Abstract
This comprehensive review examines the transformative role of metal–organic frameworks (MOFs) in advancing battery separator technology to address critical safety challenges in rechargeable lithium metal batteries. MOF-based separators leverage their highly specific surface area, tunable pore structures, and functionalized organic ligands to enable precise ion-sieving effects, uniform lithium-ion flux regulation, and dendrite suppression—significantly mitigating risks of internal short circuits and thermal runaway. We systematically analyze the mechanisms by which classical MOF families (e.g., ZIF, UiO, MIL series) enhance separator performance through physicochemical properties such as electrolyte wettability, thermal stability (>400 °C), and mechanical robustness. Furthermore, we highlight innovative composite strategies integrating MOFs with polymer matrices (e.g., PVDF, PAN) or traditional separators, which synergistically improve ionic conductivity while inhibiting polysulfide shuttling in lithium–sulfur batteries and side reactions in aqueous zinc-ion systems. Case studies demonstrate that functionalized MOF separators achieve exceptional electrochemical outcomes: Li–S batteries maintain >99% Coulombic efficiency over 500 cycles, while solid-state batteries exhibit 2400 h dendrite-free operation. Despite promising results, scalability challenges related to MOF synthesis costs and long-term stability under operational conditions require further research. This review underscores MOFs’ potential as multifunctional separator materials to enable safer, high-energy-density batteries and provides strategic insights for future material design.
1. Introduction
In recent years, the continuous advancement of battery technology has greatly promoted the development of new energy vehicles and energy storage systems, facilitating its transformation [1,2]. However, battery safety issues such as fires and short circuits have always been key challenges restricting its large-scale application. In this context, the research and application of battery separators, as one of the core components of batteries, are moving towards high performance, high safety and low cost. Their performance has a decisive impact on the safety, energy density and cycle life of batteries, and their core position in the fields of new energy vehicles and energy storage is increasingly prominent [3].
As a key component of energy storage devices, the core role of the separator is to isolate the positive and negative electrodes to prevent short [4,5]. To enhance battery performance, a good separator should have the following characteristics: electrical insulation, which can isolate the positive and negative electrodes to avoid short circuits, and high ionic conductivity [6] to specific ions after being impregnated; excellent chemical and thermal stability, not reacting with electrodes and electrolyte during cycling, maintaining physical stability within the operating temperature range of the battery without swelling, dissolution, etc.; higher mechanical strength, less likely to be punctured by dendrites, with both tensile strength and flexibility; a suitable and uniform pore structure to separate the target ions, regulate the uniform transport of ions on the electrode, and increase the ion transport flux [7]; good electrolyte wettability ensures effective ion transport [8]; appropriate thickness, too thick a separator will increase the battery’s internal resistance and reduce energy density; high safety, preventing battery thermal runaway, ensuring equipment and personal safety (Scheme 1).
Scheme 1.
The application of diaphragms in battery safety.
Currently, glass fiber (GF), cellulose filter paper, polyethylene (PP), and polypropylene (PE) are commonly used as separators in batteries. Among them, glass fiber separators have high porosity, strong hydrophilicity, high ionic conductivity (about 17.3 mS cm−1), and are compatible with electrodes at all levels. However, due to the low mechanical strength of glass fiber separators, they are prone to being punctured by dendrites, causing internal short circuits, which is the biggest obstacle to their application [9]. In addition, to achieve sufficient mechanical strength, it would result in a thickness close to 600 μm, which affects battery portability and reduces battery energy density. And it is more complex and expensive to process into uniform sheets than polymer separators [10]. Cellulose filter paper, which is mainly composed of natural cellulose, is degradable and environmentally friendly, readily available, and is one of the cheapest diaphragm materials. It has good wettability and can quickly absorb electrolytes [11], but it is very fragile and can be punctured by dendrites or torn under slight force, with a very high risk of short circuit. In addition, its pore size is large and uneven, making it difficult to suppress dendrite growth and prone to side reactions such as hydrogen evolution and corrosion [12]. Polyethylene and polypropylene separators are currently the most promising and mainstream separator options in the research and application of aqueous zinc-ion batteries (AZIBs), as they have excellent mechanical strength that can resist zinc dendrite puncture, reduce the risk of short circuit, and significantly increase cycle life. In addition, they can be made into ultrathin films that do not shrink or deform easily, which is beneficial for increasing the energy density and power density of the battery, and they have good chemical stability [13]. However, traditional polyolefin separators have insufficient wettability [14], which leads to low ionic conductivity and thus affects battery performance. In summary, traditional separators are widely used in batteries because of their mature and stable production technology, controllable cost, and electronic insulation function. However, due to their limited dendrite suppression ability and the large disordered internal pore structure that may cause uneven charge distribution, resulting in uneven deposition of metal ions, which in turn causes separator puncture and ultimately leads to battery short-circuit failure [15]. Therefore, to develop batteries with high performance, long lifespan, and high safety, it is essential to create an ultrathin separator that is low-cost, mechanically robust, highly ion-conductive, and resistant to dendrite penetration [16].
Metal–organic framework (MOF) materials, also known as porous coordination polymer materials, are structurally ordered functional porous crystal materials [17,18] and organic ligands (such as hydroxyl ligands, phosphate ligands, carboxylic acid ligands, etc.). Owing to their high porosity, large specific surface area, tunable pore channels, and abundant unsaturated metal sites [19,20], MOF materials have a wide range of applications, such as gas storage and separation [21], catalysis [22], adsorption [23], fluorescence sensors [24], drug delivery [25], energy [26], etc.
In addition, MOF materials have great potential in addressing dendrite growth in batteries. The key advantage lies in their ultra-high specific surface area and porosity, and their abundant electrochemically active sites give batteries a high capacity. The rigid framework of the MOF material provides a certain mechanical strength for the battery separator, helps prevent dendrite puncture, and shows good chemical stability in the electrolyte environment, significantly improves the wettability of the composite separator to the electrolyte, reduces interfacial impedance, and by effectively suppressing dendrites and reducing side reactions such as corrosion and HER, it can significantly enhance Coulombic efficiency and battery cycle life [27]. In addition, the high specific surface area and rich pore structure of the MOF provide transport channels for a large number of ions, facilitating the rapid transport of ions in the separator impregnated with the electrolyte. Its highly ordered and uniform microporous structure allows ions to pass through, contributing to the improvement in ionic conductivity, making the MOF material a highly promising candidate [28].
MOF separators are made by loading MOF nanoparticles onto traditional separators, usually in combination with traditional separators, and loading MOF nanoparticles on their surfaces through in situ growth, template methods, etc., to prepare new composite separators with excellent performance [27]. This modification is suitable for large-scale applications and provides a new approach to developing high-performance, safe and durable batteries [29].
The current mainstream MOFs that can be used as separator materials include the ZIF series, the UiO series, the HKUST-1 and the MIL series [30]. The ZIF series (Zeolitic Imidazolate Frameworks) are formed by coordinating imidazole ligands with metal ions such as Zn2+ and Co2+, and have excellent chemical stability in water and organic solvents [31,32]. The most typical examples are ZIF-8 and ZIF-67. The ZIF series has a large specific surface area, good electrical conductivity, and adjustable structure. Its rich porous structure and chemically active sites can effectively confine polysulfide shuttle, inhibit dendrite growth, accelerate ion transport, and enhance the specific capacity and cycle stability of the battery. Han et al. [33] coated polypropylene separators with a mixture of ZIF-67 and PVDF to prepare multifunctional PP@ZIF-67 separators, using the anion affinity and ion nanochannels of ZIF-67 to regulate ion transport, which effectively inhibited lithium dendrite growth and improved cycle stability, rate performance and safety of lithium metal batteries. Razaq et al. [34] designed a bifunctional bimetallic MOF (Fe-ZIF-8)-modified polypropylene separator to address the problems of polysulfide dissolution shuttle and lithium dendrite formation in lithium–sulfur batteries. The separator selectively blocks polysulfide migration through nanostructured pores, catalyzes polysulfide conversion at iron active sites, and achieves uniform lithium-ion transport to suppress dendrites, significantly enhancing battery cycle life and capacity performance. The UiO series (University of Oslo), with Zr4+ as the metal center and ternary acid (BDC) as the organic ligand, forms metal–organic framework structures with ultra-high chemical and thermal stability and pore size of approximately 0.5–1 nm through coordination [35]. UiO-66 and UiO-67 are representative of this series. In the case of UiO-66, its abundant electronegative sites can facilitate the rapid migration of lithium ions while repelling anion migration. Fan [36] constructed UiO-PP separators with both physical sieving and chemical adsorption barriers by coating UiO-66, which has excellent chemical and thermal stability, on commercial polypropylene separators. The separator significantly inhibited polysulfide shuttling, and the Li-S battery maintained an average capacity of approximately 720 mAh g−1 after 500 cycles at 0.5 C, with a Coulombic efficiency close to 100%. DFT calculations further confirmed the strong electrostatic adsorption between the carboxylic acid ligands of UiO-66 and Li+ and Sn2−, which effectively fixed the polysulfides. Jia et al. [37] used biodegradable bacterial cellulose (BC) as the matrix and uniformly anchored UiO-66-NH2 particles through epichlorohydrin crosslinking to construct BC/UiO-66-NH2 composite diaphragms. The microporous structure of UiO-66-NH2 stores liquid and regulates pore size, and its -NH2 can form hydrogen bonds with PF6−, increasing the lithium-ion migration number from 0.45 in PP separators to 0.62, and maintaining a discharge specific capacity of 145.4 mAh g−1 after 50 cycles, with performance significantly superior to that of a single PP separator. In addition, the nitrogen source provided by the MOF enables the composite separator to be completely degraded by soil microorganisms within 15 days, offering high safety, cycling stability and environmental friendliness. Zhao et al. [38] constructed BC@UiO-66 composite membranes by uniformly anchoring Zr-based UiO-66 on the surface of bacterial cellulose nanofibers through a room-temperature in situ growth strategy. The membrane significantly enhanced electrolyte absorption (885.5%) and ionic conductivity (11.23 mS cm−1) by means of graded pores, Lewis acid sites and high specific surface area (534 m2g−1), and induced Zn2+ level (002) crystal plane deposition, effectively suppressing zinc dendrites. The symmetrical zinc cell stably cycled at 1 mA cm−2 for 2400 h, and the full Zn/MnO2 cell maintained a capacity of 162.98 mAh g−1 after 1000 cycles of 1 A g−1, with a Coulombic efficiency of approximately 100%. HKUST-1, also known as Cu-BTC, is composed of Cu2+ coordinated with benzoic acid (BTC) and has a three-dimensional open structure with a pore size of approximately 0.9 nm. The microporous HKUST-1 can regulate Li+ distribution and increase Li+ migration number [39]. Cu2+ contains unsaturated metal sites and has a strong adsorption effect efficiently intercepts polar impurity ions [40,41]. Deng et al. [42] introduced ZIF-67 and HKUST-1 into fluorine-doped PMIA nanofiber membranes through blending electrospinning, and for the first time, constructed a gel composite separator with both high thermal stability and mechanical strength. The Co/Cu active centers of ZIF-67/HKUST-1 significantly suppressed polysulfide transmigration through enhanced chemical adsorption–catalytic synergy, resulting in a first-week discharge capacity of 1267–1272 mAh g−1 for the Li–S battery and a remaining 698–753 mAh g−1 after 500 cycles, with a Coulombic efficiency greater than 99.7%. Its microporous structure homogenizes lithium flux and effectively suppresses lithium dendrite growth.
The MIL series (Materials of Institute Lavoisier) are mostly formed with metal ions such as Fe3+ and Cr3+ and good water stability [43]. MIL-101 is a typical example of the composite separator prepared by blending it with traditional separators. It has both thermal stability and good electrolyte wettability. Its unsaturated metal sites can chemically complex with anions in the electrolyte, increasing the number of lithium-ion migrations and enhancing the electrochemical performance of lithium-ion batteries. Zhao et al. [30] first combined three morphologies (octahedron, polymeric, spherical) of MIL-101(Cr) with cellulose to form AZIBs separators and systematically compared the morphological effects. The results showed that the spherical MIL-101(Cr) separator had a maximum conductivity of 7.50 mS cm−1. The cycle life of the Zn//Zn symmetric cell was greater than 750 h, and the capacity of the Zn//MnO2 full cell remained at 83.6% after 400 cycles, all far superior to other morphologies. Jia et al. [44] formed PMIA/MIL-101 composite separators by uniformly embedding high specific surface area MIL-101(Cr) into PMIA separators through a one-step non-solvent phase conversion method. The chemical complexation of the Cr3+ active site with PF6− in the electrolyte significantly inhibited anion migration, increasing the Li+ migration number from 0.23 to 0.65, and the improvement in pore size and wettability increased ionic conductivity to 1.32 mS cm−1. After 50 cycles at 2C rate, the discharge specific capacity reached 120.4 mAh g−1, 1.5 times that of a single PP separator battery; there is no significant shrinkage at 250 °C, and it has both flame-retardant and self-extinguishing properties, achieving a synergistic improvement in safety and electrochemical performance.
Based on this, this paper mainly reviews the latest research progress of MOF separators for batteries in the field of battery separators in recent years, such as design strategies for lithium-ion batteries, aqueous zinc-ion batteries, lithium–sulfur batteries, etc., and on this basis, discusses the development prospects of functionalized separators.
