, F.; Zhang, L. ggpicrust2: An R package for PICRUSt2 predicted functional profile analysis and visualization. Bioinformatics 2023, 39, btad470. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Statist. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Cook, R.D. Detection of influential observation in linear regression. Technometrics 1977, 19, 15–18. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Schwager, E.; Weingart, G.; Bielski, C.; Huttenhower, C. CCREPE: Compositionality Corrected by Permutation and Renormalization. 2024. Available online: https://huttenhower.sph.harvard.edu/ccrepe/ (accessed on 12 February 2025).
- Doncheva, N.T.; Morris, J.H.; Holze, H.; Kirsch, R.; Nastou, K.C.; Cuesta-Astroz, Y.; Rattei, T.; Szklarczyk, D.; von Mering, C.; Jensen, L.J. Cytoscape stringApp 2.0: Analysis and Visualization of Heterogeneous Biological Networks. J. Proteome Res. 2023, 22, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Csárdi, G.; Nepusz, T.; Traag, V.; Horvát, S.; Zanini, F.; Noom, D.; Müller, K. igraph: Network Analysis and Visualization in R, 2.1.4. 2025. Available online: https://igraph.r-universe.dev/igraph (accessed on 12 February 2025).
- Hoffman, D.J.; Campos-Ponce, M.; Taddei, C.R.; Doak, C.M. Microbiome, growth retardation and metabolism: Are they related? Ann. Hum. Biol. 2017, 44, 201–207. [Google Scholar] [CrossRef]
- Ta, L.D.H.; Yap, G.C.; Tay, C.J.X.; Lim, A.S.M.; Huang, C.H.; Chu, C.W.; De Sessions, P.F.; Shek, L.P.; Goh, A.; Van Bever, H.P.S.; et al. Establishment of the nasal microbiota in the first 18 months of life: Correlation with early-onset rhinitis and wheezing. J. Allergy Clin. Immunol. 2018, 142, 86–95. [Google Scholar] [CrossRef]
- Chiang, T.Y.; Yang, Y.R.; Zhuo, M.Y.; Yang, F.; Zhang, Y.F.; Fu, C.H.; Lee, T.J.; Chung, W.H.; Chen, L.; Chang, C.J. Microbiome profiling of nasal extracellular vesicles in patients with allergic rhinitis. World Allergy Organ. J. 2022, 15, 100674. [Google Scholar] [CrossRef]
- Losol, P.; Choi, J.P.; Kim, S.H.; Chang, Y.S. The Role of Upper Airway Microbiome in the Development of Adult Asthma. Immune Netw. 2021, 21, e19. [Google Scholar] [CrossRef]
- Lynch, S.V. The Lung Microbiome and Airway Disease. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. S2), S462–S465. [Google Scholar] [CrossRef]
- Huffnagle, G.B.; Dickson, R.P.; Lukacs, N.W. The respiratory tract microbiome and lung inflammation: A two-way street. Mucosal Immunol. 2017, 10, 299–306. [Google Scholar] [CrossRef]
- Lira-Lucio, J.A.; Falfan-Valencia, R.; Ramirez-Venegas, A.; Buendia-Roldan, I.; Rojas-Serrano, J.; Mejia, M.; Perez-Rubio, G. Lung Microbiome Participation in Local Immune Response Regulation in Respiratory Diseases. Microorganisms 2020, 8, 1059. [Google Scholar] [CrossRef]
- Paudel, K.R.; Dharwal, V.; Patel, V.K.; Galvao, I.; Wadhwa, R.; Malyla, V.; Shen, S.S.; Budden, K.F.; Hansbro, N.G.; Vaughan, A.; et al. Role of Lung Microbiome in Innate Immune Response Associated With Chronic Lung Diseases. Front. Med. 2020, 7, 554. [Google Scholar] [CrossRef] [PubMed]
- Costello, E.K.; Stagaman, K.; Dethlefsen, L.; Bohannan, B.J.; Relman, D.A. The application of ecological theory toward an understanding of the human microbiome. Science 2012, 336, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.Y.; Cheng, M.L.; Chiang, M.H.; Wang, C.J.; Tsai, M.H.; Lin, G. Integrated metabolic and microbial analysis reveals host-microbial interactions in IgE-mediated childhood asthma. Sci. Rep. 2021, 11, 23407. [Google Scholar] [CrossRef] [PubMed]
- Georas, S.N.; Khurana, S. Update on asthma biology. J. Allergy Clin. Immunol. 2024, 153, 1215–1228. [Google Scholar] [CrossRef]
