Abstract
The prevalence of allergic rhinitis is rising, and it is impacting children’s growth and quality of life. To uncover unconventional treatment modalities, research was carried out to clarify the significance of novel components in the pathophysiology of allergic rhinitis. One of these elements was gut microbiota, which plays a crucial role in the development and evolution of allergic disorders. Specifically, dysbiosis, defined as impaired microbiota composition, characterizes allergic disorders. In light of this concept, probiotics (beneficial bacteria) may restore gut dysbiosis, rebalance the immune response, and indirectly influence the clinical course of allergic diseases. In this article, we discussed the role of the gut–lung axis in children and reported on new findings. We also reviewed the most relevant studies about probiotics in patients with allergic rhinitis.
Keywords:
allergic rhinitis; oral probiotics; children; allergy; treatment; prevention; microbiota; gut–lung axis 1. Introduction
Allergic rhinitis (AR) is a respiratory disease caused by an IgE-mediated inflammatory process mediated by one or more antigens (allergens) against which the subject is sensitized. The most common symptoms are rhinorrhea, sneezing, itching, nasal obstruction, and frequent conjunctivitis [1]. IgE antibodies are produced locally and in the lymphoid tissues in response to common environmental allergens. Mast cell degranulation occurs when allergens bind to specific IgE expressed over the cell surface. This results in the release of biochemical mediators including histamine, which represents the main factor in the acute allergic response [2]. A new AR classification considered the duration and severity of symptoms. According to it, AR can be subdivided into intermittent or persistent, based on the course of the symptoms, and into mild, moderate, or severe, according to the grade of clinical manifestations [3]. This classification evaluated the quality of life and the possible impact of rhinitis symptoms on the patient’s life, such as school activities and free time [1]. The diagnosis is based on the consistency between clinical history of allergic symptoms and documented sensitization (such as production of allergen-specific IgE). In other words, the exposure to sensitizing allergen causes symptoms’ occurrence. These symptoms can also negatively affect sleep quality, causing nocturnal awakenings. Diagnostic tests are aimed at demonstrating the in vivo and in vitro presence of allergen-specific IgE. The gold standard for IgE allergy testing is the skin prick test. A subsequent diagnostic step is the search for IgE antibodies directed toward specific allergenic molecules [4]. It is important to note that AR and asthma are due to type 2 inflammation of the airways, involving various cells, mainly eosinophils, mast cells, T lymphocytes, and their mediators [5]. Another important diagnostic tool can be the measurement of fractional exhaled nitric oxide, due to its ability to correlate with other parameters of airway inflammation, as demonstrated by the sharp and intense decrease in its levels when inhaled corticosteroids were administered [6,7]. The best AR management approach would be altogether avoiding the offending allergens. However, this is rarely achievable, and limited data support the efficacy of environmental control interventions. In addition, there is limited evidence that any single environmental control measure results in improved AR symptoms or other AR-related outcomes. Therefore, although allergen avoidance would be the optimal treatment of AR, the evidence supporting this approach needs to be more extensive. Several classes of effective allergy medication treat different AR symptoms [8]. Central roles are played by oral and/or intranasal H1 antihistamines, intranasal corticosteroids (INCS), and the fixed combination of INCS and H1 antihistamines [9]. Regarding the treatment of intermittent mild or intermittent AR, oral non-sedating second-generation H1 receptor antihistamine (AH) drugs are preferred over a first generation H1-antagonist, which is associated with more adverse effects, such as sedation, excessive mucosal drying, and impaired motor coordination. Conversely, for persistent moderate or severe symptoms of seasonal rhino-conjunctivitis, daily use of an intranasal corticosteroid (INCS) or an intranasal antihistamine (INAH) is the treatment of choice [10]. If an INCS, as the first choice for the treatment of SAR or PAR, is not sufficient to control symptoms, the addition of an INAH would likely be the most appropriate next option [11]. However, intranasal corticosteroids are more effective than antihistamines in controlling inflammatory events, such as nasal obstruction [12]. A combination product of fluticasone propionate plus azelastine HCl was demonstrated to have greater efficacy in reducing nasal symptoms of AR when compared with either drug alone [13]. One of the most studied and used topical corticosteroids is mometasone furoate nasal spray (MFNS). Considerable evidence supports the efficacy of MFSN, which also demonstrates a remarkable safety profile. Namely, MFNS was found to significantly reduce allergic inflammation following exposure to the allergen [14]. Montelukast, an LTD4 (leukotriene D) antagonist, may be another therapeutic strategy for selected patients with AR, but it is less effective than nasal corticosteroids. Despite good safety and tolerability, montelukast has limited efficacy for treating moderate or severe AR compared to oral antihistamines [15]. Patients with moderate to severe AR, especially if they have cross-linked allergy disorders, who do not control their symptoms with medical treatment, can be good candidates for allergen immunotherapy (AIT), which is the only available disease-modifying treatment for AR [16,17,18]. AIT reduces medication use and symptoms in patients with AR, thus improving the quality of life of these patients [19]. It is more often administrated sublingually (sublingual immunotherapy, SLIT) or subcutaneously (subcutaneous delivery, SCIT). Several clinical trials demonstrated that SLIT and SCIT were both efficient, but with a safety profile that favored SLIT [20,21]. Adverse reactions are rare and they are more often represented by local reactions, such as itching and swelling. Uncontrolled asthma, history of severe systemic reaction to immunotherapy, and eosinophilic esophagitis are the principal contraindications to AIT [16].
However, drugs used to treat AR may accompany adverse side effects (e.g., dry mouth, drowsiness, dizziness related to anti-H1 drugs). The use of probiotics as an additional option is increasing globally. The consumption of probiotics is expected to modulate immune responses in AR patients, reduce the damage caused by inflammation, and restore a balanced gut microbiota [22,23]. Gut microbiota is known to function as immunomodulator, barrier, and protective tool against infections [24]. It is constituted of more than a trillion microorganisms reunited in a complex and dynamic ecosystem, regulating the immune system and systemic physiology [25].
2. Gut–Lung Axis
The gut microbiota is connected with the respiratory tract: alterations in the quantitative composition, qualitative content (biodiversity) or the activity and function of gut microbiota, known as dysbiosis, can affect the immunity and microbiota of the lung and vice versa. This crosstalk is called the gut–lung axis. The lung is, in turn, connected with upper airways, according to the concept of ‘united airway disease’ [26]. The upper-lower airways link occurs due to anatomical, physiological, pathological, and immunological mechanisms, such as the common presence of ciliary epithelium, mucous glands, and the existence of the nose–pharyngeal–bronchial reflex [27]. This connection is essential to understand the link between the microbiota and bronchial and nasal hyperreactivity in healthy and diseased patients.
Probiotics are live microorganisms that, after oral administration, colonize the gastrointestinal tract with the goal of guaranteeing a health benefit to the host [28]. There are many probiotics, most of which can also be found naturally in the human body. They are classified into the following five species: the Lactobacillus group (e.g., L. reuteri RC-14), the Bifidobacterium group (e.g., B. bifidum), the Streptococcus group (e.g., S. fecalis), the Bacillus group (e.g., B. subtilis), and other organisms (e.g., non-pathogenic yeast Saccharomyces boulardii, Escherichia coli). They can help the respiratory, digestive, and immunological functions due to the ability to promote the maturation of the humoral responses, the IgA particularly, to improve the Th1 immune response and reduce Th2 cytokines, resulting in anti-inflammatory effects [29].
Oral probiotics can modulate the immune response of the respiratory system. They can contribute to treat, as add-on, and prevent respiratory diseases, such as asthma and AR by determining changes in gut microbiota and immune response [22]. Indeed, several studies indicated that probiotics could efficiently alleviate the symptoms of AR patients [30].
There have been promising developments in probiotics as adjuvant treatments for controlling nasal dysbiosis [31]. The use of probiotics was not only suggested to treat allergic diseases, but may be beneficial also for the immune response to viral respiratory infections, such as respiratory syncytial virus, rhinovirus [32], influenza virus [33,34], and severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). In particular, Coronavirus Disease 19 (COVID-19) is an infectious disease that affects mainly the respiratory system but also the gastrointestinal tract (GIT) [35]. The involvement of the GIT causes mild to severe symptoms, such as diarrhea, lack of appetite, abdominal pain, and vomiting [36]. During the infection, there is a reduction in biodiversity and richness of the gut microbiota, immune dysregulation, and prolonged infection may occur due to delayed SARS-CoV-2 clearance [37]. Due to the involvement of both respiratory and gastrointestinal systems and the relevant modifications that occur in local microbiota, therapies able to modulate the gut–lung axis and promote the eubiosis, such as probiotics, could be an important additional therapeutic strategy to fight COVID-19 infection [37,38].