2. Mechanisms for Improving Safety Performance Based on MOF Separators
2.1. Dendrite Inhibition Capability
Dendrite growth has always been an unavoidable serious problem during battery operation. Dendrite growth can disrupt the uniformity and integrity of the separator and reduce battery performance. When dendrites grow to a certain extent, they pierce the separator, causing direct contact between the positive and negative electrodes of the battery, resulting in a short circuit of the battery and the risk of thermal runaway [45], which studies have shown that MOF separators can effectively suppress [46,47]. On the one hand, the ordered structure of MOF provides channels for the directional migration of ions, guiding the uniform transport of ions, preventing local excessive aggregation on the anode surface and inhibiting dendrite nucleation [48]. Meanwhile, the pore size of the MOF can act as an ion sieving, allowing only ions of the appropriate size to pass through, further ensuring the uniform deposition of selectively transported ions, thereby reducing dendrite formation at the root. For example, Ma et al. [49] used MOF diaphragms to screen solvated ions through sub-nano channels, blocking the entry of hydrated zinc ions (Zn(H2O)62+) and allowing only Zn2+ to pass through, achieving desolvation and the selective transport of target ions and suppressing dendrite formation. At the same time, the amino functional groups in organic ligands are used to fix water molecules through hydrogen bonds, reduce side reactions, and synergistically promote the uniform deposition of zinc. On the other hand, some MOFs contain special functional groups that can regulate the distribution of charges and generate electrostatic interactions with ions in the electrolyte. Using this electrostatic interaction, MOFs can adsorb and transport target ions with high selectivity, suppress local concentration polarization, and repel impurity ions or molecules that cause dendrite growth. Wang et al. [46] introduced negatively charged groups (-SO3−) into the nanochannels of the MOF separator, using electrostatic repulsion to suppress anion migration while accelerating Li+ transport, thereby significantly increasing the Li+ migration number (tLi+ = 0.85) (as shown in Figure 1d). Furthermore, during the charging and discharging process of the battery, a stable solid electrolyte interface (SEI) film forms on the electrode surface. If the SEI film is unstable, it is prone to rupture, causing metal ions to deposit first at the rupture site, thereby triggering dendrite growth. However, the MOF separator can promote the formation of a stable SEI film through interaction with the electrolyte and the electrode (as shown in Figure 1e–h). For example, Cai et al. [50] prepared cobalt-based metal–organic framework (Co-MOFs)-modified graphite composites by solvothermal method and used them as the anode of lithium-ion batteries, aiming to improve the cycling stability of the graphite anode. The study found that Co-MOFs coating the surface of graphite could form a solid-state electrolyte interface (SEI) film, altering the crystal planes and defect states of graphite, with 15% co-MoFS-modified graphite anodes performing the best. The anode had initial discharge specific capacities of 368.92, 290.06, and 268.99 mAh g−1 at current densities of 100, 200, and 300 mA g−1, with a capacity retention rate of over 80% after 50 cycles, good reversibility, low charge transfer resistance, and fast lithium-ion diffusion rate. Based on a study by Liu et al. [51], a dual-electrolyte system was achieved in high-voltage lithium metal batteries using a metal–organic framework functionalized separator with solvent sieving ability (PE@MOF), which blocks the diffusion of the two electrolytes and enables the electrolyte interface between the positive and negative electrodes to be independently optimized (as shown in Figure 1a,b). By using carbonate electrolyte on the cathode side and ether electrolyte on the anode side, a robust cathode electrolyte interface (CEI) and solid electrolyte interface (SEI) were constructed on the two electrodes, respectively, significantly extending the life of the LiCoO2/Li full battery (as shown in Figure 1c).
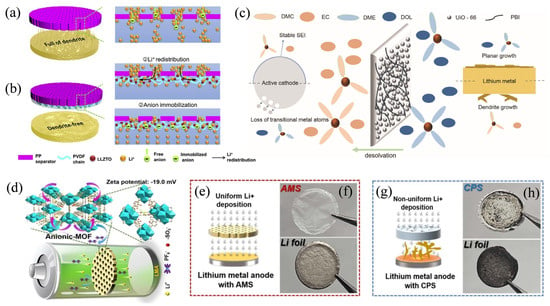
Figure 1.
Schematic illustrations of the Li+ deposition behaviors through (a) PP separator and (b) anion-immobilized PP@PLIZ separator with multiple highly conductive ion pathways. Reproduced with permission [52]. Copyright 2020 Elsevier. (c) Schematic illustration showing the working mechanism of dual electrolyte with PE@MOF. Reproduced with permission [51]. Copyright 2022 Tsinghua University Press. (d) Schematic illustration of the regulation of the transport of Li+ and anions in LMBs by the AMS. Study of the Li nucleation and growth of lithium metal anodes obtained from lithium symmetric coin cells equipped with the anionic MOF separator or a commercial polypropylene separator. (e,g) Schematic illustration of the lithium deposition mechanism of the batteries using AMS (f) and CPS (h). Reproduced with permission [46]. Copyright 2023 Royal Society of Chemistry.
2.2. Thermal Stability
Heat is generated during the operation of the battery. If the stability of the separator is poor, it may deform, shrink or even be punctured, causing a short circuit between the positive and negative terminals of the battery and triggering a safety accident [53]. MOF separators typically have good thermal stability, mainly due to the combination of high-melting-point and highly stable metal ions with rigid thermally stable organic ligands through strong coordination bonds to form highly ordered and rigid porous crystal frameworks [54,55]. This enables MOF to resist melting, contraction and pore collapse at high temperatures, ensuring the safe operation of batteries in fast-charging, high-power or extreme environments, and effectively preventing thermal runaway caused by diaphragm puncture, which is one of the important reasons why MOF materials have attracted much attention as high-performance battery separators. In the study by Zhou et al. [56], MIP-202@2320 composite separator was prepared by coating the surface of the battery separator with MIP-202 (as shown in Figure 2a), and the ionic and porous structure in MIP-202 was used to endow the separator with flame retardant properties, making the MIP-202@2320 composite separator have excellent heat resistance. The membrane retains its original size at 130 °C and has a thermal shrinkage rate of only 5% at 160 °C, indicating that the MIP-202@2320 composite separator can effectively suppress battery combustion and reduce heat release rate (as shown in Figure 2b–e). Leng et al. [57] modified Ni–Co bimetallic MOF and PAN electrospun separators to produce NCMP separators, which significantly enhanced the thermal stability of lithium–sulfur batteries at high temperatures and improved battery safety performance. The separator remained structurally intact at 200 °C, effectively suppressing thermal shrinkage and polysulfide shuttle caused by high temperatures, ensuring long-term stable operation of the battery under high-temperature conditions.
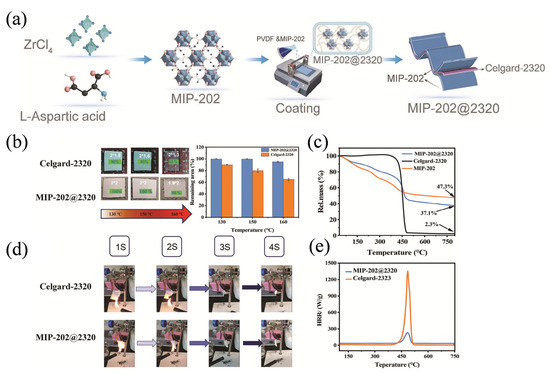
Figure 2.
(a) Schematic of the preparation process. (b) Thermal shrinkage test of bare Celgard-2320 and MIP-202@2320. (c) TGA curves of bare Celgard-2320, MIP-202, and MIP-202@2320. (d) Vertical combustion test; and (e) MCC curves of bare Celgard-2320 and MIP-202@2320. Reproduced with permission [56]. Copyright 2024 Wiley-VCH Verlag.
2.3. Mechanical Robustness
In addition to the physical isolation function, the separator also supports the battery. Therefore, whether during battery assembly or use, the separator needs to have good mechanical properties to withstand certain external forces without being damaged [58]. Some MOF materials themselves have high mechanical strength, and the mechanical properties of MOF separators can be regulated by choosing different metal ions and organic ligands, as well as by changing the reaction conditions. MOF separators can maintain a porous structure conducive to ion transport, and also have strong mechanical strength, which can resist a certain degree of external forces such as stretching and squeezing, prevent diaphragm damage due to mechanical damage, which would cause safety problems such as internal short circuits of the battery, thus ensuring safety and stability [59,60]. For example, Lan et al. [61] constructed three-dimensional multi-scale MOF separators by closely growing ZIF-8 on the surface of polyimide (PI) nanofibers through electrospinning—in situ self-assembly strategy, in response to the problems of poor electrochemical performance of lithium-ion batteries such as ease of puncture, poor electrolyte wettability and poor heat resistance of commercially available polyolefin separators. The PI@ZIF-8 separator maintained a tensile strength of 21.6 MPa in the dry state and only dropped to 21.1 MPa in the wet state after 24 h, far superior to pure PE separators. The 2000 h short-circuit-free Li//Li symmetric cycle life and 97.4% full cell capacity retention rate suggest that the PI@ZIF-8 composite separator can significantly suppress dendrites and improve interfacial stability. Gao et al. [62] fabricated rigid polybenzimidazole aramid (PBIA) nanofiber membranes by electrospinning with a high strength of 509.4 MPa and a high modulus of 10.9 GPa, and grew ZIF-67 nanosheets in situ to construct ZPBIA composite membranes with high porosity (91%) (as shown in Figure 3a), high thermal stability and high mechanical strength) (as shown in Figure 3b–g). The mechanical properties of the separator were far superior to those of commercial PP separators under high LiFePO4 loads (12 mg cm−2), effectively suppressing lithium dendrite punctures and ensuring the integrity of the separator (as shown in Figure 3h–j).
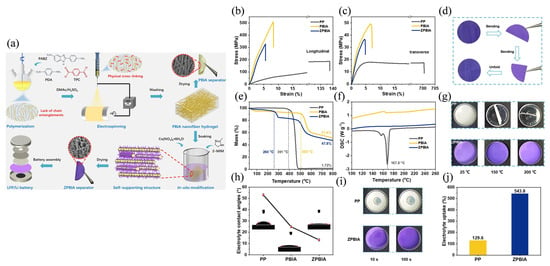
Figure 3.
(a) Illustration of the fabrication process of the ZPBIA separator. The tensile curves (b) parallel and (c) perpendicular to the oriented direction of PP, PBIA, and ZPBIA separators. (d) The flexible test of ZPBIA separator. (e) TGA curves, (f) DSC curves, and (g) the thermal dimensional stability test of PP and ZPBIA separators. (h) Liquid electrolyte contact angles of PP, PBIA, and ZPBIA separators. (i) The liquid electrolyte penetration speed and (j) uptake capacity for PP and ZPBIA separators. Reproduced with permission [62]. Copyright 2023 Elsevier B.V.
3. MOF as a Form and Strategy for Separators
3.1. Pure MOF Diaphragms
Pure MOF diaphragms are entirely composed of metal–organic frame materials, directly processed into independent films with sufficient mechanical strength, specific thickness and shape without the addition of any polymer binder or other supporting substrate.
The advantage of a single MOF as a separator material is that the MOF itself has regular and ordered channels and an extremely high specific surface area. Compared with the irregular pore structure and smaller pore size of traditional polymer separators, these unique nanoscale channels facilitate the transport of electrolyte ions, which can increase ion mobility and reduce battery resistance. Secondly, MOF materials have an ultra-high porosity. In batteries, high porosity means lower ion transport resistance, and higher ionic conductivity is beneficial for improving battery rate performance [17]. In addition, the pore size of the MOF material can be precisely regulated by choosing different metals or metal ion clusters and organic ligands, and this precise regulation can screen for the transport of specific molecules or ions. Finally, the MOF has excellent thermal and chemical stability, far exceeding that of traditional polyolefin separators (PP, PE), and can maintain a complete structure and functional integrity at high temperatures, avoiding battery short circuits caused by diaphragm contraction due to thermal runaway, significantly improving battery safety performance.
According to Kong et al. [63] research, pure MOF separators have insufficient chemical stability in aqueous electrochemical energy storage devices, are prone to structural collapse or degradation in acidic, alkaline or water environments, and pure MOF materials typically have poor electrical conductivity, making it difficult to meet the requirements of efficient ion transport and electrochemical reactions. Peng et al. [64] pointed out that pure MOF may irreversibly decompose or transform into amorphous phase during charge and discharge, disintegrate and frame collapse in water, acid and alkali environments, and its poor conductivity leads to high charge transfer resistance, which is not conducive to rate capacity and cycling stability. Yuan et al. [65] fabricated the HKUST-1@PVDF-HFP separator by in situ growth of HKUST-1 particles on a filter membrane via vacuum filtration, with PVDF-HFP employed as a binder to enhance flexibility (as shown in Figure 4a). Electrochemical evaluations revealed that this separator effectively suppressed lithium dendrite formation and the polysulfide shuttle effect, enabling a Li–S cell to retain a capacity of 802 mAh g−1 after 600 cycles (as shown in Figure 4b,c). The Cu2(CuTCPP) free-standing membrane was prepared through a wet-chemical synthesis of ultrathin nanosheets, followed by vacuum filtration to form a self-supporting film (as shown in Figure 4d). This separator delivered a capacity of 604 mAh g−1 after 900 cycles at 1 C, with an ultralow capacity decay of 0.032% per cycle, significantly outperforming conventional separators (as shown in Figure 4e,f).
