Title-Inflammatory Signaling Pathways in Allergic and Infection-Associated Lung Diseases
Abstract
:1. Introduction
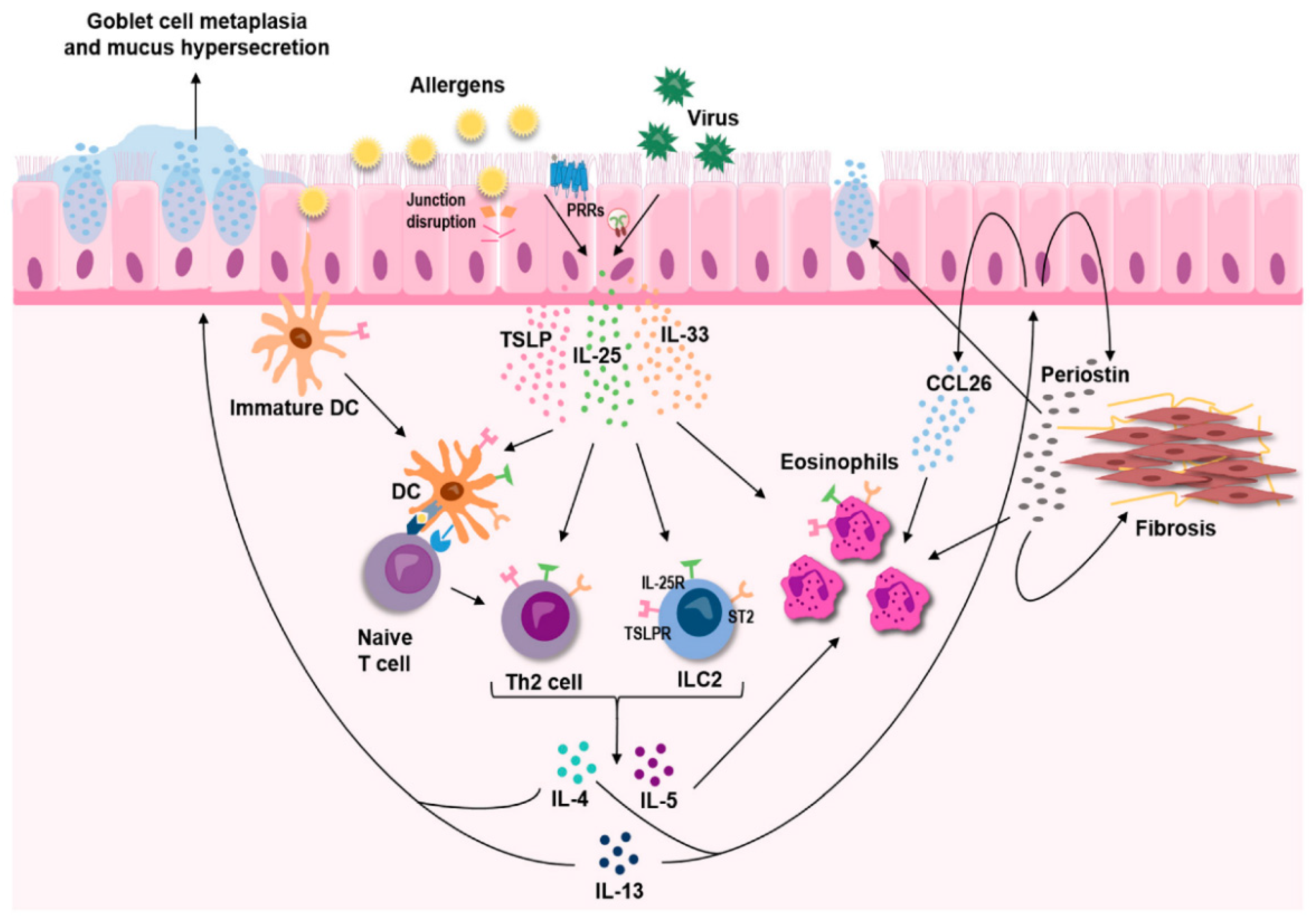
2. Etiology of Allergic Lung Disease
3. Signaling Pathways of Infection-Induced Lung Inflammation
3.1. Lung Inflammation and Receptor Signaling Pathways in Response to Bacterial Infection
3.2. Lung Inflammation and Receptor Signaling Pathways in Response to Virus Infection
3.3. Lung Inflammation and Receptor Signaling Pathways in Response to Fungal Infection
3.4. Transducers and Effectors of Receptor Signaling in Lung Inflammation
4. Signaling Pathways of Allergic Lung Inflammation and Diseases
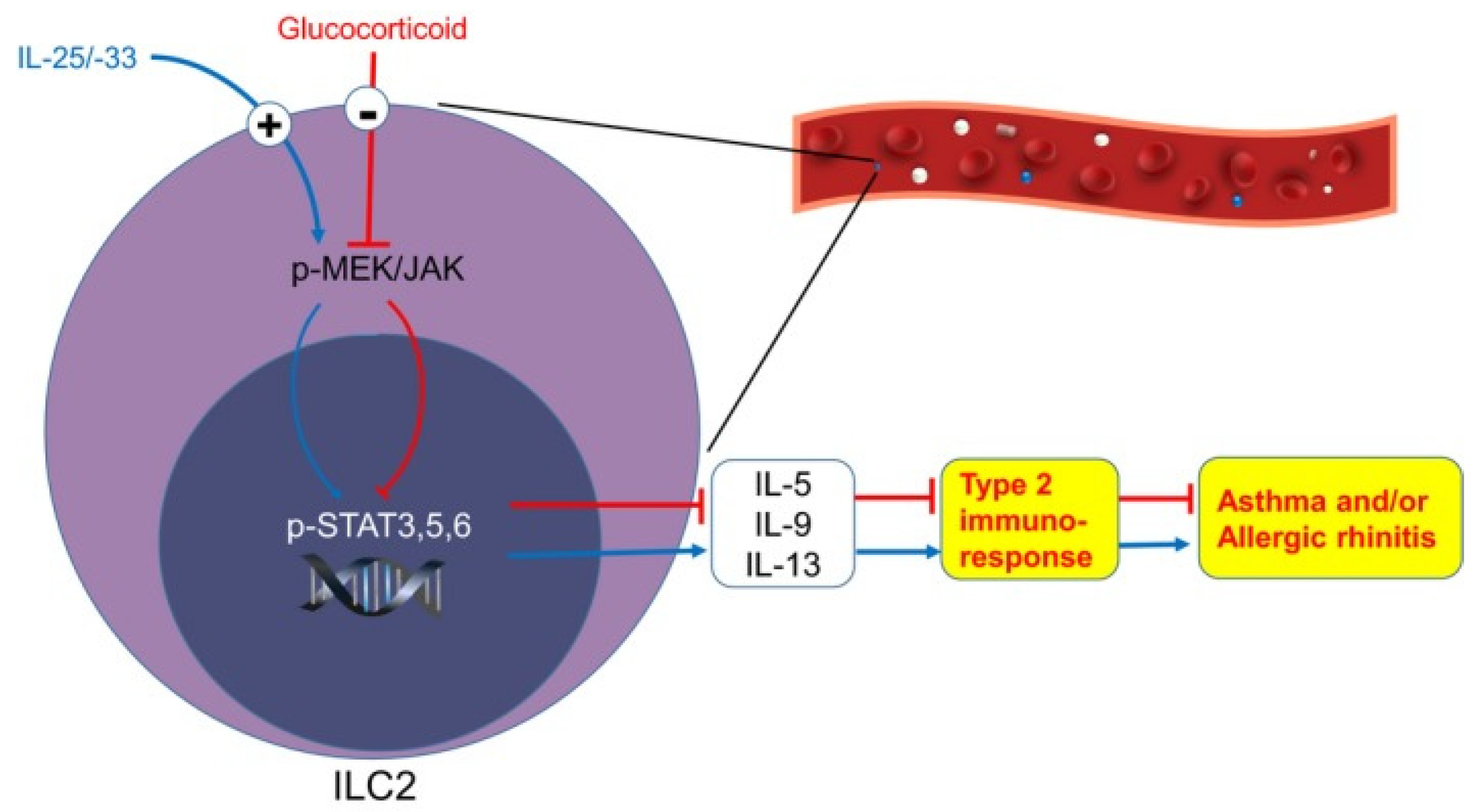
4.1. Innate Lymphoid Cells (ILCs) in Allergic Lung Inflammation
4.2. Mast Cells in Allergic Lung Inflammation
4.3. Basophils in Allergic Inflammation
4.4. T Cells and T Cell Receptor Signaling Pathways in Allergic Inflammation
5. Therapeutic Strategies for Lung Inflammation Associated with Infection
5.1. Corticosteroids
5.2. Statins
5.3. Cytokine-Targeted Therapies
5.4. Immunomodulatory Antibiotics
6. Therapeutic Strategies for Allergic Lung Inflammation
7. Soluble Receptor/Mab Therapy
7.1. Anti–IL-4 Strategies
7.2. IL-4Ra Receptor Antagonist