To date, no relevant adverse events were observed for probiotic use; thus, probiotic use appears safe.
All these data demonstrate the importance and effectiveness of administering probiotics (as single strain or mixture) to modulate the gut and respiratory microbiota, thus improving prognosis and reducing symptoms in patients with allergic diseases and respiratory viral infections, such as COVID-19 (Figure 1).
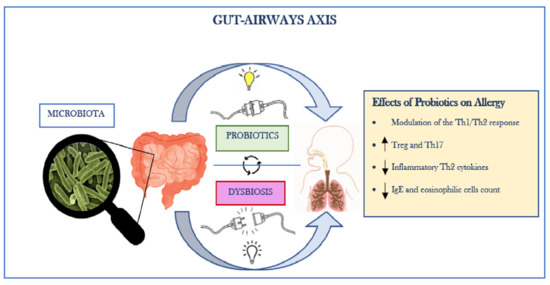
Figure 1.
The Gut–Airways Axis.
3. Probiotic Food
It is important to remember that food can also be an important source of probiotics. Dairy products, in particular yogurt, yogurt products, and milk, are excellent probiotic carriers [39]. Yogurt can be subdivided into two different classes: the standard culture yogurt, which is made with Lactobacillus bulgaricus and Streptococcus thermophilus, and bio- or probiotic yogurt, which is made by culturing additional microorganisms, generally Bifidobacteria and Lactobacillus acidophilus [40]. Many fermented food and beverages are another important source of probiotics, having several nutritional and therapeutic effects [41]. It is also possible to supplement many food products with probiotics, but their ability to deliver viable cells to the human gut may be different, because of the physical and chemical features of the food (e.g., pH, percentage of oxygen, presence of additives, titrable acidity), processing (e.g., fermentation conditions, cooling), storage (e.g., packaging materials), and microbiological parameters (e.g., strain probiotic chosen, inoculation) [42]. In the observational study conducted by Butler et al., the association between the intake of unpasteurised milk and dairy products for twelve weeks and the intestinal microbiota composition was evaluated. They enrolled twenty-four participants aged 18 to 65 years, with no chronic or current, mental or physical disease and collected their fecal samples at the beginning and end of the twelve weeks. They observed a significant increase in the presence of the genus Lactobacilli between the first and the twelfth weeks, thus demonstrating that dairy products can be a rich source of probiotic bacteria [43]. Ha-young Jeon et al. investigated the potential effects of the administration of a yogurt containing high-dose probiotics, such as Lactobacillus acidophilus and Streptococcus thermophilus, on viral respiratory infections such as influenza H1N1 and SARS-CoV-2 in an in vitro and in vivo experiment, using virus-infected animal models. They demonstrated that the administration of yogurt containing high-dose of probiotics could contribute to prevent and treat influenza H1N1 in a significant manner, reducing plaque formation in the virus-infected cells, and ameliorating the condition of influenza H1N1-infected mice. Unfortunately, the improvement effect for SARS-CoV-2 infection was less evident [44].
Commercial oral probiotic products are nowadays widely distributed, consumed and available, but there are still some concerns about their costs, efficacy, probiotic strain used, and treatment duration. It is important to remember that dietary intake has always played a major role in regulating intestinal microbiome composition and it can still represent a viable option to prevent or treat dysbiosis.
4. Probiotics and Allergic Rhinitis: Evidence and Challenges
In AR, drugs such as second-generation antihistamines or intranasal corticosteroids are prescribed for long-term control of symptoms [3]. Nevertheless, their long-term adverse effects could limit patients’ daily lives, causing drowsiness, gastrointestinal disorders, dry mouth, dizziness, headache, or infections. Moreover, these drugs’ effectiveness often depends on the time of the allergy’s onset. Therefore, the regular administration of probiotics seems to be a suitable therapeutic option because of its safety in long-term treatment regimens and because it also leads to clinical improvement in AR patients [45,46,47]. Overall, probiotic use appears safe, although a risk of infectious complications (e.g., bacteremia, endocarditis, sepsis) has been described in the literature [48,49,50]. Virulence appears to differ by species, in particular, the Lactobacillus (e.g., rhamnosus, acidophilus) and Bacillus species seem to be the most dangerous. Sepsis following probiotic usage was mostly reported in immune-deficient/malnourished patients, with important comorbidities (e.g., HIV, diabetes) [48]. Some individuals had extensive ulcerations of the mucosa of the GIT, congenital heart diseases, or had undergone antitumor chemotherapies or ionizing radiation [49]. Most patients were also treated with broad spectrum antibiotics and covered with probiotics to prevent/treat the diarrhea which often follows [50]. Therefore, since such complications were just reported sporadically, and because of their proven utility, probiotics’ use in AR seems reasonable.
5. Lactobacilli
Lactobacillus species belong to the so-called lactic acid bacteria (LAB), which also produce bacteriocins and competitively exclude potential pathogens [45]. Therefore, Lactobacilli have received considerable attention as potentially useful probiotics. Recent clinical and animal studies supported the idea that Lactobacilli, particularly some selected strains, can modify the host immune responses, leading to beneficial effects against allergic diseases [51,52,53,54]. Lactobacilli modulate the immune system by increasing Th1 cytokines [55], lowering Th2/Th1 ratios [56] or diminishing Th2 cytokines [45,57,58], and reducing IgE production and cell migration. Some strains reduce allergic nasal symptoms. For instance, some Lactobacillus species in particular (e.g., L. acidophilus) lead to nasal and ocular symptom relief, improvement of quality of life, and more extended periods of free-from-disease in children and adults suffering from AR [45]. A large variety of Lactobacillus strains exist. However, this review considered the strains that have demonstrated evidence of benefit.
L. casei (LC) is one of the most studied strains of the Lactobacillus species, not just in allergic diseases, but also in gastrointestinal disorders, as it survives the gastrointestinal tract and modulates its microbioma. Many researchers studied the application of LC. In a randomized control trial (RCT), the authors investigated the role of daily assumption of LC after one year of treatment on patients with AR. At the end of the study period, they found that children in the intervention group had fewer annual AR episodes and 33% lower occurrence of rhinitis symptoms (twice lower during the second quarter of intervention). They concluded that long-term LC consumption might improve children’s AR [59].
Additionally, L. paracasei (LP) was reported to improve the quality of life of adolescents with perennial AR, and to represent a valuable add-on option [47,60,61]. In a RCT study by Peng et al., the effects of LP 33 on AR induced by house dust mites were tested on 90 patients randomized into three treatment groups: group A was treated with the live LP33; group B with the heat-killed LP33, and group C with placebo. After 30 days, compared with the placebo group, groups A and B significantly improved their overall quality of life. There was no significant difference in the efficacy of the heat-killed LP33 compared to the live variant, supporting the notion that allergic patients could be treated with heat-killed strains instead of live variants. Notably, no side effects were reported [47]. Even though LP 33 was shown to be equally effective as cetirizine in AR children [62,63,64,65,66], its use was recommended chiefly in association with antihistamine drugs. In support of this concept, in a double-blind RCT, L. paracasei (HF.A00232) was studied as a supplementary agent to levocetirizine in children with perennial AR. Sixty patients (6–13 years old) were randomized into two groups: one receiving levocetirizine plus placebo and the other receiving regular levocetirizine plus LP (HF.A00232) for the first eight weeks, with a shift to levocetirizine as rescue treatment during the following four weeks. Clinical parameters were recorded, and physical examination and Pediatric Rhinoconjunctivitis Quality of Life Questionnaires (PRQLQs) were administered at each visit. The probiotic-treated group experienced a significant improvement in symptoms (sneezing, itchy nose, and swollen eyes), and showed significantly lower PRQLQ scores even after discontinuing regular antihistaminic use. No significant differences in cytokine levels were found between the two groups. The researchers did not observe any add-on effect of LP (HF.A00232) as a supplement to levocetirizine in managing AR in the first eight weeks. By contrast, the subgroup of probiotic-treated who did not discontinue levocetirizine use and also used more rescue levocetirizine in the following period had progressively lower PRQLQ scores in the latter part of the study. Such improvement did not occur in the other subgroup. This result can be explained as the synergistic effect of LP (HF.A00232) and levocetirizine, which implied an approximately 56% reduction in levocetirizine use [61].
The supplementation of Lactobacillus salivarius (LS) strains induced a significant increase in IL-10, which acted as an immunomodulator with anti-inflammatory effects [67,68]. In a double-blind RCT conducted by a Taiwanese group, 199 children (6–12 years old) with AR and house dust mite sensitization were randomized into two groups: one treated with placebo and the other with LS PM-A0006. They were followed for three months. LS reduced symptoms (nasal and eye symptoms) and medication scores compared with the control. Interestingly, no difference was found in specific immune and blood parameters between the probiotic and placebo group. This result was consistent with previous studies [69,70,71,72].