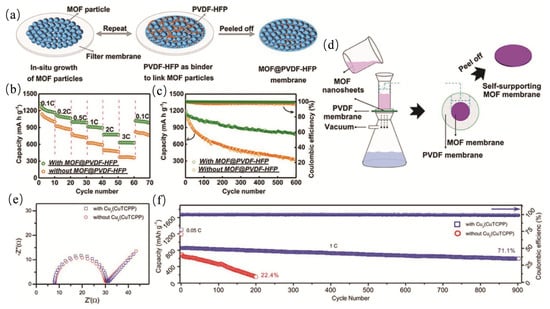
Figure 4.
(a) Schematic diagram of the formation of the HKUST−1@PVDF-HFP separator. (b) Rate capabilities of batteries (c) the long-term cyclability of batteries with and without the MOF@PVDF-HFP separator from 0.1 C to 3 C the MOF@PVDF-HFP separator. (d) Schematic illustration for the synthesis of Cu2(CuTCPP) self-supporting film. (e) EIS of the battery with Cu2(CuTCPP)−Celgard separator. (f) The long-term cyclability of theCu2(CuTCPP)−Celgard and Celgard cells at 1 C. Reproduced with permission [65]. Copyright 2021 John Wiley and Sons Ltd.
In battery applications, separators need to withstand stress during winding, bending and cycling without breaking. Although pure MOF separators can achieve a certain mechanical strength, their own brittleness remains a major obstacle to large-scale application [66]. And the contact between the pure MOF separator and the electrode interface is not as tight as that of the flexible polymer separator, which may lead to an increase in interface impedance and a reduction in the ionic conductivity of the battery, and there are still limitations in large-scale applications. Therefore, optimizing the interface remains an important research direction.
3.2. MOF Separator Materials
MOF diaphragm materials typically involve metal–organic framework materials as functional fillers or coatings, which are directly physically mixed and dispersed in polymer solutions (such as PVDF, PAN, cellulose, GF, etc.) or on the surface of traditional polymer-based diaphragms (such as PP, PE). It is a combination of the MOF and the substrate material, which combines the mechanical properties and simple preparation method of the substrate material with the excellent properties of the MOF itself, such as high porosity, adjustable pore size structure, and easy surface functionalization and stability, to enhance the overall performance of the separator and extend the cycle life of the battery through synergy.
Li et al. [67] modified carbon/copper (C/Cu) nanocomposites on 20 μm thick cellulose nanofiber (CNF) membranes and designed a functional ultrathin separator (FUS) that is only 23 μm thick, taking advantage of the rich zinc-philic low-barrier nucleation sites provided by the Cu-MOF-derived C/Cu layer and the uniform ion flux of the CNF substrate, To significantly suppress zinc dendrites and greatly enhance the cycling stability of zinc anodes, Xu et al. [68] coated a commercial glass fiber separator (GF) with a ZIF-8 zeolite imidazolide skeleton material to prepare a GF@ZIF-8 composite functional separator. By taking advantage of the high porosity and negative charge of ZIF-8, sodium ion flux can be regulated to achieve uniform deposition of sodium metal, thereby significantly suppressing sodium dendrites and improving the cycling stability and rate performance of sodium metal batteries. Samy et al. [69] constructed ZIF-8@GF composite separators by loading ZIF-8 nanoparticles on the surface of glass fiber separators (GF) through in situ growth strategies and used them in conjunction with NiFe2O4@graphene (NiFe2O4@GNS) composite catalytic electrodes to effectively suppress the REDOX shuttle effect of I3− in Li–O2 batteries. The composite system gave the battery a cycle life of 501 times at a current density of 300 mA g−1 and a capacity limit of 500 mAh g−1, much more than 209 times higher for the unmodified separator, and the polarization voltage was reduced to 0.61 V, compared with 1.00 V for the unmodified one.
Liu et al. [70] fabricated the ZIF-8/PAN composite separator by uniformly embedding ZIF-8 nanoparticles into polyacrylonitrile (PAN) fibers using an electrospinning-hot-pressing integrated process (as shown in Figure 5a,b). The composite separator demonstrated high electrolyte wettability with a contact angle of only 3°, a wider electrochemical stability window of 5.04 V, and a higher Li+ migration number of 0.306, enabling the stable cycling of Li-symmetric batteries for more than 600 h with a voltage lag of only 30 mV; The Li//LiFePO4 full cell maintained a capacity of 142 mAh g−1 after 270 cycles at 0.5 C, with a Coulombic efficiency of more than 99.5%, significantly superior to a single commercial PP separator (as shown in Figure 5c–e).
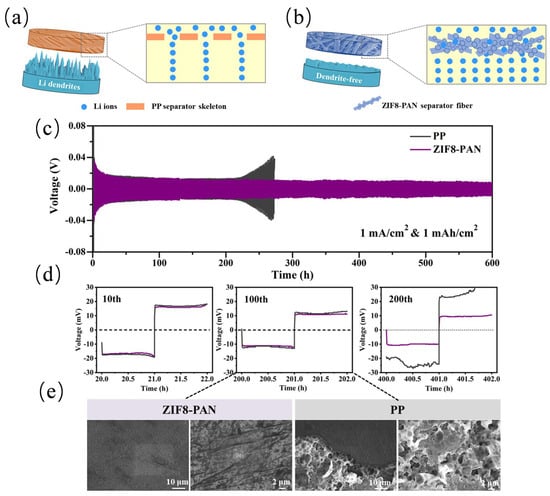
Figure 5.
Schematic diagram of (a) non-uniform deposition of Li ions after passing through PP separator. (b) Uniform deposition of Li ions after redistribution through the obtained ZIF8-PAN separator. (c) The cycling stability of the Li//Li symmetric cells assembled with PP and PAN separators under current density of 1.0 mA cm−2 with area capacity of 1.0 mAh cm−2 at 25 °C. (d) Corresponding magnified voltage profiles at the 10th, 100th and 200th cycle. (e) The SEM analysis of 100th cycled Li electrodes tested in Li//Li symmetric cells using a certain separator. Reproduced with permission [70]. Copyright 2023 Multidisciplinary Digital Publishing Institute.
It is notable that when MOF particles are directly mixed into a polymer solution, MOF particle agglomeration is prone to occur, resulting in local stress concentration, which in turn makes the membrane brittle and clogs the channels, reducing the performance of the MOF separator. In addition, the high production cost of MOF has prevented it from being applied on a large scale, which is also a major factor restricting its development.
3.3. Functional Modification of MOF Separator Materials
Functionally modified MOF diaphragms are achieved by introducing specific functional groups or structures into a metal–organic framework to precisely regulate ions and sieve specific ions (such as Na+, Li+, Zn2+, etc.) while suppressing side reaction ions (such as polysulfides, anions, etc.), and these functional groups can accelerate ion migration and enhance rate performance. Secondly, it enhances interfacial stability, induces the formation of a stable solid electrolyte interface (SEI), and inhibits dendrite growth.
Lin et al. [71] introduced four lithium sulfonate (-SO3Li) groups into the UiO-66 skeleton to synthesize UiO-66(SO3Li)4 as a separator modification material (as shown in Figure 6a) and formed a layer of MOF coating on the surface of the PP separator by post-synthesis two-step oxidation–lithiation (as shown in Figure 6b). The composite separator significantly inhibited polysulfide shuttling and accelerated Li+ transport, resulting in a Li–S battery capacity of 1493.3 mAh g−1 at 0.1 C, 730.1 mAh g−1 at 2 C, and a capacity decay of only 0.053% after 1000 cycles at 1 C. At a high sulfur load of 4.83 mg cm−2, it still reached 838.6 mAh g−1 (as shown in Figure 6c–i). Yu et al. [72] constructed (CF3)2-UiO-66@GF composite diaphragms by in situ loading (CF3)2-UiO-66 on glass fiber diaphragms in a one-step hydrothermal process. The diaphragm regulates the Zn2+ flux by strong electronegativity and hydrophobicity and suppresses the hydrogen evolution side reaction, enabling the Zn symmetric cell to operate stably for more than 2000 h at 2 mA cm−2/1 mAh cm−2 and over 800 h at 5 mA cm−2/2 mAh cm−2. The capacity retention rate of the full cell is 92.3% after 1000 cycles at 5 A g−1. Pang et al. [73] constructed highly selective separators by coating sulfonic acid-functionalized Al-MIL-101-NH2 (S-MIL-101) on the surface of Celgard 2500 separators, using sulfonic acid group negative charges to repulse polysulfide anions and accelerate Li+ conduction. The Li–S battery had an initial capacity of 1444.6 mAh g−1 at 0.2 C and maintained 990.4 mAh g−1 after 100 cycles when the separator was used; even at a high sulfur loading of 4.7 mg cm−2, the initial capacity still reached 895.7 mAh g−1, and after 500 1C cycles, it still maintained 510.4 mAh g−1, significantly suppressing the shuttle effect and improving cycle stability.
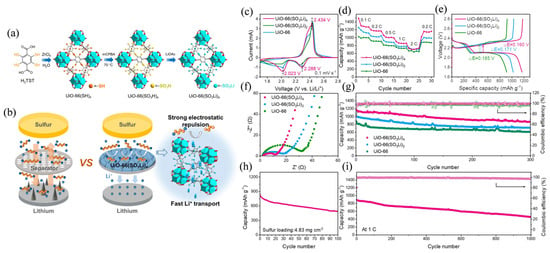
Figure 6.
(a) Preparation route for the UiO-66(SO3Li)4. (b) Mechanistic illustration of UiO-66(SOLi)4-modified PP separator for Li–S batteries. (c) CV profiles and (d) rate performance of the Li–S cells with UiO-66(SOLi)4 and UiO-66(SOLi)2 as well as primitive UiO-66-modified separators. (e) Galvanostatic charge–discharge profiles at 0.2 C. (f) EIS spectra of different cells. (g) Cycling performance at 0.5 C. Cycling performance of the UiO-66(SO3Li)4-based cell (h) at a high areal sulfur loading (4.83 mg cm−2) and (i) at a high rate of 1 C. Reproduced with permission [71]. Copyright 2023 Elsevier B.V.
Table 1 compares the core performance of three types of diaphragms: pure MOF, MOF composite, and functionalized MOF. This comparison provides a clear performance–cost reference framework for the selection and design of MOF separators for different battery systems.

Table 1.
Performance comparison of pure MOF, MOF composite, and functionalized MOF.
4. MOF Separator Design for Safety-Critical Battery Systems
Metal–organic framework (MOF)-based separators have emerged as a promising class of next-generation membrane materials for advanced rechargeable batteries. Their superiority originates from the precisely tunable pore architecture, abundant surface functionalities, and exceptional chemical/thermal robustness inherent to MOF crystals. Integrating MOFs into battery separators not only reinforces mechanical integrity and thermal endurance relative to conventional polyolefin membranes but also enables selective ion transport, interfacial electric-field homogenization, and active-species adsorption/catalysis, collectively leading to markedly enhanced safety, prolonged cycle life, and superior rate capability. These attributes render MOF separators broadly applicable to lithium-ion, lithium–sulfur, aqueous zinc-ion, and solid-state lithium metal battery chemistries.
4.1. Lithium-Ion Battery (LIB)
In electrochemical energy storage technology, lithium-ion batteries are currently the mainstream energy storage technology due to their low redox potential (compared to the standard hydrogen electrode at −3.04 V) and high theoretical specific capacity (3860 mA h g−1) [74,75]. However, the thermal stability and electrolyte wettability of traditional polyolefin separators are insufficient. At high temperatures, they may cause the polyolefin separators to contract and melt, leading to a risk of thermal runaway, which significantly limits the development of lithium-ion batteries.
As a new type of functional molecular crystal material, MOF, with its ultra-high specific surface area, regularly adjustable nanoscale pores and excellent thermal stability, can significantly enhance the key performance of the separator when combined with traditional separators, providing a new option for the composite separator of the next generation of lithium-ion batteries.
Li et al. [76] designed a binder-free ZIF-67 array directly epitaxially grown on copper foil as a multifunctional current collector for long-cycle lithium-poor metal batteries (LLMBs). The high specific surface area and abundant lithium-philic sites of the ZIF-67 nanosheets promote uniform lithium deposition, and the lithium–nitrogen-philic sites they contain also facilitate the formation of an inorganic SEI rich in LiF/Li3N, enhancing interfacial stability (as shown in Figure 7a), lithium-ion flux and ionic conductivity for dendrite-free dense (as shown in Figure 7b–d) and smooth lithium deposition (as shown in Figure 7e,f). It enables lithium-poor metal batteries to exhibit excellent rate performance and cycle stability under high cathode loading and poor electrolyte conditions (as shown in Figure 7g–i). Mei et al. [77] prepared PU/PAN lithium-ion battery separators with PU as the base material and PAN as the additive by centrifugal spinning and found that when the mass fraction was 18% and PU:PAN was 7:3, the separators had a 3D porous structure with a porosity of 83.9% and a liquid absorption rate of 493%. The size was stable at 160 °C and the electrochemical stability window was up to 5.2 V. The separator provides an efficient channel for lithium-ion migration, increasing ionic conductivity to 1.79 mS cm−1. LiFePO4 batteries equipped with this separator maintain 95.8% capacity after 50 cycles at 0.2 C and exhibit high discharge capacity at different rates, significantly improving cycle stability and rate performance.
