Nasal Administration of Lipopolysaccharide Exacerbates Allergic Rhinitis through Th2 Cytokine Production from Mast Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Immunization Protocol and Treatment
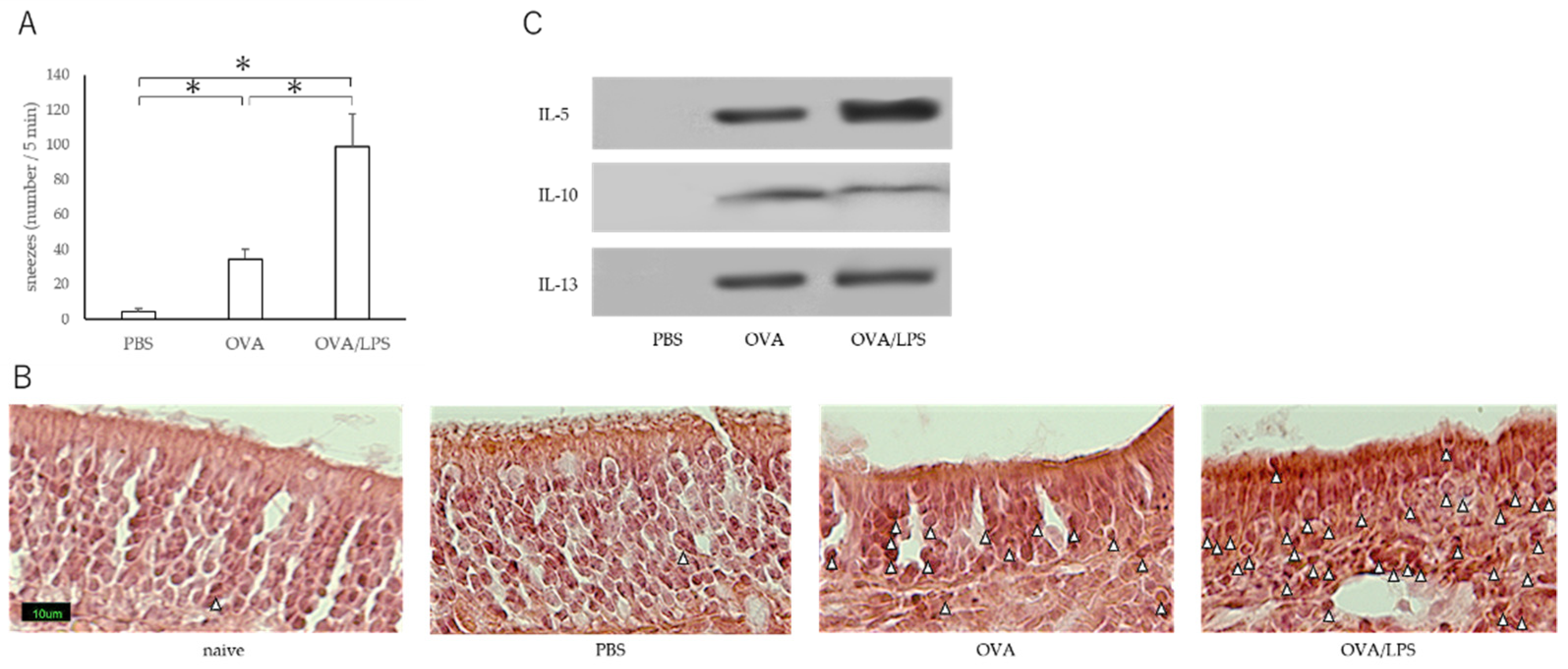
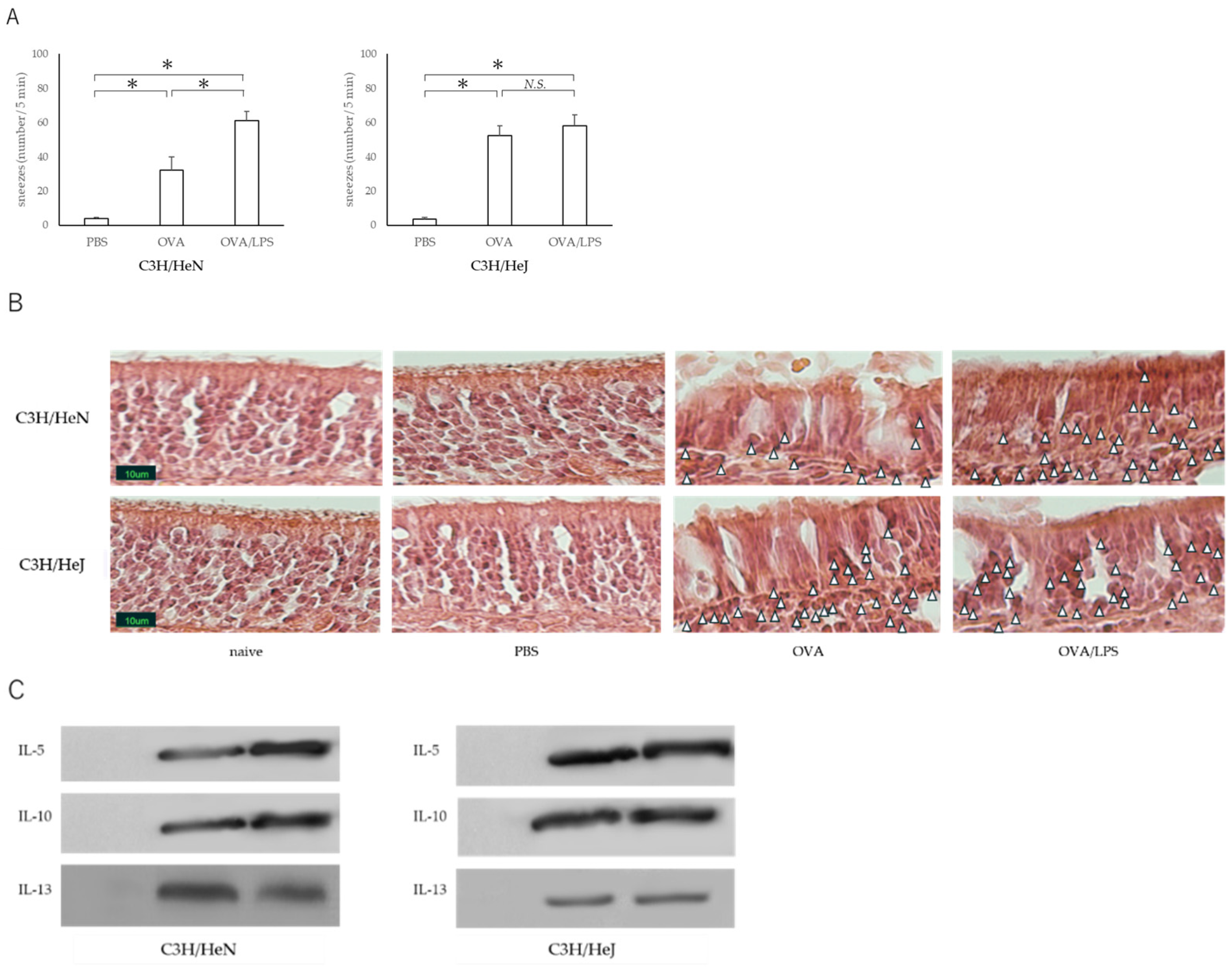
2.3. Evaluation of Nasal Signs
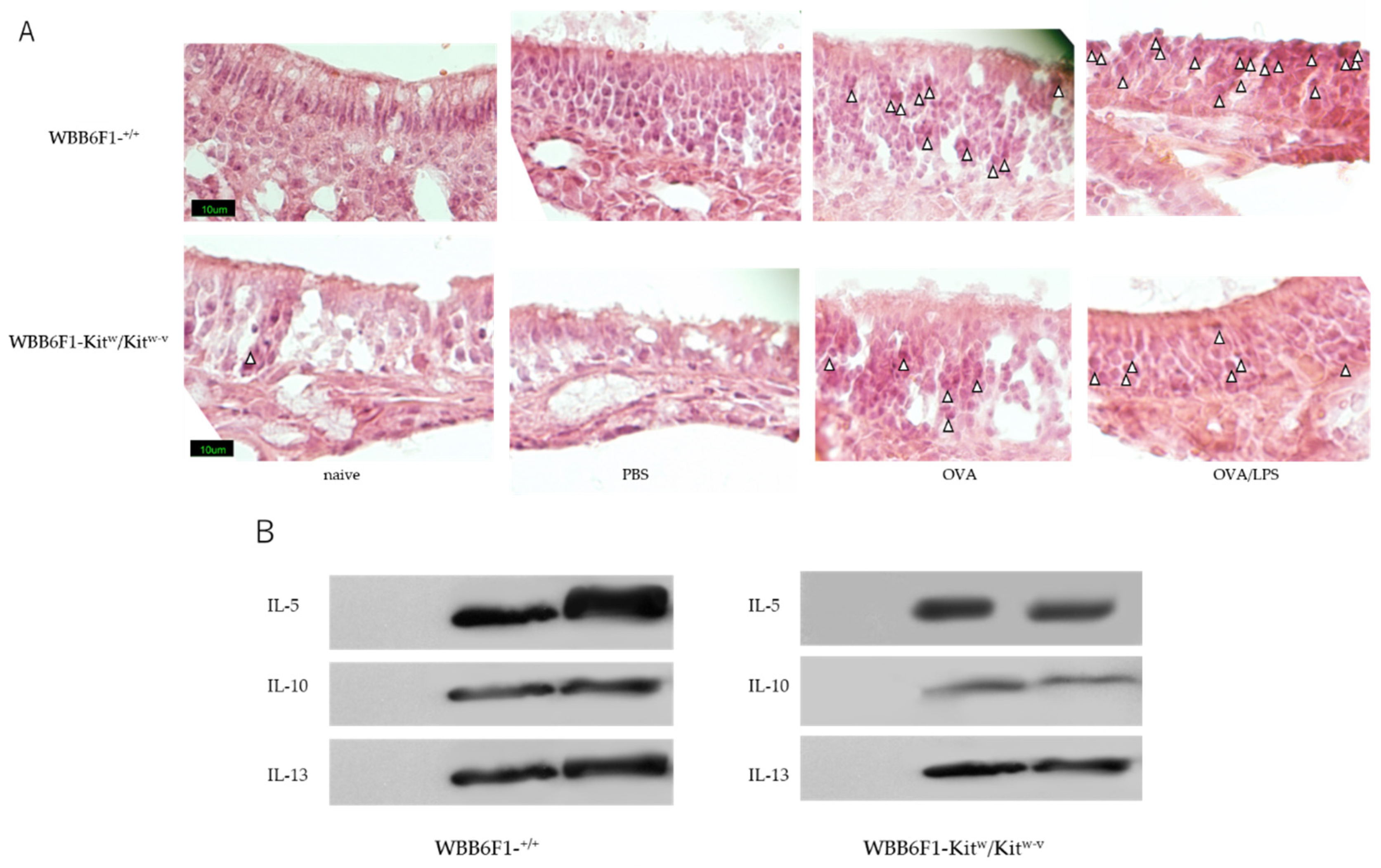
2.4. Histological Examination
2.5. Western Blot Analysis
2.6. Preparation of Murine Bone Marrow–Derived Mast Cells
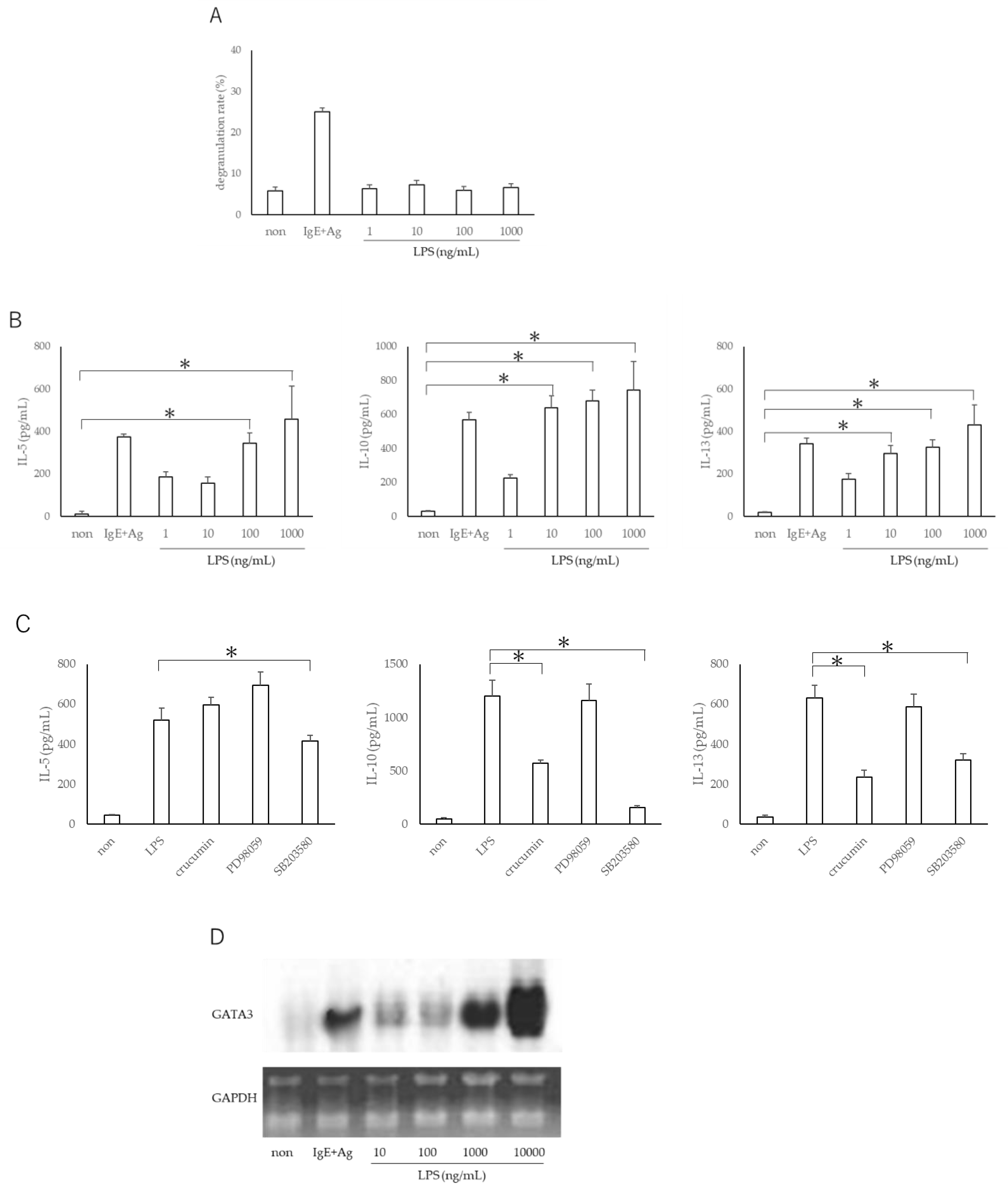
2.7. Measurement of Cytokine Productions from BMMCs
2.8. Measurement of Degranulation of BMMCs
2.9. Northern Blot Analyses
2.10. Statistical Analysis
3. Results
3.1. Nasal Administration of LPS Together with the Antigen Exacerbates Nasal Allergy via TLR4 of Mast Cells
3.2. LPS Does Not Exacerbate Mast Cell Degranulation but Promotes TH2 Production from Mast Cells via Expression of GATA3 Gene
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Darveaux, J.I.; Lemanske, R.F., Jr. Infection-related asthma. J. Allergy Clin. Immunol. Pract. 2014, 2, 658–663. [Google Scholar] [CrossRef] [Green Version]
- Reed, C.E.; Milton, D.K. Endotoxin-stimulated innate immunity: A contributing factor for asthma. J. Allergy Clin. Immunol. 2001, 108, 157–166. [Google Scholar] [CrossRef]