L. helveticus (LH) was examined in the study of Tamashita and co-workers. These authors demonstrated that LH2171 decreased the eosinophil counts in patients with symptomatic perennial AR. In addition, the LH2171-treated group experienced a significant clinical improvement, mainly concerning the stuffy nose, compared to the placebo group [73].
It was demonstrated that L. reuteri (LR) can also influence the immune system. LR CCFM1040, in particular, modulated type 2 inflammation and gut microbiota [74,75]. The mechanism through which it worked included modulating gut microbiota and metabolizing endogenous tryptophan to balance systemic and mucosal immune reactivity, thereby inhibiting airway inflammation [76,77,78]. In a recent RCT study, the supplementation with CCFM1040 decreased total symptom score (TSS), RQLQ, nasal congestion, watery eyes, rhinorrhoea, and sleep quality, and significantly improved eye symptoms in patients with AR. No difference in the blood and urine parameters and adverse effects were observed [79].
Most of the studies concerning L. plantarum, gasseri, and rhamnosus were carried out in animal models. Only a few studied the use of these strains in AR patients. Some were, in fact, conducted on mice, as in an experimental study carried out by Choi et al.; they demonstrated that the oral administration of Lactobacillus plantarum CJLP133 and CJLP243 in mice alleviated the symptoms of birch pollen (BP)-induced AR by reducing airway hyperresponsiveness, the histological scores, and the number of infiltrated cells in the nasal cavity and lungs. This probiotic mixture also restored the Th1/Th2 balance by enhancing the type 1 immune response [80]. Other studies were conducted on guinea pigs. A Japanese study investigated antigen-sensitized animals to demonstrate the improvement of nasal blockage measuring nasal airway resistance [81]. They proved that the oral administration of L. rhamnosus GG (LGG) and L. gasseri TMC0356 (LG TMC0356) significantly ameliorated the antigen-induced nasal blockage. Surprisingly, oral administration of LGG and TMC0356 did not substantially change the levels of serum antigen-specific IgG1, IgG2, and IgE or the numbers of inflammatory cells from nasal lavage fluid (NCLF) [54]. Other strains of LG were studied for their possible involvement in AR treatment. In a clinical trial conducted by Chen et al., 105 patients with asthma and AR were divided into two groups: one was treated with the LG strain A5 and the other was treated with placebo. After eight weeks, the airway function, clinical symptoms, and immunoregulatory cytokine production improved significantly in the probiotic group compared with the placebo group [82]. Some LG strains were also evaluated in association with other Lactobacilli. For example, it was proved that the daily assumption of L. coryniformis (LC) CECT5711 and LG CECT5714 in fermented products could modulate immunological parameters in healthy adults and children [83,84,85,86]. A Spanish randomized double-blinded trial conducted on children suffering from allergic rhinitis demonstrated that the consumption of products containing LG CECT5714 and LC CECT5711 reduced the plasma level of IgE and increased T-regulatory cells [58]. In addition, the administration of LP NCC 2461 resulted in beneficial effects in subjects with AR to grass pollen [87,88].
However, other studies did not show significant effects. Ouwenhand et al. observed that AR patients treated with L. acidophilus NCFMTM had a reduction in nasal eosinophil infiltration, but symptom severity did not significantly change [89]. Another study tested the effects of 8-week LA NCC 2461 supplementation in AR patients with grass allergy [90]. The probiotic administration did not significantly improve quality of life, IgE levels, total nasal symptom score (TNSS), total ocular symptom score (TOSS), and drug use. Finally, a Finnish RCT evaluated respiratory and eye symptoms and medication use in patients treated with Lactobacillus rhamnosus (LR) or placebo [91]. The 5.5-month treatment did not affect any clinical parameter (Table 1).

Table 1.
RCTs on Lactobacilli in children with AR.
6. Bifidobacteria
In the human gastrointestinal tract, Bifidobacteria are the dominant bacterial population. Bifidobacteria have an anti-bacterial function, protecting the organism from the proliferation of pathogenic bacteria, such as Helicobacter pylori [92]. Additionally, they are involved in the production of short-chain fatty acids and vitamins, and in the regulation of the immune response in allergic, autoimmune, and inflammatory bowel diseases [92]. Bifidobacterium bifidum, B. longum, B. infantis, and B. breve are the most prevalent species in breastfed infants. B. adolescentis, B. animalis, and B. lactis appear later [93].
It was shown that Bifidobacterium longum, alone and in association with Lactobacillus plantarum, alleviated AR symptoms and restored Th2/Treg balance in mice [94]. Similar results were observed with the supplementation of B. breve at 107 CFU or higher [95] and B. bifidum [96]. In humans, B longum supplementation significantly reduced nasal allergic symptoms and Th2-polarized immune response [71]. Bifidobacteria strains were frequently reduced in atopic children [78] and adults [79], and the BB12 strain, in particular, showed anti-Th2 properties by dampening allergic inflammation [80].
In a study performed by Di Pierro et al., pollen-allergic children aged 2–14 years were randomly assigned to three groups: untreated, preventive, and treated arm. The prophylactic and treated groups assumed a probiotic mixture, containing Bifidobacterium animalis subsp. lactis BB12, and Enterococcus faecium L3, for three months before the pollen season or during the pollen season, respectively [97]. The BB12 and L3 strains significantly decreased rhinitis symptoms, watery eyes, and cough/wheezing in the prophylactic group compared to the control arm. However, when the mixture was administered during the pollen season, there was lower efficacy. In addition, medication use was reduced. In another recent study, children with seasonal AR, aged between 4–17 years, were randomly assigned to two groups: placebo-treated and actively-treated with a supplementation containing a Bifidobacteria mixture (B. longum BB536, B. infantis M-63, and B. breve M-16 V) [98]. After two months, children who received the probiotic mixture showed a substantial improvement in symptoms and quality of life, while the use of rescue medications overlapped in the two groups.
7. Enterococci
Enterococci are among the first bacterial colonists after birth and can survive in large and small intestines. One of the strains with the highest relevance is Enterococcus faecium (EF), which is mainly used to contrast pathogenic intestinal bacteria and boost the effectiveness of other probiotic strains. E. faecium modulates the type 2 inflammation, as evidenced by ex vivo studies, and alleviated nasal symptoms and eosinophilia in mouse models [99,100]. In addition, Enterococcus faecium L3 (L3) promotes the preservation of endogenous colonic Bifidobacteria in children [101]. Regarding its potential use in AR, it was demonstrated that when administered as prophylactic treatment in AR patients, L3 strains significantly reduced the development of nasal, ocular, and bronchial symptoms [74]. A RCT study provided confirming results [102]. This trial included 250 patients (6 to 17 years old) affected by AR; they were randomly divided into an intervention group (treated with a daily oral administration of a probiotic mixture containing BB12 DSM 15,954 and EF L3 LMG P-27496 strain), and a placebo group. Treatment was administered during the three months preceding the typical onset of the symptoms. Only 203 children completed the study. At the end of the study, the nasal symptoms score (NSS) was significantly improved in the intervention group, and the intake of medications (oral antihistamines and local corticosteroids) was significantly reduced.
8. Saccharomyces
A Chinese RCT enrolled 90 children with AR to evaluate the efficacy of the combination of Saccharomyces Boulardii (SB) and cetirizine, compared to the use of levocetirizine only. Serum IFN-γ and interleukin-4 (IL-4) levels were measured. Thirty non-AR children were then enrolled as the healthy control group. The study was carried out for four weeks. Before the treatment, serum IFN-γ levels were significantly lower in allergic subjects compared to the healthy group. In contrast, IL-4 was significantly higher in the two allergic groups than in the healthy group. At the end of the study period, the symptom scores of the two allergic groups were significantly reduced. The observational group showed indeed significantly lower nasal congestion, sneezing, nasal itching, and runny nose as compared to the control group. Additionally, INF-γ levels were considerably lower and IL-4 significantly higher in the observational group than in the control group [103].