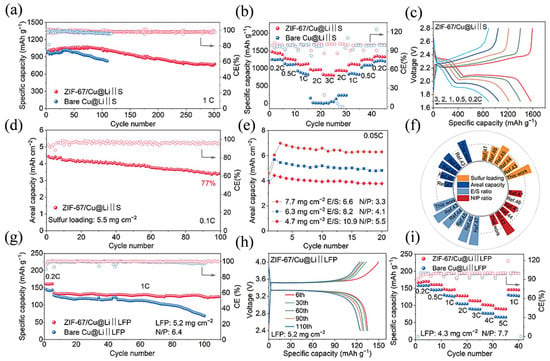
Figure 7.
(a) Cycling stability comparison of Li–S full cells based on bare Cu@Li and ZIF-67/Cu@Li anodes at 1 C. (b) Rate capabilities and (c) corresponding charge/discharge profiles of Li–S full cells at different current densities. (d) Cycling performance of ZIF-67/Cu@Li/S full cells with sulfur loading of 5.5 mg cm−2 at 0.1 C. (e) Cycling performance of ZIF-67/Cu@Li/S full cells at 0.05 C. (f) Comparison of Nyquist diagram for the Li–S full cells. (g) Cycling performance comparison and (h) corresponding charge/discharge profiles of LiFePO4 full cells based on bare Cu@Li and ZIF-67/Cu@Li anodes at 1 C. (i) Rate capabilities of LiFePO4 full cells based on different Li anodes at various current densities. Reproduced with permission [76]. Copyright 2025 Elsevier B.V.
MOFs play a pivotal role in suppressing dendrite growth and regulation. Du et al. [78] fabricated continuous electronegative nanochannels through in situ growth of fluorine-functionalized UiO-66F metal–organic framework (MOF) nanoparticles on the surface of polyimide (PI) separators. By leveraging the electrostatic attraction–repulsion effect, these nanochannels selectively facilitate the homogeneous migration of Li+ and impede the migration of anions. As a result, the ion flux at the electrode–electrolyte interface is homogenized, alleviating the dendrite growth induced by concentration gradients. Subsequently, LiNO3 was encapsulated within the pores of UiO-66F, enabling its controlled and sustained release in carbonate electrolytes. This preferentially reduces to form a Li3N-enriched solid electrolyte interface (SEI) characterized by high ionic conductivity and remarkable mechanical toughness. Consequently, the interface impedance and lithium deposition overpotential are further diminished. The concerted action of the electronegative MOF channels and the Li3N-rich SEI induce a dense and spherical lithium deposition morphology. This effectively inhibits the formation of dendrites and dead lithium. As a consequence, the Li//Li symmetric cell can stably cycle for more than 1000 h at a high current density of 10 mA cm−2, and the cycle life of high-voltage Li//NCM811 full cells is significantly enhanced. Liu et al. [79] employed a “crystal-face engineering-anion anchoring” strategy. They directionally synthesized ZIF-8 nanocrystals with exposed (110) crystal faces and uniformly coated them on the surface of commercial polypropylene separators to fabricate a functionalized separator. The (110) crystal face is abundant in coordination-unsaturated Zn2+ Lewis acid sites. These sites can selectively adsorb and immobilize TFSI− anions, preventing their migration towards the lithium anode. This, in turn, weakens the space–charge layer and homogenizes the Li+ flux at the interface. Simultaneously, the three-dimensional microporous network of ZIF-8 offers spatial confinement, reducing the local current density and promoting homogeneous lithium nucleation. Experimental findings demonstrate that this strategy gives rise to a dense and smooth lithium deposition, effectively suppressing dendrite growth. The Li//Cu cell can stably cycle for over 1400 cycles under conditions of 2 mA cm−2/1 mAh cm−2. Moreover, this strategy exhibits excellent fast-charging and discharging stability in high-rate, high-loading LFP and NCM811 full cells (retaining 99.9% Coulombic efficiency after 3000 cycles at 5C). Thus, it provides a scalable interface regulation approach for the construction of high-safety, fast-charging lithium metal batteries.
4.2. Lithium–Sulfur Batteries (Li-S)
Lithium–sulfur batteries (Li–S) have extremely high theoretical energy density (2600 W h kg−1), high theoretical specific capacity (1675 mAh g−1), and a wide operating temperature range (−30 to 60 °C) [80,81,82]. In addition, lithium–sulfur batteries have become ideal energy storage devices due to their high safety performance. Although lithium–sulfur batteries have broad application prospects, there are still some problems that limit their development, such as complex manufacturing process, poor rate performance, lithium dendrite growth, slow sulfur REDOX kinetics, but most importantly, the shuttle effect [83,84]. Theoretically, lithium–sulfur batteries can achieve excellent electrochemical performance through REDOX reactions, but soluble polysulfides are produced during charging and discharging (as shown in Figure 8a–d). These polysulfides have high solubility in the electrolyte and can freely migrate between the cathode and anode. If they interact with lithium metal, it will cause Li2S2 and Li2S to accumulate on the surface of the lithium anode and aggregate as “dead sulfur”. It leads to loss of active material, corrosion and passivation of the lithium anode, severe self-discharge behavior and poor cycle life, ultimately resulting in capacity degradation of lithium–sulfur batteries [85,86].
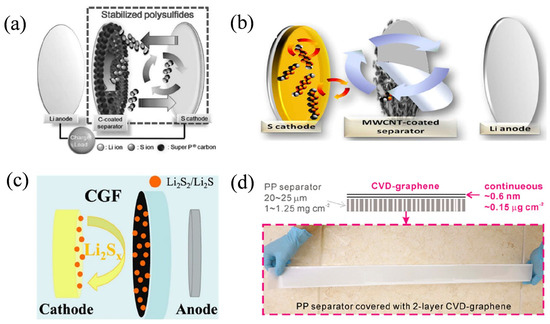
Figure 8.
Functions and preparation principles of different modified separators. (a) Schematic configuration of an LSB with the Super P-coated separator. (b) A schematic cell configuration of the Li–S cell employing the MWCNT-coated separator. (c) Schematic showing the role of the CGF separator in an LSB. (d) Schematic of the prepared separator. Reproduced with permission [86]. Copyright 2025 Elsevier B.V.
The separator, as an important component of the battery, is the main part that prevents the shuttle of PS [87]. Therefore, it plays a crucial role in determining the electrochemical performance of the Li–S batteries. Conventional separators are typically composed of macroporous polymer membranes, such as PP/PE separators with a pore size of 50–600 nm, while polysulfides (Li2Sn, 4 ≤ n ≤ 8) have a molecular size of 1.2–1.7 nm [88,89,90]. Therefore, it is urgent to design a separator that promotes lithium-ion transport, inhibits lithium dendrite growth, and also suppresses PS shuttle.
MOF is an ideal separator material because of its highly ordered structure, adjustable pore size and abundant porosity. Usually, the pore size of MOF is significantly smaller than that of PS [91,92]. Therefore, MOF diaphragms enable rapid and efficient transport of lithium ions on both sides of the diaphragm while successfully intercepting the shuttle of PS.
In previous reports, Chen et al. [93] developed the FJU-90 MOF-modified diaphragm by reconstructing the pore network and chemical environment of metal–organic frameworks (MOFs) using a pore space partitioning strategy. The membrane synergistically promotes lithium-ion conduction, suppresses polysulfide shuttling and accelerates its catalytic conversion through reasonable pore size, large specific surface area and abundant catalytic sites. Lithium–sulfur batteries based on this separator exhibit excellent rate performance and cycle stability at high sulfur loads, with a capacity retention rate of 79.0% after 500 cycles. Wang et al. [94] prepared Ti3C2Tx nanosheets, grew UiO-66-NH2 in situ at room temperature by hydrothermal method, and then coated UiO-66-NH2 mixed with conductive carbon and PVDF on the surface of Celgard-2400 to produce the Ti3C2Tx-UiO-66–2NH2/PP composite separator. The separator gave the Li–S battery an initial capacity of 1247 mAh g−1 at 0.1 C and a decay of only 0.04093% per cycle after 1500 cycles; at 2.5 C and 8.2 mg cm−2 high sulfur loads, it can operate stably for 600 cycles.
4.3. Aqueous Zinc-Ion Batteries (AZIBs)
Aqueous zinc-ion batteries are promising for the next generation of energy storage devices [95]. Currently, the mainstream porous separator materials for aqueous zinc-ion batteries available on the market are glass fiber separators (GF) and commercial polyolefin separators (PP/PE), among which the large pores of GF separators facilitate the rapid transport of Zn2+, but GF separators have low mechanical strength and are easily pierced by zinc dendrites [96]. Traditional PP/PE separators have good chemical stability and inertness, but their thermal stability is insufficient. When the temperature exceeds the melting point of the PP/PE separator, the separator will shrink [97]. And these diaphragms are unable to suppress the growth of zinc dendrites and the occurrence of side reactions (such as hydrogen evolution and corrosion) [98]. Zinc dendrites grow to a certain extent and pierce the separator, causing direct contact between the positive and negative electrodes of the battery and also causing a short circuit in the battery. The occurrence of the hydrogen evolution reaction (HER), which involves two reactions on the surface of the anode: 2H2O + 2e− → H2 + 2OH− and Zn2+ + 2e− → Zn, competes for electrons that should have taken place in the zinc deposition reaction, resulting in some being consumed before the deposition reaction takes place, significantly reducing the Coulombic efficiency (CE) of zinc deposition [6]. Zinc corrosion also involves two reactions: Zn + 2H2O → Zn(OH)2, Zn + 2H+ → Zn2+ + H2⬆, which lead to the continuous dissolution of zinc at rest, capacity degradation of aqueous zinc-ion batteries [99]. At the same time, its synergy with HER will exacerbate swelling and may even lead to battery explosion. Therefore, developing a suitable separator material to delay the growth of zinc dendrites and suppress the occurrence of side reactions is a key challenge at present.
Due to the unique structure and physicochemical properties of MOF diaphragms, they can fundamentally solve the problems faced by traditional diaphragms such as being easily punctured by zinc dendrites and being unable to suppress side reactions. Because the regular nanochannels of MOF are typically 0.6–1.1 nm, they can precisely screen zinc ions, allow zinc ions to migrate directionally through the channels, and make zinc ions evenly distributed. According to a characterization by Mohamed et al. [100], XRD comparison of the crystal plane orientation of zinc anodes after cycling revealed that the Zn(002) peaks corresponding to UiO-66-GF and UiO-67-GF were significantly enhanced, and the intensity ratio of (002)/(101) was significantly increased, demonstrating that MOF-induced zinc preferentially grows along (002) horizontally. There was no short circuit in the symmetrical cell cycle for 400 h, and SEM showed dense horizontal lamellar rather than vertical dendrites on the zinc surface, indicating that the horizontal (002) crystal plane structure effectively prevented dendrites from penetrating the separator, suggesting that the functional groups on the MOF surface could induce zinc deposition to grow preferentially along the (002) crystal plane, forming a horizontal structure that was less likely to be punctured by zinc dendrites. Electrochemical measurements further corroborate that the MOF effectively modulates the interfacial electrochemical environment and suppresses parasitic reactions (as shown in Figure 9a–d). Beyond the coordination between functional groups and Zn2+, the orientational regulation of zinc deposition by metal–organic frameworks (MOFs) can be rationalized within the coupled frameworks of crystal-growth kinetics and interfacial thermodynamics. Classical nucleation theory posits that the crystallographic orientation of metal deposition is governed not solely by the ion-flux distribution, but rather by the synergistic interplay between interfacial energy and the surface free energies of individual crystallographic facets. Zr-MOFs exemplified by UiO-66 possess surfaces densely terminated with carboxylate (–COO−) moieties that electrostatically generate weakly coordinated Zn2+ intermediates; these intermediates preferentially adsorb onto the Zn(002) plane [101], thereby lowering its effective surface energy and thermodynamically promoting the (002) orientation as the dominant growth facet that facilitates uniform deposition. Concomitantly, the well-defined pore architecture and tailored coordination environment of the MOF modulate the desolvation kinetics and migration trajectory of Zn2+, kinetically activating the (002) facet, fostering dense deposition, and effectively suppressing dendrite proliferation [102]. In previous reports, Zhou et al. [103] prepared a zinc-functionalized UiO-66 (UiO-dcbdt-Zn)-modified separator (UiO-dcbdt-Zn@GF) by functionalizing sulfonic acid onto UiO-66. They loaded it onto glass fibers using vacuum filtration. They loaded it onto glass fibers using vacuum filtration. The unique [S—Zn–S−] zinc-responsive sites in this modified separator can regulate the flux of Zn2+ and accelerate ion transport. The modified separator promotes the desolvation of hydrated zinc ions, inhibits water-induced side reactions, reduces zinc electrode corrosion and dendrite growth, and enables the zinc-symmetric cell to stably cycle for 2200 h at 1 mA cm−2. In the Zn/MnO2 full cell, the separator gave the cell a capacity retention rate of 88% after 2500 cycles at 1 A g−1, an initial capacity of 153.3 mAh g−1, and a Coulombic efficiency of up to 99.5%.