7.3. Anti–IL-5 mAbs
7.4. Anti–IL-13 mAb
8. Immune Cell Treatment
9. Stem Cell Treatment
10. Transcription Factor Inhibition
10.1. Syk Kinase Inhibitors
10.2. Peroxisome Proliferator-Activated Receptor (PPAR)γ Agonists
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Passalacqua, G.; Ciprandi, G. Allergy and the lung. Clin. Exp. Immunol. 2008, 153, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Costabel, U.; Miyazaki, Y.; Pardo, A.; Koschel, D.; Bonella, F.; Spagnolo, P.; Guzman, J.; Ryerson, C.J.; Selman, M. Hypersensitivity pneumonitis. Nat. Rev. Dis. Primers 2020, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of Asthma in Children and Adults. Front. Pediatr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Schatz, M.; Rosenwasser, L. The Allergic Asthma Phenotype. Allergy Clin. Immunol. Pract. 2014, 2, 645–648. [Google Scholar] [CrossRef]
- Holgate, S.T. Pathogenesis of Asthma. Clin. Exp. Allergy 2008, 38, 872–897. [Google Scholar] [CrossRef]
- Akinbami, L.J.; Moorman, J.E.; Bailey, C.; Zahran, H.S.; King, M.; Johnson, C.A.; Liu, X. Trends in Asthma Prevalence, Health Care Use, and Mortality in the United States, 2001–2010; US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Hyattsville, MD, USA, 2012; pp. 1–8.