9. Butyric Acid Producing Bacteria
Butyric acid producing bacteria (BAPD) belong to the Gram-positive Firmicute phylum. The most prevalent species are Eubacterium rectale/Roseburia spp. and Faecalibacterium prausnitzii. The functions of these bacteria in the gut and their impact on health are currently being uncovered [104]. Butyric acid’s anti-inflammatory benefits are widely known. A plausible reason is the inhibition of deacetylase activity, which leads to hyperacetylation of histones and, as a result, suppression of nuclear factor-kappa B activation [105]. Decreases in members of BAPD have been reproducibly reported in the gut of intestinal bowel diseases (IBD) patients [106]. It was demonstrated that BAPD administration had a beneficial effect in IBD patients’ inflamed intestinal mucosa [107]. Regarding allergic diseases, high levels of butyrate in early life were associated with protection against atopy [108]. The latest studies on the gut microbiota in children with allergic diseases supported the hypothesis that dysbiosis characterized by fewer BAPD leads to fewer regulatory T cells, resulting in cow milk protein allergy, food allergy, and asthma [109]. Therefore, BAPD supplementation alone or in combination with other probiotics could represent a new dietary option for infants and children with allergic diseases [110]. However, the majority of research was conducted on mice. The therapeutic and preventative role of BAPD may be uncovered by implementing additional research in humans in the near future.
10. The Role of Probiotics in the Prevention of Allergy during Pregnancy
The World Allergy Organization supports probiotic supplementation in pregnant women and infants at high risk of allergy [111]. In this regard, it is important to note that during pregnancy, there is an increase in the bacterial load and alterations in the maternal gut microbiota, such as the major representation of Actinobacteria and Proteobacteria and reduced the presence of Faecalibacterium and other short-chain fatty-acid producers [112,113]. These changes in maternal gut microbiota may have consequences in terms of immunity, health, and growth of the fetus [114]. It is known that maternal microbiota has a role in shaping the offspring’s immune system in terms of immune gene expression and the number of innate immunity cells [115]. Furthermore, many studies showed the role of microbial exposure during pregnancy in preventing allergic disease in the offspring [116]. Creating an appropriate intestinal microbiota in neonates is crucial for guaranteeing them protection from enteric pathogens and local and systemic inflammation. This process is influenced by the infant’s diet, maternal microbiome, and environment. Pregnancy and the period from birth to 24 months (B-24) are sensitive windows during which diet has a powerful influence on the life trajectory of health [117]. A recent analysis of four randomized, double-blind, placebo-controlled clinical trials found that administration of perinatal L. rhamnosus was associated with a decrease in allergic disease in infants with no safety concern [118]. In this regard, a meta-analysis performed by Zuccotti et al. suggested that the administration of probiotics during pregnancy prevented atopic dermatitis in children [64]. Accordingly, Bertelsen et al. showed that probiotic Lactobacilli and Bifidobacteria during pregnancy decreased the incidence of atopic dermatitis and rhinoconjunctivitis in children [119]. Another meta-analysis of seventeen randomized controlled trials performed by Du et al. demonstrated that supplementation with probiotics in pre- and postnatal periods successfully prevented asthma, but the effects depended on the type of probiotic mixture used [120]. It is important to note that probiotic supplementation may also have a protective role against preeclampsia, vaginal infections, gestational diabetes, later childhood disease, and maternal and infant weight gain [121]. These data provide compelling evidence that the maternal microbiome influences the infant microbiome, which subsequently affects childhood health, and that the administration of probiotics during pregnancy, lactation, and postnatal life could be a safe and effective strategy to modify both the maternal and neonatal microbiota, thus improving pregnancy and neonatal outcomes [122]. On the other hand, some studies reported discordant results on the benefit of the use of probiotics in pregnancy, possibly due to the use of different strains of probiotics, study period, other methods of administration and follow-ups. In this regard, a randomized study by Boyle et al. recruited 250 pregnant women carrying infants at high risk of allergy disease. They administered to 125 women a probiotic supplementation with Lactobacillus GG each morning for thirty-six weeks of gestation until delivery, and to the other 125 women, they administered a maltodextrin placebo. They found no evidence that prenatal treatment with LGG prevented eczema [123]. A study by Simpson et al. recruited 415 pregnant women. They were randomized in a double-blind study to receive probiotic milk or placebo from thirty-six weeks of gestation until three months postpartum. The probiotic milk contained Lactobacillus rhamnosos GG, L. acidophilus La-5, and Bifidobacterium animalis subsp. Lactis Bb-12. Afterwards, they evaluated their children through clinical examinations and family questionnaires. The results suggested that there was no significant reduction in the prevalence of asthma, atopic sensitization, and allergic rhinoconjunctivitis, but only reduction in atopic dermatitis [124].
11. Conclusions
Most human studies showed that, compared to a placebo, probiotics alone or in combination with antihistamines can alleviate allergic symptoms and reduce the frequency and duration of AR episodes in the pediatric population [59,61,66,72,82,89]. In addition, there were no noticeable adverse reactions. However, most studies did not detect significant differences in immunological parameters and blood eosinophil count between the active and control groups.
It must be underlined that the duration of treatments, measure variables, strains employed, and clinical and functional characteristics of participants considerably differed across investigations. Most of the studies investigated Lactobacillus species and their modulatory effects on immunologic parameters in allergic disorders. By reviewing the literature, we found that no strain has emerged as the most effective as their effects seem to be strain-specific.
There has yet to be an agreement on the best Lactobacillus candidate to be used in AR human trials [45,79]. Although most of the studies proved the efficacy of probiotics in AR treatment, there are studies where their assumption did not show significant effects [58,89,90,91,125]. Therefore, their use is still controversial. Although it is currently exactly unknown how lactic acid bacteria affect the immune system, prevent the onset of allergies, or alleviate allergic symptoms. At present, the International Consensus Statement on Allergy and Rhinology reads: “Allergic Rhinitis: recommended to consider probiotics as adjuvant therapy, such as add-on, for patients with AR thanks to their ability to alleviate symptoms and enhance the quality of life without causing adverse effects” [126].
Author Contributions
A.K. drafted the sections on probiotics and allergy, conclusions, Figure 1, and Table 1. A.S. drafted the section on probiotics and allergy and Table 1. G.D. carried out the methodology and revision. C.I. and F.D. supervised the study. M.C. drafted the section on the Introduction, Gut-Lung Axis, Allergic Rhinitis, Probiotic Food, and Pregnancy. S.V. drafted the section on the Introduction, Allergic Rhinitis, and Pregnancy. G.C. was responsible for the revision. M.M.d.G. was responsible for the conceptualization, revision, and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Del Giudice, M.M.; Marseglia, A.; Leonardi, S.; La Rosa, M.; Salpietro, C.; Brunese, F.; Arrigo, T.; Perrone, L. Allergic Rhinitis and Quality of Life in Children. Int. J. Immunopathol. Pharmacol. 2011, 24 (Suppl. 4), 25–28. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, M.M.; Allegorico, A.; Marseglia, G.L.; Martelli, A.; Calvani, M.; Cardinale, F.; Duse, M.; Chiappini, E.; Manti, S.; Cravidi, C.; et al. Allergic Rhinoconjunctivitis. Acta Bio Medica Atenei Parm. 2020, 91 (Suppl. 11), 1–3. [Google Scholar] [CrossRef]