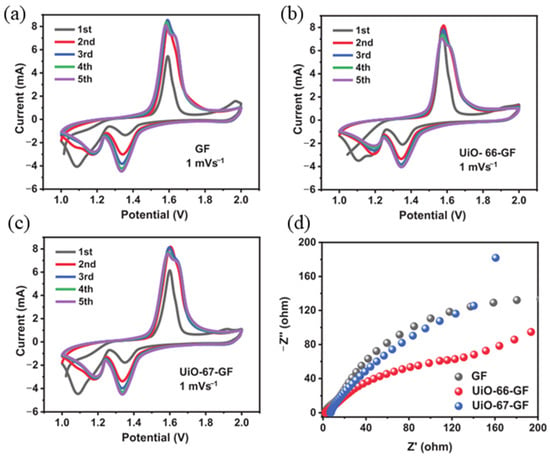
Figure 9.
(a) CV curves for Zn/GF/MnO2 collected over the first five cycles at a scan rate of 1 mV per second. (b) The initial five cycles of the Zn/UiO-66-GF/MnO2 CV curves, calculated at a scan rate of 1 mV per second. (c) The CV curves of Zn/UiO-67-GF/MnO2 were scanned at a rate of 1mV per second. (d) The EIS curves for Zn/GF/MnO2, Zn/UiO-66-GF/MnO2, and Zn/UiO-67-GF/MnO2 batteries. Reproduced with permission [100]. Copyright 2025 Wiley—VCH Verlag GmbH & CO. KGaA.
4.4. Solid-State Lithium Metal Batteries
Solid-state lithium metal batteries (SS-LMB) are an important direction for the next generation of battery technology, replacing the organic liquid electrolyte in traditional lithium-ion batteries with a solid-state electrolyte, a change that significantly improves battery performance. Because solid-state electrolytes are non-flammable, the most prominent characteristic is that they fundamentally solve the problem of flammable and explosive liquid electrolytes and significantly reduce the risk [104]. Secondly, some solid electrolytes, especially inorganic ceramic ones, have higher mechanical strength, which can more effectively prevent lithium dendrite puncture and avoid internal short circuits of the battery, further enhancing the safety [105]. In addition, solid-state electrolytes typically have a wider electrochemical window [106], which can be matched with higher-voltage cathode materials [107], such as high-nickel, lithium-rich manganese-based ones, to boost the voltage. And metallic lithium has an extremely high theoretical specific capacity, up to 3860 mAh g−1, which is ten times that of graphite. Because the solid electrolytes are less prone to side reactions with the electrode materials, its stability is also better compared to the liquid electrolyte [108,109], and it has a longer cycle life.
But the interface problem remains a major technical bottleneck hindering its large-scale development. The liquid electrolyte can wet the electrode, while the solid-state electrolyte has rigid contact with the electrode, which has a much smaller contact area, resulting in high [110,111]. By integrating the porous structure, ordered crystal structure and customizable framework structure of metal–organic frameworks (MOFs) into solid electrolytes (SSEs), the electrochemical performance of SSEs can be improved and the interface between SSEs and electrodes can be optimized, resulting in SSBs with excellent performance.
According to previous reports, Chen et al. [112] coated Cd2500 diaphragms with modified PVDF-HFP polymer and bimetallic MOF nanocapsules to construct MDPH@Cd2500 composite diaphragms with 1.3 × 10−3 S cm−1 high Li+ conductance and uniform ion flux. The structure synergistically suppressed lithium dendrites and stabilized the SEI layer, enabling the solid-state lithium metal battery to have a cycle life of 2400 h, and the capacity retention rates of the LFP//Li and LCO//Li full battery after 500 cycles at 1 C were 85.6% and 67.2%, respectively (as shown in Figure 10a–g).
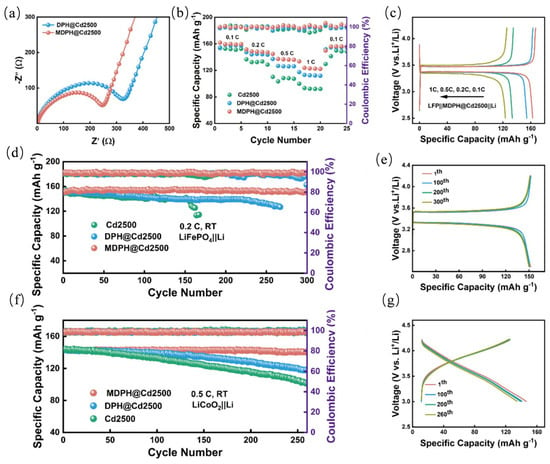
Figure 10.
Electrochemical performance of full cells with different separators. (a) EIS spectra of LFP//Li batteries with DPH@Cd2500 and MDPH@Cd2500 separators. (b) Rate capability comparison of batteries with different separators. (c) Charge–discharge voltage profiles of the MDPH@Cd2500 separator at different rates. (d) Cycle performance of batteries with different separators at 0.2 C over 300 cycles. (e) Charge–discharge voltage curves of the MDPH@Cd2500 separator (f) Cycle performance of LCO: Li batteries with different separators at 0.5 C over 260 cycles. (g) Charge–discharge voltage profiles of the MDPH@Cd2500 separator during cycling. Reproduced with permission [112]. Copyright 2025 Elsevier.
Across different battery systems, MOF-based separators tackle the key challenges of each chemistry by virtue of their tunable pore architectures and tailorable surface functionalities. In lithium-ion batteries, they suppress dendrite proliferation; in lithium–sulfur cells they block the polysulfide shuttle, thereby extending cycle life and sulfur utilization. For aqueous zinc-ion systems, they guide uniform Zn2+ deposition, mitigating dendrite formation and parasitic reactions, whereas in solid-state lithium metal batteries, they serve as composite electrolyte fillers that improve interfacial contact and ion-transport kinetics. Despite this broad applicability, intrinsically low ionic conductivity, long-term chemical stability, and scalable, cost-effective fabrication remain critical hurdles to be overcome. Table 2 systematically benchmarks the core safety demands, pivotal functions, and prevailing technical bottlenecks of MOF-based separators across four representative battery chemistries. By delineating a clear “performance-vs-challenge” map, this comparison offers a rational blueprint for the future design of tailored MOF topologies, surface functionalization strategies, and composite engineering protocols.

Table 2.
A comparison of the core roles of MOF-based separators in different battery systems.
5. Scalability and Manufacturing Challenges
5.1. Advanced Manufacturing Technology
The ideal diaphragm should have high ionic conductivity, good electrolyte wettability, excellent stability and strong mechanical properties, etc. Electrospinning is a simple method in the preparation of MOF diaphragms [113], which involves dispersing MOF particles proportionally in a polymer spinning solution and then obtaining a composite [114]. At present, preparing MOF-based nanofibers containing MOF particles and polymers by electrospinning is the main approach in this field [40,115]. For example, Leng [57] synthesized Ni–Co MOF by hydrothermal method and then combined it with PAN nanofibers by electrospinning to form a Ni–Co MOF@PAN three-dimensional porous structure. Lithium–sulfur batteries with NCMP separators had an initial capacity of 1560 mAh g−1 at 0.1 C and still maintained 794 mAh g−1 after 500 cycles, with a capacity retention rate of 84.1% and a capacity decay rate of only 0.032% per cycle. Chen et al. [40] produced HKUST-1/PAN composite nanofiber separators by in situ crystallization and encapsulation of HKUST-1 in polyacrylonitrile (PAN) nanofibers in a one-pot electrospinning process. When tested with a 45 μm ultrathin lithium anode and a high-load NMC811 cathode full cell, the capacity retention rate of the separator was 83.1% after 200 cycles, significantly better than pure PAN and commercial PP separators. Lan et al. [61] fabricated PI@ZIF-8 composite nanofiber separators by constructing three-dimensional multi-scale ZIF-8 networks on the surface of polyimide (PI) nanofibers using electrospinning—in situ self-assembly strategies. The separator was stably cycled for 2000 h in lithium symmetric cells with a polarization voltage of only 37.5 mV, and after 300 cycles in LFP full cells with a capacity retention rate of 97.4%, an ionic conductivity of 2.40 mS cm−1, and a Li+ migration number of 0.88 (as shown in Figure 11a–f).
Figure 11.
Electrochemical properties of the LMBs with different separators. (a) Cyclic voltammograms, (b) rate performance, (c) long-term cycle performance at 1C, and (d) electrochemical impedances before and after cycling of LFP//PE//Li LFP//PI//Li and LFP//PI@ZIF-8//Li cells. (e) LSV curves of the three separators. (f) Cycling performance of NCM811//Li cells at 0.5 C. Reproduced with permission [61]. Copyright 2025 Royal Society of Chemistry.
The composite diaphragms prepared by this method have a synergistic effect of the porous structure and functional properties of the MOF and the mechanical properties of the polymer, making the diaphragms have a suitable porous structure, good mechanical properties, excellent ionic conductivity and excellent thermal stability, effectively inhibiting dendrite growth [116,117]. It is worth noting that the mechanical strength of the composite diaphragm varies with the MOF content, and when the MOF loading is too high, the flexibility of the composite diaphragm may decrease and create additional thermal activation steps [115].
5.2. Cost–Safety Trade-Offs
MOF separators, with their unique structural and performance advantages, have broad application prospects in enhancing battery safety, effectively suppressing dendrite growth, enhancing thermal stability, and stabilizing the composition and properties of the electrolyte, etc. However, the current high cost has severely restricted its large-scale commercial application, mainly including the synthesis of MOF materials and the raw materials required for the process of preparing the separator, etc. Therefore, researchers are currently focusing on choosing low-cost raw materials, exploring new synthetic methods to increase the yield of MOF, optimizing the structure of MOF, etc., which can, to some extent, reduce costs and improve battery safety.
First, a wide variety of metal salts and organic ligands are needed to synthesize MOF, and some rare metal salts and organic ligands are expensive. At the same time, if high-purity MOF materials are to be prepared, the cost of raw materials will also be higher. The cost of preparing MOF diaphragms will increase accordingly if there is a shortage or price increase in raw materials. Currently, common methods for synthesizing MOF include solvothermal, hydrothermal, and in situ synthesis, which typically require high temperature, high pressure, and long reaction time conditions with high energy consumption, resulting in an increase in the cost of the synthesis process. In the case of in situ synthesis, the reaction usually takes more than 12 h and the reaction temperature is generally between 100 and 400 °C, which not only takes time but also has certain requirements for the experimental equipment. In the preparation of MOF diaphragms, auxiliary materials such as binders and dispersants are needed to ensure that the MOF can be evenly dispersed on the surface of the traditional diaphragm matrix. The use of these additives increases the material cost and may also have a certain impact on the performance of the diaphragm.
In response to these issues, researchers attempted to use low-cost but similar-performance raw materials instead of expensive metal salts and organic ligands to reduce raw material costs while ensuring the basic performance of the MOF material. For example, using common metal ions such as iron, nickel, and cobalt instead of precious metal ions. For instance, Boukayouht et al. [118] utilized stainless steel scraps as the source of metals Cr, Ni, and Fe, and PET plastic to provide ligands to prepare CrNiFe-MOF. They used water as the sole solvent and completed the reaction at room temperature in only 5–10 min, without the need for high-temperature or high-pressure equipment, thus simplifying the process. Suresh et al. [119] modulated the electronic structure of NiCo-MOF using a dual ligand of melamine and H3BTC, without the need for precious metals such as Pt or Ru, and reduced the overpotential by 120 mV. Developing novel, efficient and low-energy synthesis methods is key to reducing the cost of MOF materials. An et al. [120] added MOF crystals to water without using organic solvents, skipped the induction period, and synthesized 11 kinds of MOF at room temperature with 60% lower energy consumption. Xiao et al. [121] synthesized multi-module MOFs in 2 h using solvent-free heating with C2H2/CO2 selective separation, a method with zero solvent cost. In addition, the structure of the MOF can be optimized, such as by adjusting the porosity and the way the MOF material is dispersed in the separator, to further improve the safety performance of the battery. Yang [122] modified the metal sites on the surface of the MOF with carboxylic acid-containing crown ethers (CEC) to achieve super-uniform dispersion in 11 solvents such as polyimide and polysulfone and six polymer matrices. When applied in lithium–sulfur battery separators, the MOF particles did not aggregate, effectively adsorbed polysulfides, and effectively inhibited dendrite growth. The symmetric battery stably cycled for 2500 h at 1 mA cm−2 with an 8 s delay in the combustion test, indicating a significant improvement in thermal stability. Li et al. [123] pre-crosslinked the carboxyl groups of MOF-801 to form covalent ester bonds with the hydroxyl groups of polyvinyl alcohol (PVA) to prevent particle aggregation. EDS spectra confirmed the uniform distribution of Zr elements. Meanwhile, the thermal stability of the separator was improved, and there was no performance degradation after 92 h of operation at a high temperature of 343K, reducing the risk of thermal runaway of lithium batteries.
