Highlights
Main findings
- Our study provides structural and functional insights into the cross-reactivity between house dust mite (HDM) allergens and helminth proteins, highlighting that tropomyosins and proteases displayed highly conserved structural regions that are critical for IgE binding in both organisms.
- The discovery of conserved epitopes in HDM and helminth proteins opens the possibility of developing more specific diagnostic tools, suggesting that serological tests could be fine-tuned to avoid false positives due to cross-reactivity.
- The use of protein modeling and molecular docking tools offers a pathway to systematically identify allergen–ortholog pairs with potential cross-reactivity, which could be vital in regions where both mites and helminths are prevalent.
- Further in vitro and in vivo studies are needed to confirm the identified IgE cross-reactive regions, allowing not only a full understanding of the HDM and helminth allergens involved in cross-reactivity, but also the development of improved therapeutic and diagnostical strategies in the allergy field.
Abstract
Updated notions about the so-called hygiene hypothesis consider now that helminths may have influence in the training of the immune system during childhood. Considering the similar type of immune response between helminth infections and allergic illnesses, the objective of this study was to evaluate how structural and functional conservation between house-dust mite allergens and their helminth orthologs might contribute to the cross-induction of IgE responses in allergies and helminthiasis. Amino acid sequences from group-1, -2, -5, -9, -10, -18, -21, and -23 allergens of the house dust mite Dermatophagoides pteronyssinus were retrieved from curated databases, and orthologs were identified in other mite species and different helminth parasites. We also assessed structural, conservational, functional, and immunologic relationships between these major mite allergens and their helminth counterparts. De novo 3D-modelling, B-cell epitopes prediction, structural conservation, and docking analyses were analyzed by Robetta platform, ElliPro and CBTope, RaptorX, and Z-Dock, respectively. Our results extend previous findings on structural conservations between major allergens and parasite proteins and show that these conservations go beyond the well-known conservations and may account for the observed immunological cross-reactions. This understanding can contribute in the near future to the development of more specific serological testing for mite-induced allergies and helminthiasis.
1. Introduction
An allergen can be defined as a component able to induce hypersensitivity reactions and take part in the immune reactions of allergy [1]. It is suggested that allergens usually lead to type 2 immunity (Th2), though recent reports showed that Th17 cells, as well as GATA-3+ type 2 innate lymphoid cells (ILC2s), are also involved in the development of allergies [2,3,4]. House dust mites (HDM) are important sources of allergens, and among them, Dermatophagoides pteronyssinus is the most widespread species worldwide, with up to 34 acknowledged allergens [5] and thereby have a well-established role in the induction of persistent allergic diseases [6,7].
Current knowledge suggests the existence of more than one single factor leading to the successful induction of an allergic response [8,9]. Among these factors, some are related to the allergen itself, such as molecular size, structure, charge, stability, biological function, and resistance to denaturation [8,10,11]. These particular features are found within only a small portion of all known protein families and therefore represent a very limited number of biochemical functions [12]. On the other hand, there are factors related to the patients, such as genetic and environmental factors and concomitant or previous diseases, that play a significant role in the development of allergic reactions and diseases like asthma [13].
Exactly 35 years ago, an inverse association between hay fever and the number of older siblings was observed by David Strachan [14]. The outcome was defined as the so-called ‘hygiene hypothesis’, which was widely adopted in subsequent years to explain the rise of allergic and autoimmune diseases as a consequence of lifestyle changes in industrialized countries [15]. Over the past few years, associations with protection from allergic diseases in both humans and experimental models have been proposed for some viral [16], bacterial [17,18], protozoan [19], and helminth infections [20,21].
The particular case of helminth infections is intriguing, as some parasites are able to modulate allergic responses even though they promote Th2 responses with production of pro-allergenic interleukins and IgE [21,22,23]. Therefore, one might expect an enhancement of allergic reactions in these infections. However, these parasites, having co-evolved with mammals for millions of years, have developed mechanisms to modulate their hosts’ immune systems, shaping not only immune features but also hosts’ microbiota, raising, in turn, this anti-inflammatory aspect of chronic parasitism; this mechanism allows them to survive in their host while causing minimal or no disease [1,10]. Nevertheless, helminths are a source of allergens, which are highly cross-reactive with mite-allergen orthologs but may be responsible for cross-reactivity with seafood allergens, such as shrimps, as well [24,25,26].
Not surprisingly, it has been proposed that the immunological cross-reactivity between allergens from commonly analyzed sources and their helminth-protein orthologs contributes to allergic sensitization associated with the acute and early stages of helminthiasis [25,27,28,29]. This cross-reactivity has been observed not only with helminth proteins, but also with other invertebrates, which increases the associated problems not only for respiratory allergies, but also for food allergies [30,31,32]. This fact has crucial impacts on allergy diagnosis, as was thoroughly shown by other authors, leading to false positive results in allergy tests [25,27,33]. Although cross-reactivity between HDM and helminths tropomyosins has dominated the discussion about cross-sensitization, several other allergens have orthologs in helminths that may be involved in this phenomenon [29,34]. An important point to consider in understanding these cross-reactions is the identification of IgE epitopes from both sources of allergens. While IgE epitopes for mite allergens have been identified by some authors [35,36,37,38,39], for helminth allergens, this type of experiment is still scarce [40].
In order to clarify the relationship between HDM allergens and helminth orthologs in the cross-sensitization process, in silico tools may aid in elucidating the evolutionary and structural relationships between these proteins [10,41]. Furthermore, refined analysis can be performed due to the growing number of available protein sequences and the advancements in bioinformatics methods [12,42,43]. In particular, protein family databases that are linked to protein sequence databases, such as the UniProtKB-InterPro integrated database, provide the basis for improved classifications of allergens [44,45]. In this study, we employed a structural bioinformatics approach to analyze the levels of structural and functional conservation between major HDM allergens and helminth proteins. Our results suggest a link between structural, functional, and molecular levels and cross-reactivity between HDM allergens and helminth proteins.
2. Materials and Methods
2.1. Identification of Most Relevant Groups of Mite Allergens
The identification and selection of relevant groups of mite allergens were performed through a literature search of studies involving allergens and the prevalence of IgE reactivity in different populations [34,46,47,48,49]. Moreover, we also included previous studies that have assessed cross-reactivity between allergens and helminth proteins [25,26,28,29]. The HDM allergen groups chosen for study were groups 1, 2, 5, 9, 10, 11, 18, 21, and 23.
2.2. Protein Sequences Retrieval and Functional Analysis
Database entries containing information about Dermatophagoides pteronyssinus allergens (groups 1, 2, 5, 9, 11, 18, 21, 23) were searched on the relational database Allergome, a platform for allergen knowledge [43] (http://www.allergome.org/, accessed on 14 June 2020). The entries in Allergome contain links to the sequences in the curated protein database UniProtKB [50] (http://www.uniprot.org/, accessed on 20 June 2020) which is integrated with the motifs and function database InterPro [51] (https://www.ebi.ac.uk/interpro/, accessed on 20 June 2020). InterPro allows access to information about function, protein motifs, and signal peptides. Protein sequences present in the form of pre-propeptides or propeptides were manually trimmed using UniProtKB/InterPro annotations.
2.3. Search for Orthologs in other Organisms
The search for orthologs was performed using the allergens of Dermatophagoides pteronyssinus as queries with tools based on hidden Markov models (HMM), Hmmer (https://www.ebi.ac.uk/Tools/hmmer/, accessed on 15 July 2020), and JackHmmer (https://www.ebi.ac.uk/Tools/hmmer/search/jackhmmer, accessed on 15 July 2020) [52], both with default parameters. The E-value cut-offs were set to 1 × 10−3 for Hmmer and 1 × 10−10 for JackHmmer. The Hmmer server provides results with higher e-values, but when no hits were found in the search for orthologs in helminths, the search was performed on the JackHmmer server, which uses an iterative approach. Both tools are linked to UniProtKB, which has direct links to the sequences of mite allergens and helminth orthologs. On these platforms, the sequences previously obtained from Dermatophagoides pteronyssinus were used to perform custom searches in the genomes of the following organisms: Dermatophagoides farinae, Blomia tropicalis, Ascaris lumbricoides, Loa loa, Schistosoma mansoni, Toxocara canis, and Trichuris trichiura. The helminth species were chosen based on the prevalence of their infections in the Brazilian population (except for Loa loa, which is rare in Brazil) [27,53,54,55].
2.4. Sequence Alignment and Similarity Levels
Multiple protein-sequence alignments were performed through the PRABI (NPS@) Server [56] (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_clustalw.html, accessed on 30 July 2020) and the T-Coffee server [57] (http://tcoffee.crg.cat/apps/tcoffee/index.html, accessed on 30 July 2020); alignments were analyzed in the ESPript 3.0 server [58] (http://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi, accessed on 5 August 2020), which gives information about amino acid consensus. Similarity levels were calculated with SIAS (Sequence Identity and Similarity) (http://imed.med.ucm.es/Tools/sias.html, accessed on 20 August 2020) using the BLOSUM62 Matrix and default parameters.