- Brożek, J.L.; Bousquet, J.; Baena-Cagnani, C.E.; Bonini, S.; Canonica, G.W.; Casale, T.B.; van Wijk, R.G.; Ohta, K.; Zuberbier, T.; Schünemann, H.J. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 Revision. J. Allergy Clin. Immunol. 2010, 126, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, F.; Mastrorilli, C.; Tripodi, S.; Ricci, G.; Perna, S.; Panetta, V.; Asero, R.; Dondi, A.; Bianchi, A.; Maiello, N.; et al. Diagnostic relevance of IgE sensitization profiles to eight recombinant Phleum pratense molecules. Allergy 2017, 73, 673–682. [Google Scholar] [CrossRef]
- Kay, A. Asthma and inflammation. J. Allergy Clin. Immunol. 1991, 87, 893–910. [Google Scholar] [CrossRef]
- Lanz, M.J.; Leung, D.Y.; White, C.W. Comparison of exhaled nitric oxide to spirometry during emergency treatment of asthma exacerbations with glucocorticoids in children. Ann. Allergy Asthma Immunol. 1999, 82, 161–164. [Google Scholar] [CrossRef]
- Kharitonov, S.A.; Yates, D.H.; Barnes, P.J. Inhaled glucocorticoids decrease nitric oxide in exhaled air of asthmatic patients. Am. J. Respir. Crit. Care Med. 1996, 153, 454–457. [Google Scholar] [CrossRef]
- Cox, L. Approach to Patients with Allergic Rhinitis: Testing and Treatment. Med. Clin. N. Am. 2019, 104, 77–94. [Google Scholar] [CrossRef]
- Bousquet, J.; Schünemann, H.J.; Togias, A.; Bachert, C.; Erhola, M.; Hellings, P.W.; Klimek, L.; Pfaar, O.; Wallace, D.; Ansotegui, I.; et al. Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. J. Allergy Clin. Immunol. 2019, 145, 70–80.e3. [Google Scholar] [CrossRef]
- Bernstein, D.I.; Schwartz, G.; Bernstein, J.A. Allergic Rhinitis: Mechanisms and Treatment. Immunol. Allergy Clin. N. Am. 2016, 36, 261–278. [Google Scholar] [CrossRef]
- Bousquet, J.; Schunemann, H.J.; Fonseca, J.; Samolinski, B.; Bachert, C.; Canonica, G.W.; Casale, T.; Cruz, A.A.; Demoly, P.; Hellings, P.; et al. MACVIA-ARIA Sentinel NetworK for allergic rhinitis (MASK-rhinitis): The new generation guideline implementation. Allergy 2015, 70, 1372–1392. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Anto, J.M.; Bachert, C.; Baiardini, I.; Bosnic-Anticevich, S.; Canonica, G.W.; Melén, E.; Palomares, O.; Scadding, G.K.; Togias, A.; et al. Allergic rhinitis. Nat. Rev. Dis. Prim. 2020, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Carr, W.; Bernstein, J.; Lieberman, P.; Meltzer, E.; Bachert, C.; Price, D.; Munzel, U.; Bousquet, J. A novel intranasal therapy of azelastine with fluticasone for the treatment of allergic rhinitis. J. Allergy Clin. Immunol. 2012, 129, 1282–1289.e10. [Google Scholar] [CrossRef]
- Indolfi, C.; Dinardo, G.; Umano, G.R.; Klain, A.; Contieri, M.; Decimo, A.; Decimo, F.; Ciprandi, G.; Del Giudice, M.M. Mometasone furoate nasal spray in Italian children with seasonal allergic rhinitis: A comprehensive assessment. Allergol. Immunopathol. 2022, 50, 61–67. [Google Scholar] [CrossRef]
- Grainger, J.; Drake-Lee, A. Montelukast in allergic rhinitis: A systematic review and meta-analysis. Clin. Otolaryngol. 2006, 31, 360–367. [Google Scholar] [CrossRef]
- Tenero, L.; Vaia, R.; Ferrante, G.; Maule, M.; Venditto, L.; Piacentini, G.; Senna, G.; Caminati, M. Diagnosis and Management of Allergic Rhinitis in Asthmatic Children. J. Asthma Allergy 2023, 16, 45–57. [Google Scholar] [CrossRef]
- Lin, C.-F.; Lin, Y.-T.; Liao, C.-K.; Yeh, T.-H. Recent Updates of Immunotherapy for Allergic Rhinitis in Children. Curr. Otorhinolaryngol. Rep. 2023, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tosca, M.A.; Licari, A.; Olcese, R.; Castagnoli, R.; Marseglia, A.; Marseglia, G.L.; del Giudice, M.M.; Martelli, A.; Calvani, M.; Caffarelli, C.; et al. Allergen immunotherapy in children and adolescents with respiratory diseases. Acta Bio Medica Atenei Parm. 2020, 91 (Suppl. 11), 1–4. [Google Scholar] [CrossRef]
- Ponda, P.; Carr, T.; Rank, M.A.; Bousquet, J. Nonallergic Rhinitis, Allergic Rhinitis, and Immunotherapy: Advances in the Last Decade. J. Allergy Clin. Immunol. Pract. 2022, 11, 35–42. [Google Scholar] [CrossRef]
- Liu, W.; Zeng, Q.; He, C.; Chen, R.; Tang, Y.; Yan, S.; Luo, X.; Luo, R. Compliance, efficacy, and safety of subcutaneous and sublingual immunotherapy in children with allergic rhinitis. Pediatr. Allergy Immunol. 2020, 32, 86–91. [Google Scholar] [CrossRef]
- Del Giudice, M.M.; Licari, A.; Brambilla, I.; Tosca, M.; Ciprandi, G. Allergen Immunotherapy in Pediatric Asthma: A Pragmatic Point of View. Children 2020, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Hu, T.; Kang, C.; Liu, J.; Zhang, J.; Ran, H.; Zeng, X.; Qiu, S. Research Advances in the Treatment of Allergic Rhinitis by Probiotics. J. Asthma Allergy 2022, 15, 1413–1428. [Google Scholar] [CrossRef]
- Capponi, M.; Gori, A.; De Castro, G.; Ciprandi, G.; Anania, C.; Brindisi, G.; Tosca, M.; Cinicola, B.L.; Salvatori, A.; Loffredo, L.; et al. (R)Evolution in Allergic Rhinitis Add-On Therapy: From Probiotics to Postbiotics and Parabiotics. J. Clin. Med. 2022, 11, 5154. [Google Scholar] [CrossRef]
- Klain, A.; Dinardo, G.; Salvatori, A.; Indolfi, C.; Contieri, M.; Brindisi, G.; Decimo, F.; Zicari, A.M.; del Giudice, M.M. An Overview on the Primary Factors That Contribute to Non-Allergic Asthma in Children. J. Clin. Med. 2022, 11, 6567. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef]
- De Benedictis, F.; del Giudice, M.; Severini, S.; Bonifazi, F. Rhinitis, sinusitis and asthma: One linked airway disease. Paediatr. Respir. Rev. 2001, 2, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Klain, A.; Indolfi, C.; Dinardo, G.; Licari, A.; Cardinale, F.; Caffarelli, C.; Manti, S.; Ricci, G.; Pingitore, G.; Tosca, M.; et al. United Airway Disease. Acta Bio Medica Atenei Parm. 2021, 92 (Suppl 7), 2021526. [Google Scholar] [CrossRef]
- Probiotics in Food Health and Nutritional Properties and Guidelines for Evaluation FAO Food and Nutrition Paper. Available online: https://books.google.rs/books/about/Probiotics_in_Food.html?id=kNxxQgAACAAJ&redir_esc=y (accessed on 13 December 2022).
- Hajavi, J.; Esmaeili, S.; Varasteh, A.; Vazini, H.; Atabati, H.; Mardani, F.; Momtazi-Borojeni, A.A.; Hashemi, M.; Sankian, M.; Sahebkar, A. The immunomodulatory role of probiotics in allergy therapy. J. Cell. Physiol. 2018, 234, 2386–2398. [Google Scholar] [CrossRef]
- Farahmandi, K.; Mohr, A.E.; McFarland, L.V. Effects of Probiotics on Allergic Rhinitis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Am. J. Rhinol. Allergy 2022, 36, 440–450. [Google Scholar] [CrossRef]
- Luo, C.; Peng, S.; Li, M.; Ao, X.; Liu, Z. The Efficacy and Safety of Probiotics for Allergic Rhinitis: A Systematic Review and Meta-Analysis. Front. Immunol. 2022, 13, 848279. [Google Scholar] [CrossRef]
- Luoto, R.; Ruuskanen, O.; Waris, M.; Kalliomäki, M.; Salminen, S.; Isolauri, E. Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: A randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2014, 133, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Waki, N.; Matsumoto, M.; Fukui, Y.; Suganuma, H. Effects of probiotic Lactobacillus brevis KB290 on incidence of influenza infection among schoolchildren: An open-label pilot study. Lett. Appl. Microbiol. 2014, 59, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Namba, K.; Hatano, M.; Yaeshima, T.; Takase, M.; Suzuki, K. Effects of Bifidobacterium longum BB536 Administration on Influenza Infection, Influenza Vaccine Antibody Titer, and Cell-Mediated Immunity in the Elderly. Biosci. Biotechnol. Biochem. 2010, 74, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.S.; Hung, I.F.N.; Chan, P.P.Y.; Lung, K.C.; Tso, E.; Liu, R.; Ng, Y.Y.; Chu, M.Y.; Chung, T.W.H.; Tam, A.R.; et al. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples from a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology 2020, 159, 81–95. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, X.; Zhang, Z.; Huang, S.; Zhang, Z.; Fang, Z.; Gu, Z.; Gao, L.; Shi, H.; Mai, L.; et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020, 69, 997–1001. [Google Scholar] [CrossRef]
- Liu, Y.; Kuang, D.; Li, D.; Yang, J.; Yan, J.; Xia, Y.; Zhang, F.; Cao, H. Roles of the gut microbiota in severe SARS-CoV-2 infection. Cytokine Growth Factor Rev. 2022, 63, 98–107. [Google Scholar] [CrossRef]