In the future, the development of MOF separators will still need to overcome the technical challenge of green, low-cost and large-scale production. With the continuous exploration of related solutions, it will show broad prospects in reducing costs and improving safety performance, thereby driving battery technology towards higher safety, lower cost and higher performance.
6. Conclusions and Views
Metal–organic framework (MOF) materials, with their unique structures and chemically active sites, offer new avenues for battery separator innovation and demonstrate significant advantages in enhancing battery safety performance.
Relying on the high specific surface area, regular pores and excellent stability of the MOF structure, it is introduced into the traditional diaphragm system through coating or composite means. The MOF material itself forms a stable framework through the interaction of metal ions and rigid organic ligands, endowing the MOF diaphragm with excellent thermal stability and effectively enhancing its high-temperature resistance, thus avoiding the problem of contact short circuit between the positive and negative electrodes caused by the melting of the separator during battery heating; at the same time, the ordered channels of the MOF can guide electrolyte ions to achieve uniform migration, significantly inhibit dendrite growth, and promote the formation of a stable solid electrolyte interface (SEI), preventing the dendrite puncture of the separator from the root and further ensuring the safety of the battery during cycling. In addition, some of the MOF materials themselves have high mechanical strength, and when combined with the polymer substrate, they can further enhance the tensile and puncture resistance of the separator, ensuring the structural stability of the battery during assembly and cycling. These mechanisms work together to significantly enhance the safety and cycle life of MOF separators in a variety of systems, including lithium-ion batteries, lithium–sulfur batteries, aqueous zinc-ion batteries, and solid-state lithium metal batteries.
However, despite the significant improvement in battery safety performance of MOF separators, their large-scale application still faces key bottlenecks: First, the synthesis of MOF materials relies on some precious metals and organic ligands, and traditional synthesis methods often require reaction conditions such as high temperature, high pressure, and long duration, with complex processes and high costs, and the preparation process needs to be further optimized to reduce production costs. Secondly, there are differences in compatibility between different MOF structures and battery systems. Functional MOF separators need to be designed for different battery types to improve the overall performance of the battery by reasonably regulating the MOF structure while ensuring safety performance.
However, with the continuous maturation of the preparation process and the continuous optimization of material performance, MOF materials are expected to drive innovation in battery safety technology and achieve large-scale breakthroughs in the application of MOF separators in battery energy storage.
In the future, integrating the experimental synthesis of MOFs with theoretical computations is expected to enable the precise, targeted construction of MOF architectures bearing designated functional groups, tunable pore sizes, and optimized interfacial properties, thereby realizing “tailor-made” separators for diverse battery chemistries.
Author Contributions
Conceptualization, T.Z. and Y.C.; resources, T.Z., Y.B. and Y.C.; writing—original draft preparation, Y.B., J.C., J.Y. and S.P.; writing—review and editing, T.Z., Y.C., Y.B. and F.L.; supervision, T.Z. and Y.C.; funding acquisition, T.Z. and Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the National Natural Science Foundation of China (52073086, 51802094), the Natural Science Foundation of Hunan Province (2024JJ7164, 2023JJ60447), the Hunan Provincial Innovation Foundation for Postgraduate (CX20240908) and Scientific Research and Innovation Foundation of Hunan University of Technology (CX2413).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No data was used for the research described in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, Y.; Li, Q.; Wang, Z. Recent progress about transmission electron microscopy characterizations on lithium-ion batteries. J. Energy Chem. 2024, 95, 39–56. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, W.; Li, S.; Shen, X.; Xu, H. Design strategies and energy storage mechanisms of MOF-based aqueous zinc ion battery cathode materials. Energy Storage Mater. 2024, 69, 103436. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, J.; Xiao, P.; Nie, S.; Luo, F.; Chen, Y. Exploring metal-organic frameworks in battery electrodes, separators, and electrolytes: A comprehensive review. Coord. Chem. Rev. 2025, 532, 216501. [Google Scholar] [CrossRef]
- Song, Y.; Sheng, L.; Wang, L.; Xu, H.; He, X. From separator to membrane: Separators can function more in lithium ion batteries. Electrochem. Commun. 2021, 124, 106948. [Google Scholar] [CrossRef]
- Xue, Y.; Li, W.; Liu, R.; Lv, Y.; Li, J.; Li, D.; Guo, Y.; Hao, R.; He, H. Functionalized separator strategies accelerate the development of Zinc-Ion batteries. iScience 2025, 28, 112787. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Wu, F.; Ma, L.; Feng, J.; Yin, X.; Wang, Y.; Dai, X.; Liu, W.; Shi, W.; Cao, X. A review on advanced optimization strategies of separators for aqueous zinc-ion batteries. Funct. Mater. Lett. 2023, 16, 2340017. [Google Scholar] [CrossRef]
- Zong, Y.; He, H.; Wang, Y.; Wu, M.; Ren, X.; Bai, Z.; Wang, N.; Ning, X.; Dou, S.X. Functionalized separator strategies toward advanced aqueous Zinc-ion batteries. Adv. Energy Mater. 2023, 13, 2300403. [Google Scholar] [CrossRef]
- Li, L.; Jia, S.; Zhang, C.C. Improved strategies for separators in Zinc-ion batteries. ChemSusChem 2023, 16, e202202330. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Cheng, Z.; Zhang, T.; Zhang, X.; Ma, Y.; Guo, Y.; Wang, X.; Zheng, Z.; Hou, Z.; Zi, Z. High efficient recycling of glass fiber separator for sodium-ion batteries. Ceram. Int. 2023, 49, 23598–23604. [Google Scholar] [CrossRef]
- Ma, X.; Chen, Z.; Zhang, T.; Zhang, X.; Ma, Y.; Guo, Y.; Wei, Y.; Ge, M.; Hou, Z.; Zi, Z. Efficient utilization of glass fiber separator for low-cost sodium-ion batteries. Int. J. Miner. Metall. Mater. 2023, 30, 1878–1886. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Z.; Feng, Y.; Cai, S.; Gao, H.; Wei, Z.; Zhao, Y. Cellulose-based separators for lithium batteries: Source, preparation and performance. Chem. Eng. J. 2023, 471, 144593. [Google Scholar] [CrossRef]
- Liu, W.; Dang, Y.; Xie, W.; Tang, A. Weakening of mechanical properties of cellulose separator caused by electrolyte immersion and elevated temperature. Polym. Compos. 2019, 40, 3857–3865. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, C.; Dong, F.; Xie, H.; Sun, L. Multilayer polyethylene separator with enhanced thermal properties for safe lithium-ion batteries. Particuology 2024, 91, 29–37. [Google Scholar] [CrossRef]
- Man, Y.; Nan, H.; Ma, J.; Li, Z.; Zhou, J.; Wang, X.; Li, H.; Xue, C.; Yang, Y. Functionalized γ-boehmite covalent grafting modified polyethylene for lithium-ion battery separator. Materials 2024, 17, 2162. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, M.; Chen, Z.; Zhang, Z.; Qiu, T.; Hu, Z.; Xiang, H.; Zhu, L.; Xu, G.; Zhu, M. Advances in nonwoven-based separators for lithium-ion batteries. Adv. Fiber Mater. 2023, 5, 1827–1851. [Google Scholar] [CrossRef]
- Tong, B.; Li, X. Towards separator safety of lithium-ion batteries: A review. Mater. Chem. Front. 2024, 8, 309–340. [Google Scholar] [CrossRef]
- Cheng, Z.; Lian, J.; Zhang, J.; Xiang, S.; Chen, B.; Zhang, Z. Pristine MOF materials for separator application in Lithium–Sulfur battery. Adv. Sci. 2024, 11, 2404834. [Google Scholar] [CrossRef] [PubMed]
- Phung, J.; Zhang, X.; Deng, W.; Li, G. An overview of MOF-based separators for lithium-sulfur batteries. Sustain. Mater. Technol. 2022, 31, e00374. [Google Scholar] [CrossRef]
- Gao, X.; Wang, L.; Cheng, J.; Zhao, J.; Liu, X. Manufacturing process of MOF-based separator for lithium sulfur batteries: A mini review. Chin. Chem. Lett. 2025, 36, 110247. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Atik, A.; Tosun, M.; Zhang, X. MOF@ Nanofiber separators for Lithium-ion batteries. J. Appl. Polym. Sci. 2025, 142, e56562. [Google Scholar] [CrossRef]
- Jia, T.; Gu, Y.; Li, F. Progress and potential of metal-organic frameworks (MOFs) for gas storage and separation: A review. J. Environ. Chem. Eng. 2022, 10, 108300. [Google Scholar] [CrossRef]
- Felix Sahayaraj, A.; Joy Prabu, H.; Maniraj, J.; Kannan, M.; Bharathi, M.; Diwahar, P.; Salamon, J. Metal–Organic Frameworks (MOFs): The next generation of Materials for catalysis, gas storage, and separation. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1757–1781. [Google Scholar] [CrossRef]
- Li, X.; Chen, K.; Guo, R.; Wei, Z. Ionic liquids functionalized MOFs for adsorption. Chem. Rev. 2023, 123, 10432–10467. [Google Scholar] [CrossRef]
- Wang, L.-B.; Wang, J.-J.; Yue, E.-L.; Li, J.-F.; Bai, C.; Tang, L.; Wang, X.; Hou, X.-Y.; Zhang, Y. Fluorescence sensing and anti-counterfeiting application based a heterometallic Cd (II)–Na (I)-MOF. J. Solid State Chem. 2022, 309, 123026. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, J.; Wen, N.; Xiong, H.; Cai, S.; He, Q.; Hu, Y.; Peng, D.; Liu, Z.; Liu, Y. Metal-organic frameworks for stimuli-responsive drug delivery. Biomaterials 2020, 230, 119619. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, G.; Peng, Y.; Li, Y.; Chi, H.; Pang, H. Recent progress in 1D MOFs and their applications in energy and environmental fields. Adv. Colloid Interface Sci. 2023, 321, 103022. [Google Scholar] [CrossRef]
- Zhao, T.; Luo, F.; Xiao, P.; Nie, S.; Chen, J.; Chen, Y. Strategies for the preparation of MOFs-based composites and their research progress in electrocatalysis. Microporous Mesoporous Mater. 2025, 389, 113560. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Yuan, H.; Yu, Y.; Tan, Y. Recent progress and challenge in Metal–Organic Frameworks for Lithium–Sulfur battery separators. Adv. Funct. Mater. 2024, 34, 2405890. [Google Scholar] [CrossRef]
- Zhao, T.; Xiao, P.; Nie, S.; Luo, M.; Zou, M.; Chen, Y. Recent progress of Metal-Organic Frameworks based high performance batteries separators: A review. Coord. Chem. Rev. 2024, 502, 215592. [Google Scholar] [CrossRef]
- Zhao, T.; Nie, S.; Xiao, P.; Peng, S.; Chen, J.; Yu, J.; Luo, F.; Chen, Y. Exploring the influence of MIL-101(Cr) morphologies on the efficacy of cellulose separators for zinc ion battery performance. J. Membr. Sci. 2025, 720, 123788. [Google Scholar] [CrossRef]
- Huang, Q.; Zhao, C.; Li, X. Enhanced electrolyte retention capability of separator for lithium-ion battery constructed by decorating ZIF-67 on bacterial cellulose nanofiber. Cellulose 2021, 28, 3097–3112. [Google Scholar] [CrossRef]
- Tang, G.; Song, X.; Qiu, X.; Wang, J.; Sun, X.; Chen, F.; Cao, Z.; Gao, S. A ZIF-8 modified aramid nanofiber separator against the shuttle effect for high performance lithium-sulfur batteries. J. Alloys Compd. 2024, 971, 172698. [Google Scholar] [CrossRef]