- Braun-Fahrländer, C.; Riedler, J.; Herz, U.; Eder, W.; Waser, M.; Grize, L.; Maisch, S.; Carr, D.; Gerlach, F.; Bufe, A.; et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N. Engl. J. Med. 2002, 347, 869–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klintberg, B.; Berglund, N.; Lilja, G.; Wickman, M.; van Hage-Hamsten, M. Fewer allergic respiratory disorders among farmers’ children in a closed birth cohort from Sweden. Eur. Respir. J. 2001, 17, 1151–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, T.; Akira, S. TLR signaling. Semin. Immunol. 2007, 19, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Gerhold, K.; Blümchen, K.; Bock, A.; Seib, C.; Stock, P.; Kallinich, T.; Löhning, M.; Wahn, U.; Hamelmann, E. Endotoxins prevent murine IgE production, TH2 immune responses, and development of airway eosinophilia but not airway hyperreactivity. J. Allergy Clin. Immunol. 2002, 110, 110–116. [Google Scholar] [CrossRef]
- Murakami, D.; Yamada, H.; Yajima, T.; Masuda, A.; Komune, S.; Yoshikai, Y. Lipopolysaccharide inhalation exacerbates allergic airway inflammation by activating mast cells and promoting Th2 responses. Clin. Exp. Allergy 2007, 37, 339–347. [Google Scholar] [CrossRef]
- von Mutius, E.; Smits, H.H. Primary prevention of asthma: From risk and protective factors to targeted strategies for prevention. Lancet 2020, 396, 854–866. [Google Scholar] [CrossRef]
- Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of asthma in children and adults. Front. Pediatr. 2019, 7, 246. [Google Scholar] [CrossRef]
- Schuijs, M.J.; Willart, M.A.; Vergote, K.; Gras, D.; Deswarte, K.; Ege, M.J.; Madeira, F.B.; Beyaert, R.; van Loo, G.; Bracher, F.; et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science 2015, 349, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhin 2020, 58, 1–464. [Google Scholar] [CrossRef]
- Kawauchi, H.A.; Aoi, N.; Morikura, I.; Fuchiwaki, T.; Shimizu, Y.; Shimizu, K.; Hotta, Y.; Infei, Q.; Yamada, T.; Prokopakis, E. Oxatomide inhibits interleukin-8 release from respiratory epithelial cells. RHINOL 2018, 1, 50–56. [Google Scholar] [CrossRef]
- Xu, H.; Shu, H.; Zhu, J.; Song, J. Inhibition of TLR4 inhibits allergic responses in murine allergic rhinitis by regulating the NF-κB pathway. Exp. Ther. Med. 2019, 18, 761–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renkonen, J.; Toppila-Salmi, S.; Joenväärä, S.; Mattila, P.; Parviainen, V.; Hagström, J.; Haglund, C.; Lehtonen, M.; Renkonen, R. Expression of toll-like receptors in nasal epithelium in allergic rhinitis. APMIS 2015, 123, 716–725. [Google Scholar] [CrossRef]