- De Oliveira, G.L.V.; Oliveira, C.N.S.; Pinzan, C.F.; de Salis, L.V.V.; Cardoso, C.R.D.B. Microbiota Modulation of the Gut-Lung Axis in COVID-19. Front. Immunol. 2021, 12, 635471. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.; Kachrimanidou, V.; Bosnea, L.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Hadjimbei, E.; Botsaris, G.; Chrysostomou, S. Beneficial Effects of Yoghurts and Probiotic Fermented Milks and Their Functional Food Potential. Foods 2022, 11, 2691. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Sağiroğlu, A.; Özdemir, N.; Çon, A.H. Multifunctional Potentials of Lactic Acid Bacterial Isolates from Turkish Traditional Fermented Foods. Lett. Appl. Microbiol. 2023, 76, 1–14. [Google Scholar] [CrossRef]
- Butler, M.I.; Bastiaanssen, T.F.S.; Long-Smith, C.; Berding, K.; Morkl, S.; Cusack, A.-M.; Strain, C.; Busca, K.; Porteous-Allen, P.; Claesson, M.J.; et al. Recipe for a Healthy Gut: Intake of Unpasteurised Milk Is Associated with Increased Lactobacillus Abundance in the Human Gut Microbiome. Nutrients 2020, 12, 1468. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.-Y.; Kim, K.-S.; Kim, S. Effects of yogurt containing probiotics on respiratory virus infections: Influenza H1N1 and SARS-CoV-2. J. Dairy Sci. 2023, 106, 1549–1561. [Google Scholar] [CrossRef] [PubMed]
- Steiner, N.C.; Lorentz, A. Probiotic Potential of Lactobacillus Species in Allergic Rhinitis. Int. Arch. Allergy Immunol. 2021, 182, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Matsumoto, K.; Matsumoto, N.; Kobatake, E.; Kabuki, T. Anti-allergic effect of Lactobacillus helveticus SBT2171 on murine model of pollen allergy. Funct. Foods Health Dis. 2019, 9, 166. [Google Scholar] [CrossRef]
- Peng, G.-C.; Hsu, C.-H. The efficacy and safety of heat-killed Lactobacillus paracasei for treatment of perennial allergic rhinitis induced by house-dust mite. Pediatr. Allergy Immunol. 2005, 16, 433–438. [Google Scholar] [CrossRef]
- Haghighat, L.; Crum-Cianflone, N.F. The potential risks of probiotics among HIV-infected persons: Bacteraemia due to Lactobacillus acidophilus and review of the literature. Int. J. STD AIDS 2016, 27, 1223–1230. [Google Scholar] [CrossRef]
- Joshi, S.; Udani, S.; Sen, S.; Kirolikar, S.; Shetty, A. Bacillus Clausii Septicemia in a Pediatric Patient After Treatment with Probiotics. Pediatr. Infect. Dis. J. 2019, 38, e228–e230. [Google Scholar] [CrossRef]
- Vahabnezhad, E.; Mochon, A.B.; Wozniak, L.; Ziring, D.A. Lactobacillus Bacteremia Associated with Probiotic Use in a Pediatric Patient With Ulcerative Colitis. J. Clin. Gastroenterol. 2013, 47, 437–439. [Google Scholar] [CrossRef]
- Ishida, Y.; Nakamura, F.; Kanzato, H.; Sawada, D.; Hirata, H.; Nishimura, A.; Kajimoto, O.; Fujiwara, S. Clinical Effects of Lactobacillus acidophilus Strain L-92 on Perennial Allergic Rhinitis: A Double-Blind, Placebo-Controlled Study. J. Dairy Sci. 2005, 88, 527–533. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Salminen, S.; Arvilommi, H.; Kero, P.; Koskinen, P.; Isolauri, E. Probiotics in primary prevention of atopic disease: A randomised placebo-controlled trial. Lancet 2001, 357, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; He, F.; Kubota, A.; Hata, J.-Y.; Kobayakawa, S.-I.; Hiramatsu, M. Inhibitory Effect of Lactobacillus gasseri TMC0356 and Lactobacillus GG on Enhanced Vascular Permeability of Nasal Mucosa in Experimental Allergic Rhinitis of Rats. Biosci. Biotechnol. Biochem. 2006, 70, 3025–3030. [Google Scholar] [CrossRef]
- Kawase, M.; He, F.; Kubota, A.; Harata, G.; Hiramatsu, M. Orally Administrated Lactobacillus gasseri TMC0356 and Lactobacillus GG Alleviated Nasal Blockage of Guinea Pig with Allergic Rhinitis. Microbiol. Immunol. 2007, 51, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Majamaa, H.; Isolauri, E. Probiotics: A novel approach in the management of food allergy. J. Allergy Clin. Immunol. 1997, 99, 179–185. [Google Scholar] [CrossRef]
- Morita, H.; He, F.; Kawase, M.; Kubota, A.; Hiramatsu, M.; Kurisaki, J.-I.; Salminen, S. Preliminary Human Study for Possible Alteration of Serum Immunoglobulin E Production in Perennial Allergic Rhinitis with Fermented Milk Prepared with Lactobacillus gasseri TMC0356. Microbiol. Immunol. 2006, 50, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Nabe, T.; Mizutani, N.; Osaki, S.; Sugahara, S.; Takenaka, H.; Kohno, S. Comparison of Cedar Pollen-Induced Allergic Rhinitis in Passively and Actively Sensitized Guinea Pigs. Jpn. J. Pharmacol. 2001, 85, 409–415. [Google Scholar] [CrossRef]
- Martínez-Cañavate, A.; Sierra, S.; Lara-Villoslada, F.; Romero, J.; Maldonado, J.; Boza, J.; Xaus, J.; Olivares, M. A probiotic dairy product containing L. gasseri CECT5714 and L. coryniformis CECT5711 induces immunological changes in children suffering from allergy. Pediatr. Allergy Immunol. 2009, 20, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, M.; Agostoni, C.; Riva, E.; Salvini, F.; Ruscitto, A.; Zuccotti, G.V.; Radaelli, G. A Randomized Prospective Double Blind Controlled Trial on Effects of Long-Term Consumption of Fermented Milk Containing Lactobacillus casei in Pre-School Children with Allergic Asthma and/or Rhinitis. Pediatr. Res. 2007, 62, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.F.; Lin, H.C.; Wang, Y.Y.; Hsu, C.H. Treatment of perennial allergic rhinitis with lactic acid bacteria. Pediatr. Allergy Immunol. 2004, 15, 152–158. [Google Scholar] [CrossRef]
- Lin, W.-Y.; Fu, L.-S.; Lin, H.-K.; Shen, C.-Y.; Chen, Y.-J. Evaluation of the Effect of Lactobacillus paracasei (HF.A00232) in Children (6–13 years old) with Perennial Allergic Rhinitis: A 12-week, Double-blind, Randomized, Placebo-controlled Study. Pediatr. Neonatol. 2014, 55, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Vliagoftis, H.; Kouranos, V.; Betsi, G.I.; Falagas, M.E. Probiotics for the treatment of allergic rhinitis and asthma: Systematic review of randomized controlled trials. Ann. Allergy Asthma Immunol. 2008, 101, 570–579. [Google Scholar] [CrossRef]
- Costa, D.J.; Marteau, P.; Amouyal, M.; Poulsen, L.K.; Hamelmann, E.; Cazaubiel, M.; Housez, B.; Leuillet, S.; Stavnsbjerg, M.; Molimard, P.; et al. Efficacy and safety of the probiotic Lactobacillus paracasei LP-33 in allergic rhinitis: A double-blind, randomized, placebo-controlled trial (GA2LEN Study). Eur. J. Clin. Nutr. 2014, 68, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Zuccotti, G.V.; Meneghin, F.; Aceti, A.; Barone, G.; Callegari, M.L.; Di Mauro, A.; Fantini, M.P.; Gori, D.; Indrio, F.; Maggio, L.; et al. Probiotics for prevention of atopic diseases in infants: Systematic review and meta-analysis. Allergy 2015, 70, 1356–1371. [Google Scholar] [CrossRef] [PubMed]
- Vilà-Nadal, G.; Phillips-Anglés, E.; Domínguez-Ortega, J. The Use of Probiotics in Respiratory Allergy. J. Pharm. Nutr. Sci. 2016, 6, 89–94. [Google Scholar] [CrossRef]
- Ahmed, M.; Billoo, A.G.; Iqbal, K. Efficacy of probiotic in perennial allergic rhinitis under five year children: A randomized controlled trial. Pak. J. Med. Sci. 2019, 35, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Aste-Amezaga, M.; Valiante, N.M.; Ma, X.; Kubin, M.; Trinchieri, G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 1993, 178, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Groux, H.; Bigler, M.; EDe Vries, J.E.; Roncarolo, M.G. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J. Exp. Med. 1996, 184, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Nakamura, F.; Kanzato, H.; Sawada, D.; Yamamoto, N.; Kagata, H.; Oh-Ida, M.; Takeuchi, H.; Fujiwara, S. Effect of Milk Fermented with Lactobacillus acidophilus Strain L-92 on Symptoms of Japanese Cedar Pollen Allergy: A Randomized Placebo-Controlled Trial. Biosci. Biotechnol. Biochem. 2005, 69, 1652–1660. [Google Scholar] [CrossRef]
- Tamura, M.; Shikina, T.; Morihana, T.; Hayama, M.; Kajimoto, O.; Sakamoto, A.; Kajimoto, Y.; Watanabe, O.; Nonaka, C.; Shida, K.; et al. Effects of Probiotics on Allergic Rhinitis Induced by Japanese Cedar Pollen: Randomized Double-Blind, Placebo-Controlled Clinical Trial. Int. Arch. Allergy Immunol. 2006, 143, 75–82. [Google Scholar] [CrossRef]