- Han, G.; Hu, Q.; Xia, Y.; Gao, K.; Wang, Y.; Yao, J. Stabilizing dendrite-free lithium metal batteries via an anionphilic ZIF-67 modified separator. J. Alloys Compd. 2025, 1036, 181713. [Google Scholar] [CrossRef]
- Razaq, R.; Din, M.M.U.; Småbråten, D.R.; Eyupoglu, V.; Janakiram, S.; Sunde, T.O.; Allahgoli, N.; Rettenwander, D.; Deng, L. Synergistic effect of bimetallic MOF modified separator for long cycle life lithium-sulfur batteries. Adv. Energy Mater. 2024, 14, 2302897. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Wu, T.; Luo, X. MOF particles (UiO-66 and UiO-66(Ce))/cellulose nanocomposite separators with regulating ion transport controllably for lithium battery. J. Electroanal. Chem. 2023, 946, 117708. [Google Scholar] [CrossRef]
- Fan, Y.; Niu, Z.; Zhang, F.; Zhang, R.; Zhao, Y.; Lu, G. Suppressing the Shuttle Effect in Lithium–Sulfur Batteries by a UiO-66-Modified Polypropylene Separator. ACS Omega 2019, 4, 10328–10335. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Chen, Z.; Li, Y.; Li, C.; Duan, C.; Lim, K.H.; Kawi, S. Construction of greenly biodegradable bacterial cellulose/UiO-66-NH2 composite separators for efficient enhancing performance of lithium-ion battery. Int. J. Biol. Macromol. 2024, 269, 131988. [Google Scholar] [CrossRef]
- Zhao, T.; Xiao, P.; Nie, S.; Yu, J.; Peng, S.; Chen, J.; Luo, F.; Chen, Y. Innovative bacterial cellulose and UiO-66 composites for superior zinc ion battery separator performance. Green Chem. 2025, 27, 9541–9558. [Google Scholar] [CrossRef]
- Sun, X.; Yu, X.; Zhou, Y.; Hu, Z.; Mao, W.; Ai, G.; Li, D. Biomimic membrane-like separator toward ultra-stable high-rate lithium metal anodes. J. Energy Storage 2025, 111, 115394. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, W.; Zhu, H.; Li, B.-G.; Lu, Y.; Zhu, S. Fabrication of metal-organic framework-based nanofibrous separator via one-pot electrospinning strategy. Nano Res. 2021, 14, 1465–1470. [Google Scholar] [CrossRef]
- Sun, K.; Wu, H.; Huang, H.; Huang, X.; Xu, Y.; Xu, F. In-situ growth strategy to design metal-organic frameworks separators for efficient Zn-ion hybrid supercapacitors. Chem. Eng. J. 2025, 511, 162004. [Google Scholar] [CrossRef]
- Deng, N.; Wang, L.; Feng, Y.; Liu, M.; Li, Q.; Wang, G.; Zhang, L.; Kang, W.; Cheng, B.; Liu, Y. Co-based and Cu-based MOFs modified separators to strengthen the kinetics of redox reaction and inhibit lithium-dendrite for long-life lithium-sulfur batteries. Chem. Eng. J. 2020, 388, 124241. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Y.; Fu, S.; Li, H.; Huang, J. Regulating the non-effective carriers transport for high-performance lithium metal batteries. J. Energy Chem. 2024, 92, 132–141. [Google Scholar] [CrossRef]
- Jia, S.; Li, Y.; Chen, Z.; Li, C.; Duan, C.; Shen, B. Synthesis of PMIA/MIL-101(Cr) composite separators with high Li+ transmission for boosting safety and electrochemical performance of lithium-ion batteries. J. Colloid Interface Sci. 2023, 647, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Tan, R.; Li, J.; Huang, J.; Song, W. Coatings on Lithium battery separators: A Strategy to Inhibit Lithium Dendrites Growth. Molecules 2023, 28, 7788. [Google Scholar] [CrossRef]
- Wang, C.; Hao, Z.; Hu, Y.; Wu, Y.; Liu, J.; Jin, Y.; Wang, H.; Zhang, Q. Enabling dendrite-free and high-rate lithium anode with a self-standing anionic-MOF separator. J. Mater. Chem. A 2023, 11, 8131–8140. [Google Scholar] [CrossRef]
- Cheng, N.-C.; Wu, Y.C.; Chu, Y.-C.; Hsu, H.-Y.; Chen, W.-C.; Wang, P.-H.; Chang, T.-L.; Chang, J.-K.; Wang, C.-Y. A modulated MOF as a modification layer on copper foil for lithium dendrite suppression. J. Mater. Chem. A 2024, 12, 8474–8486. [Google Scholar] [CrossRef]
- Deng, L.; Cai, C.; Huang, Y.; Fu, Y. In-situ MOFs coating on 3D-channeled separator with superior electrolyte uptake capacity for ultrahigh cycle stability and dendrite-inhibited lithium-ion batteries. Microporous Mesoporous Mater. 2022, 329, 111544. [Google Scholar] [CrossRef]
- Ma, H.; Yu, J.; Chen, M.; Han, X.; Chen, J.; Liu, B.; Shi, S. Amino-enabled desolvation sieving effect realizes dendrite-inhibiting thin separator for durable aqueous Zinc-ion batteries. Adv. Funct. Mater. 2023, 33, 2307384. [Google Scholar] [CrossRef]
- Cai, K.; Liu, Y.; Lang, X.; Li, L.; Zhang, Q.; Xu, T.; Chen, D. Design of cobalt-based metal-organic frameworks like solid electrolyte interphase (SEI) film for chemically stable graphite anode of lithium-ion batteries. Ionics 2020, 26, 61–67. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Hong, Y.; Xu, Y.; Chen, Z.; Zhao, W.; Hu, Z.; Wang, J.; Wu, H.B. Solvent sieving separators implement dual electrolyte for high-voltage lithium-metal batteries. Nano Res. 2023, 16, 4901–4907. [Google Scholar] [CrossRef]
- Huo, H.; Li, X.; Chen, Y.; Liang, J.; Deng, S.; Gao, X.; Doyle-Davis, K.; Li, R.; Guo, X.; Shen, Y.; et al. Bifunctional composite separator with a solid-state-battery strategy for dendrite-free lithium metal batteries. Energy Storage Mater. 2020, 29, 361–366. [Google Scholar] [CrossRef]
- Ren, W.; Liang, J.; Huang, M.; Qian, W.; Zhao, M.; Qiao, F.; Cui, L.; Zhang, Z.; Wang, J.; Zhou, C.; et al. Thermally-stable and flame-retardant metal-organic framework-based separator for high-performance lithium-ion batteries. Chem. Eng. J. 2024, 498, 155811. [Google Scholar] [CrossRef]
- Huang, D.; Liang, C.; Chen, L.; Tang, M.; Zheng, Z.; Wang, Z. MOF composite fibrous separators for high-rate lithium-ion batteries. J. Mater. Sci. 2021, 56, 5868–5877. [Google Scholar] [CrossRef]
- Song, X.; Huang, W.; Wang, H.; Lu, Y.M.; Xing, J. Polyimide/Metal–Organic Framework-5 separator with excellent electrolyte wettability and thermal stability for high performance Lithium-ion battery. ACS Appl. Polym. Mater. 2025, 7, 6312–6325. [Google Scholar] [CrossRef]
- Zhou, L.; Pan, H.; Yin, G.; Xiang, Y.; Tan, P.; Li, X.; Jiang, Y.; Xu, M.; Zhang, X. Tailoring the function of battery separators via the design of MOF coatings. Adv. Funct. Mater. 2024, 34, 2314246. [Google Scholar] [CrossRef]
- Leng, X.; Zeng, J.; Yang, M.; Li, C.; Vattikuti, S.V.P.; Chen, J.; Li, S.; Shim, J.; Guo, T.; Ko, T.J. Bimetallic Ni–Co MOF@PAN modified electrospun separator enhances high-performance lithium-sulfur batteries. J. Energy Chem. 2023, 82, 484–496. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, M.; Xu, T.; Liu, K.; Wang, Y.; Zhang, H.; Wang, X.; Yuan, Z.; Si, C. Nanocellulose/metal-organic frameworks composites for advanced energy storage of electrodes and separators. Chem. Eng. J. 2024, 500, 157318. [Google Scholar] [CrossRef]
- Li, Y.; Peng, X.; Li, X.; Duan, H.; Xie, S.; Dong, L.; Kang, F. Functional Ultrathin Separators Proactively Stabilizing Zinc Anodes for Zinc-Based Energy Storage. Adv. Mater. 2023, 35, 2300019. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Pei, F.; Yao, M.; Fu, Z.; Lin, L.; Wu, G.; Xu, G.; Kitagawa, H.; Fang, X. Ultrathin MOF nanosheet assembled highly oriented microporous membrane as an interlayer for lithium-sulfur batteries. Energy Storage Mater. 2019, 21, 14–21. [Google Scholar] [CrossRef]
- Lan, F.; Zhao, H.; Jiang, Y.; Jin, C.; Zhao, G.; Li, L. A thermomechanically stable nanofiber separator with multiscale MOF networks towards high-efficiency ion transport. J. Mater. Chem. A 2025, 13, 7357–7370. [Google Scholar] [CrossRef]
- Gao, K.; Luo, J.; Li, X.; Fan, K.; Luo, L.; Liu, X. Electrospun heterocycle aramid nanofiber separator with MOF-supported porous structure enabled excellent cycling stability for lithium metal batteries with high LiFePO4 loading. J. Alloys Compd. 2023, 966, 171549. [Google Scholar] [CrossRef]
- Kong, L.; Cheng, M.; Huang, H.; Pang, J.; Liu, S.; Xu, Y.; Bu, X.-H. Metal-organic frameworks for advanced aqueous ion batteries and supercapacitors. EnergyChem 2022, 4, 100090. [Google Scholar] [CrossRef]
- Peng, Y.; Xu, J.; Ma, J.; Bai, Y.; Cao, S.; Zhang, S.; Pang, H. Metal-organic framework (MOF) composites as promising materials for energy storage applications. Adv. Colloid Interface Sci. 2022, 307, 102732. [Google Scholar] [CrossRef]
- Yuan, N.; Sun, W.; Yang, J.; Gong, X.; Liu, R. Multifunctional MOF-based separator materials for advanced Lithium–Sulfur batteries. Adv. Mater. Interfaces 2021, 8, 2001941. [Google Scholar] [CrossRef]
- Wu, J.; Ma, Y.; Zhang, H.; Xie, H.; Hu, J.; Shi, C.; Chen, B.; He, C.; Zhao, N. Regulating metal centers of MOF-74 promotes PEO-based electrolytes for all-solid-state Lithium-metal batteries. ACS Appl. Mater. Interfaces 2024, 16, 16351–16362. [Google Scholar] [CrossRef]
- Ngo, N.M.; Nguyen, M.H.; Song, S.-W.; Park, S. Bimetallic metal organic Framework-modified glass fiber as composite separator for lithium sulfur batteries. Mater. Lett. 2025, 382, 137835. [Google Scholar] [CrossRef]
- Xu, S.; Liu, C.; Yang, Y.; Yao, Y.; Yang, H.; Rui, X.; Yu, Y. ZIF-8 functionalized separator regulating Na-ion flux and enabling high-performance sodium-metal batteries. Small Methods 2025, 9, 2402084. [Google Scholar] [CrossRef] [PubMed]
- Samy, A.; Palani, R.; Wu, Y.-S.; Wu, S.-H.; Chang, J.-K.; Numan, A.; Jose, R.; Yang, C.-C. Suppressing the redox shuttling effect based on the anion trapping composite separator enhances the performance of Li-O2 batteries. ACS Appl. Energy Mater. 2025, 8, 6407–6422. [Google Scholar] [CrossRef]
- Liu, T.; Hu, X.; Zhang, Y.; He, T.; Zhou, J.; Qiao, J. Ion transport regulated Lithium metal batteries achieved by electrospun ZIF/PAN composite separator with suitable electrolyte wettability. Batteries 2023, 9, 166. [Google Scholar] [CrossRef]
- Lin, S.; Dong, J.; Chen, R.; Zhang, G.; Huang, T.; Li, J.; Zhou, H.; Chung, L.-H.; Hu, X.; He, J. Lithium sulfonate-rich MOF modified separator enables high performance lithium–sulfur batteries. J. Alloys Compd. 2023, 965, 171389. [Google Scholar] [CrossRef]
- Yu, M.; Li, J.; Li, Z.; Mu, J.; Si, W.; Li, T.; Zheng, W.; Dai, Y.; Li, X.; He, G. Regulating Zn2+ flux and transport behavior with CF3-functionalized UiO-66 modified separator for high-performance aqueous zinc-ion batteries. J. Colloid Interface Sci. 2025, 701, 138636. [Google Scholar] [CrossRef]
- Pang, S.; Liu, Y.; Zhang, Z.; Li, Y.; Li, C.; Shi, Z.; Feng, S. Sulfonic acid functionalized Al-based MIL-101-NH2 modified separator for lithium-sulfur batteries. Microporous Mesoporous Mater. 2024, 365, 112892. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ding, X.; Liu, T.; Nai, J.; Wang, Y.; Liu, Y.; Liu, C.; Tao, X. A review of concepts and contributions in lithium metal anode development. Mater. Today 2022, 53, 173–196. [Google Scholar] [CrossRef]
- Li, S.; Sun, L.; Gao, H.; Long, Y.; Guo, S.; Jia, Q.; Jiang, Y.; Ye, Z. Direct epitaxial growth of MOF arrays on Cu foils to regulate Li deposition toward lean-lithium metal batteries. Chem. Eng. J. 2025, 512, 162370. [Google Scholar] [CrossRef]
- Mei, S.; Liu, T.; Chen, L.; Wang, Y. Preparation and performance of a PU/PAN Lithium-ion battery separator based on a centrifugal spinning method. Appl. Sci. 2023, 13, 6682. [Google Scholar] [CrossRef]