- Possa, S.; Leick, E.; Prado, C.; Martins, M.; Tibério, I. Eosinophilic Inflammation in Allergic Asthma. Front. Pharmacol. 2013, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Bogaert, P.; Tournoy, K.G.; Naessens, T.; Grooten, J. Where asthma and hypersensitivity pneumonitis meet and differ: Noneosinophilic severe asthma. Am. J. Pathol. 2009, 174, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Bochner, B.S.; Undem, B.J.; Lichtenstein, L.M. Immunological Aspects of Allergic Asthma. Annu. Rev. Immunol. 1994, 12, 295–335. [Google Scholar] [CrossRef]
- Selman, M.; Pardo, A.; King, T.E., Jr. Hypersensitivity pneumonitis: Insights in diagnosis and pathobiology. Am. J. Respir. Crit. Care Med. 2012, 186, 314–324. [Google Scholar] [CrossRef]
- Backman, H.; Räisänen, P.; Hedman, L.; Stridsman, C.; Andersson, M.; Lindberg, A.; Lundbäck, B.; Rönmark, E. Increased prevalence of allergic asthma from 1996 to 2006 and further to 2016-results from three population surveys. Clin. Exp. Allergy 2017, 47, 1426–1435. [Google Scholar] [CrossRef]
- Stern, J.; Pier, J.; Litonjua, A.A. Asthma epidemiology and risk factors. Semin. Immunopathol. 2020, 42, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, M.L.; Gu, T.; Murray, S.; Gross, B.H.; Chughtai, A.; Sayyouh, M.; Kazerooni, E.A.; Myers, J.L.; Lagstein, A.; Konopka, K.E.; et al. Hypersensitivity Pneumonitis: Radiologic Phenotypes Are Associated With Distinct Survival Time and Pulmonary Function Trajectory. Chest 2019, 155, 699–711. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Assessing National Capacity for the Prevention and Control of Noncommunicable Diseases: Report of the 2019 Global Survey; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Beasley, R.; Crane, J.; Lai, C.K.W.; Pearce, N. Prevalence and etiology of asthma. J. Allergy Clin. Immunol. 2000, 105, S466–S472. [Google Scholar] [CrossRef]
- Pakkasela, J.; Ilmarinen, P.; Honkamäki, J.; Tuomisto, L.E.; Andersén, H.; Piirilä, P.; Hisinger-Mölkänen, H.; Sovijärvi, A.; Backman, H.; Lundbäck, B.; et al. Age-specific incidence of allergic and non-allergic asthma. BMC Pulm. Med. 2020, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Perez, E.R.; Kong, A.M.; Raimundo, K.; Koelsch, T.L.; Kulkarni, R.; Cole, A.L. Epidemiology of Hypersensitivity Pneumonitis among an Insured Population in the United States: A Claims-based Cohort Analysis. Ann. Am. Thorac. Soc. 2018, 15, 460–469. [Google Scholar] [CrossRef]
- Bang, K.M.; Weissman, D.N.; Pinheiro, G.A.; Antao, V.C.; Wood, J.M.; Syamlal, G. Twenty-three years of hypersensitivity pneumonitis mortality surveillance in the United States. Am. J. Ind. Med. 2006, 49, 997–1004. [Google Scholar] [CrossRef]
- Murdoch, J.R.; Lloyd, C.M. Chronic inflammation and asthma. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2010, 690, 24–39. [Google Scholar] [CrossRef]
- Cano-Jiménez, E.; Acuña, A.; Botana, M.I.; Hermida, T.; González, M.G.; Leiro, V.; Martín, I.; Paredes, S.; Sanjuán, P. Revisión de la enfermedad del pulmón de granjero. Arch. De Bronconeumol. 2016, 52, 321–328. [Google Scholar] [CrossRef]
- Wollin, L.; Distler, J.H.W.; Redente, E.F.; Riches, D.W.H.; Stowasser, S.; Schlenker-Herceg, R.; Maher, T.M.; Kolb, M. Potential of nintedanib in treatment of progressive fibrosing interstitial lung diseases. Eur. Respir. J. 2019, 54. [Google Scholar] [CrossRef]
- Just, J.; Deschildre, A.; Lejeune, S.; Amat, F. New perspectives of childhood asthma treatment with biologics. Pediatr. Allergy Immunol. 2019, 30, 159–171. [Google Scholar] [CrossRef]
- Agache, I.; Rocha, C.; Beltran, J.; Song, Y.; Posso, M.; Solà, I.; Alonso-Coello, P.; Akdis, C.; Akdis, M.; Canonica, G.W.; et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab and omalizumab) for severe allergic asthma: A systematic review for the EAACI Guidelines-recommendations on the use of biologicals in severe asthma. Allergy 2020, 75, 1043–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, A.; Price, D. Treatment Adherence in Adolescents with Asthma. J. Asthma Allergy 2020, 13, 39–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, P.J. Efficacy of inhaled corticosteroids in asthma. J. Allergy Clin. Immunol. 1998, 102, 531–538. [Google Scholar] [CrossRef]