2.5. Post Translational Modifications and Analysis of Evolutionary Conservation
Previous studies have shown that post-translational modifications (PTM) enhance the allergenicity of allergens derived from fungi and plants [59,60]. D. pteronyssinus allergen sequences were analyzed with ScanProsite [61] (http://prosite.expasy.org/scanprosite/, accessed on 10 September 2020) to identify any possible PTM and determine their conservation level and presence in the epitope regions. Analysis of evolutionary conservation was performed using Protein Residue Conservation Prediction (http://compbio.cs.princeton.edu/conservation/index.html, accessed on 25 September 2020) [62]. Results are shown as a score ranging from 0 (no conservation) to 1 (conserved) in a line chart.
2.6. Prediction, Refinement, Validation, Structural Alignment, and Visualizationof Protein Tertiary Structure
All sequences of D. pteronyssinus allergens and their orthologs in other mites and helminths were submitted to protein-tertiary-structure-prediction server Robetta (de novo modeling) [63] (http://robetta.bakerlab.org/, accessed on 8 October 2020). The resulting file is a protein structure in high resolution in the pdb format, which has yet to be refined. The refinement was performed with the interactive platform FoldIt Standalone [64], which refines both sidechains and backbone based on free energy/score in kcal/mol. Structure quality assessment and validation were carried out in the QMEAN (Qualitative Model Energy ANalysis) server [65] (https://swissmodel.expasy.org/qmean/, accessed on 2 February 2021). QMEAN searches for proteins with similar size and molecular weight in the protein databank RCSB (https://www.rcsb.org/pdb/home/home.do, accessed on 2 February 2021) were carried out to evaluate the quality of the model. A normal distribution was generated with the data for the known proteins, and the server native cut-off (QMEAN4 value) at which models were considered validated is between 2 and −2. Protein-structure alignment was executed to evaluate the structural conservation between mite allergens and helminth orthologs. Analysis and measurement of the RMSD (root-mean-square deviation of atomic positions) values were carried out using RaptorX multiple-structure alignment [66] (http://raptorx.uchicago.edu/DeepAlign/submit/, accessed on 14 March 2021). RMSD values below 3Å show proteins with conserved structure and function. Above this value, further analyses were needed. Refined 3D structures were visualized with FoldIt Standalone and with PyMOL Molecular Graphics System (https://pymol.org/, accessed on 30 April 2020).
2.7. B Cell Epitope Prediction and Representation
Prediction of B cell epitopes was performed using two different servers, the results of which were combined to produce a consensus. D. pteronyssinus allergens sequences were submitted to ElliPro [67] (http://tools.iedb.org/ellipro/, accessed on 15 April 2021) which utilizes parameters such as ellipsoid propensity and protein regions density; and to CBTope [68] (http://osddlinux.osdd.net/raghava/cbtope/submit.php, accessed on 17 April 2021), which utilizes physicochemical parameters such as amino acid proportion, charge and accessibility to solvents. After a consensus was established, similarity levels were determined for the previously generated alignments. A cut-off value of 0.600 was used for ElliPro results. In order to represent nucleic-acid conservation in epitope regions between proteins from helminths and HDM, we submitted alignments to the WebLogo 3 server (http://weblogo.threeplusone.com/, accessed on 3 July 2021) [69].