- Hu, Q.; Jin, L.; Zeng, J.; Wang, J.; Zhong, S.; Fan, W.; Liao, W. Tryptophan metabolite-regulated Treg responses contribute to attenuation of airway inflammation during specific immunotherapy in a mouse asthma model. Hum. Vaccin. Immunother. 2020, 16, 1891–1899. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Cheng, M.L.; Chiang, M.H.; Wang, C.J.; Tsai, M.H.; Lin, G. Metabolomic Analysis Reveals Distinct Profiles in the Plasma and Urine Associated with IgE Reactions in Childhood Asthma. J. Clin. Med. 2020, 9, 887. [Google Scholar] [CrossRef]
- Samra, M.S.; Lim, D.H.; Han, M.Y.; Jee, H.M.; Kim, Y.K.; Kim, J.H. Bacterial Microbiota-derived Extracellular Vesicles in Children With Allergic Airway Diseases: Compositional and Functional Features. Allergy Asthma Immunol. Res. 2021, 13, 56–74. [Google Scholar] [CrossRef]
- Li, K.J.; Chen, Z.L.; Huang, Y.; Zhang, R.; Luan, X.Q.; Lei, T.T.; Chen, L. Dysbiosis of lower respiratory tract microbiome are associated with inflammation and microbial function variety. Respir. Res. 2019, 20, 272. [Google Scholar] [CrossRef]
- Al Bataineh, M.T.; Hamoudi, R.A.; Dash, N.R.; Ramakrishnan, R.K.; Almasalmeh, M.A.; Sharif, H.A.; Al-Hajjaj, M.S.; Hamid, Q. Altered respiratory microbiota composition and functionality associated with asthma early in life. BMC Infect. Dis. 2020, 20, 697. [Google Scholar] [CrossRef]
- Perez-Losada, M.; Castro-Nallar, E.; Garcia-Huidobro, J.; Boechat, J.L.; Delgado, L.; Rama, T.A.; Oliveira, M. Characterization of the oral mycobiome of Portuguese with allergic rhinitis and asthma. Curr. Res. Microb. Sci. 2024, 7, 100300. [Google Scholar] [CrossRef]
- Perez-Losada, M.; Castro-Nallar, E.; Garcia-Huidobro, J.; Boechat, J.L.; Delgado, L.; Rama, T.A.; Oliveira, M. The nasal mycobiome of individuals with allergic rhinitis and asthma differs from that of healthy controls in composition, structure and function. Front. Microbiol. 2024, 15, 1464257. [Google Scholar] [CrossRef] [PubMed]
- Faust, K.; Raes, J. Microbial interactions: From networks to models. Nat. Res. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Faust, K.; Sathirapongsasuti, J.F.; Izard, J.; Segata, N.; Gevers, D.; Raes, J.; Huttenhower, C. Microbial co-occurrence relationships in the human microbiome. PLoS Comput. Biol. 2012, 8, e1002606. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontol 2000 2021, 87, 107–131. [Google Scholar] [CrossRef]
- Dickson, R.P.; Erb-Downward, J.R.; Huffnagle, G.B. Towards an ecology of the lung: New conceptual models of pulmonary microbiology and pneumonia pathogenesis. Lancet. Respir. Med. 2014, 2, 238–246. [Google Scholar] [CrossRef]
- Hooks, K.B.; O’Malley, M.A. Dysbiosis and Its Discontents. mBio 2017, 8, e01492–e01517. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Lynch, M.D.; Neufeld, J.D. Ecology and exploration of the rare biosphere. Nat. Rev. Microbiol. 2015, 13, 217–229. [Google Scholar] [CrossRef]
- Litchman, E.; Villeger, S.; Zinger, L.; Auguet, J.C.; Thuiller, W.; Munoz, F.; Kraft, N.J.B.; Philippot, L.; Violle, C. Refocusing the microbial rare biosphere concept through a functional lens. Trends Ecol. Evol. 2024, 39, 923–936. [Google Scholar] [CrossRef]
- Reyman, M.; Clerc, M.; van Houten, M.A.; Arp, K.; Chu, M.; Hasrat, R.; Sanders, E.A.M.; Bogaert, D. Microbial community networks across body sites are associated with susceptibility to respiratory infections in infants. Commun. Biol. 2021, 4, 1233. [Google Scholar] [CrossRef]
- Hilton, S.K.; Castro-Nallar, E.; Perez-Losada, M.; Toma, I.; McCaffrey, T.A.; Hoffman, E.P.; Siegel, M.O.; Simon, G.L.; Johnson, W.E.; Crandall, K.A. Metataxonomic and Metagenomic Approaches vs. Culture-Based Techniques for Clinical Pathology. Front. Microbiol. 2016, 7, 484. [Google Scholar] [CrossRef]
- Perez-Losada, M.; Narayanan, D.B.; Kolbe, A.R.; Ramos-Tapia, I.; Castro-Nallar, E.; Crandall, K.A.; Dominguez, J. Comparative Analysis of Metagenomics and Metataxonomics for the Characterization of Vermicompost Microbiomes. Front. Microbiol. 2022, 13, 854423. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).