- Vasakova, M.; Selman, M.; Morell, F.; Sterclova, M.; Molina-Molina, M.; Raghu, G. Hypersensitivity Pneumonitis: Current Concepts of Pathogenesis and Potential Targets for Treatment. Am. J. Respir. Crit. Care Med. 2019, 200, 301–308. [Google Scholar] [CrossRef]
- Lacasse, Y.; Selman, M.; Costabel, U.; Dalphin, J.C.; Ando, M.; Morell, F.; Erkinjuntti-Pekkanen, R.; Muller, N.; Colby, T.V.; Schuyler, M.; et al. Clinical diagnosis of hypersensitivity pneumonitis. Am. J. Respir. Crit. Care Med. 2003, 168, 952–958. [Google Scholar] [CrossRef]
- Moldoveanu, B.; Otmishi, P.; Jani, P.; Walker, J.; Sarmiento, X.; Guardiola, J.; Saad, M.; Yu, J. Inflammatory mechanisms in the lung. J. Inflamm. Res. 2009, 2, 1–11. [Google Scholar]
- Shibata, S.; Furusawa, H.; Inase, N. Pirfenidone in chronic hypersensitivity pneumonitis: A real-life experience. Sarcoidosis Vasc. Diffus. Lung Dis. 2018, 35, 139–142. [Google Scholar] [CrossRef]
- Bartlett, J.G. Anaerobic bacterial infection of the lung. Anaerobe 2012, 18, 235–239. [Google Scholar] [CrossRef]
- Nogueira, R.; Melo, N.; Novais, E.B.H.; Martins, N.; Delgado, L.; Morais, A.; P, C.M. Hypersensitivity pneumonitis: Antigen diversity and disease implications. Pulmonology 2019, 25, 97–108. [Google Scholar] [CrossRef]
- Christensen, L.T.; Schmidt, C.D.; Robbins, L. Pigeon breeders’ disease—A prevalence study and review. Clin. Allergy 1975, 5, 417–430. [Google Scholar] [CrossRef]
- Eddens, T.; Kolls, J.K. Host defenses against bacterial lower respiratory tract infection. Curr. Opin. Immunol. 2012, 24, 424–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartlett, J.G. Diagnostic tests for agents of community-acquired pneumonia. Clin. Infect. Dis. 2011, 52 (Suppl. S4), S296–S304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muruganandah, V.; Kupz, A. Immune responses to bacterial lung infections and their implications for vaccination. Int. Immunol. 2021, 34, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Balamayooran, T.; Balamayooran, G.; Jeyaseelan, S. Review: Toll-like receptors and NOD-like receptors in pulmonary antibacterial immunity. Innate Immun. 2010, 16, 201–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opitz, B.; van Laak, V.; Eitel, J.; Suttorp, N. Innate immune recognition in infectious and noninfectious diseases of the lung. Am. J. Respir. Crit. Care Med. 2010, 181, 1294–1309. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Mindru, C.; Botha, J.F.; Grant, W.J.; Mercer, D.F.; Olivera, M.A.; McCartan, M.A.; McCashland, T.M.; Langnas, A.N.; Florescu, D.F. Risk of cytomegalovirus disease in high-risk liver transplant recipients on valganciclovir prophylaxis: A systematic review and meta-analysis. Liver Transpl. 2012, 18, 1440–1447. [Google Scholar] [CrossRef]
- Pritt, B.S.; Aubry, M.C. Histopathology of viral infections of the lung. Semin. Diagn. Pathol. 2017, 34, 510–517. [Google Scholar] [CrossRef]
- Jennings, L.C.; Anderson, T.P.; Beynon, K.A.; Chua, A.; Laing, R.T.; Werno, A.M.; Young, S.A.; Chambers, S.T.; Murdoch, D.R. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax 2008, 63, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Kawai, T.; Akira, S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009, 21, 317–337. [Google Scholar] [CrossRef] [Green Version]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [Green Version]
- Coro, E.S.; Chang, W.L.; Baumgarth, N. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J. Immunol. 2006, 176, 4343–4351. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, K.; Oropallo, M.A.; Cancro, M.P.; Marshak-Rothstein, A. Role of type I interferons in the activation of autoreactive B cells. Immunol. Cell Biol. 2012, 90, 498–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crouse, J.; Kalinke, U.; Oxenius, A. Regulation of antiviral T cell responses by type I interferons. Nat. Rev. Immunol. 2015, 15, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Crouse, J.; Xu, H.C.; Lang, P.A.; Oxenius, A. NK cells regulating T cell responses: Mechanisms and outcome. Trends Immunol. 2015, 36, 49–58. [Google Scholar] [CrossRef]