2.8. Computational Docking of Mite and Helminth Proteins with Antibodies
To confirm that our predictions correctly identified antibody-binding regions that could contribute to cross-reactivity, we performed molecular-docking analyses. Specifically, we used Z-Dock server (https://zdock.wenglab.org/, accessed on 17 May 2024) [70] with the default setup, testing the binding capacity of an antibody against two proteins in silico. The tertiary structure of this antibody was previously determined by crystallography [37,39], and we used the pbd file of a modelled antibody. Specifically, we tested only the binding capacity of the 4C1 antibody [37,39] against Der p 1 and its Ascaris lumbricoides orthologue, using the pbd file of the modelled two proteins. The retrieved pdb file from Z-Dock was then visualized in Mol* 3D Viewer (https://www.rcsb.org/3d-view, accessed on 17 May 2024) to better identify the amino acid residues forming the cross-reactive regions.
3. Results
3.1. Allergens with Protease Activity (Groups 1 and 9)
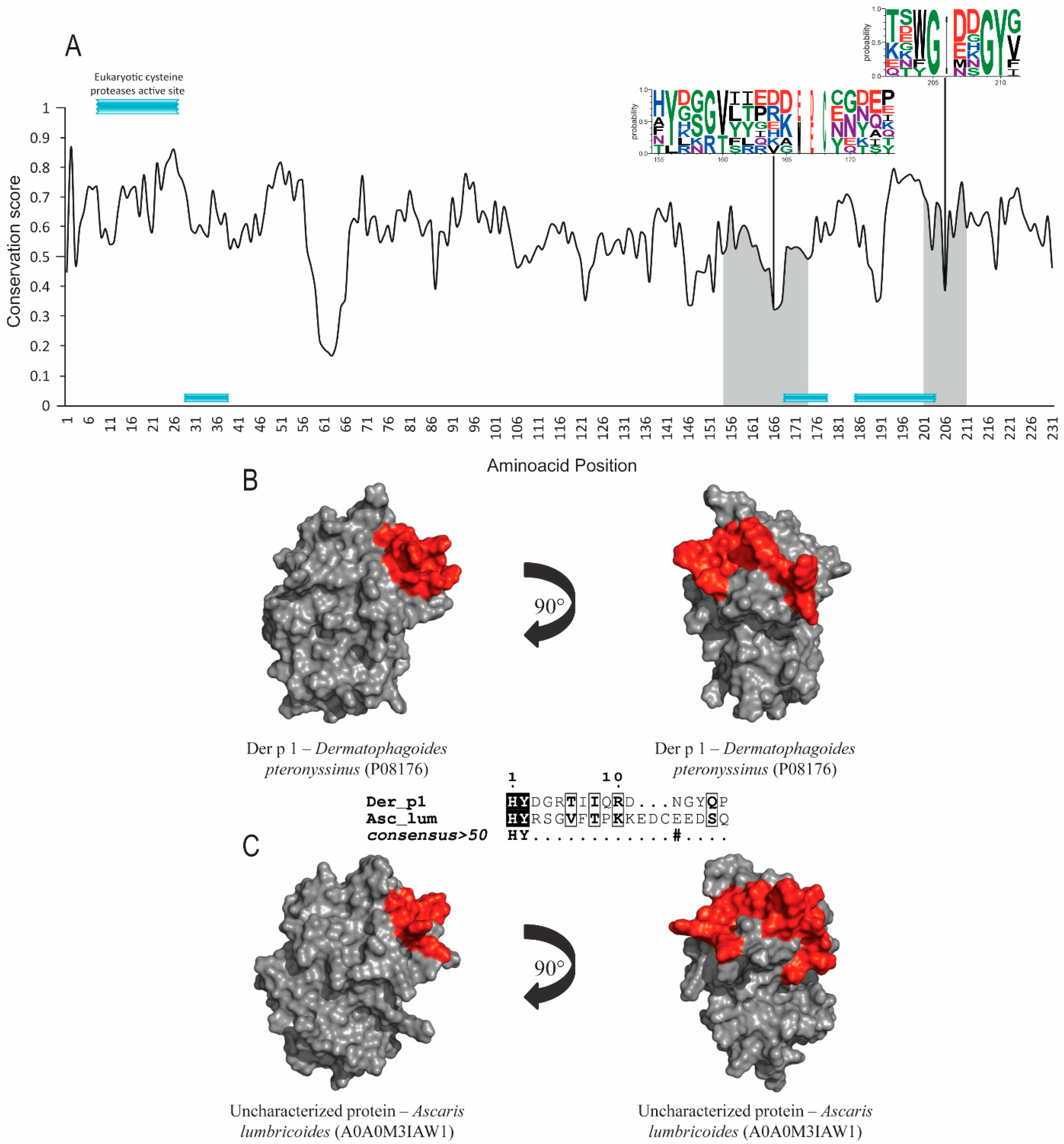
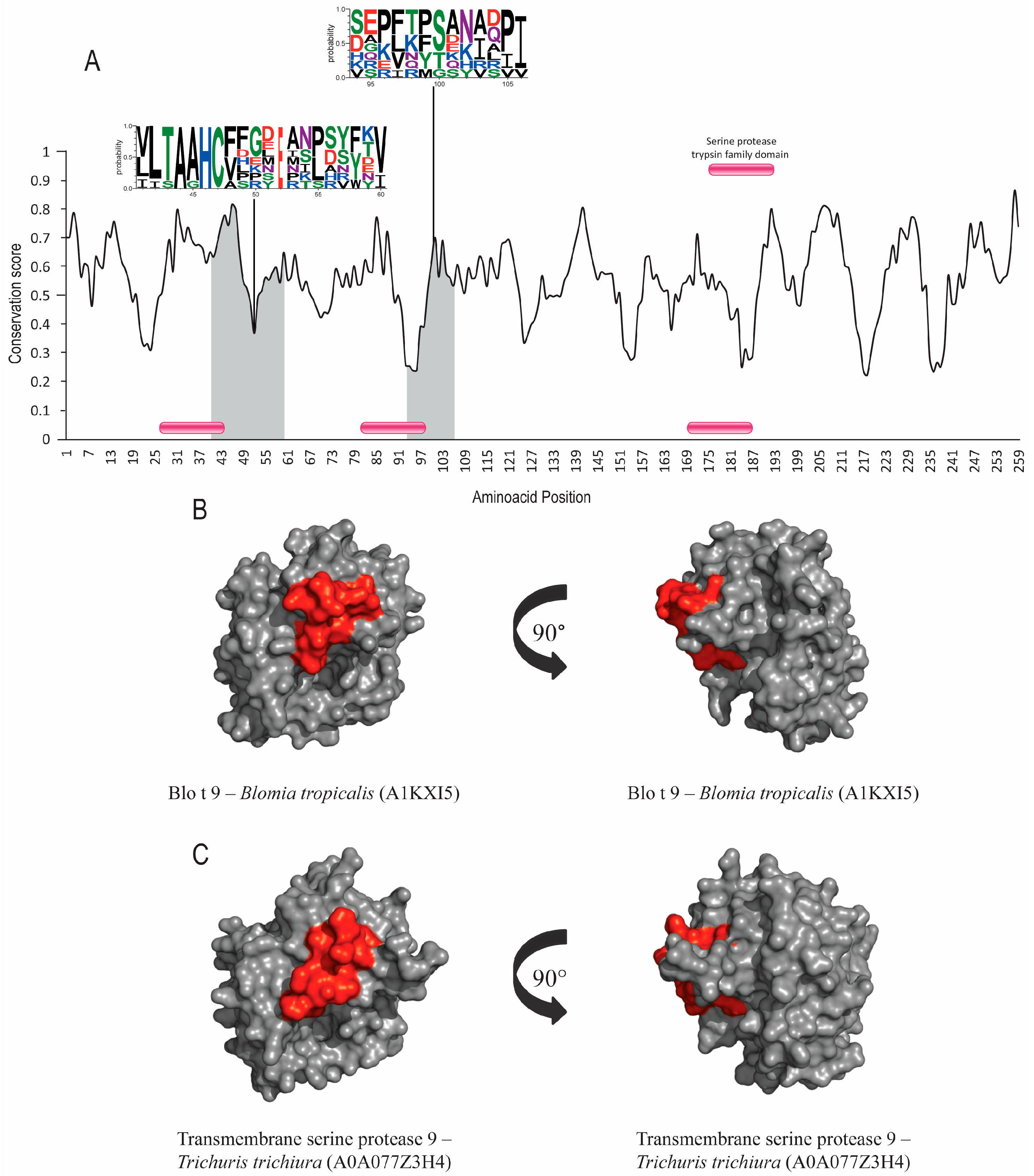
Group 1 and 9 allergens possess cysteine-protease and serine-protease activities, respectively. Signal-peptide and protease active-site inhibitors were found in both groups, but no post-translational modifications were identified. Der p 1, Der p 9 and their orthologs’ sequence accession numbers are shown in Table 1. The evolutionary conservation results for Der p 1 (Figure 1A), Der p 9 (Figure 2A) and the corresponding orthologs showed predicted epitopes in conserved areas for both allergen groups and high similarity levels among the helminth orthologs (around 40%). SIAS results (Table 1) show a high level of similarity among Der p 1, Der p 9 and their orthologs in helminths, ranging from around 37 to 41% and from 39% to 41%, respectively. There was also a high level of structural conservation, with RMSD values of 1.40Å for Der p 1 and its orthologs and 1.29Å for Der p 9 and its orthologs in multiple structural alignments (Table 1; Figure 1B,C and Figure 2B,C).

Table 1.
Dermatophagoides pteronyssinus orthologs in helminths species and other house dust mites.
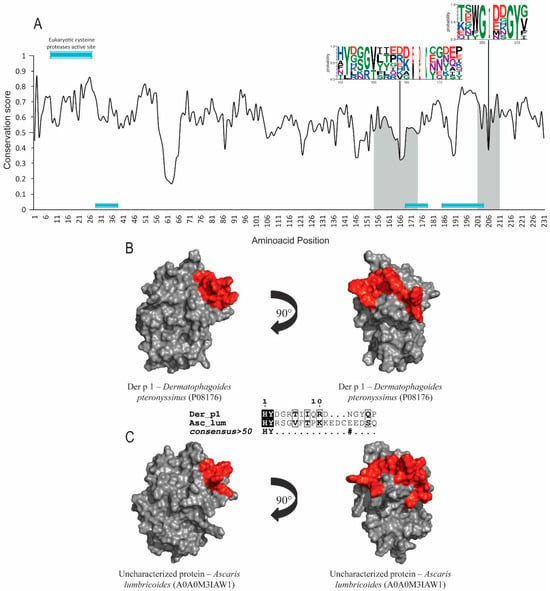
Figure 1.
Evolutionary-conservation analysis and predicted B-cell epitopes in group-1 allergens and their orthologs. (A)Evolutionary-conservation analysis shows conservation throughout the protein alignment of HDM allergens from group 1 and their orthologs in helminths. Conservation in epitope regions is shown as WebLogo format. Domain regions are shown as boxes. (B) Tertiary structure representation of predicted IgE epitope region of Der p 1. (C) Tertiary structure representation of the predicted IgE epitope region of Der p1 in the corresponding ortholog in Ascaris lumbricoides. Epitope regions are shown.5.
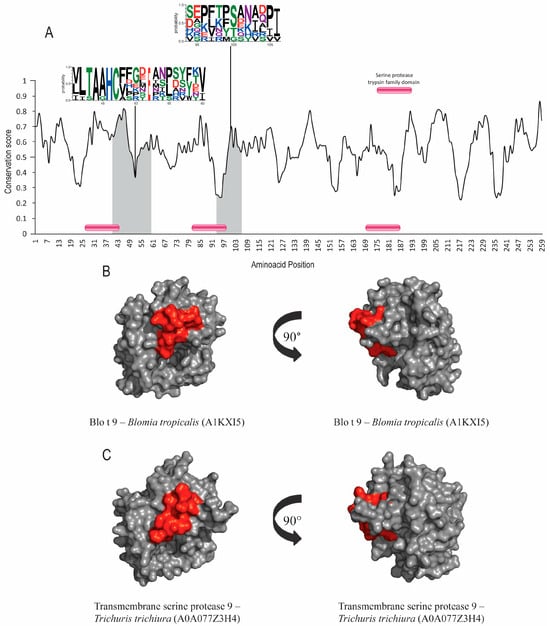
Figure 2.
Evolutionary-conservation analysis and predicted B-cell epitopes in group-9 allergens and their orthologs. (A) Evolutionary-conservation analysis shows conservation throughout the protein alignment of HDM allergens from group 9 and their orthologs in helminths. Conservation in epitope regions is shown in WebLogo format. Domain regions are shown as boxes. (B) Tertiary structure representation of the predicted IgE epitope region of Blo t 9. (C) Tertiary structure representation of predicted IgE epitope region of Blo t 9 in the correspondent ortholog in Trichuris trichiura. Epitope regions are shown.