- Xiao, J.-Z.; Kondo, S.; Yanagisawa, N.; Takahashi, N.; Odamaki, T.; Iwabuchi, N.; Miyaji, K.; Iwatsuki, K.; Togashi, H.; Enomoto, K. Probiotics in the treatment of Japanese cedar pollinosis: A double-blind placebo-controlled trial. Clin. Exp. Allergy 2006, 36, 1425–1435. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Chen, C.-J.; Chen, L.-K.; Wen, S.-H.; Jan, R.-H. Effect of probiotics on allergic rhinitis in Df, Dp or dust-sensitive children: A randomized double blind controlled trial. Indian Pediatr. 2013, 50, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Miyoshi, M.; Iwai, M.; Takeda, R.; Ono, T.; Kabuki, T. Lactobacillus helveticus SBT2171 Alleviates Perennial Allergic Rhinitis in Japanese Adults by Suppressing Eosinophils: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2020, 12, 3620. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fang, Z.; Liu, X.; Hu, W.; Lu, W.; Lee, Y.-K.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus reuteri attenuated allergic inflammation induced by HDM in the mouse and modulated gut microbes. PLoS ONE 2020, 15, e0231865. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fang, Z.; Liu, Z.; Zhao, J.; Zhang, H.; Wang, S.; He, J.; Lu, W.; Chen, W. Lactobacillus reuteri CCFM1072 and CCFM1040 with the role of Treg cells regulation alleviate airway inflammation through modulating gut microbiota in allergic asthma mice. J. Funct. Foods 2020, 76, 104286. [Google Scholar] [CrossRef]
- Cervantes-Barragan, L.; Chai, J.N.; Tianero, M.D.; Di Luccia, B.; Ahern, P.P.; Merriman, J.; Cortez, V.S.; Caparon, M.G.; Donia, M.S.; Gilfillan, S.; et al. Lactobacillus reuteri induces gut intraepithelial CD4+CD8αα+T cells. Science 2017, 357, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef]
- Kepert, I.; Fonseca, J.; Müller, C.; Milger, K.; Hochwind, K.; Kostric, M.; Fedoseeva, M.; Ohnmacht, C.; Dehmel, S.; Nathan, P.; et al. D-tryptophan from probiotic bacteria influences the gut microbiome and allergic airway disease. J. Allergy Clin. Immunol. 2017, 139, 1525–1535. [Google Scholar] [CrossRef]
- Li, L.; Fang, Z.; Lee, Y.-K.; Zhao, J.; Zhang, H.; Peng, H.; Zhang, Y.; Lu, W.; Chen, W. Efficacy and Safety of Lactobacillus reuteri CCFM1040 in Allergic Rhinitis and Asthma: A Randomized, Placebo-Controlled Trial. Front. Nutr. 2022, 9, 862934. [Google Scholar] [CrossRef]
- Choi, S.-P.; Oh, H.-N.; Choi, C.-Y.; Ahn, H.; Yun, H.; Chung, Y.; Kim, B.; Lee, S.; Chun, T. Oral administration of Lactobacillus plantarum CJLP133 and CJLP243 alleviates birch pollen-induced allergic rhinitis in mice. J. Appl. Microbiol. 2017, 124, 821–828. [Google Scholar] [CrossRef]
- Pennock, B.E.; Cox, C.P.; Rogers, R.M.; Cain, W.A.; Wells, J.H. A noninvasive technique for measurement of changes in specific airway resistance. J. Appl. Physiol. 1979, 46, 399–406. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Lin, Y.-L.; Jan, R.-L.; Chen, H.-H.; Wang, J.-Y. Randomized placebo-controlled trial of lactobacillus on asthmatic children with allergic rhinitis. Pediatr. Pulmonol. 2010, 45, 1111–1120. [Google Scholar] [CrossRef]
- Olivares, M.; Díaz-Ropero, M.P.; Gómez, N.; Lara-Villoslada, F.; Sierra, S.; Maldonado, J.A.; Martín, R.; Lopez-Huertas, E.; Rodríguez, J.; Xaus, J. Oral administration of two probiotic strains, Lactobacillus gasseri CECT5714 and Lactobacillus coryniformis CECT5711, enhances the intestinal function of healthy adults. Int. J. Food Microbiol. 2006, 107, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Díaz-Ropero, M.P.; Gómez, N.; Lara-Villoslada, F.; Sierra, S.; Maldonado, A.J.; Martín, R.; Rodríguez, J.M.; Xaus, J. The consumption of two new probiotic strains, Lactobacillus gasseri CECT 5714 and Lactobacillus coryniformis CECT 5711, boosts the immune system of healthy humans. Int. Microbiol. 2006, 9, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Díaz-Ropero, M.P.; Gómez, N.; Sierra, S.; Lara-Villoslada, F.; Martín, R.; Rodríguez, J.M.; Xaus, J. Dietary deprivation of fermented foods causes a fall in innate immune response. Lactic acid bacteria can counteract the immunological effect of this deprivation. J. Dairy Res. 2006, 73, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Lara-Villoslada, F.S.S. [Beneficial Effects of Consumption of a Dairy Product Containing Two Probiotic Strains, Lactobacillus coryniformis CECT5711 and Lactobacillus gasseri CECT5714 in Healthy Children]-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/17650892/ (accessed on 24 November 2022).
- Wassenberg, J.; Nutten, S.; Audran, R.; Barbier, N.; Aubert, V.; Moulin, J.; Mercenier, A.; Spertini, F. Effect of Lactobacillus paracasei ST11 on a nasal provocation test with grass pollen in allergic rhinitis. Clin. Exp. Allergy 2011, 41, 565–573. [Google Scholar] [CrossRef]
- Perrin, Y.; Nutten, S.; Audran, R.; Berger, B.; Bibiloni, R.; Wassenberg, J.; Barbier, N.; Aubert, V.; Moulin, J.; Singh, A.; et al. Comparison of two oral probiotic preparations in a randomized crossover trial highlights a potentially beneficial effect of Lactobacillus paracasei NCC2461 in patients with allergic rhinitis. Clin. Transl. Allergy 2014, 4, 1. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Nermes, M.; Collado, M.C.; Rautonen, N.; Salminen, S.; Isolauri, E. Specific probiotics alleviate allergic rhinitis during the birch pollen season. World J. Gastroenterol. 2009, 15, 3261–3268. [Google Scholar] [CrossRef]
- Nembrini, C.; Singh, A.; De Castro, C.A.; Mercenier, A.; Nutten, S. Oral administration of Lactobacillus paracasei NCC 2461 for the modulation of grass pollen allergic rhinitis: A randomized, placebo-controlled study during the pollen season. Clin. Transl. Allergy 2015, 5, 41. [Google Scholar] [CrossRef]
- Helin, T.; Haahtela, S. No effect of oral treatment with an intestinal bacterial strain, Lactobacillus rhamnosus (ATCC 53103), on birch-pollen allergy: A placebo-controlled double-blind study. Allergy 2002, 57, 243–246. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Delgado, S.; Ruiz, L.; Ruas-Madiedo, P.; Sánchez, B.; Margolles, A. Bifidobacteria and Their Health-Promoting Effects. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Ficara, M.; Pietrella, E.; Spada, C.; Muttini, E.D.C.; Lucaccioni, L.; Iughetti, L.; Berardi, A. Changes of intestinal microbiota in early life. J. Matern. Neonatal Med. 2018, 33, 1036–1043. [Google Scholar] [CrossRef]
- Kim, W.-G.; Kang, G.-D.; Kim, H.; Han, M.; Kim, D.-H. Bifidobacterium longum IM55 and Lactobacillus plantarum IM76 alleviate allergic rhinitis in mice by restoring Th2/Treg imbalance and gut microbiota disturbance. Benef. Microbes 2019, 10, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhao, Y.; Huang, S.; Lv, D.; Yang, F.; Lou, L.; Zheng, Y.; Zhang, J.; Liu, S.; Zhang, N.; et al. Immunomodulatory effect of Bifidobacterium breve on experimental allergic rhinitis in BALB/c mice. Exp. Ther. Med. 2018, 16, 3996–4004. [Google Scholar] [CrossRef]
- Tsunemine, S.; Isa, Y.; Shimakawa, M.; Ohno, H.; Yamamura, H. Effects of Bifidobacterium bifidum G9-1 on Nasal Symptoms in a Guinea Pig Model of Experimental Allergic Rhinitis. Biosci. Microflora 2011, 30, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Basile, I.; Danza, M.L.; Venturelli, L.; Contini, R.; Risso, P.; Colombo, M. Use of a probiotic mixture containing Bifidobacterium animalis subsp. lactis BB12 and Enterococcus faecium L3 in atopic children. Minerva Pediatr. 2018, 70, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, M.M.; Indolfi, C.; Capasso, M.; Maiello, N.; Decimo, F.; Ciprandi, G. Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma. Ital. J. Pediatr. 2017, 43, 25. [Google Scholar] [CrossRef]
- Rho, M.-K.; Kim, Y.-E.; Rho, H.-I.; Kim, T.-R.; Kim, Y.-B.; Sung, W.-K.; Kim, T.-W.; Kim, D.-O.; Kang, H. Enterococcus faecium FC-K Derived from Kimchi Is a Probiotic Strain That Shows Anti-Allergic Activity. J. Microbiol. Biotechnol. 2017, 27, 1071–1077. [Google Scholar] [CrossRef]
- Luping, Z.; Takashi, S.; Ruoxi, C.; Meiping, L.; Qingzhao, Z.; Wenmin, L.; Min, Y.; Tadao, E.; Lei, C. Effects of lysed Enterococcus faecalis FK-23 on experimental allergic rhinitis in a murine model. J. Biomed. Res. 2012, 26, 226–234. [Google Scholar] [CrossRef]
- Lo Skiavo LA, G.N. [Dynamics of Contamination and Persistence of Clostridium difficile in Intestinal Microbiota in Newborn Infants during Antibiotic Therapy and Use of Probiotic Strain Enterococcus Faecium L3]-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/24734423/ (accessed on 24 November 2022).