- Aoi, N.; Morikura, I.; Fuchiwaki, T.; Yamada, T.; Prokopakis, E.; Kawauchi, H. OK-432 administration inhibits murine allergic rhinitis at the induction phase, through the macrophage activation with TLR2 signaling pathway. Med. Sci. 2018, 6, 107. [Google Scholar] [CrossRef] [Green Version]
- Snella, M.C. Effects of bacterial endotoxin inhalation. Rev. Epidemiol. Sante Publique 1981, 29, 209–216. [Google Scholar] [PubMed]
- Supajatura, V.; Ushio, H.; Nakao, A.; Okumura, K.; Ra, C.; Ogawa, H. Protective roles of mast cells against enterobacterial infection are mediated by toll-like receptor 4. J. Immunol. 2001, 167, 2250–2256. [Google Scholar] [CrossRef] [Green Version]
- Enerbäck, L.; Pipkorn, U.; Olofsson, A. Intraepithelial migration of mucosal mast cells in hay fever: Ultrastructural observations. Int. Arch. Allergy Appl. Immunol. 1986, 81, 289–297. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.Z.M.; Jamur, M.C.; Oliver, C. Mast cell function: A new vision of an old cell. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef]
- Zhang, D.H.; Yang, L.; Ray, A. Differential responsiveness of the IL-5 and IL-4 genes to transcription factor GATA-3. J. Immunol. 1998, 161, 3817–3821. [Google Scholar]
- Rosewich, M.; Lee, D.; Zielen, S. Pollinex Quattro: An innovative four injections immunotherapy in allergic rhinitis. Hum. Vaccines Immunother. 