3.2. Lipid-Binding Proteins (Group 2)
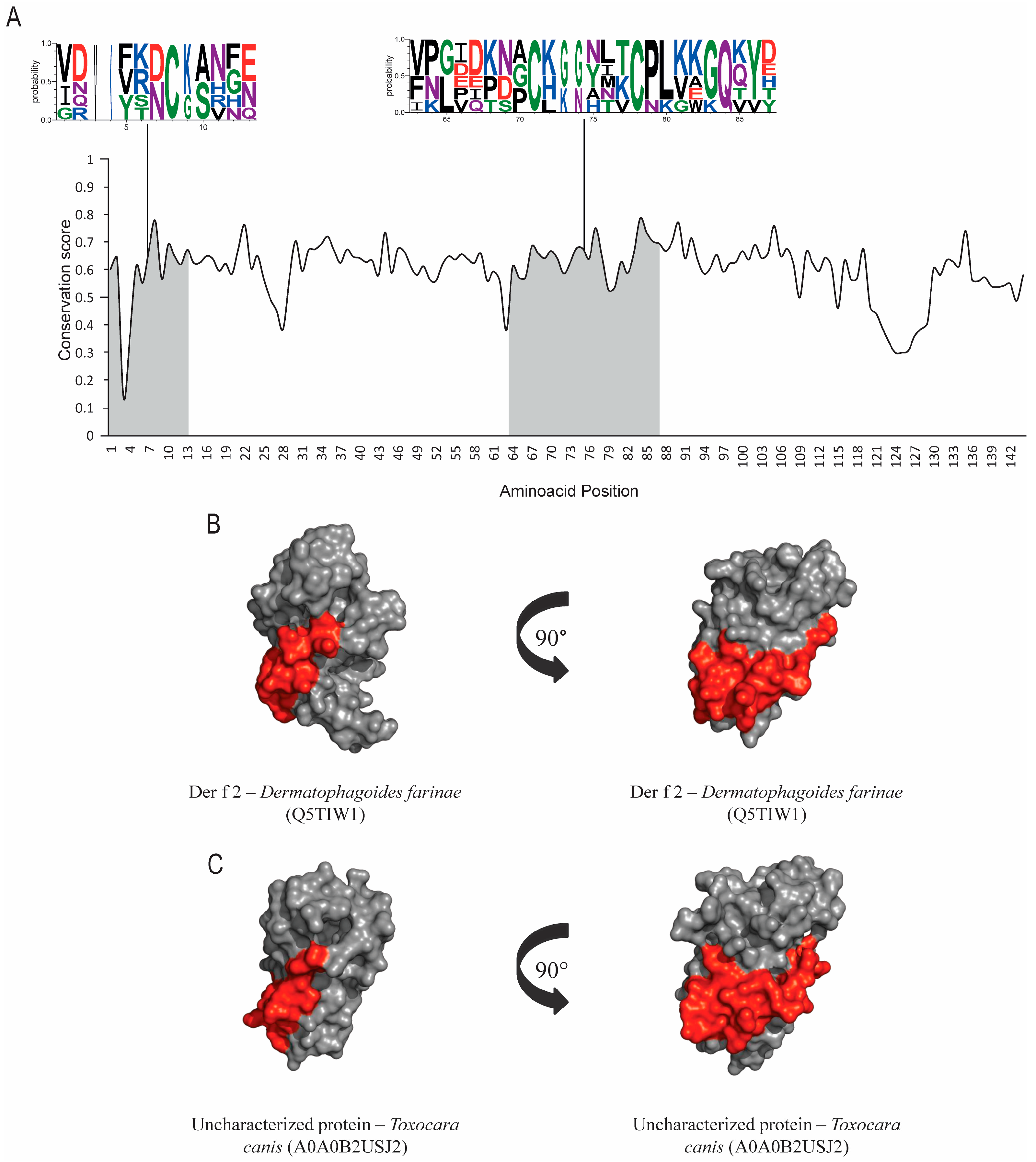
Der p 2 and its orthologs sequences accession numbers from UniProtKB are found in the Table 1. Results from group-2 allergens show low conservation levels among helminths. No hit was found between Der p 2 and any possible homologs in helminths using pHmmer. Further searches were performed with the JackHmmer server, which showed lower e-values; however, no hit was found on Ascaris lumbricoides and Schistosoma mansoni. ScanProsite did not find post-translational modification sites. Evolutionary conservation results (Figure 3A) show epitopes with conserved residues; however, structural-alignment results present a high value of RMSD (Table 1). SIAS results (Table 1) show a moderate level of similarity between Der p 2 and its orthologs in helminths, ranging from around 29 to 33%. Structural and epitope regions are compared in Figure 3B,C.
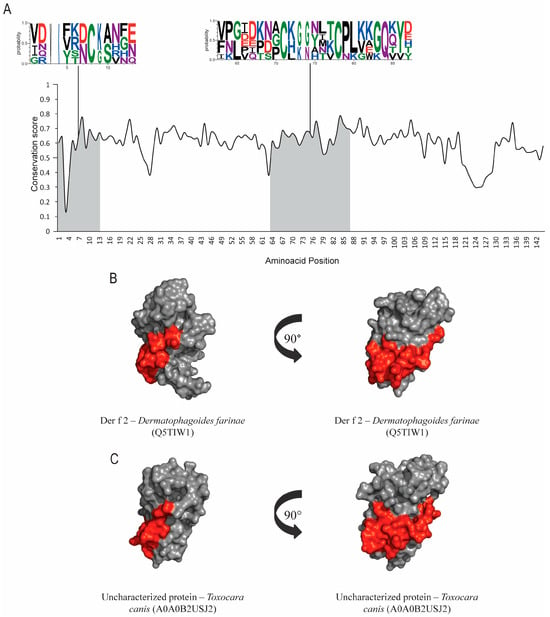
Figure 3.
Evolutionary-conservation analysis and predicted B-cell epitopes in group-2 allergens and their orthologs. (A) Evolutionary-conservation analysis shows conservation throughout the protein alignment of HDM allergens from group 2 and their orthologs in helminths. Conservation in epitope regions is shown in WebLogo format. Domain regions are shown as boxes. (B) Tertiary-structure representation of the predicted IgE epitope region of Der f 2. (C)Tertiary-structure representation of the predicted IgE epitope region of Der f 2 in the correspondent ortholog in Toxocara canis. Epitope regions are shown.
3.3. Unknown-Function Proteins (Groups 5 and 21)
The primary sequences and structures of allergens from groups 5 and 21 are very similar. A search for orthologs in helminths was performed, but we were not able to identify related proteins in any of target organisms.
3.4. Tropomyosins (Group 10)
Tropomyosins are the most-studied allergen group in terms of cross-reactivity with helminth orthologs. It is involved in invertebrate muscle contractions. Accession numbers for sequences of Der p 10 and its orthologs from UniProtKB are found in Table 1. This allergen group showed the highest levels of similarity between mite allergens and its helminth orthologs; no post-translational modification sites were identified. In this particular case, epitopes were mapped but different results were found, as shown in Table S1. However, at least one of the five common IgE epitopes for these pan-allergens were identified by our search. We found only one IgE epitope (RLEDELVHEKEKYKSISDELDQTFSVQKLQK) that was also conserved in the C-terminal region based on previous experimental determination of IgE epitopes for Der p 10. However, our prediction failed to find other IgE epitopes that were highly conserved in shrimp tropomyosin, such as VAALNRRIQLLEEDLERSEER and ESKIVELEEELRVVG.
Given the predicted epitope regions of the mite allergens from groups 10 and 11, structural conservation between them and helminth orthologs was quantified (RMSD < 2), and similarity results are presented in Table 1. SIAS results show a high level of similarity between Der p 10 and its orthologs in helminths, ranging from around 56 to 81%.
3.5. Chitin-Binding Domains (Groups 18 and 23)
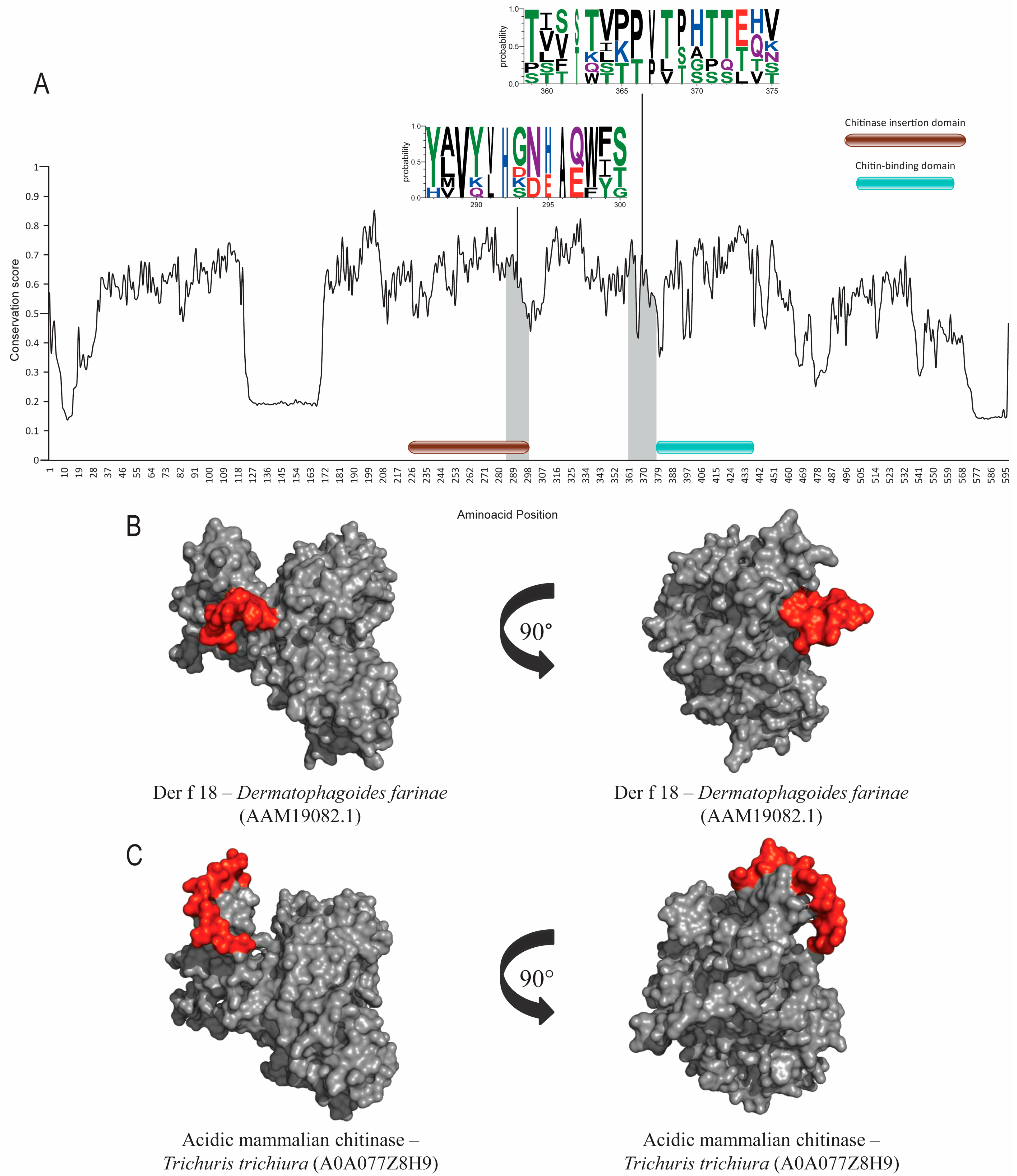
Both groups of allergens have a chitin-binding domain but very different functions. ScanProsite did not find post-translational modification sites in either allergen group. Epitope regions were found within conserved and non-conserved regions in Der p 18 (Figure 4A–C), and with Der p 23, the prediction was compromised due to its small size of 70 amino acids. SIAS results (Table 1) show a high level of similarity between Der p 18 and its orthologs in helminths, ranging from 30 to 37% and taking into account the size of this class of protein (500–600 amino acids).