- Newton, A.H.; Cardani, A.; Braciale, T.J. The host immune response in respiratory virus infection: Balancing virus clearance and immunopathology. Semin. Immunopathol. 2016, 38, 471–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latgé, J.P. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 1999, 12, 310–350. [Google Scholar] [CrossRef] [Green Version]
- Kronstad, J.W.; Attarian, R.; Cadieux, B.; Choi, J.; D’Souza, C.A.; Griffiths, E.J.; Geddes, J.M.; Hu, G.; Jung, W.H.; Kretschmer, M.; et al. Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nat. Rev. Microbiol. 2011, 9, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Lu, G.; Meng, G. Pathogenic Fungal Infection in the Lung. Front. Immunol. 2019, 10, 1524. [Google Scholar] [CrossRef] [Green Version]
- Sorrell, T.C.; Chen, S.C. Fungal-derived immune modulating molecules. Adv. Exp. Med. Biol. 2009, 666, 108–120. [Google Scholar] [CrossRef]
- Hatinguais, R.; Willment, J.A.; Brown, G.D. PAMPs of the Fungal Cell Wall and Mammalian PRRs. Curr. Top. Microbiol. Immunol. 2020, 425, 187–223. [Google Scholar] [CrossRef]
- Osterholzer, J.J.; Milam, J.E.; Chen, G.H.; Toews, G.B.; Huffnagle, G.B.; Olszewski, M.A. Role of dendritic cells and alveolar macrophages in regulating early host defense against pulmonary infection with Cryptococcus neoformans. Infect. Immun. 2009, 77, 3749–3758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gazendam, R.P.; van de Geer, A.; Roos, D.; van den Berg, T.K.; Kuijpers, T.W. How neutrophils kill fungi. Immunol. Rev. 2016, 273, 299–311. [Google Scholar] [CrossRef] [PubMed]
- McDermott, A.J.; Klein, B.S. Helper T-cell responses and pulmonary fungal infections. Immunology 2018, 155, 155–163. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Pullamsetti, S.S.; Seeger, W.; Savai, R. Classical IL-6 signaling: A promising therapeutic target for pulmonary arterial hypertension. J. Clin. Investig. 2018, 128, 1720–1723. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef]
- Pellegrini, E.; Desfosses, A.; Wallmann, A.; Schulze, W.M.; Rehbein, K.; Mas, P.; Signor, L.; Gaudon, S.; Zenkeviciute, G.; Hons, M.; et al. RIP2 filament formation is required for NOD2 dependent NF-kappaB signalling. Nat. Commun. 2018, 9, 4043. [Google Scholar] [CrossRef] [Green Version]
- Saxena, M.; Yeretssian, G. NOD-Like Receptors: Master Regulators of Inflammation and Cancer. Front. Immunol. 2014, 5, 327. [Google Scholar] [CrossRef] [Green Version]
- Rehwinkel, J.; Gack, M.U. RIG-I-like receptors: Their regulation and roles in RNA sensing. Nat. Rev. Immunol. 2020, 20, 537–551. [Google Scholar] [CrossRef]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef] [Green Version]
- Colgan, J.D.; Hankel, I.L. Signaling pathways critical for allergic airway inflammation. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 42–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wills-Karp, M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev. Immunol. 1999, 17, 255–281. [Google Scholar] [CrossRef] [PubMed]
- Calven, J.; Ax, E.; Radinger, M. The Airway Epithelium-A Central Player in Asthma Pathogenesis. Int. J. Mol. Sci. 2020, 21, 8907. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, I.; Steer, C.A.; Takei, F. Lung ILC2s link innate and adaptive responses in allergic inflammation. Trends Immunol. 2015, 36, 189–195. [Google Scholar] [CrossRef]
- Toki, S.; Goleniewska, K.; Zhang, J.; Zhou, W.; Newcomb, D.C.; Zhou, B.; Kita, H.; Boyd, K.L.; Peebles, R.S., Jr. TSLP and IL-33 reciprocally promote each other’s lung protein expression and ILC2 receptor expression to enhance innate type-2 airway inflammation. Allergy 2020, 75, 1606–1617. [Google Scholar] [CrossRef]
- Barnes, H.; Troy, L.; Lee, C.T.; Sperling, A.; Strek, M.; Glaspole, I. Hypersensitivity pneumonitis: Current concepts in pathogenesis, diagnosis, and treatment. Allergy 2021. [Google Scholar] [CrossRef]
- Girard, M.; Israel-Assayag, E.; Cormier, Y. Impaired function of regulatory T-cells in hypersensitivity pneumonitis. Eur. Respir. J. 2011, 37, 632–639. [Google Scholar] [CrossRef]