- Du, M.; He, Z.; Zhang, Y.; Cai, Y.-P.; Zheng, Q. Sustainable release of LiNO3 from a fluorine-decorated Metal–Organic Framework separator to enable high-performance Li-metal batteries in carbonate electrolytes. Adv. Energy Mater. 2025, 15, 2403674. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.; Wang, Z.; Jin, Z.; Deng, T.; Yang, Z.; Han, D.; Su, Y.; Wang, Y.; Cao, Z.; et al. Crystal facet-engineered anion regulation enables fast-charging stability in Lithium metal batteries. Angew. Chem. Int. Ed. 2025, 64, e202512742. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, X.-Q.; Huang, J.-Q.; Liu, Q.; Zhang, Q. Lithium-anode protection in Lithium–Sulfur batteries. Trends Chem. 2019, 1, 693–704. [Google Scholar] [CrossRef]
- Yin, Y.-X.; Xin, S.; Guo, Y.-G.; Wan, L.-J. Lithium–Sulfur batteries: Electrochemistry, materials, and prospects. Angew. Chem. Int. Ed. 2013, 52, 13186–13200. [Google Scholar] [CrossRef]
- Su, Y.; Wang, W.; Wang, A.; Huang, Y.; Guan, Y. Cerium-Based MOF as a separator coating for high-performance Lithium-Sulfur batteries. J. Electrochem. Soc. 2022, 169, 030528. [Google Scholar] [CrossRef]
- Deng, N.; Kang, W.; Liu, Y.; Ju, J.; Wu, D.; Li, L.; Hassan, B.S.; Cheng, B. A review on separators for lithiumsulfur battery: Progress and prospects. J. Power Sources 2016, 331, 132–155. [Google Scholar] [CrossRef]
- Vignesh, K.; Mathivanan, T.; Ganeshbabu, M.; Prasanna Naga Puneeth, N.; Ramkumar, B.; Lee, Y.S.; Kalai Selvan, R. Nature-inspired three-dimensional foam-like porous carbon surface modified separator for high-performance Li-S batteries. Carbon Trends 2025, 20, 100545. [Google Scholar] [CrossRef]
- Quan, K.; Zhang, J.; Lin, W.; Tong, Q.; Yan, R.; Ye, D.; Weng, J.; Zhu, M. Multiple dimensional engineering of MOF-related materials in separators for Lithium-Sulfur batteries: A Review. J. Electrochem. Soc. 2022, 169, 120519. [Google Scholar] [CrossRef]
- Xia, S.; Xu, X.; Wu, W.; Chen, Y.; Liu, L.; Wang, G.; Fu, L.; Zhang, Q.; Wang, T.; He, J.; et al. Advancements in functionalized high-performance separators for lithium-sulfur batteries. Mater. Sci. Eng. R Rep. 2025, 163, 100924. [Google Scholar] [CrossRef]
- Xiao, H.; Qin, J.; Wang, H.; Lai, X.; Shi, P.; Chen, C.; Sun, D. MOF-Derived CeO2 Nanorod as a Separator Coating Enabling Enhanced Performance for Lithium–Sulfur Batteries. Molecules 2024, 29, 1852. [Google Scholar] [CrossRef]
- Liang, F.; Zhu, Y.; Wang, N.; Zhu, M.; He, H.; Zhu, Y.; Shen, P.; Zhu, J. Recent advances in copper-based materials for robust lithium polysulfides adsorption and catalytic conversion. Chin. Chem. Lett. 2024, 35, 109461. [Google Scholar] [CrossRef]
- Wang, W.; Xi, K.; Li, B.; Li, H.; Liu, S.; Wang, J.; Zhao, H.; Li, H.; Abdelkader, A.M.; Gao, X.; et al. A sustainable multipurpose separator directed against the shuttle effect of polysulfides for high-performance Lithium–Sulfur batteries. Adv. Energy Mater. 2022, 12, 2200160. [Google Scholar] [CrossRef]
- Sun, X.; Zheng, J.; Yang, T.; Liu, Y.; Wu, X.; Xiao, J.; Cai, X.; Song, Y.; He, C. Regulating capture and conversion of polysulfides by BiOCl/PAN nanofibers separator to realize longevous lithium-sulfur batteries. J. Energy Storage 2025, 129, 117364. [Google Scholar] [CrossRef]
- Kang, X.; He, T.; Dang, H.; Li, X.; Wang, Y.; Zhu, F.; Ran, F. Designing amino functionalized Titanium-Organic Framework on separators toward sieving and redistribution of polysulfides in Lithium-Sulfur batteries. Nano-Micro Lett. 2025, 17, 277. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Chen, Y.; Lian, J.; Chen, X.; Xiang, S.; Chen, B.; Zhang, Z. Interface engineering of MOF nanosheets for accelerated redox kinetics in Lithium-Sulfur batteries. Angew. Chem. Int. Ed. 2025, 64, e202421726. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Pan, H.; Zhang, J.; Xiang, S.; Cheng, Z.; Zhang, Z. Pore-space-partitioned MOF separator promotes high-sulfur-loading Li–S batteries with intensified rate capability and cycling life. J. Mater. Chem. A 2021, 9, 26929–26938. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, R.; Chen, L.; Yang, Y.; Yu, H.; Qiu, X. Fabrication of MXene/MOF composite separators for high performance lithium-sulfur batteries. Chem. Eng. J. 2025, 512, 162305. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Q.; Lin, X.; Luo, C.; Shen, Y.; Huang, X. Ion-confinement effect for zinc anode of aqueous zinc ion batteries. J. Energy Storage 2023, 73, 109085. [Google Scholar] [CrossRef]
- Wu, B.; Wu, Y.; Lu, Z.; Zhang, J.; Han, N.; Wang, Y.; Li, X.-m.; Lin, M.; Zeng, L. A cation selective separator induced cathode protective layer and regulated zinc deposition for zinc ion batteries. J. Mater. Chem. A 2021, 9, 4734–4743. [Google Scholar] [CrossRef]
- Xiong, B.; Chen, R.; Zeng, F.; Kang, J.; Men, Y. Thermal shrinkage and microscopic shutdown mechanism of polypropylene separator for lithium-ion battery: In-situ ultra-small angle X-ray scattering study. J. Membr. Sci. 2018, 545, 213–220. [Google Scholar] [CrossRef]
- Liu, T.; Hong, J.; Wang, J.; Xu, Y.; Wang, Y. Uniform distribution of zinc ions achieved by functional supramolecules for stable zinc metal anode with long cycling lifespan. Energy Storage Mater. 2022, 45, 1074–1083. [Google Scholar] [CrossRef]
- Huang, J.-Q.; Chong, W.G.; Zhang, B. Tackling the challenges of aqueous Zn-ion batteries via functional separator design. J. Power Sources 2024, 594, 234036. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Hussain, A.; Hardianto, Y.P.; Faheem, M.; Mirghni, A.A.; Helal, A.; Aziz, M.A. Synergistic Role of UiO-66 and UiO-67 MOF-Modified Glass Fiber Separators for Dendrite-Free and Long-Life Zinc-Ion Batteries. Asian J. Org. Chem. 2025, 14, e202500204. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Z.; Meng, Z.; Zhang, J.; Lv, W.; Guo, C.; Lv, Z.; Huang, S.; Yang, Y.; Liu, Z.; et al. Epitaxy Orientation and Kinetics Diagnosis for Zinc Electrodeposition. ACS Nano 2025, 19, 736–747. [Google Scholar] [CrossRef]
- Zhang, R.; Feng, Y.; Ni, Y.; Zhong, B.; Peng, M.; Sun, T.; Chen, S.; Wang, H.; Tao, Z.; Zhang, K. Bifunctional interphase with target-distributed desolvation sites and directionally depositional ion flux for sustainable zinc anode. Angew. Chem. Int. Ed. 2023, 62, e202304503. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, R.; Jiang, Z.; Li, J.; Liang, H.; Chung, L.-H.; Hu, X.; He, J. Zn-thiocatecholate functionalized MOF modified separator stabilizing zinc anodes for long-life aqueous zinc-ion batteries. Chem. Commun. 2025, 61, 10808–10811. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Z.; Liu, Y.; Li, Y.; Zhang, H.; He, X. Safety perceptions of solid-state lithium metal batteries. eTransportation 2023, 16, 100239. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; Dong, R.; Zhang, T.; Yuan, J.; Liu, Y.; Liu, Y.; Sun, Y.; Zhong, B.; Chen, Y.; et al. A high strength asymmetric polymer–inorganic composite solid electrolyte for solid-state Li-ion batteries. Electrochim. Acta 2022, 404, 139701. [Google Scholar] [CrossRef]
- Fu, X.; Wei, Q.; Zheng, W.; Bai, H.; Liu, X.; Hao, S.; Lu, W. Plasticized composite electrolytes with mesoporous silica nanoparticle-reinforced PVDF–HFP for solid-state lithium–metal batteries. New J. Chem. 2025, 49, 14710–14717. [Google Scholar] [CrossRef]
- Li, J.; Ji, Y.; Song, H.; Chen, S.; Ding, S.; Zhang, B.; Yang, L.; Song, Y.; Pan, F. Insights into the interfacial degradation of high-voltage all-solid-state Lithium batteries. Nano-Micro Lett. 2022, 14, 191. [Google Scholar] [CrossRef]
- Wang, S.; Fang, R.; Li, Y.; Liu, Y.; Xin, C.; Richter, F.H.; Nan, C.-W. Interfacial challenges for all-solid-state batteries based on sulfide solid electrolytes. J. Mater. 2021, 7, 209–218. [Google Scholar] [CrossRef]
- Shi, X.; Pang, Y.; Wang, B.; Sun, H.; Wang, X.; Li, Y.; Yang, J.; Li, H.W.; Zheng, S. In situ forming LiF nanodecorated electrolyte/electrode interfaces for stable all-solid-state batteries. Mater. Today Nano 2020, 10, 100079. [Google Scholar] [CrossRef]
- Choi, W.; Ku, J.H.; Kim, Y.; Gwon, H.; Yoon, G.; Yu, D.; Kim, J.-S. Formulating interfacial impedances for designing high-energy and high-power all-solid-state battery cathodes. ACS Appl. Mater. Interfaces 2024, 16, 26066–26078. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Bo, S.-H.; Kim, J.C.; Miara, L.J.; Ceder, G. Understanding interface stability in solid-state batteries. Nat. Rev. Mater. 2020, 5, 105–126. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, G.; Xu, H.; Jiang, K.; Zhao, Y. A polymer-modified separator with lithiophilic nanocapsules for high-performance lithium metal batteries. J. Energy Storage 2025, 131, 117512. [Google Scholar] [CrossRef]
- Zhang, W.; Tu, Z.; Qian, J.; Choudhury, S.; Archer, L.A.; Lu, Y. Design Principles of Functional Polymer Separators for High-Energy, Metal-Based Batteries. Small 2018, 14, 1703001. [Google Scholar] [CrossRef]
- Yang, L.-Y.; Cao, J.-H.; Cai, B.-R.; Liang, T.; Wu, D.-Y. Electrospun MOF/PAN composite separator with superior electrochemical performances for high energy density lithium batteries. Electrochim. Acta 2021, 382, 138346. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, L.; Shen, J.; Liu, F.; Chen, G.; Tao, R.; Ma, S.; Peng, Y.; Lu, Y. Anion-Sorbent Composite Separators for High-Rate Lithium-Ion Batteries. Adv. Mater. 2019, 31, 1808338. [Google Scholar] [CrossRef]
- Ju, Y.; Liu, H.; Chen, Y.; Sheng, J.; Zhai, Y.; Dong, B.; Cheng, R.; Zhou, Y.; Li, L. An ultrathin Zn-BDC MOF nanosheets functionalized polyacrylonitrile composite separator with anion immobilization and Li+ redistribution for dendrite-free Li metal battery. Compos. Commun. 2023, 37, 101449. [Google Scholar] [CrossRef]
- Liu, J.-j.; Huang, Y.-h.; Zhang, X.-j.; Ding, Y.-X.; Liu, H.; Gui, X.-F. MOF-silsesquioxane synergistic modified hybrid porous membrane for high-performance and high-safety lithium battery. Mater. Lett. 2024, 361, 136162. [Google Scholar] [CrossRef]
- Boukayouht, K.; Bazzi, L.; Daouli, A.; Maurin, G.; El Hankari, S. Ultrarapid and sustainable synthesis of trimetallic-based MOF (CrNiFe-MOF) from stainless steel and disodium terephthalate-derived PET wastes. ACS Appl. Mater. Interfaces 2024, 16, 2497–2508. [Google Scholar] [CrossRef]
- Suresh, P.; Shakina, J.; Pandian, M.S.; Tharmaraj, P.; John Jeya Kamaraj, J.J. Cost-effective bimetallic MOF electrocatalyst with dual-linker strategy for enhanced oxygen and hydrogen evolution reactions. Mater. Sci. Eng. B 2025, 321, 118510. [Google Scholar] [CrossRef]
- An, H.-T.; Zhang, X.; Dong, C.; Lu, M.-Y.; Li, R.; Xie, Y.; Xie, L.-H.; Li, J.-R. Seed-aided green synthesis of metal-organic frameworks in water. Green Chem. Eng. 2023, 4, 64–72. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, Y.; Hong, A.N.; Bu, X.; Feng, P. Solvent-free synthesis of multi-module pore-space-partitioned Metal-Organic Frameworks for gas separation. Angew. Chem. Int. Ed. 2023, 62, e202300721. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, Q.; Liu, Y.; Nian, M.; Xie, M.; Xie, S.; Yang, Q.; Wang, S.; Wei, H.; Duan, J.; et al. Metal-Organic Framework nanoparticles with universal dispersibility through crown ether surface coordination for phase-transfer catalysis and separation membranes. Angew. Chem. Int. Ed. 2023, 62, e202303280. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, C.; Ying, Y.; Zhang, W. Improve MOF-801 dispersibility in PVA membranes by a pre-crosslinking strategy for enhanced pervaporation performance. J. Membr. Sci. 2023, 687, 122043. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).