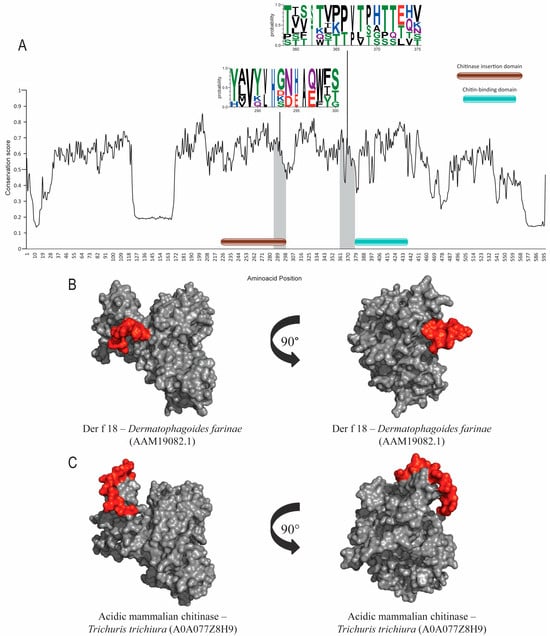
Figure 4.
Evolutionary-conservation analysis and predicted B-cell epitopes in group-18 allergens and its orthologs. (A) Evolutionary-conservation analysis shows conservation throughout the protein alignment of HDM allergens from group 18 and their orthologs in helminths. Conserved areas in epitope regions are shown in WebLogo format. Domain regions are shown as boxes. (B) Tertiary-structure representation of the predicted IgE epitope region of Der f 18. (C) Tertiary-structure representation of predicted IgE epitope region of Der f 18 in the corresponding ortholog in Trichuris trichiura. Epitope regions are shown.
Group-23 allergens are peritrophin-like proteins, with most of the ortholog proteins in found in insects but none found in helminths. Moreover, the searches performed with Hmmer and JackHmmer showed no significant results. Peritrophin-like proteins have a chitin-biding domain, and the protein group that has this domain in helminths is the chitinases (already studied with group-18 allergens).
3.6. Confirmation of Predicted Regions by Docking Analyses
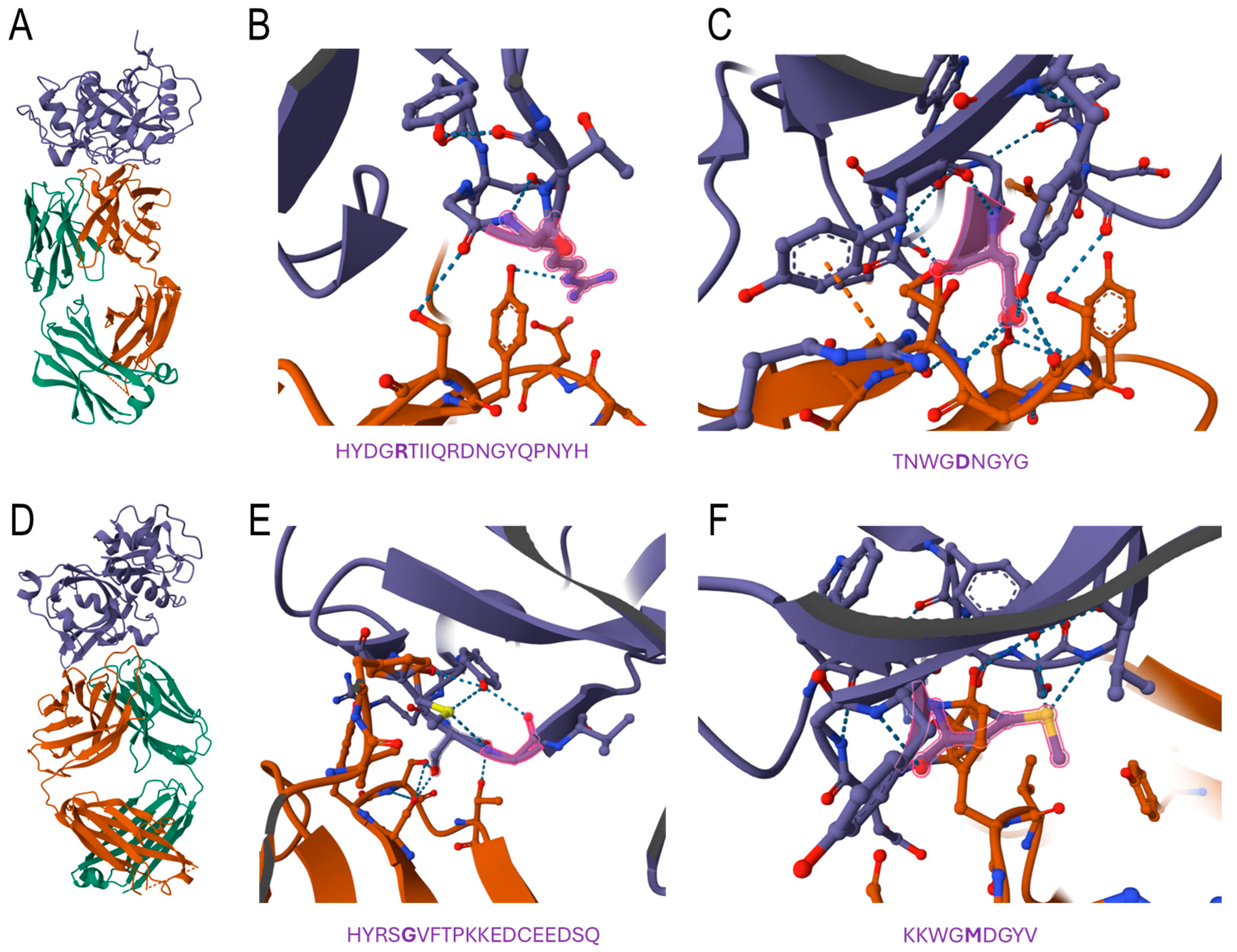
Figure 5 shows that the predicted epitope regions for both Der p 1 and its A. lumbricoides orthologue (Figure 1) interacted with the crystallographically determined structure of the 4C1 antibody, whose binding has been reported to overlap partially with the IgE-binding epitopes of the mite allergen. As displayed in Figure 5B,C, the interaction of Der p 1 with the 4C1 antibody occurred through some amino acid residues, but especially with Arg 159 and Asp 208, a finding that agrees with previous determinations. Figure 5E,F shows that compared to Der p 1, for the A. lumbricoides orthologues, the first region seemed to display more interactions. Moreover, the substitutions in the orthologue were highlighted, showing interactions with residues Gly 159 and Met 208 (Figure 5E,F).
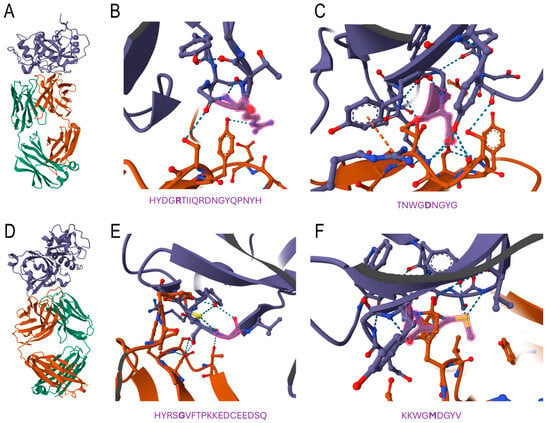
Figure 5.
Computational docking of the 4C1 antibody with Der p 1 and its ortholog protein in A. lumbricoides. (A) Modelled tertiary structure of in silico interaction between 4C1 antibody (light chain in orange and heavy chain in green) and Der p 1 (purple). Zoomed-in view of the first (B) and second (C) predicted region for cross-reactivity in Der p 1. The predicted amino acid residues are below the zoomed-in tertiary structure and the key residues (Arg 159 and Asp 208), with highlights in red (tertiary structure) and bold (sequence). (D) Modelled tertiary structure of in silico interaction between the 4C1 antibody (light chain in orange and heavy chain in green) and Der p 1 (purple). Zoomed-on view of the first (E) and second (F) predicted regions for cross-reactivity to an A. lumbricoides orthologue. The predicted amino acid residues are below the zoomed tertiary structure and possible key residues (Gly 159 and Met 208), with highlights in red (tertiary structure) and bold (sequence).