- Barron, L.; Wynn, T.A. Fibrosis is regulated by Th2 and Th17 responses and by dynamic interactions between fibroblasts and macrophages. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G723–G728. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Kim, H.Y.; Chang, Y.J.; DeKruyff, R.H.; Umetsu, D.T. Innate lymphoid cells and asthma. J. Allergy Clin. Immunol. 2014, 133, 943–950. [Google Scholar] [CrossRef]
- Halim, T.Y.; Krauss, R.H.; Sun, A.C.; Takei, F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity 2012, 36, 451–463. [Google Scholar] [CrossRef] [Green Version]
- Bartemes, K.R.; Iijima, K.; Kobayashi, T.; Kephart, G.M.; McKenzie, A.N.; Kita, H. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J. Immunol. 2012, 188, 1503–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Q.N.; Guo, Y.B.; Li, X.; Li, C.L.; Tan, W.P.; Fan, X.L.; Qin, Z.L.; Chen, D.; Wen, W.P.; Zheng, S.G.; et al. ILC2 frequency and activity are inhibited by glucocorticoid treatment via STAT pathway in patients with asthma. Allergy 2018, 73, 1860–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrova, T.; Pesic, J.; Pardali, K.; Gaestel, M.; Arthur, J.S.C. p38 MAPK signalling regulates cytokine production in IL-33 stimulated Type 2 Innate Lymphoid cells. Sci. Rep. 2020, 10, 3479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burrows, B.; Martinez, F.D.; Halonen, M.; Barbee, R.A.; Cline, M.G. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N. Engl. J. Med. 1989, 320, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Manabe, T.; Niki, Y.; Matsushima, T. Summer-type hypersensitivity pneumonitis in a patient with a positive skin test (15 minutes) for Trichosporon mucoides and a high serum IgE level. Nihon Kyobu Shikkan Gakkai Zasshi 1996, 34, 1168–1173. [Google Scholar] [PubMed]
- Siraganian, R.P. Mast cell signal transduction from the high-affinity IgE receptor. Curr. Opin. Immunol. 2003, 15, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Nakae, S.; Suto, H.; Iikura, M.; Kakurai, M.; Sedgwick, J.D.; Tsai, M.; Galli, S.J. Mast cells enhance T cell activation: Importance of mast cell costimulatory molecules and secreted TNF. J. Immunol. 2006, 176, 2238–2248. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.C. Immunoglobulin E receptor signaling and asthma. J. Biol. Chem. 2011, 286, 32891–32897. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Kurashima, Y. Two Sides of the Coin: Mast Cells as a Key Regulator of Allergy and Acute/Chronic Inflammation. Cells 2021, 10, 1615. [Google Scholar] [CrossRef]
- Rivera, J.; Fierro, N.A.; Olivera, A.; Suzuki, R. New insights on mast cell activation via the high affinity receptor for IgE. Adv. Immunol. 2008, 98, 85–120. [Google Scholar] [CrossRef] [Green Version]
- Huber, M.; Helgason, C.D.; Damen, J.E.; Liu, L.; Humphries, R.K.; Krystal, G. The src homology 2-containing inositol phosphatase (SHIP) is the gatekeeper of mast cell degranulation. Proc. Natl. Acad. Sci. USA 1998, 95, 11330–11335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, J.T. Basophils beyond effector cells of allergic inflammation. Adv. Immunol. 2009, 101, 123–161. [Google Scholar] [CrossRef] [PubMed]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Sharma, J.; Raju, R.; Palapetta, S.M.; Prasad, T.S.; Huang, T.C.; Yoda, A.; Tyner, J.W.; van Bodegom, D.; Weinstock, D.M.; et al. TSLP signaling pathway map: A platform for analysis of TSLP-mediated signaling. Database 2014, 2014, bau007. [Google Scholar] [CrossRef]
- Markovic, I.; Savvides, S.N. Modulation of Signaling Mediated by TSLP and IL-7 in Inflammation, Autoimmune Diseases, and Cancer. Front. Immunol. 2020, 11, 1557. [Google Scholar] [CrossRef]
- Nakajima, H.; Iwamoto, I.; Tomoe, S.; Matsumura, R.; Tomioka, H.; Takatsu, K.; Yoshida, S. CD4+ T-lymphocytes and interleukin-5 mediate antigen-induced eosinophil infiltration into the mouse trachea. Am. Rev. Respir. Dis. 1992, 146, 374–377. [Google Scholar] [CrossRef]
- Smith-Garvin, J.E.; Koretzky, G.A.; Jordan, M.S. T cell activation. Annu. Rev. Immunol. 2009, 27, 591–619. [Google Scholar] [CrossRef]