- Anania, C.; Di Marino, V.; Olivero, F.; De Canditiis, D.; Brindisi, G.; Iannilli, F.; De Castro, G.; Zicari, A.; Duse, M. Treatment with a Probiotic Mixture Containing Bifidobacterium animalis Subsp. Lactis BB12 and Enterococcus faecium L3 for the Prevention of Allergic Rhinitis Symptoms in Children: A Randomized Controlled Trial. Nutrients 2021, 13, 1315. [Google Scholar] [CrossRef]
- Fang, G.X.; Li, Z.; Su, C.; Hu, G.H. Clinical observation of saccharomyces boulardii combined with cetirizine hydrochloride in children allergic rhinitis. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2017, 31, 1649–1652. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Van Immerseel, F.; Ducatelle, R.; De Vos, M.; Boon, N.; Van De Wiele, T.; Verbeke, K.; Rutgeerts, P.; Sas, B.; Louis, P.; Flint, H.J. Butyric acid-producing anaerobic bacteria as a novel probiotic treatment approach for inflammatory bowel disease. J. Med. Microbiol. 2010, 59, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humaran, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, M.; Akagawa, S.; Akagawa, Y.; Nakai, Y.; Yamanouchi, S.; Kimata, T.; Hashiyada, M.; Akane, A.; Tsuji, S.; Kaneko, K. Decreased butyric acid-producing bacteria in gut microbiota of children with egg allergy. Allergy 2021, 76, 2279–2282. [Google Scholar] [CrossRef] [PubMed]
- Roduit, C.; Frei, R.; Ferstl, R.; Loeliger, S.; Westermann, P.; Rhyner, C.; Schiavi, E.; Barcik, W.; Rodriguez-Perez, N.; Wawrzyniak, M.; et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 2018, 74, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Akagawa, S.; Kaneko, K. Gut microbiota and allergic diseases in children. Allergol. Int. 2022, 71, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Sangwan, N.; Stefka, A.; Nocerino, R.; Paparo, L.; Aitoro, R.; Calignano, A.; Khan, A.A.; Gilbert, J.; Nagler, C.R. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 2016, 10, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Pawankar, R.; Cuello-Garcia, C.; Ahn, K.; Al-Hammadi, S.; Agarwal, A.; Beyer, K.; Burks, W.; Canonica, G.W.; Ebisawa, M.; et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): Probiotics. World Allergy Organ. J. 2015, 8, 1–4. [Google Scholar] [CrossRef]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host Remodeling of the Gut Microbiome and Metabolic Changes during Pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Obuchowska, A.; Gorczyca, K.; Standyło, A.; Obuchowska, K.; Kimber-Trojnar, Ż.; Wierzchowska-Opoka, M.; Leszczyńska-Gorzelak, B. Effects of Probiotic Supplementation during Pregnancy on the Future Maternal Risk of Metabolic Syndrome. Int. J. Mol. Sci. 2022, 23, 8253. [Google Scholar] [CrossRef]
- Kuperman, A.A.; Koren, O. Antibiotic use during pregnancy: How bad is it? BMC Med. 2016, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gomez de Agüero, M.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The maternal microbiota drives early postnatal innate immune development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Neuman, H.; Koren, O. The Pregnancy Microbiome. Nestle Nutr. Inst. Workshop Ser. 2017, 88, 1–9. [Google Scholar] [CrossRef]
- Aagaard, K.; Riehle, K.; Ma, J.; Segata, N.; Mistretta, T.-A.; Coarfa, C.; Raza, S.; Rosenbaum, S.; Veyver, I.V.D.; Milosavljevic, A.; et al. A Metagenomic Approach to Characterization of the Vaginal Microbiome Signature in Pregnancy. PLoS ONE 2012, 7, e36466. [Google Scholar] [CrossRef] [PubMed]
- Lundelin, K.; Poussa, T.; Salminen, S.; Isolauri, E. Long-term safety and efficacy of perinatal probiotic intervention: Evidence from a follow-up study of four randomized, double-blind, placebo-controlled trials. Pediatr. Allergy Immunol. 2016, 28, 170–175. [Google Scholar] [CrossRef]
- Bertelsen, R.J.; Brantsæter, A.L.; Magnus, M.C.; Haugen, M.; Myhre, R.; Jacobsson, B.; Longnecker, M.P.; Meltzer, H.M.; London, S.J. Probiotic milk consumption in pregnancy and infancy and subsequent childhood allergic diseases. J. Allergy Clin. Immunol. 2013, 133, 165–171.e8. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wang, L.; Wu, S.; Yuan, L.; Tang, S.; Xiang, Y.; Qu, X.; Liu, H.; Qin, X.; Liu, C. Efficacy of probiotic supplementary therapy for asthma, allergic rhinitis, and wheeze: A meta-analysis of randomized controlled trials. Allergy Asthma Proc. 2019, 40, 250–260. [Google Scholar] [CrossRef]
- Arango, L.F.G.; Barrett, H.L.; Callaway, L.K.; Nitert, M.D. Probiotics and Pregnancy. Curr. Diabetes Rep. 2014, 15, 1–9. [Google Scholar] [CrossRef]
- Sohn, K.; Underwood, M.A. Prenatal and postnatal administration of prebiotics and probiotics. Semin. Fetal Neonatal Med. 2017, 22, 284–289. [Google Scholar] [CrossRef]
- Boyle, R.J.; Ismail, I.H.; Kivivuori, S.; Licciardi, P.V.; Robins-Browne, R.M.; Mah, L.-J.; Axelrad, C.; Moore, S.; Donath, S.; Carlin, J.B.; et al. Lactobacillus GG treatment during pregnancy for the prevention of eczema: A randomized controlled trial. Allergy 2010, 66, 509–516. [Google Scholar] [CrossRef]
- Simpson, M.R.; Dotterud, C.K.; Storrø, O.; Johnsen, R.; Øien, T. Perinatal probiotic supplementation in the prevention of allergy related disease: 6 year follow up of a randomised controlled trial. BMC Dermatol. 2015, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.L.; Dunstan, J.A.; Prescott, S.L. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: A randomized controlled trial. J. Allergy Clin. Immunol. 2007, 119, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Wise, S.K.; Lin, S.Y.; Toskala, E.; Orlandi, R.R.; Akdis, C.A.; Alt, J.A.; Azar, A.; Baroody, F.M.; Bachert, C.; Canonica, G.W.; et al. International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis. Int. Forum Allergy Rhinol. 2018, 8, 108–352. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).