4. Discussion
Helminth infections and allergy have evolutionary and clinical relationships. Our results showed the high levels of structural and evolutionary conservation between selected HDM allergens and their orthologs in helminths. However, it was only observed in protein groups that are conserved among other species as well, suggesting that conservation is inherent to the protein function. Among the allergens analyzed in this study, group -1, -9, -10, and -18 allergen orthologs are known to play a role in eliciting IgE cross-reactivity due to their high conservation in both amino acid sequence and structural conformation. Nevertheless, the percentage similarities determined in this study were not always high (> 80%), although lower similarity would not lead to cross-reactivity. For sure, higher similarity would increase the chance of finding cross-reactive regions, but sequence similarity is sometimes more important in certain regions, such as the one containing exposed amino acid residues.
Allergens from group 10 (tropomyosin) are the most-studied examples of cross-reactivity between mites, helminths and other organisms and give insight into our result regarding its functional conservation. Allergens with such features are specially classified as pan-allergens [71,72]. Tropomyosins from HDM have significant homology with tropomyosins from different species and are frequently characterized as involved in the cross-reactivity between mites, shrimp, insects, and helminths [26,28,29,34,47,48,72]. A study in Brazil found patients who were highly IgE-reactive to shrimp tropomyosin but had never eaten shrimp or other crustaceans [73]. This study showed how tropomyosins are conserved and relevant in the cross-reactivity process.
In our group-10 epitope-mapping analyses, we found only one epitope for Der p 10 [74], which has also highly conserved counterparts in shrimp [75,76,77]. However, our prediction failed to find other IgE epitopes that were highly conserved in shrimp tropomyosin [75,76]. Therefore, it is highly unlikely that those identical sequences are not Der p 10 IgE epitopes too, highlighting that ElliPro and CBTope may have difficulties in finding epitopes from a single coil structure due to the use of surface-exposure criteria. However, experimental determination will be needed to confirm our inferences by using peptides from both allergenic sources and sera of sensitized patients.
Aside from tropomyosins, proteases (allergens from groups 1 and 9) show conserved function and structures along with some different domains; this may be due to each protease, organism, and specific substrate [78,79]. Nevertheless, structural conservation between proteases from different organisms can also play a relevant role in the cross-reactivity process. In fact, our predictions of cross-reactive epitopes of group-1 mite allergens and other cysteine proteases has found at least two regions, which were previously determined by crystallography to contain residues involved in IgE epitopes for Der p 1 [37,39]. Our prediction confirms that residues Arg 156 and Asp 198 of Der p 1 [37,39] were conserved in the sequences of all evaluated mites, Trichuris trichiura, and Schistosoma mansoni. Of note, our docking analyses confirmed the interaction with the 4C1 antibody, even in the case of non-conserved amino acid residues, as was the case for the Ascaris lumbricoides orthologue. These findings with molecular docking contribute to validation of epitope predictions.
Allergens from group 2 belong to the ML-domain lipid-binding protein family, which also includes the Neimann-Pick type C2 (NPC2) proteins [49], but its function remains yet to be elucidated. Moreover, our results show that cross-reactivity may occur with less conserved proteins like the group-2 allergens. However, our prediction has failed to find the same epitope regions for group-2 mite allergens, which had been previously identified as the ones involved in IgE binding [35,36,38]. As related before, functional conservation is believed to be a crucial factor in the cross-reactivity process, and proteins with strict functions in mites (or related organisms such as insects) do not have orthologs in other organisms. Results from groups 5, 21 and 23 suggest that proteins with strict functions play no role in the cross-reactivity process and are good candidates for inclusion in hypoallergenic cross-reactivity-free immunotherapy and diagnosis.
Besides the factors previously cited as conserved in structure, epitopes, and function, there are other factors to be considered. In a recent study in the USA, scientists analyzed the cross-reactivity between HDM glutathione S-transferase allergens (group 8), cockroach GST, and helminth GST. The results showed a lack of significant IgE cross-reactivity among the GSTs, a finding in agreement with the low shared amino acid identity at the molecular surfaces of these proteins [80]. Meanwhile, in the tropics, several studies showed that GSTs from helminths, cockroaches, and mites show high levels of cross-reactivity [25,34,81]. Both results suggest that geographic location and the human population might influence the results.
Although in silico methods for epitope prediction have limitations, tools for analyzing conservation of amino acid sequences and structures provide important information that contributes to the accurate investigation of the similarity between allergens and helminth proteins. Our study shows how function and structural conservation may be key factors in IgE cross-reactivity. Further studies can be done with novel allergens and organisms, like cockroaches, storage dust mites, and shrimps, and have the potential to support analysis of the different allergen structural groups to identify meaningful thresholds for shared similarity, as it remains to be identified whether there is a single shared feature of proteins that makes them allergens.
5. Conclusions
Our results extend previous findings on structural conservations among allergenic proteins from mites and helminths. In particular, we found epitope regions in helminth orthologs of mite allergens that correlate well with predicted IgE-epitope regions of the allergens, even in the absence of a high overall sequence similarity between the proteins. The real contribution to IgE-cross-reactivity of these identified helminth proteins and epitope sequences remains to be determined by future studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/allergies4020006/s1, Table S1: Predicted epitopes for Der p 10.
Author Contributions
Conceptualization, L.G.C.P., E.S.d.S. and N.M.A.-N.; methodology, A.B.P.L., E.S.d.S., C.S.P. and L.G.C.P.; formal analysis, A.B.P.L. and E.S.d.S.; investigation, L.G.C.P., C.S.P., A.B.P.L., E.R.G.R.A. and E.S.d.S..; resources, L.G.C.P., E.S.d.S. and N.M.A.-N.; execution of assays, A.B.P.L.; writing-original draft preparation A.B.P.L. and E.S.d.S.; writing-review and editing, A.B.P.L., E.R.G.R.A., E.S.d.S., N.M.A.-N., C.S.P. and L.G.C.P.; supervision, E.S.d.S., C.S.P. and L.G.C.P.; project administration, L.G.C.P. and E.S.d.S.; funding acquisition, L.G.C.P., C.S.P. and N.M.A.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by the following research grant: FAPESB/CNPq—PRONEM PNE 007/2014; CAPES/PROCAD—071/2013.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors. The data are not publicly available, due to issues in our university regarding cloud availability, which is not existent at the moment.
Acknowledgments
A.B.P.L. was recipient of a scholarship from the Coordination for the Improvement of Higher Education Personnel (CAPES). L.G.C.P. is the recipient of a research fellowship from National Council for Scientific and Technological Development (CNPq). E.S.d.S. was recipient of a scholarship from CAPES (grant 88887.803528/2023-00). We are grateful for Andrés Felipe Sánchez help in the latest in silico experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Scheurer, S.; Toda, M.; Vieths, S. What makes an allergen? Clin. Exp. Allergy 2015, 45, 1150–1161. [Google Scholar] [CrossRef]
- Bajoriuniene, I.; Malakauskas, K.; Lavinskiene, S.; Jeroch, J.; Sakalauskas, R. Th17 response to Dermatophagoides pteronyssinus is related to late-phase airway and systemic inflammation in allergic asthma. Int. Immunopharmacol. 2013, 17, 1020–1027. [Google Scholar] [CrossRef]