- Deindl, S.; Kadlecek, T.A.; Brdicka, T.; Cao, X.; Weiss, A.; Kuriyan, J. Structural basis for the inhibition of tyrosine kinase activity of ZAP-70. Cell 2007, 129, 735–746. [Google Scholar] [CrossRef] [Green Version]
- Müller-Redetzky, H.; Lienau, J.; Suttorp, N.; Witzenrath, M. Therapeutic strategies in pneumonia: Going beyond antibiotics. Eur. Respir. Rev. 2015, 24, 516–524. [Google Scholar] [CrossRef]
- Blum, C.A.; Nigro, N.; Briel, M.; Schuetz, P.; Ullmer, E.; Suter-Widmer, I.; Winzeler, B.; Bingisser, R.; Elsaesser, H.; Drozdov, D.; et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2015, 385, 1511–1518. [Google Scholar] [CrossRef]
- Pliakos, E.E.; Andreatos, N.; Tansarli, G.S.; Ziakas, P.D.; Mylonakis, E. The Cost-Effectiveness of Corticosteroids for the Treatment of Community-Acquired Pneumonia. Chest 2019, 155, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Remmelts, H.H.; Meijvis, S.C.; Biesma, D.H.; van Velzen-Blad, H.; Voorn, G.P.; Grutters, J.C.; Bos, W.J.; Rijkers, G.T. Dexamethasone downregulates the systemic cytokine response in patients with community-acquired pneumonia. Clin. Vaccine Immunol. 2012, 19, 1532–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihailidou, I.; Pelekanou, A.; Pistiki, A.; Spyridaki, A.; Tzepi, I.M.; Damoraki, G.; Giamarellos-Bourboulis, E.J. Dexamethasone Down-Regulates Expression of Triggering Receptor Expressed on Myeloid Cells-1: Evidence for a TNFα-Related Effect. Front. Public Health 2013, 1, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalmers, J.D.; Short, P.M.; Mandal, P.; Akram, A.R.; Hill, A.T. Statins in community acquired pneumonia: Evidence from experimental and clinical studies. Respir. Med. 2010, 104, 1081–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parihar, S.P.; Guler, R.; Brombacher, F. Statins: A viable candidate for host-directed therapy against infectious diseases. Nat. Rev. Immunol. 2019, 19, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Lukan, N. “Cytokine storm”, not only in COVID-19 patients. Mini-review. Immunol. Lett. 2020, 228, 38–44. [Google Scholar] [CrossRef]
- Geiler, J.; Michaelis, M.; Naczk, P.; Leutz, A.; Langer, K.; Doerr, H.W.; Cinatl, J. N-acetyl-L-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza A virus. Biochem. Pharm. 2010, 79, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Kanoh, S.; Rubin, B.K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin. Microbiol. Rev. 2010, 23, 590–615. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowska, B.; Maślińska, M. Macrolide therapy in chronic inflammatory diseases. Mediat. Inflamm. 2012, 2012, 636157. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.; Dong, C. Therapeutic antibodies that target inflammatory cytokines in autoimmune diseases. Int. Immunol. 2016, 28, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Tauber, P.A.; Pickl, W.F. Pharmacological targeting of allergen-specific T lymphocytes. Immunol. Lett. 2017, 189, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Therapeutic strategies for allergic diseases. Nature 1999, 402, B31–B38. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Botran, R.; Crespo, F.A.; Sun, X. Soluble cytokine receptors in biological therapy. Expert Opin. Biol. 2002, 2, 585–605. [Google Scholar] [CrossRef] [PubMed]
- Borish, L.C.; Nelson, H.S.; Lanz, M.J.; Claussen, L.; Whitmore, J.B.; Agosti, J.M.; Garrison, L. Interleukin-4 receptor in moderate atopic asthma. A phase I/II randomized, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 1999, 160, 1816–1823. [Google Scholar] [CrossRef]
- Borish, L.C.; Nelson, H.S.; Corren, J.; Bensch, G.; Busse, W.W.; Whitmore, J.B.; Agosti, J.M.; Group, I.-R.A.S. Efficacy of soluble IL-4 receptor for the treatment of adults with asthma. J. Allergy Clin. Immunol. 2001, 107, 963–970. [Google Scholar] [CrossRef]
- Corren, J.; Busse, W.; Meltzer, E.O.; Mansfield, L.; Bensch, G.; Fahrenholz, J.; Wenzel, S.E.; Chon, Y.; Dunn, M.; Weng, H.H.; et al. A randomized, controlled, phase 2 study of AMG 317, an IL-4Ralpha antagonist, in patients with asthma. Am. J. Respir. Crit. Care Med. 2010, 181, 788–796. [Google Scholar] [CrossRef]