2013, 9, 1523–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaar, O.; Barth, C.; Jaschke, C.; Hörmann, K.; Klimek, L. Sublingual allergen-specific immunotherapy adjuvanted with monophosphoryl lipid A: A phase I/IIa study. Int. Arch. Allergy Immunol. 2011, 154, 336–344. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoi, N.; Fuchiwaki, T.; Morikura, I.; Kawauchi, H.; Sakamoto, T. Nasal Administration of Lipopolysaccharide Exacerbates Allergic Rhinitis through Th2 Cytokine Production from Mast Cells. Allergies 2021, 1, 216-224. https://doi.org/10.3390/allergies1040020
Aoi N, Fuchiwaki T, Morikura I, Kawauchi H, Sakamoto T. Nasal Administration of Lipopolysaccharide Exacerbates Allergic Rhinitis through Th2 Cytokine Production from Mast Cells. Allergies. 2021; 1(4):216-224. https://doi.org/10.3390/allergies1040020
Chicago/Turabian StyleAoi, Noriaki, Takafumi Fuchiwaki, Ichiro Morikura, Hideyuki Kawauchi, and Tatsunori Sakamoto. 2021. "Nasal Administration of Lipopolysaccharide Exacerbates Allergic Rhinitis through Th2 Cytokine Production from Mast Cells" Allergies 1, no. 4: 216-224. https://doi.org/10.3390/allergies1040020
APA StyleAoi, N., Fuchiwaki, T., Morikura, I., Kawauchi, H., & Sakamoto, T. (2021). Nasal Administration of Lipopolysaccharide Exacerbates Allergic Rhinitis through Th2 Cytokine Production from Mast Cells. Allergies, 1(4), 216-224. https://doi.org/10.3390/allergies1040020