- Kubo, M. Innate and adaptive type 2 immunity in lung allergic inflammation. Immunol. Rev. 2017, 278, 162–172. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Zhang, H.; Hu, L.; Liu, J.; Wang, L.; Wang, T.; Zhang, H.; Cong, L.; Wang, Q. Pathogenesis of allergic diseases and implications for therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Vrtala, S. Allergens from house dust and storage mites. Allergo J. Int. 2022, 31, 267–271. [Google Scholar] [CrossRef]
- Calderon, M.A.; Casale, T.B.; Nelson, H.S.; Demoly, P. An evidence-based analysis of house dust mite allergen immunotherapy: A call for more rigorous clinical studies. J. Allergy Clin. Immunol. 2013, 132, 1322–1336. [Google Scholar] [CrossRef]
- Aggarwal, P.; Senthilkumaran, S. Dust Mite Allergy. In StatPearls; Ineligible Companies: Treasure Island, FL, USA, 2024. [Google Scholar]
- Poulsen, L.K. What makes an allergen more than an allergen? Clin. Exp. Allergy 2009, 39, 623–625. [Google Scholar] [CrossRef]
- Aglas, L.; Gilles, S.; Bauer, R.; Huber, S.; Araujo, G.R.; Mueller, G.; Scheiblhofer, S.; Amisi, M.; Dang, H.H.; Briza, P.; et al. Context matters: T(H)2 polarization resulting from pollen composition and not from protein-intrinsic allergenicity. J. Allergy Clin. Immunol. 2018, 142, 984–987.e986. [Google Scholar] [CrossRef]
- Daschner, A.; Gonzalez Fernandez, J. Allergy in an Evolutionary Framework. J. Mol. Evol. 2020, 88, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Bannon, G.A. What makes a food protein an allergen? Curr. Allergy Asthma Rep. 2004, 4, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Radauer, C.; Breiteneder, H. Evolutionary biology of plant food allergens. J. Allergy Clin. Immunol. 2007, 120, 518–525. [Google Scholar] [CrossRef]
- Poulsen, L.K.; Hummelshoj, L. Triggers of IgE class switching and allergy development. Ann. Med. 2007, 39, 440–456. [Google Scholar] [CrossRef] [PubMed]
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef] [PubMed]
- Scudellari, M. News Feature: Cleaning up the hygiene hypothesis. Proc. Natl. Acad. Sci. USA 2017, 114, 1433–1436. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Gaitan, M.I. Multiple sclerosis and environmental factors: The role of vitamin D, parasites, and Epstein-Barr virus infection. Acta Neurol. Scand. 2015, 132, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Miftahussurur, M.; Nusi, I.A.; Graham, D.Y.; Yamaoka, Y. Helicobacter, Hygiene, Atopy, and Asthma. Front. Microbiol. 2017, 8, 1034. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Endo, A.; Isolauri, E.; Scalabrin, D. Early Gut Colonization With Lactobacilli and Staphylococcus in Infants: The Hygiene Hypothesis Extended. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.M.C.; Carneiro, V.L.; Galvao, A.A.; Fonseca, T.R.; Vitor, R.W.A.; Alcantara-Neves, N.M.; Cruz, A.A.; Figueiredo, C.A. Toxoplasma gondii protects from IgE sensitization and induces Th1/Th2 immune profile. Parasite Immunol. 2020, 42, e12694. [Google Scholar] [CrossRef] [PubMed]
- Versini, M.; Jeandel, P.Y.; Bashi, T.; Bizzaro, G.; Blank, M.; Shoenfeld, Y. Unraveling the Hygiene Hypothesis of helminthes and autoimmunity: Origins, pathophysiology, and clinical applications. BMC Med. 2015, 13, 81. [Google Scholar] [CrossRef]
- Yazdanbakhsh, M.; Kremsner, P.G.; van Ree, R. Allergy, parasites, and the hygiene hypothesis. Science 2002, 296, 490–494. [Google Scholar] [CrossRef]
- Cruz, A.A.; Cooper, P.J.; Figueiredo, C.A.; Alcantara-Neves, N.M.; Rodrigues, L.C.; Barreto, M.L. Global issues in allergy and immunology: Parasitic infections and allergy. J. Allergy Clin. Immunol. 2017, 140, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Maizels, R.M. Parasitic helminth infections and the control of human allergic and autoimmune disorders. Clin. Microbiol. Infect. 2016, 22, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.; Huang, C.H.; Lee, B.W. Shellfish and House Dust Mite Allergies: Is the Link Tropomyosin? Allergy Asthma Immunol. Res. 2016, 8, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, L.; Acevedo, N. Allergy in the tropics: The impact of cross-reactivity between mites and ascaris. Front Biosci. 2011, 3, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, N.; Sanchez, J.; Erler, A.; Mercado, D.; Briza, P.; Kennedy, M.; Fernandez, A.; Gutierrez, M.; Chua, K.Y.; Cheong, N.; et al. IgE cross-reactivity between Ascaris and domestic mite allergens: The role of tropomyosin and the nematode polyprotein ABA-1. Allergy 2009, 64, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.B.; Amor, A.L.M.; Santos, L.N.; Galvao, A.A.; Oviedo Vera, A.V.; Silva, E.S.; Barbosa, C.G.; Goncalves, M.S.; Cooper, P.J.; Figueiredo, C.A.; et al. Risk factors for Toxocara spp. seroprevalence and its association with atopy and asthma phenotypes in school-age children in a small town and semi-rural areas of Northeast Brazil. Acta Trop. 2017, 174, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Santiago, H.C.; Bennuru, S.; Boyd, A.; Eberhard, M.; Nutman, T.B. Structural and immunologic cross-reactivity among filarial and mite tropomyosin: Implications for the hygiene hypothesis. J. Allergy Clin. Immunol. 2011, 127, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, L.; Coronado, S. Parasite allergens. Mol. Immunol. 2018, 100, 113–119. [Google Scholar] [CrossRef]
- Shen, C.Y.; Tsai, J.J.; Liao, E.C. Cross-reactivity of sIgE to mite and shrimp induced allergies in different age groups and clinical profiles of shrimp sIgE in vegetarians. Sci. Rep. 2019, 9, 12548. [Google Scholar] [CrossRef]
- Aalberse, R.C. Allergens from mites: Implications of cross-reactivity between invertebrate antigens. Allergy 1998, 53, 47–48. [Google Scholar] [CrossRef]
- Sidenius, K.E.; Hallas, T.E.; Poulsen, L.K.; Mosbech, H. Allergen cross-reactivity between house-dust mites and other invertebrates. Allergy 2001, 56, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Amoah, A.S.; Boakye, D.A.; Yazdanbakhsh, M.; van Ree, R. Influence of Parasitic Worm Infections on Allergy Diagnosis in Sub-Saharan Africa. Curr. Allergy Asthma Rep. 2017, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Santiago Hda, C.; Ribeiro-Gomes, F.L.; Bennuru, S.; Nutman, T.B. Helminth infection alters IgE responses to allergens structurally related to parasite proteins. J. Immunol. 2015, 194, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Mueller, G.A.; Glesner, J.; Daniel, J.L.; Zhang, J.; Hyduke, N.; Richardson, C.M.; DeRose, E.F.; Chapman, M.D.; Peebles, R.S., Jr.; Smith, A.S.; et al. Mapping Human Monoclonal IgE Epitopes on the Major Dust Mite Allergen Der p 2. J. Immunol. 2020, 205, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Khatri, K.; Richardson, C.M.; Glesner, J.; Kapingidza, A.B.; Mueller, G.A.; Zhang, J.; Dolamore, C.; Vailes, L.D.; Wunschmann, S.; Peebles, R.S., Jr.; et al. Human IgE monoclonal antibody recognition of mite allergen Der p 2 defines structural basis of an epitope for IgE cross-linking and anaphylaxis in vivo. PNAS Nexus 2022, 1, pgac054. [Google Scholar] [CrossRef] [PubMed]
- Glesner, J.; Vailes, L.D.; Schlachter, C.; Mank, N.; Minor, W.; Osinski, T.; Chruszcz, M.; Chapman, M.D.; Pomes, A. Antigenic Determinants of Der p 1: Specificity and Cross-Reactivity Associated with IgE Antibody Recognition. J. Immunol. 2017, 198, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Glesner, J.; Kapingidza, A.B.; Godzwon, M.; Offermann, L.R.; Mueller, G.A.; DeRose, E.F.; Wright, P.; Richardson, C.M.; Woodfolk, J.A.; Vailes, L.D.; et al. A Human IgE Antibody Binding Site on Der p 2 for the Design of a Recombinant Allergen for Immunotherapy. J. Immunol. 2019, 203, 2545–2556. [Google Scholar] [CrossRef] [PubMed]
- Chruszcz, M.; Pomes, A.; Glesner, J.; Vailes, L.D.; Osinski, T.; Porebski, P.J.; Majorek, K.A.; Heymann, P.W.; Platts-Mills, T.A.; Minor, W.; et al. Molecular determinants for antibody binding on group 1 house dust mite allergens. J. Biol. Chem. 2012, 287, 7388–7398. [Google Scholar] [CrossRef]
- Garcia Alonso, M.; Caballero, M.L.; Umpierrez, A.; Lluch-Bernal, M.; Knaute, T.; Rodriguez-Perez, R. Relationships between T cell and IgE/IgG4 epitopes of the Anisakis simplex major allergen Ani s 1. Clin. Exp. Allergy 2015, 45, 994–1005. [Google Scholar] [CrossRef]
- McClain, S. Bioinformatic screening and detection of allergen cross-reactive IgE-binding epitopes. Mol. Nutr. Food Res. 2017, 61, 1600676. [Google Scholar] [CrossRef]
- Hayes, M.; Rougé, P.; Barre, A.; Herouet-Guicheney, C.; Roggen, E.L. In silico tools for exploring potential human allergy to proteins. Drug Discov. Today Dis. Models 2015, 17–18, 3–11. [Google Scholar] [CrossRef]