- Nagase, H.; Ueki, S.; Fujieda, S. The roles of IL-5 and anti-IL-5 treatment in eosinophilic diseases: Asthma, eosinophilic granulomatosis with polyangiitis, and eosinophilic chronic rhinosinusitis. Allergol. Int. 2020, 69, 178–186. [Google Scholar] [CrossRef]
- Kips, J.C.; O’Connor, B.J.; Langley, S.J.; Woodcock, A.; Kerstjens, H.A.; Postma, D.S.; Danzig, M.; Cuss, F.; Pauwels, R.A. Effect of SCH55700, a humanized anti-human interleuki.in-5 antibody, in severe persistent asthma: A pilot study. Am. J. Respir. Crit. Care Med. 2003, 167, 1655–1659. [Google Scholar] [CrossRef]
- Flood-Page, P.T.; Menzies-Gow, A.N.; Kay, A.B.; Robinson, D.S. Eosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am. J. Respir. Crit. Care Med. 2003, 167, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Kane, B.; Molfino, N.A.; Faggioni, R.; Roskos, L.; Woodcock, A. A phase 1 study evaluating the pharmacokinetics, safety and tolerability of repeat dosing with a human IL-13 antibody (CAT-354) in subjects with asthma. BMC Pulm. Med. 2010, 10, 3. [Google Scholar] [CrossRef] [Green Version]
- Alvaro-Lozano, M.; Akdis, C.A.; Akdis, M.; Alviani, C.; Angier, E.; Arasi, S.; Arzt-Gradwohl, L.; Barber, D.; Bazire, R.; Cavkaytar, O.; et al. EAACI Allergen Immunotherapy User’s Guide. Pediatr. Allergy Immunol. 2020, 31 (Suppl. S25), 1–101. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M.; Akitsu, K.; Kubota, K. Effect of Sublingual Immunotherapy on Airway Inflammation and Airway Wall Thickness in Allergic Asthma. J. Allergy Clin. Immunol. Pr. 2019, 7, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- Xian, M.; Feng, M.; Dong, Y.; Wei, N.; Su, Q.; Li, J. Changes in CD4+CD25+FoxP3+ Regulatory T Cells and Serum Cytokines in Sublingual and Subcutaneous Immunotherapy in Allergic Rhinitis with or without Asthma. Int. Arch. Allergy Immunol. 2020, 181, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Skuljec, J.; Chmielewski, M.; Happle, C.; Habener, A.; Busse, M.; Abken, H.; Hansen, G. Chimeric Antigen Receptor-Redirected Regulatory T Cells Suppress Experimental Allergic Airway Inflammation, a Model of Asthma. Front. Immunol. 2017, 8, 1125. [Google Scholar] [CrossRef] [Green Version]
- Mirershadi, F.; Ahmadi, M.; Rezabakhsh, A.; Rajabi, H.; Rahbarghazi, R.; Keyhanmanesh, R. Unraveling the therapeutic effects of mesenchymal stem cells in asthma. Stem Cell Res. 2020, 11, 400. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, E.O.; Berkowitz, R.B.; Grossbard, E.B. An intranasal Syk-kinase inhibitor (R112) improves the symptoms of seasonal allergic rhinitis in a park environment. J. Allergy Clin. Immunol. 2005, 115, 791–796. [Google Scholar] [CrossRef] [PubMed]

| Infectious Agent | PAMPs | PRR |
|---|---|---|
| Bacteria | LPS | TLR4 |
| Lipoproteins, peptidoglycan | TLR2,6 | |
| Flagellin | TLR5 | |
| Bacterial DNA (CpG) | TLR9 | |
| Muramyl dipeptide (MDP) | NOD2 | |
| Diaminopimelic acid | NOD1 | |
| Viruses | Haemagglutinin (HA) | TLR2 |
| DsRNA | TLR3 | |
| Short dsRNA | RIG-1 | |
| Long dsRNA | MDA-5 | |
| ssRNA | TLR7/8 | |
| dsDNA | TLR9, NLRP3 | |
| Fungi | β-Glucan | TLR2, Dectin-1 |
| phospholipomannon | TLR2 | |
| Mannose | Mannose receptors |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upadhyay, M.; Nehme, A.; Wasnik, S. Title-Inflammatory Signaling Pathways in Allergic and Infection-Associated Lung Diseases. Allergies 2022, 2, 57-74. https://doi.org/10.3390/allergies2020006
Upadhyay M, Nehme A, Wasnik S. Title-Inflammatory Signaling Pathways in Allergic and Infection-Associated Lung Diseases. Allergies. 2022; 2(2):57-74. https://doi.org/10.3390/allergies2020006
Chicago/Turabian StyleUpadhyay, Mala, Antoine Nehme, and Samiksha Wasnik. 2022. "Title-Inflammatory Signaling Pathways in Allergic and Infection-Associated Lung Diseases" Allergies 2, no. 2: 57-74. https://doi.org/10.3390/allergies2020006
APA StyleUpadhyay, M., Nehme, A., & Wasnik, S. (2022). Title-Inflammatory Signaling Pathways in Allergic and Infection-Associated Lung Diseases. Allergies, 2(2), 57-74. https://doi.org/10.3390/allergies2020006