- Mari, A.; Rasi, C.; Palazzo, P.; Scala, E. Allergen databases: Current status and perspectives. Curr. Allergy Asthma Rep. 2009, 9, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Wasmuth, E.V.; Lima, C.D. The Rrp6 C-terminal domain binds RNA and activates the nuclear RNA exosome. Nucleic Acids Res. 2017, 45, 846–860. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Attwood, T.K.; Babbitt, P.C.; Bateman, A.; Bork, P.; Bridge, A.J.; Chang, H.Y.; Dosztanyi, Z.; El-Gebali, S.; Fraser, M.; et al. InterPro in 2017-beyond protein family and domain annotations. Nucleic Acids Res. 2017, 45, D190–D199. [Google Scholar] [CrossRef] [PubMed]
- Walton, S.F.; Slender, A.; Pizutto, S.; Mounsey, K.E.; Oprescu, F.; Thomas, W.R.; Hales, B.J.; Currie, B.J. Analysis of IgE binding patterns to house dust mite allergens in scabies-endemic communities: Insights for both diseases. Clin. Exp. Allergy 2016, 46, 508. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Caldas, E.; Puerta, L.; Caraballo, L.; Lockey, R.F. Mite allergens. Clin. Allergy Immunol. 2008, 21, 161–182. [Google Scholar] [PubMed]
- Bessot, J.C.; Pauli, G. Mite allergens: An overview. Eur. Ann. Allergy Clin. Immunol. 2011, 43, 141–156. [Google Scholar]
- Cui, Y. Structural biology of mite allergens. Mol. Biol. Rep. 2013, 40, 681–686. [Google Scholar] [CrossRef] [PubMed]
- UniProt, C. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef]
- Mitchell, A.L.; Attwood, T.K.; Babbitt, P.C.; Blum, M.; Bork, P.; Bridge, A.; Brown, S.D.; Chang, H.Y.; El-Gebali, S.; Fraser, M.I.; et al. InterPro in 2019: Improving coverage, classification and access to protein sequence annotations. Nucleic Acids Res. 2019, 47, D351–D360. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.Q.; Junqueira, A.C.; Abellana, R.; Barrio, P.C.; Terrazas, W.C.; Sodre, F.C.; Boia, M.N.; Ascaso, C. Prevalence of intestinal parasites and risk factors forspecific and multiple helminth infections in a remote city of the Brazilian Amazon. Rev. Soc. Bras. Med. Trop. 2016, 49, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Chammartin, F.; Guimaraes, L.H.; Scholte, R.G.; Bavia, M.E.; Utzinger, J.; Vounatsou, P. Spatio-temporal distribution of soil-transmitted helminth infections in Brazil. Parasit. Vectors 2014, 7, 440. [Google Scholar] [CrossRef] [PubMed]
- Casavechia, M.T.; Lonardoni, M.V.; Venazzi, E.A.; Campanerut-Sa, P.A.; da Costa Benalia, H.R.; Mattiello, M.F.; Menechini, P.V.; Dos Santos, C.A.; Teixeira, J.J. Prevalence and predictors associated with intestinal infections by protozoa and helminths in southern Brazil. Parasitol. Res. 2016, 115, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Combet, C.; Blanchet, C.; Geourjon, C.; Deleage, G. NPS@: Network protein sequence analysis. Trends Biochem. Sci. 2000, 25, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Notredame, C.; Higgins, D.G.; Heringa, J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 2000, 302, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef] [PubMed]
- Lang-Yona, N.; Shuster-Meiseles, T.; Mazar, Y.; Yarden, O.; Rudich, Y. Impact of urban air pollution on the allergenicity of Aspergillus fumigatus conidia: Outdoor exposure study supported by laboratory experiments. Sci. Total Environ. 2016, 541, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.; Carlsson, M.C.; Madsen, C.B.; Brand, S.; Moller, S.R.; Olsen, C.E.; Vakhrushev, S.Y.; Brimnes, J.; Wurtzen, P.A.; Ipsen, H.; et al. Glycoproteomic analysis of seven major allergenic proteins reveals novel post-translational modifications. Mol. Cell Proteom. 2015, 14, 191–204. [Google Scholar] [CrossRef]
- de Castro, E.; Sigrist, C.J.; Gattiker, A.; Bulliard, V.; Langendijk-Genevaux, P.S.; Gasteiger, E.; Bairoch, A.; Hulo, N. ScanProsite: Detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 2006, 34, W362–W365. [Google Scholar] [CrossRef]
- Capra, J.A.; Singh, M. Predicting functionally important residues from sequence conservation. Bioinformatics 2007, 23, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; DiMaio, F.; Wang, R.Y.; Kim, D.; Miles, C.; Brunette, T.; Thompson, J.; Baker, D. High-resolution comparative modeling with RosettaCM. Structure 2013, 21, 1735–1742. [Google Scholar] [CrossRef]
- Kleffner, R.; Flatten, J.; Leaver-Fay, A.; Baker, D.; Siegel, J.B.; Khatib, F.; Cooper, S. Foldit Standalone: A video game-derived protein structure manipulation interface using Rosetta. Bioinformatics 2017, 33, 2765–2767. [Google Scholar] [CrossRef]
- Benkert, P.; Tosatto, S.C.; Schomburg, D. QMEAN: A comprehensive scoring function for model quality assessment. Proteins 2008, 71, 261–277. [Google Scholar] [CrossRef]
- Wang, S.; Ma, J.; Peng, J.; Xu, J. Protein structure alignment beyond spatial proximity. Sci. Rep. 2013, 3, 1448. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, J.; Bui, H.H.; Li, W.; Fusseder, N.; Bourne, P.E.; Sette, A.; Peters, B. ElliPro: A new structure-based tool for the prediction of antibody epitopes. BMC Bioinform. 2008, 9, 514. [Google Scholar] [CrossRef]
- Ansari, H.R.; Raghava, G.P. Identification of conformational B-cell Epitopes in an antigen from its primary sequence. Immunome Res. 2010, 6, 6. [Google Scholar] [CrossRef]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Pierce, B.G.; Wiehe, K.; Hwang, H.; Kim, B.H.; Vreven, T.; Weng, Z. ZDOCK server: Interactive docking prediction of protein-protein complexes and symmetric multimers. Bioinformatics 2014, 30, 1771–1773. [Google Scholar] [CrossRef]
- Papia, F.; Bellia, C.; Uasuf, C.G. Tropomyosin: A panallergen that causes a worldwide allergic problem. Allergy Asthma Proc. 2021, 42, e145–e151. [Google Scholar] [CrossRef]
- McKenna, O.E.; Asam, C.; Araujo, G.R.; Roulias, A.; Goulart, L.R.; Ferreira, F. How relevant is panallergen sensitization in the development of allergies? Pediatr. Allergy Immunol. 2016, 27, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.F.; Mendonca, T.N.; Melo, J.M.; Moreno, A.S.; Januario, Y.C.; DaSilva, L.L.; Dias, M.M.; Meireles, P.R.; Santos, K.S.; Yang, A.C.; et al. Reactions to Shrimp Including Severe Anaphylaxis in Mite- and Cockroach-Allergic Patients Who Have Never Eaten Shrimp: Clinical Significance of IgE Cross-Reactivity to Tropomyosins From Different Sources. J. Investig. Allergol. Clin. Immunol. 2019, 29, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Figo, D.D.; Cordeiro Macedo, P.R.; Gadermaier, G.; Remuzgo, C.; Castro, F.F.M.; Kalil, J.; Galvao, C.E.S.; Santos, K.S. IgE and IgG4 Epitopes of Dermatophagoides and Blomia Allergens before and after Sublingual Immunotherapy. Int. J. Mol. Sci. 2023, 24, 4173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.M.; Xiao, H.; Nowak-Wegrzyn, A.; Zhou, P. IgE-binding epitope mapping of tropomyosin allergen (Exo m 1) from Exopalaemon modestus, the freshwater Siberian prawn. Food Chem. 2020, 309, 125603. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, X.M.; Wen, Y.Q.; Zhao, J.L.; Xu, T.C.; Yong, L.; Lin, H.; Zhang, H.W.; Li, Z.X. Comparison of tropomyosin released peptide and epitope mapping after in vitro digestion from fish (Larimichthys crocea), shrimp (Litopenaeus vannamei) and clam (Ruditapes philippinarum) through SWATH-MS based proteomics. Food Chem. 2023, 403, 134314. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.Y.; Leung, N.Y.; Ho, M.H.; Gershwin, L.J.; Shu, S.A.; Leung, P.S.; Chu, K.H. Immunization with Hypoallergens of shrimp allergen tropomyosin inhibits shrimp tropomyosin specific IgE reactivity. PLoS ONE 2014, 9, e111649. [Google Scholar] [CrossRef] [PubMed]
- Takai, T.; Kato, T.; Yasueda, H.; Okumura, K.; Ogawa, H. Analysis of the structure and allergenicity of recombinant pro- and mature Der p 1 and Der f 1: Major conformational IgE epitopes blocked by prodomains. J. Allergy Clin. Immunol. 2005, 115, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; McKerrow, J. Erratum to “Cysteine proteases of parasitic organisms”: [Mol. Biochem. Parasitol. 120 (2002) 1–21]. Mol. Biochem. Parasitol. 2002, 121, 159. [Google Scholar] [CrossRef]
- Mueller, G.A.; Pedersen, L.C.; Glesner, J.; Edwards, L.L.; Zakzuk, J.; London, R.E.; Arruda, L.K.; Chapman, M.D.; Caraballo, L.; Pomes, A. Analysis of glutathione S-transferase allergen cross-reactivity in a North American population: Relevance for molecular diagnosis. J. Allergy Clin. Immunol. 2015, 136, 1369–1377. [Google Scholar] [CrossRef]
- Santiago, H.C.; LeeVan, E.; Bennuru, S.; Ribeiro-Gomes, F.; Mueller, E.; Wilson, M.; Wynn, T.; Garboczi, D.; Urban, J.; Mitre, E.; et al. Molecular mimicry between cockroach and helminth glutathione S-transferases promotes cross-reactivity and cross-sensitization. J. Allergy Clin. Immunol. 2012, 130, 248–256.e249. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).